Abstracts
We studied 12 Hb C carriers: 4 homozygotic Hb CC and 8 heterozygotic. We observed the presence of free crystals in the peripheral blood of the homozygotes but in none of the heterozygotes. However, after incubation with 3% NaCl we were able to detect crystals in the heterozygotes (Hb AC and Hb SC), and in the homozygotes (Hb CC). In patient 04 (P04) less crystals formation occurred due to inhibition of the process by the presence of elevated levels of Hb F (12.2%). All the homozygotic patients had a splenomegaly of 3 to 6 fingerbreadths.We believe that the spleen wears off with time, thus allowing the passage of crystals to the peripheral blood. This finding might be associated with splenic insufficiency without a reduction of its dimensions. Finally, the finding of crystals in the peripheral blood permitted the diagnosis of Hb C obviating the need for electrophoresis.
Hemoglobin C; Crystal; Non-splenectomized
Estudamos 12 pacientes portadores de Hb C, sendo 4 homozigotos Hb CC e 8 heterozigotos. Observamos a presença de cristais livres no sangue periférico dos homozigotos e nenhum nos heterozigotos. Quando incubamos com NaCl 3%, detectamos cristais quer nos heterozigotos, Hb AC e Hb SC, quanto nos homozigotos, Hb CC. No paciente 4 (P04) houve menor formação de cristais devido a inibição pela Hb Fetal que se encontrava aumentada (12,2%). Todos os pacientes homozigotos mostraram esplenomegalia de 3 a 6 dedos. Emitimos a hipótese de que ocorra no baço um desgaste com o progredir da idade, permitindo a passagem dos cristais para o sangue periférico. Este fato poderia estar associado à uma insuficiência esplênica sem redução do baço. A presença dos cristais no sangue periférico permitiu o diagnóstico de Hb C mesmo sem a realização da eletroforese de Hb.
J.T. de ARAÚJO(1)(1) Médico Hematologista, Disciplina de Hematologia, HCFMUSP, Chefe responsável do Laboratório de Diagnóstico e Investigação das Hemoglobinopatias e Talassemias do Instituto de Medicina Tropical de São Paulo HCFMUSP. Apoio: Fundação Faculdade de Medicina da USP, São Paulo, SP, Brasil.(2) Farmacêutica-Bioquímica, aprimoranda do Laboratório de Diagnóstico e Investigação de Hemoglobinopatias e Talassemias do IMT-HCFMUSP, São Paulo, SP, Brasil.(3) Biomédica do Laboratório de Diagnóstico e Investigação de Hemoglobinopatias e Talassemias do IMT-HCFMUSP, São Paulo, SP, Brasil., A.C. BATISSOCO(2)(1) Médico Hematologista, Disciplina de Hematologia, HCFMUSP, Chefe responsável do Laboratório de Diagnóstico e Investigação das Hemoglobinopatias e Talassemias do Instituto de Medicina Tropical de São Paulo HCFMUSP. Apoio: Fundação Faculdade de Medicina da USP, São Paulo, SP, Brasil.(2) Farmacêutica-Bioquímica, aprimoranda do Laboratório de Diagnóstico e Investigação de Hemoglobinopatias e Talassemias do IMT-HCFMUSP, São Paulo, SP, Brasil.(3) Biomédica do Laboratório de Diagnóstico e Investigação de Hemoglobinopatias e Talassemias do IMT-HCFMUSP, São Paulo, SP, Brasil. & L. BODEMEIER(3)(1) Médico Hematologista, Disciplina de Hematologia, HCFMUSP, Chefe responsável do Laboratório de Diagnóstico e Investigação das Hemoglobinopatias e Talassemias do Instituto de Medicina Tropical de São Paulo HCFMUSP. Apoio: Fundação Faculdade de Medicina da USP, São Paulo, SP, Brasil.(2) Farmacêutica-Bioquímica, aprimoranda do Laboratório de Diagnóstico e Investigação de Hemoglobinopatias e Talassemias do IMT-HCFMUSP, São Paulo, SP, Brasil.(3) Biomédica do Laboratório de Diagnóstico e Investigação de Hemoglobinopatias e Talassemias do IMT-HCFMUSP, São Paulo, SP, Brasil.
SUMMARY
We studied 12 Hb C carriers: 4 homozygotic Hb CC and 8 heterozygotic. We observed the presence of free crystals in the peripheral blood of the homozygotes but in none of the heterozygotes. However, after incubation with 3% NaCl we were able to detect crystals in the heterozygotes (Hb AC and Hb SC), and in the homozygotes (Hb CC). In patient 04 (P04) less crystals formation occurred due to inhibition of the process by the presence of elevated levels of Hb F (12.2%). All the homozygotic patients had a splenomegaly of 3 to 6 fingerbreadths.We believe that the spleen wears off with time, thus allowing the passage of crystals to the peripheral blood. This finding might be associated with splenic insufficiency without a reduction of its dimensions. Finally, the finding of crystals in the peripheral blood permitted the diagnosis of Hb C obviating the need for electrophoresis.
KEYWORDS: Hemoglobin C; Crystal; Non-splenectomized
INTRODUCTION
In 1950 ITANO & NEEL10 described a new hemoglobin and named it hemoglobin C (Hb C). In 1953 SPAET et al.16 described a second case and soon thereafter RANNEY et al.13 report the third one with the curious observation of many target red blood cells in the patient's peripheral blood smear.
DIGGS et al.6 in 1954 described intra-erythrocytic crystals in the peripheral blood of Hb C bearers, thus emphasizing that target cells were in reality crystals of Hb C and the presence of free rods identified as crystals also caught his attention.
The main defect of Hb C is the substitution of the glutamic acid residue at position 6 of the N-terminal of the beta chain by a lysine, whilst in sickle cell disease the presence of Hb S is due to the substitution of this same residue by a valine.
This phenomenon explains the morphological variations of the erythrocytes: forming crystals in Hb C, and sickle cells in Hb S, thus yielding it possible to identify these two different diseases by simply looking at peripheral blood smears.
In our opinion, the red cells described by RANNEY et al.13 contained Hb C crystals within them and were morphologically different from the ones seen in Hb E, thalassemia, and certain liver diseases which are real target cells. Based on our experience it is possible to identify wether an Hb is of the C or E type simply by analyzing peripheral blood smears using a common optical microscope. In Hb E, thalassemia, and certain liver diseases typical target cells can be seen which differ from the ones observed in Hb C were both intra and extra-erythrocytic crystals are found even with the spleen present. FABRY et al.7, mention that only in smears of splenectomized Hb C patients can the crystals be seen, as these crystals would be all removed by the spleen. Therefore, when splenectomy is performed, the resistance of the splenic network certainly disappears and consequently liberates these crystals. It is also hypothesized that as the splenic network wears off with time, splenic function in Hb C patients diminishes which leads to increasing amounts of the crystals in the peripheral blood with age. In sickle cell disease patients we know that fibrosis followed by splenic involution does occur, many times reducing the spleen to a single nodule. However, in patients homozygotic for Hb C, although a reduction in splenic size does not occur, the progressive decrease in function is a fact that explains the finding of crystals in peripheral blood.
ADACHI & ASAKURA, 19791 studied Hb C crystals using a concentrated fosfate buffer and were able to identify numerous intra and extraerythrocytic crystals of this hemoglobin in the blood of Hb C carriers. FABRY et al., 19817 performed the same study using a 3% NaCl solution, and the resulting crystals were named by ROZENBEG14 as sickling for Hb C, in contrast to red cells containing Hb S.
Although both Hb C and Hb S arise from the African continent, SUTCHARITCHAN et al.15 performing molecular studies, were able to identify the Hb C gene in Thailandese natives, thus demonstrating a non-African origin for Hb C.
The incidence of Hb C is 17 to 28 % in Western Africa, in the vicinities of Northern Ghana. Up to the present date, no reasonable explanation accounts for this high occurrence17.
In Brazil, ARAUJO et al.3, reported an incidence of 0.6 to 1.0% of Hb C by means of crystals demonstration. He also described rare cases of Hb C in Italian and Portuguese descendants.
METHODS AND MATERIALS
During our analysis we identified 4 non-splenectomized carriers of Hb CC, 4 of Hb AC, and also 4 of Hb SC. We studied Hb C crystals in all the carriers, both after incubation with a phosphate buffer and a 3% NaCl solution.
We also analyzed 50 normal blood samples as a negative control for the crystals.
The blood samples were collected with anticoagulant (EDTA), washed 3 times in saline, hemolysed to a 10 g% hemoglobin concentration, and then analyzed by pH 8.6 cellulose acetate electrophoresis, and pH 6.2 agar gel. The fractions were measured after elution15, and Hb F was quantitated by the Betke method5.
In order to demonstrate the crystals, we used a hypertonic 3% NaCl solution, and also a phosphate buffer with a pH of 7.4 and 1.8M9.
The technique consisted of placing one drop of total blood and 2 drops of the 3% NaCl solution7 a test tube and then incubating at 37 °C for 4 hours. An identical procedure was performed using the phosphate buffer with a pH of 7.4 and l.8M9.
The blood smears were stained with the Leishman method, and the crystals could be easily identified with the common optical microscope (Figure 1).
RESULTS
In the 50 samples of normal donors (negative controls) no intra or extra-erythrocytic crystals formation was observed.
The highest amount of crystals formation was seen in the cases homozygotic for Hb C, whilst the lower amount occurred in the heterozygotes for Hb AC. An amount of crystals intermediate between Hb CC and Hb AS was found in the Hb SC cases (Fig. 1 and Fig. 2).
- "In vitro" demonstration of tetrahedrical crystals of Hb CC in the peripheral blood of non-splenectomized patients. Arrows indicate one crystal out of erythrocyte and many crystals inside erythrocytes.
Also, a lower amount of crystals was present in those homozygotes for Hb C that had higher levels of Fetal Hb (Table 1).
TABLE 1
Concentration of hemoglobin in both states homozygotic and heterozygotic, and the presence of crystals
Visualization of the crystals was better with the phosphate buffer at pH 7.4 and 1.8M than with the 3% NaCl solution.
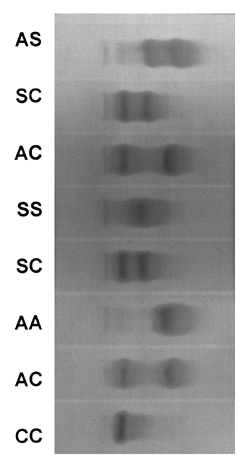
The concentration of Hb C measured by electrophoresis was of 30 to 40% for AC heterozygotes and of 40 to 50% for the SC. As for the homozygotes the concentration of Hb C was of 98% with the remaining 2% consisting of Fetal Hb, excepting those cases where there is an increase in Hb F which reaches a concentration of 12.2 % (Fig. 3).
Hemoglobin electrophoresis by pH 8.6 agar gel demonstrating the differences in mobility of the hemoglobin fraction.
DISCUSSION
The spleen's clearing function, also known as "culling", refers to the organ's capacity of removing aged, dysmorphic, deformed, agglutinated, antibody or complement coated erythrocytes, and erythrocytic corpuscles (or bodies)11.
This is the reason why a normal spleen would remove any free Hb C crystals occasionally present in the circulation. However, even a normal splenic function might permit a few crystals containing erythrocytes to reach the circulation, and this accounts for the finding of these crystals in the peripheral blood of homozygotic Hb C carriers.
When an individual undergoes splenectomy, the peripheral blood may show red cells with crystals similar to sickling forms, free crystals, and also Heinz and Howell-Jolly bodies11.
DIGGS et al.6 in 1954, and posteriorly FABRY et al., 19817 demonstrated the presence of Hb C crystals in the peripheral blood of splenectomized and non-splenectomized patients. This is probably due to the fact the splenic network is many times unable to retain all the red cells with crystals, thus permitting their identification in the peripheral blood.
ROZENBERG14, using photographs, demonstrated the presence of crystals in the peripheral blood of individuals with Hb C, and also confirmed the presence of these in the non-splenectomized carriers of Hb C by means of the 3% NaCl solution technique.
At our laboratory, we routinely examine the peripheral blood smears without prior knowledge of the diagnosis. In all cases, the crystals allowed us to identify the smears as proceeding from Hb C patients, which was further confirmed by electrophoresis. Due to this fact, we concluded that the spleen is unable to retain all the crystals containing red cells or even the free crystals (Figure 2). We believe that over time, as the spleen retains the crystals of Hb C the network becomes insufficient and thus allows the crystals to appear in the peripheral blood.
The spleen undergoes regression in sickle cell disease, in contrast to homozygotic Hb C disease where the spleen remains enlarged, and no regression occurs. This does not mean, however, that damage due to crystals deposition does not occur. In fact, the crystals can be seen in simple X-Rays of the spleen.
Also, lower amounts of crystals are formed when comparing patients with Hb C that present with high levels of Fetal hemoglobin in relation to those with normal levels.
HIRSCH et al.8 demonstrated that Hb F inhibits the crystallization of Hb C when compared to Hb A. The same is true for A2 (a 2d 2) although to a lesser extent than Hb F (a 2g 2). These authors performed a study comparing the inhibitory potentials of the gama (g ) delta (d ) chains. These chains both differ from the beta (b ) chain at 12 substitution sites, whilst having 4 residues in common located at positions 9, 50, 22, and 87. The two former residues are probably not responsible for the phenomenon, as they involve substitutions exhibiting similar proprieties. Position 22, however, although a common point in both gama (g ) and delta (d ) chains, involves different substitutions. The gama (g ) chain posseses an Asp substituting a Glu, whilst at the delta (d ) chain the Glu is substituted by a Val. We are left with position 87 were Thr is substituted for Gli in both the gama (g ) and delta (d ) chains, and is therefore responsible for the crystallization of Hb C. In addition, this is the same residue that inhibits the polymerization of Hb S by Hb A2 andHb F8.
Hb Lepore-Washington originates from a mutation where a fusion between the beta and delta chains occurs, with substitution of only 6 residues. The resulting Hb is also able to inhibit Hb C crystals formation albeit in lesser degrees if compared to Hb A2 and Hb F. Consequently, this observation suggests that the Gli 87 residue is in fact responsible for the inhibition of Hb C crystals formation. Nevertheless, in agreement with HIRSCH et al.8, the varied intensities of inhibition among the different hemoglobins enable us to conclude that there may be two possible explanations: the first is that this variation is probably due to the differences in hemoglobin molecular conformations, and the second is that residue 87 is not the only culprit. In this case, the other residues located between positions 88 and 146 might merit consideration.
Therefore, Fetal Hb with the g Gli 87 residue partially inhibits Hb C crystallization, whilst Hb S speeds up the process. This fact definetely demonstrates the existence of intra-erythrocytic crystals in the oxygenated form of the red blood cells of Hb SC individuals. Hb S might speed up crystallization due to the fact the phosphate buffer renders it insoluble9.
The best visualization of the crystals occurred with the 1.8M phosphate buffer at a pH of 7.4 using the common optical microscope, due to the fact Hb C is less soluble than Hb A in these conditions.
RESUMO
Cristais de hemoglobina C demonstráveis "in vivo" e "in vitro" em pacientes não esplenectomizados
Estudamos 12 pacientes portadores de Hb C, sendo 4 homozigotos Hb CC e 8 heterozigotos.
Observamos a presença de cristais livres no sangue periférico dos homozigotos e nenhum nos heterozigotos. Quando incubamos com NaCl 3%, detectamos cristais quer nos heterozigotos, Hb AC e Hb SC, quanto nos homozigotos, Hb CC. No paciente 4 (P04) houve menor formação de cristais devido a inibição pela Hb Fetal que se encontrava aumentada (12,2%).
Todos os pacientes homozigotos mostraram esplenomegalia de 3 a 6 dedos. Emitimos a hipótese de que ocorra no baço um desgaste com o progredir da idade, permitindo a passagem dos cristais para o sangue periférico. Este fato poderia estar associado à uma insuficiência esplênica sem redução do baço.
A presença dos cristais no sangue periférico permitiu o diagnóstico de Hb C mesmo sem a realização da eletroforese de Hb.
Correspondence to: Prof. Dr. João Targino de Araújo, Instituto de Medicina Tropical de São Paulo, Av. Dr. Enéas de Carvalho Aguiar 470, 05403-000 São Paulo, SP, Brasil.
Received: 28 May 1999
Accepted: 10 August 1999
- 1. ADACHI, K. & ASAKURA, T. - The solubility of sickle cell and non sickle hemoglobin in concentrate phosphate buffer. J. biol. Chem., 254: 4079-4084, 1979.
- 2. ARAÚJO, J.T. de - Hemoglobinas anormais em Săo Paulo. Métodos de estudo. Incidęncia. J. bras. Med., 9: 1264-1283, 1965.
- 3. ARAÚJO, J.T. de; RIBEIRO, V.S. & ARAÚJO, R.A.T. - Hemoglobinopatias: aspectos moleculares, genéticos e clínicos. Rev. Hosp. Clín. Fac. Med. S. Paulo, 42: 260-266, 1964.
- 4. BETKE, K.; MARTI, H.R. & SCHLICHT, I. - Estimation of small percentages of foetal haemoglobin. Nature (Lond.), 184: 1877-1878, 1959.
- 5. CHARACHE, S.; CONLEY, C.L.; WAUGH, D.F.; UGORETZ, R.J. & SPURRELL, J.R. - Pathogenesis of hemolytic anemia in homozygous hemoglobin C disease. J. clin. Invest., 46: 1795-1811, 1967.
- 6. DIGGS, L.W.; KRAUS, A.P.; MORRISSON, D.B. & RUDNICKI, R.P.T. - Intraerythrocytic crystals in a white patient with hemoglobin C in the absence of other types of hemoglobin. Blood, 9: 1172-1184, 1954.
- 7. FABRY, M.E.; KAUL, D.K.; ROVENTOS, C. et al - Some aspects of the pathophysiology of homozygous Hb CC erythrocytes. J. clin. Invest., 67: 1284-1291, 1981.
- 8. HIRSH, R.E.; LIN, M.J. & NAGEL, R.L. - The inhibition of hemoglobin C and crystallization by haemoglobin F. J. biol. Chem., 263: 5936-5939, 1988.
- 9. HIRSH, R.E.; WITKOWSKA, H.E.; SHAFER, F. et al - HbC compound heterozygotes [HbC/Hb Riyadh and HbC/Hb N-Baltimore] with opposing effects upon Hb C crystallization. Brit. J. Haemat., 97: 259-265, 1997.
- 10. ITANO, H.A. & NEEL, J. - New inherited abnormality of hemoglobin. Proc. nat. Acad. Sci. (Wash.), 36: 613-617, 1950.
- 11. JAMRA, M. & LORENZI, T. - Baço Rio de Janeiro, Medsi, 1988.
- 12. NAOUM, P.C. - Hemoglobinopatias e talassemias Săo Paulo, Sarvier, 1997.
- 13. RANNEY, H.M.; LARSON, P.L. & McCORNACK JR., G.H. - Some clinical biochemical and genetic observations on hemoglobin C. J. clin. Invest., 32: 1277, 1953.
- 14. ROZENBERG, G. - Microscopic Haematology: a practical guide for the laboratory Sydney, Harwood Acad., 1996.
- 15. SUTCHARITCHAN, P.; NGO, H.; FUCHAROEN, S. et al - The first report of Hb CE disease: diagnostic considerations posed by a b c gene gone astray. In: Congress of the International Society of Haematology, 26, Singapore, 1996. (Int. J. Haemat., 64(suppl.1): S80, 1996).
- 16. SPAET, T.H.; ALWAY, R.H. & WARD, G. - Homozygote type C hemoglobin. Pediactris, 12: 483-490, 1953.
- 17. WILLIAMS, W.J.; BEUTLER, E.; ERSLEV, A..J. & LICHTMAN, M.A. Hematology 4. ed. New York, McGraw-Hill, 1990. p. 314.
Publication Dates
-
Publication in this collection
10 Nov 1999 -
Date of issue
July 1999
History
-
Accepted
10 Aug 1999 -
Received
28 May 1999