Abstracts
This report describes a preliminary characterization of proteolytic activity of proteins isolated from lysate of Giardia trophozoites of an axenic Brazilian strain. Fractions obtained by high-performance liquid chromatography (FPLC) were tested in SDS-polyacrylamide gel for the protein profiles, and the proteases activity was analyzed using gelatin impregnated SDS-PAGE. The proteases characterization was based on inhibition assays employing synthetic inhibitors for cysteine (E-64, IAA), serine (PMSF, TPCK, TLCK, and elastatinal), metalo (EDTA) and aspartic (pepstatin) proteases. Among thirty eluted fractions, polypeptide bands were observed in eight of them, however, proteolytic activity was detected in four ones (F23, F24, F25 and F26). Protein profiles of these fractions showed a banding pattern composed by few bands distributed in the migration region of 45 to < 18 kDa. The zymograms revealed proteolytic activity in all the four fractions assayed, mainly distributed in the migration region of 62 to 35 kDa. Among the profiles, the main pronounced zones of proteolysis were distinguished at 62, 55, 53, 50, 46 and 40 kDa. In inhibition assays, the protease activities were significantly inhibited by cysteine (E-64) and serine proteases (TPCK, TLCK and elastatinal) inhibitors. Gels incubated with other cysteine and serine protease inhibitors, IAA and PMSF, respectively, showed a decrease in the intensity of hydrolysis zones. Indeed, in the assays with the inhibitors EDTA for metalloproteases and pepstatin for aspartic proteases, none inhibition was detected against the substrate. These observations are relevants, especially if we consider that to define the real role of the proteases in host-parasite interaction, the purification of these enzymes for detailed studies may be warranted.
Giardia duodenalis; Axenic strain; Protein fractionation; Protease activity
O presente estudo consiste em uma caracterização preliminar da atividade proteolítica de frações de proteínas purificadas a partir de lisados de trofozoítos de cepa isolada e axenizada no Brasil. Frações obtidas por cromatografia líquida (FPLC) foram analisadas quanto ao perfil eletroforético em géis de poliacrilamida (SDS-PAGE) e a atividade proteolítica foi avaliada em géis contendo gelatina como substrato. A caracterização das enzimas foi realizada a partir da análise do efeito de inibidores sintéticos de cisteína-proteases (E-64, IAA), serina-proteases (PMSF), serina e cisteína-proteases (TPCK, TLCK, elastatinal), metalo-proteases (EDTA) e aspartil proteases (pepstatina) sobre a degradação do substrato. Entre 30 frações eluídas, bandas de proteínas foram observadas em oito delas, entretanto, atividade proteolítica foi detectada apenas nas frações 23, 24, 25 e 26. O perfil eletroforético das proteínas revelou poucas bandas distribuídas na faixa de 45 a 18 kDa. Os zimogramas revelaram zonas de proteólise na faixa de aproximadamente 62 a 35 kDa, entretanto destacaram-se as bandas de hidrólise de 62, 55, 53, 50, 46 e 40 kDa. Nos ensaios de inibição, a proteólise foi marcantemente inibida por E-64, TPCK, TLCK e elastatinal. Redução discreta da proteólise foi observada com IAA e PMSF, enquanto que EDTA e pepstatina não promoveram alteração dos perfis de hidrólise. Estas observações são relevantes, especialmente se considerarmos que para elucidar o envolvimento das proteases na relação parasita-hospedeiro, a purificação dessas moléculas é um requisito importante.
BRIEF COMMUNICATION
Partial characterization of proteolytic activity in Giardia duodenalis purified proteins
Caracterização parcial da atividade proteolítica de frações de proteínas purificadas de trofozoítos de Giardia duodenalis
Erica Boarato DavidI; Semíramis GuimarãesI; Paulo Eduardo Martins RibollaI; Silvana Torossian CoradiII; Diego Peres AlonsoI;
IDepartamento de Parasitologia, Instituto de Biociências, Universidade Estadual Paulista (Unesp), Botucatu, SP, Brazil
IIDepartamento de Ciências Biológicas e da Saúde, Universidade do Sagrado Coração (Usc), Bauru, SP, Brazil
Correspondence to Correspondence to: Semíramis Guimarães Depto. de Parasitologia/IBB/Unesp 18618-000 Botucatu, SP, Brasil E-mail: sgviana@ibb.unesp.br
SUMMARY
This report describes a preliminary characterization of proteolytic activity of proteins isolated from lysate of Giardia trophozoites of an axenic Brazilian strain. Fractions obtained by high-performance liquid chromatography (FPLC) were tested in SDS-polyacrylamide gel for the protein profiles, and the proteases activity was analyzed using gelatin impregnated SDS-PAGE. The proteases characterization was based on inhibition assays employing synthetic inhibitors for cysteine (E-64, IAA), serine (PMSF, TPCK, TLCK, and elastatinal), metalo (EDTA) and aspartic (pepstatin) proteases. Among thirty eluted fractions, polypeptide bands were observed in eight of them, however, proteolytic activity was detected in four ones (F23, F24, F25 and F26). Protein profiles of these fractions showed a banding pattern composed by few bands distributed in the migration region of 45 to < 18 kDa. The zymograms revealed proteolytic activity in all the four fractions assayed, mainly distributed in the migration region of 62 to 35 kDa. Among the profiles, the main pronounced zones of proteolysis were distinguished at 62, 55, 53, 50, 46 and 40 kDa. In inhibition assays, the protease activities were significantly inhibited by cysteine (E-64) and serine proteases (TPCK, TLCK and elastatinal) inhibitors. Gels incubated with other cysteine and serine protease inhibitors, IAA and PMSF, respectively, showed a decrease in the intensity of hydrolysis zones. Indeed, in the assays with the inhibitors EDTA for metalloproteases and pepstatin for aspartic proteases, none inhibition was detected against the substrate. These observations are relevants, especially if we consider that to define the real role of the proteases in host-parasite interaction, the purification of these enzymes for detailed studies may be warranted.
Keywords:Giardia duodenalis; Axenic strain; Protein fractionation; Protease activity.
RESUMO
O presente estudo consiste em uma caracterização preliminar da atividade proteolítica de frações de proteínas purificadas a partir de lisados de trofozoítos de cepa isolada e axenizada no Brasil. Frações obtidas por cromatografia líquida (FPLC) foram analisadas quanto ao perfil eletroforético em géis de poliacrilamida (SDS-PAGE) e a atividade proteolítica foi avaliada em géis contendo gelatina como substrato. A caracterização das enzimas foi realizada a partir da análise do efeito de inibidores sintéticos de cisteína-proteases (E-64, IAA), serina-proteases (PMSF), serina e cisteína-proteases (TPCK, TLCK, elastatinal), metalo-proteases (EDTA) e aspartil proteases (pepstatina) sobre a degradação do substrato. Entre 30 frações eluídas, bandas de proteínas foram observadas em oito delas, entretanto, atividade proteolítica foi detectada apenas nas frações 23, 24, 25 e 26. O perfil eletroforético das proteínas revelou poucas bandas distribuídas na faixa de 45 a 18 kDa. Os zimogramas revelaram zonas de proteólise na faixa de aproximadamente 62 a 35 kDa, entretanto destacaram-se as bandas de hidrólise de 62, 55, 53, 50, 46 e 40 kDa. Nos ensaios de inibição, a proteólise foi marcantemente inibida por E-64, TPCK, TLCK e elastatinal. Redução discreta da proteólise foi observada com IAA e PMSF, enquanto que EDTA e pepstatina não promoveram alteração dos perfis de hidrólise. Estas observações são relevantes, especialmente se considerarmos que para elucidar o envolvimento das proteases na relação parasita-hospedeiro, a purificação dessas moléculas é um requisito importante.
Recent advances in biochemistry and parasite molecular biology have pointed to the proteases involvement in the host-parasite relationship. Thus, investigations have implied that proteases of parasitic organisms are related to tissue and cellular invasion, morphogenesis during life cycle, catabolism of host proteins, pathogenicity, virulence and stimulation and evasion of immune response. Indeed, these molecules have been exploited as chemotherapeutic targets12.
In relation to the intestinal protozoan Giardia, proteases have been characterized and may play role in metabolism and physiologic process such as nutrition, excystation, pathogenesis and evasion of the host immune response2-5,9-11,15.
Although these investigations, relatively little is known about Giardia proteases. At this moment, few studies have identified and characterized trophozoite proteases of strains isolated in endemic areas. Proteolytic activity in trophozoites of two Brazilian axenic strains and the predominance of cysteine proteases were firstly reported by GUIMARÃES et al.3. More recently, CORADI & GUIMARÃES2, employing cell lysates of five strains isolated and axenized in Brazil observed differences in the Giardia proteases ability to hydrolyse different protein substrates and the inhibition of enzymatic activity was substrate dependent.
In general, different functions of Giardia proteases have been speculated, but to define the real role of these enzymes, the purification and characterization of the molecules can provide more details about their mechanisms of action. Thus, the present study reports a partial purification of Giardia proteases and it describes a preliminary characterization of these enzymes isolated from trophozoites of an axenic Brazilian strain.
For this study, Giardia duodenalis trophozoites, BTU-11 strain, were cultivated axenically in filter-sterilized TYI-S-33 medium7. The strain isolated in Brazil, at the Giardiasis Laboratory (IB/UNESP) in Botucatu, São Paulo, was recovered from cysts in the feces of a symptomatic individual presenting diarrhea, flatulence and abdominal cramps.
Trophozoites harvested in log-phase growth within 72 to 96 h postinoculation, after chilling in bath ice for 15 min, were collected by centrifugation at 500 x g for 20 min at 4 ºC. Pooled trophozoites in each sample were resuspended in 0.25 M sucrose, washed thrice by centrifugation (50 x g, 20 min, 4 ºC) and the total number of cells were counted in a hemocytometer and adjusted to approximately 2x108 parasites/mL. Then, suspensions were freezed at -80 ºC, thawed at room temperature and cells were disrupted at 4 ºC by six 30 sec periods of ultrasonic treatment. Finally, the cell lysate was centrifuged (5000 x g, 20 min, 4 ºC) and supernatants recovered were stored at -80 ºC until used. The protein concentration was estimated by the method of bicinconinic acid (BCA, Protein Assay Reagent Kit, Pierce), according to manufacture's instructions, employing bovine serum albumin (BSA) as standard.
About 1.0 mL of supernatant was applied to an anion exchange column (Source column - Q) in FPLC system (Amersham Pharmacia Biotech) equilibrated with 9.0 mL of 10 mM Tris/HCl buffer (pH 8.0), and eluted with 0-1 mM NaCl gradient at a rate of 1.0 mL/min. Each eluted material was pooled separately and listed by fractions.
The protein profiles of lysates and chromatographic fractions were analyzed by 7-14% and 10% SDS-PAGE gels8, respectively. The gels were stained with ammoniacal silver solution17.
Proteinase activities were examined using polyacrylamide gels containing 0.2% copolymerized gelatin. Forty microliters (40 µL) of each fraction were mixed without boiling with sample buffer (1:4) and applied at gels. After electrophoresis at 90 volts at 4 ºC in Hoefer mini VE system (Amersham Pharmacia Biotech), gels were renatured twice with 2.5% Triton X-100 for one hour at room temperature, washed with distilled water and then incubated for 15 h in the following activation buffer (0.1 M phosphate buffer, pH 5.5 with 3 mM DTT). Proteolysis was detected as clear zones against a blue background following amido black blue staining1.
In order to verify proteolysis inhibition, the following protease inhibitors were tested in the assays: l-trans-epoxysuccinyl-l-leucylamido- (4-guanidino)-butane (E-64, 100 µM), iodoacetamide (IAA, 1 mM); tosyl-l-phenylalanyl chloromethylketone (TPCK, 1 mM), tosyl-l-lysil chloromethylketone (TLCK, 1 mM), phenylmethylsulfonylfluoride (PMSF, 1 mM), ethylenediamine tetraacetic acid (EDTA, 5 mM), elastatinal (100 µM) and pepstatin A (100 µM). After electrophoresis and Triton X-100 treatment, the gels were incubated in the activation buffer supplemented with inhibitors.
Thirty chromatographic fractions were obtained by supernatant elution on anion-exchange column. In general, eluted materials are appropriated pooled according to elution profile. Considering that biochemical characterization of fractionated proteins from trophozoites of a Brazilian strain was not further conducted, we decided to analyze each fraction separately.
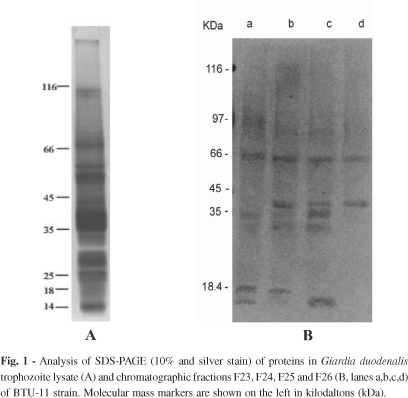
Among all eluted fractions, polypeptide bands were observed in eight of them, however, proteolytic activity was detected in four ones (F23, F24, F25 and F26). Figure 1 (1A and 1B) shows the SDS-PAGE gels of BTU-11 lysate and fractions. Lysate profile revealed approximately 25 bands with molecular masses ranging from 116 to < 18 kDa, most of them visualized between 75 to < 18 kDa (Fig. 1A). The fractions 23, 24, 25 and 26 showed a banding pattern composed by few bands mainly distributed in the migration region of 45 to < 18 kDa (Fig. 1B). The bands at approximately 39, 25, 23 and < 18 kDa were detected in the F23, F24 and F25 profiles. A 27 kDa band was detected in F24 and F25. In fraction 26, two protein bands of 39 and 27 kDa were identified.
Fractionated proteases promote degradation of protein substrate gelatin (Fig. 2B). Analysis of gelatin gel electrophoresis revealed protease activity in all the four fractions assayed. In contrast to lysate zymogram, high molecular weight hydrolysis bands were not exhibited in the profiles of these fractions (Fig. 2A, B). The F23 proteolytic profile revealed four faint hydrolysis bands at 62, 50, 46 and 40 kDa. Fraction 24 showed evident proteolytic bands at 62 kDa and 53 kDa, while in the fraction 24 the most evident band was approximately at 55 kDa. Besides this, in both fractions, hydrolysis was also visualized at a diffuse and weak zone ranging from 62 to 35 kDa. In F26 zymogram, a single and sharp hydrolysis band at 55 kDa was distinguished. Among all the profiles, the most pronounced zone of hydrolysis was the 50 kDa band observed in the fractions 25 and 26.
In relation to inhibition assays (Fig. 2C, D, E), the activities of all proteases were completely blocked by E-64 and elastatinal, cysteine and serine protease inhibitors, respectively. Gel incubation with TPCK and TLCK promoted a marked inhibition, whereas in the presence of IAA, showed a decrease in the intensity of hydrolysis zones. In the presence of PMSF, only protease activities of F23 were inhibited. Indeed, in the assays with the inhibitors EDTA for metalloproteases and pepstatin for aspartic proteases, none inhibition was detected against the substrate.
Considering the evidences highlighted in different investigations2-5,9-11,15, it is clear that the protozoan Giardia contains many proteins with proteolytic activities and that these molecules may be involved in the host-parasite relationship. Most of the reported observations have focused on proteolysis in the trophozoites of the standard strain Portland 1, whereas few studies have been identified and characterized proteases of other axenic strains.
In order to know more about strains isolated and axenized in endemic areas, recently, in our laboratory, a study undertaken to identify and characterize trophozoite proteases of five axenic strains isolated in Brazil and the reference strain Portland 1 revealed that cysteine proteases of all assayed strains promoted degradation of protein substrates such as gelatin, collagen, BSA and azocasein, while the activity was predominantly due to serine proteases against hemoglobin2. Indeed, differences on the hydrolysis pattern were observed and the more evident differences were observed in the BTU-11 enzymatic patterns, mainly in the assays employing hemoglobin as a substrate.
Moreover, considering that Giardia proteinases have not been fully characterized, the purification of these enzymes is an important step for detailed analysis. Although some proteins of Giardia trophozoites have been purified, little is known about their proteolytic activities. In previous investigations9,15, cysteine proteases of 95 and 35 kDa and 38 kDa were purified from homogenates of trophozoites of WB and Portland 1 strains. In view of these aspects and in attempt to have a more detailed characterization of the Giardia proteases, we analyzed the proteolytic activities in purified fractions from the autochthonous strain BTU-11.
Here, we observed that among fractions eluted from an anion-exchange column, only fractions 23, 24, 25 and 26 were able to hydrolyze gelatin and a major proteolytic activity migrating at 62 to 35 kDa was detected by gelatin-SDS-PAGE analysis. Among the profiles, the main pronounced zones of proteolysis were distinguished at 62, 55, 53, 50, 46 and 40 kDa.
In contrast to lysate profile, the fractionated proteases did not promote substrate hydrolysis at high molecular weight range in the gelatin gel. The lack of proteases activity at this range lead us to think about the possibility of protein degradation. As any protein, the proteases are molecules susceptible to changes in their physical and chemical properties. Many times, these changes can be induced during biochemical protocols, for instance the protein purification by chromatography. In view of this, it is important to take account that each protein possesses a unique set of physical characteristics, and conditions which are suitable for the purification of one protein not be suitable for others. Considering that, it is not ease to maintain the best conditions for all the proteins enclosed in a complex preparation. Thus, during the purification protocols, some proteins may suffer changes in their molecular structure leading to complete loss of activity.
The zymograms performed in the presence of proteases inhibitors revealed a striking inhibition of proteolysis by E-64, a class-specific inhibitor of cysteine proteases, and a decrease in the intensity of hydrolysis zones by IAA, another cysteine proteinase inhibitor. The activity was also significantly inhibited by TPCK and TLCK, known to inhibit both serine and cysteine proteases. These observations are in concordance with previous reports2,3-5,9,16 that have demonstrated proteolysis inhibition by the thiol proteases inhibitors E-64 and IAA and by inhibitors with effect on serine and some cysteine proteases as TPCK, TLCK and elastatinal. Interestingly, the assays with PMSF, an irreversible inhibitor of serine proteases, showed a decrease in the intensity of hydrolysis zones. The activity was predominantly due to cysteine proteases against gelatin substrate, but the evidence of serine proteases was also indicated.
Most investigations on proteases in Giardia reported cysteine proteases to be the major protease class2-5,9-11,15,16 which may be implicated in metabolic and physiologic processes14. Despite of the preliminary observations presented here, we reported the first attempt to purify and characterize proteases isolated from an axenic Brazilian strain. It is important to emphasize that, further experiments applying improved protocols for the isolation of proteases and enzymatic assays employing different substrates as the synthetic ones are needed to know more about the biochemical characteristics of these molecules and to elucidate which proteases play roles in Giardia trophozoites processes.
Received: 4 December 2006
Accepted: 18 June 2007
- 1. BONALDO, M.C.; D' ESCOFFIER, L.N.; SALLES, J.M. & GOLDENBERG, S. - Characterization and expression of proteases during Trypanossoma cruzi metacyclogenesis. Exp. Parasit., 73: 44-51, 1991.
- 2. CORADI, S.T. & GUIMARÃES, S. - Giardia duodenalis: protein substrates degradation by trophozoite proteases. Parasit. Res., 99: 131-136, 2006.
- 3. GUIMARÃES, S.; SOGAYAR, M.I.L. & FRANCO, M.F. - Protease activity in Giardia duodenalis trophozoites of axenic strains isolated from symptomatic and asymptomatic patients. Mem. Inst. Oswaldo Cruz, 98: 77-81, 2003.
- 4. HARE, D.F.; JARROLL, E.L. & LINDMARK, D.G. - Giardia lamblia: characterization of proteinase activity in trophozoites. Exp. Parasit., 68: 168-175, 1989.
- 5. JIMÉNEZ, J.C.; UZCANGA, G.; ZAMBRANO, A.; DI PRISCO, M.C. & LYNCH, N.R. - Identification and partial characterization of excretory/secretory products with proteolytic activity in Giardia intestinalis J. Parasit., 86: 859-862, 2000.
- 6. KAUR, H.; GHOSH, S.; SAMRA, H.; VINAYAK, V.K. & GANGULY, N.K. - Identification and characterization of an excretory-secretory product from Giardia lamblia Parasitology, 123: 347-356, 2001.
- 7. KEISTER, D.B. - Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans. roy. Soc. trop. Med. Hyg., 77: 487-488, 1983.
- 8. LAEMMLI, U.K. - Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature, 277: 680-685, 1970.
- 9. PARENTI, D.M. - Characterization of a thiol proteinase in Giardia lamblia J. infect. Dis., 160: 1076-1080, 1989.
- 10. ROBERTSON, C.D.; IRVINE, J.W.; BROWN, K. et al. - Proteinases of Giardia lamblia trophozoites. Trans. roy. Soc. trop. Hyg., 85: 844, 1991.
- 11. RODRÍGUEZ-FUENTES, G.B.; CEDILLO-RIVERA, R.; FONSECA-LIÑAN, R. et al.- Giardia duodenalis: analysis of secreted proteases upon trophozoite-epithelial cell interaction in vitro Mem. Inst. Oswaldo Cruz, 101: 693-696, 2006.
- 12. SAJID, M. & MCKERROW, J.H. - Cysteine proteases of parasitic organisms. Mol. Biochem. Parasit., 120: 1-21, 2002.
- 13. SHANT, J.; BHATTACHARYYA, S.; GHOSH, S.; GANGULY, N.K. & MAJUMDAR, S. - A potentially important excretory-secretory product of Giardia lamblia Exp. Parasit., 102: 178-186, 2002.
- 14. TOUZ, M.C.; NORES, M.J.; SLAVIN, I. et al. - Membrane-associated dipeptidyl IV is involved in encystations-specific gene expression during Giardia differentation. Biochem. J., 364: 703-710, 2002.
- 15. WERRIES, E.; FRANZ, A.; HIPPE, H. & ACIL, Y. - Purification and substrate especificity of two cysteine proteinases from Giardia lamblia. J. Protozool., 38: 378-383, 1991.
- 16. WILLIAMS, A.G. & COOMBS, G.H. - Multiple protease activities in Giardia intestinalis trophozoites. Int. Parasit., 25: 771-778, 1995.
- 17. WRAY, W.; BOULIKAS, T.; WRAY, V.P. & KANCOCK, J.R. - Silver staining of proteins in polyacrilamide gels. Analyt. Biochem., 118: 197-203, 1981.
Publication Dates
-
Publication in this collection
20 Dec 2007 -
Date of issue
Dec 2007
History
-
Received
04 Dec 2006 -
Accepted
18 June 2007