Abstracts
The aim of this study was to assess the antioxidant and anti-schistosomal activities of the garlic extract (AGE) and Nigella sativa oil (NSO) on normal and Schistosoma mansoni-infected mice. AGE (125 mg kg-1, i.p.) and NSO (0.2 mg kg-1, i.p.) were administrated separately or in combination for successive 28 days, starting from the 1st day post infection (pi). All mice were sacrificed at weeks 7 pi. Hematological and biochemical parameters including liver and kidney functions were measured to assess the progress of anemia, and the possibility of the tissue damage. Serum total protein level, albumin, globulin and cholesterol were also determined. Malondialdehyde (MDA) and glutathione (GSH) levels were determined in the liver tissues as biomarkers for oxidative and reducing status, respectively. The possible effect of the treatment regimens on Schistosoma worms was evaluated by recording percentage of the recovered worms, tissue egg and oogram pattern. Result showed that, protection with AGE and NSO prevented most of the hematological and biochemical changes and markedly improved the antioxidant capacity of schistosomiasis mice compared to the infected-untreated ones. In addition, remarkable reduction in worms, tissue eggs and alteration in oogram pattern were recorded in all the treated groups. The antioxidant and antischistosomal action of AGE and NSO was greatly diverse according to treatment regimens. These data point to these compounds as promising agents to complement schistosomiasis specific treatment.
Medicinal plant; Hematology; Enzymes; Oxidative stress; Antioxidant; Parasite burden
O propósito deste estudo foi verificar os efeitos anti-oxidantes e anti-esquistossômicos do extrato de alho (AGE) e do óleo da Nigella sativa (NSO) em camundongos normais e infectados com S. mansoni. AGE (125 mg/kg, i.p. ) e NSO (0,2 mg/kg, i.p.) foram administrados separadamente ou em combinação por 28 dias sucessivos começando do primeiro dia pós infecção (p.i.). Todos os camundongos foram sacrificados sete semanas p.i. Parâmetros hematológicos e bioquímicos incluindo funções renais e hepáticas foram medidos para avaliar o progresso da anemia e a possibilidade de dano tecidual. O nível total de proteínas séricas, albumina, globulina e colesterol foram também medidos. Níveis de malondialdeído (MDA) e glutationa (GSH) foram determinados em tecido hepático como biomarcações para o estado oxidativo e redutor, respectivamente. O possível efeito dos tratamentos sobre os vermes de Schistosoma foram avaliados através do percentual de vermes recuperados, ovos no tecido e o oograma. Resultados mostraram que a proteção com AGE e NSO preveniu a maior parte das alterações hematológicas e bioquímicas e melhoraram bastante a capacidade anti-oxidante de camundongos com esquistossomose comparados com aqueles infectados e não tratados. Adicionalmente, foi registrado uma acentuada redução nos vermes, ovos no tecido e alterações do oograma. A ação anti-oxidante e anti-esquistossômica do AGE e NSO foi diferente de acordo com os vários tratamentos. Estes dados mostram que estes compostos são agentes promissores para complementar o tratamento específico da esquistossomose.
SCHISTOSOMIASIS
The effect of antioxidant properties of aqueous garlic extract and Nigella sativa as anti-schistosomiasis agents in mice
O efeito das propriedades antioxidantes do extrato aquoso do alho e da Nigella sativa como agentes anti-esquistossômicos no camundongo
Nahla S. EL Shenawy; Maha F. M. Soliman; Shimaa I. Reyad
Correspondence to Correspondence to: Nahla S. El Shenawy Zoology Department, Faculty of Science Suez Canal University, Ismailia, Egypt E-mail: elshenawy_nahla@hotmail.com
SUMMARY
The aim of this study was to assess the antioxidant and anti-schistosomal activities of the garlic extract (AGE) and Nigella sativa oil (NSO) on normal and Schistosoma mansoni-infected mice. AGE (125 mg kg-1, i.p.) and NSO (0.2 mg kg-1, i.p.) were administrated separately or in combination for successive 28 days, starting from the 1st day post infection (pi). All mice were sacrificed at weeks 7 pi. Hematological and biochemical parameters including liver and kidney functions were measured to assess the progress of anemia, and the possibility of the tissue damage. Serum total protein level, albumin, globulin and cholesterol were also determined. Malondialdehyde (MDA) and glutathione (GSH) levels were determined in the liver tissues as biomarkers for oxidative and reducing status, respectively. The possible effect of the treatment regimens on Schistosoma worms was evaluated by recording percentage of the recovered worms, tissue egg and oogram pattern. Result showed that, protection with AGE and NSO prevented most of the hematological and biochemical changes and markedly improved the antioxidant capacity of schistosomiasis mice compared to the infected-untreated ones. In addition, remarkable reduction in worms, tissue eggs and alteration in oogram pattern were recorded in all the treated groups. The antioxidant and antischistosomal action of AGE and NSO was greatly diverse according to treatment regimens. These data point to these compounds as promising agents to complement schistosomiasis specific treatment.
Keywords: Medicinal plant; Hematology; Enzymes; Oxidative stress; Antioxidant; Parasite burden.
RESUMO
O propósito deste estudo foi verificar os efeitos anti-oxidantes e anti-esquistossômicos do extrato de alho (AGE) e do óleo da Nigella sativa (NSO) em camundongos normais e infectados com S. mansoni. AGE (125 mg/kg, i.p. ) e NSO (0,2 mg/kg, i.p.) foram administrados separadamente ou em combinação por 28 dias sucessivos começando do primeiro dia pós infecção (p.i.). Todos os camundongos foram sacrificados sete semanas p.i. Parâmetros hematológicos e bioquímicos incluindo funções renais e hepáticas foram medidos para avaliar o progresso da anemia e a possibilidade de dano tecidual. O nível total de proteínas séricas, albumina, globulina e colesterol foram também medidos. Níveis de malondialdeído (MDA) e glutationa (GSH) foram determinados em tecido hepático como biomarcações para o estado oxidativo e redutor, respectivamente. O possível efeito dos tratamentos sobre os vermes de Schistosoma foram avaliados através do percentual de vermes recuperados, ovos no tecido e o oograma. Resultados mostraram que a proteção com AGE e NSO preveniu a maior parte das alterações hematológicas e bioquímicas e melhoraram bastante a capacidade anti-oxidante de camundongos com esquistossomose comparados com aqueles infectados e não tratados. Adicionalmente, foi registrado uma acentuada redução nos vermes, ovos no tecido e alterações do oograma. A ação anti-oxidante e anti-esquistossômica do AGE e NSO foi diferente de acordo com os vários tratamentos. Estes dados mostram que estes compostos são agentes promissores para complementar o tratamento específico da esquistossomose.
INTRODUCTION
Parasitic helminths of genus Schistosoma are the causative agents of schistosomiasis, an infectious disease affecting humans and animals. Schistosomiasis is a parasitic disease that has attracted increased focus and funding for control16. For humans, it is one of the most prevalent parasitism in the world, second behind malaria40. The World Health Organization (WHO) indicated that more than 200 million people are infected worldwide. Schistosomiasis tops all the endemic parasitic diseases world-wide particularly in Egypt11. In the last two decades ambitious efforts have been made to develop an effective vaccine against schistosomes, but without resounding success30. In addition, there is a pressing need to develop new antihelminthics due to the potential emerging resistance against the commonly used drug, praziquantel32. Most pathology in Schistosoma-infected animals is attributed to the host's reaction to the eggs, which is maximal by the 8th week of infection. The toxic egg material destroys the host tissue cells and the antigenic material stimulates the development of large inflammatory reactions (granuloma) around the egg14. This granuloma is considered to serve as a protective barrier by sequestering the toxic and antigenic substances secreted continuously from Schistosoma eggs. Moreover, high rate of oxidative processes, formation of hepatic malondialdehyde (MDA) due to the peroxidative damage to the liver microsomal membrane lipid and impairment of the antioxidant defense characterize schistosomiasis14,15. Among the antioxidant defense mechanisms is glutathione (GSH) that removes reactive oxygen species once formed7.
It has been reported that the oil extracted from Nigella sativa (NSO), one of the most important medicinal plants belonging to the Family Ranumculaceae24, possesses anticestode and antinematode actions. Besides, it produced a hepatoprotective effect in some models of liver toxicity. Nowadays, there is an increased demand for using plants in therapy "back to nature" instead of using synthetic drugs which may have adverse effects that may be more dangerous than the disease itself24. Many effects have been described for the seeds of Nigella sativa and their constituents including its antioxidant role8,23 especially against hepatotoxicity12,26. One of the mechanisms responsible for antioxidant potentials of NSO could be the inhibition of 5-lipoxygenase18,32,. Also, it possesses a fairly good activity against earthworms and tape worms1,2. Recently, NSO has been found to have antihelminthic activity against human parasitic infections (S. mansoni and S. hematobium)4,14 and Fasciola hepatica24.
Garlic has been used as a folk remedy for a variety of ailments since ancient times. In the past few years, it has been found in certain models that garlic preparations including aged garlic prevented cardiovascular diseases20, liver damage27, and aging26 which are considered to be associated with oxygen radical and lipid peroxidation. The aqueous garlic extracts (AGE)28 and some garlic constituents32 have been widely documented in vivo19 and in vitro31. Antioxidant properties of garlic compounds representing the four main chemical classes, alliin, allyl cysteine, allyl disulfide, and allicin, prepared by chemical synthesis or purification were reported9. Although, garlic has been reported to reduce free radical-induced oxidative damage in experimental models and human, there are no reports about its anti-schistosomal effects.
Accordingly, in this study, we investigated the antioxidant and anti-schistosomal effects of AGE and NSO alone and in combination to on normal and Schsitosoma mansoni_infected mice to determine the possible interaction between the two treatments using hematological, biochemical, oxidative, antioxidant, and parasitological parameters.
MATERIALS AND METHODS
Preparation of aqueous garlic extract: Peeled garlic (30 g) was crushed with distilled water in a mortar. The crushed material was carefully decanted by pressing and 60 mL of aqueous extract was extracted. One milliliter of aqueous extract contained 500 mg of garlic materials34.
Experimental design: Eighty male Swiss albino mice weighing 20-22 g were obtained from experimental research center of Theodor Bilharz Institute, Cairo, Egypt. They housed in polypropylene cages at 25 ± 2 °C with 12 h/12 h light/dark cycle, and had free access to pelletal food with tap water ad libitum. The animals were randomly divided into eight groups with; ten in each, according the experimental design shown in Table 1. Four groups of mice were infected transcutaneously by exposing them to 50 S. mansoni cercariae (Egyptian strain) / mouse. The animals were treated by intraperitoneal route (i.p.) with different regime (Table 1) for 28 days (three times per week) starting from 1st day post infection (pi)
Parasitological study: At day 49 post infection, mice were killed by decapitation according the ethical rules and Animal Experimentation Committee of our Institution. Animals were dissected and the whole liver was put into a 20×20 cm plastic folder and compressed between two 21×21 cm glass plates until the parenchyma was evenly dispersed into a thin transparent layer, then examined under a stereomicroscope. The adult worms were counted and sexed39. The distal part of small intestine was placed in a Petri dish and under a stereomicroscope; the male and female worms in the mesenteric veins were removed and counted. Number of Schistosoma eggs per gram of liver was estimated21. For oogram study, the proportion of eggs in various stages of maturity was estimated29. One hundred eggs per oogram were randomly chosen, qualified by microscopic examination, and classified as dead, viable immature and mature in all infected and treated groups.
Determination of hematological parameters: At day 49 post infection, mice were killed by decapitation and blood was collected for hematological and biochemical parameters. Blood samples used for hematological analysis were collected into polyethylene tubes containing an anticoagulant, ethylene diamine tetraacetic acid (EDTA). Erythrocytes (RBCs), total leucocytes counts (WBCs) Hemoglobin (Hb) %, hematocrit value (PCV) and absolute values of erythrocyte indices were determined using the cell counter (ADVIA 60 \Cell Dyne counting, ABOTT1800, Ireland).
Determination of serum biochemical parameters: Blood samples of mice were collected using capillary tubes (Micro Hematocrit Capillaries, Mucaps) introduced into the medial retro-orbital venous plexus under light ether anesthesia. Serum was separated in an electric centrifuge at 300 xg for 10 min.
Serum lactate dehydrogenase (LDH) activity was measured as a marker of tissue injury with kits (Boehringer Mannheim, GmbH, Mannheim, Germany). The determination of LDH activity with the kit was based on the formation of diformazan by reduction of nitroblue tetrazolium in a reaction catalyzed by diaphorase with NADH. NADH was formed from NAD used as a cofactor in the oxidation of L-lactate to pyruvate, which was catalyzed by LDH. Absorbance at 560 nm was measured with a spectrophotometer (U-2000; Hitachi Ltd.). LDH activity of the medium, from which background LDH activity of the control, sample-free medium incubated for the same period as that from experimental groups was subtracted, was considered to be the liberated LDH activity. Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were determined to assess liver function using commercial kits (Roche Diagnostics, GmbH, D-68298, Mannheim, Germany). Both enzyme activities were determined photometrically in which the decrease in NADH levels was directly proportional to enzyme activities. Serum total protein contents were determined by colorimetric method using bovine serum albumin as standard (Stanbio Laboratory, USA). Serum albumin level was determined to indicate the tissue damage and exudation using commercial kit supplied by Diamond, RA50, Ireland. Serum cholesterol was determined using a kit from Stanbio Laboratory, USA. Serum urea and creatinine were determined to assess kidney function using kits from Quimica Clinica Aplicada S.A., Spain and from Diamond, RA50, Ireland, respectively.
Tissue malondialdehyde (MDA) and glutathione (GSH) assays: The hepatic reduced glutathione (GSH) level was determined by the method of Ellman6. Briefly, after 0.2 g liver tissues were homogenized in 4 mL of 0.02 M EDTA Na2 (using an all glass Ten-Broeck homogenizer in an ice bath). 2.5 mL tissue homogenates (aliquots) were mixed with 2.0 mL of distilled water and 0.2 mL of 50 % TCA. All tubes were shaken intermittently for 10-15 min and centrifuged for 15 min at approximately 3000 ×g. 2.0 mL of 0.4 M Tris buffer (pH 8.9) and 0.1 mL of 0.01 M 5,5'-dithiobis- 2-nitrobenzoic acid (DTNB) were added to 2.0 mL of tissue supernatant, and the sample was shaken. The absorbance was read within five min of the addition of DTNB at 412 nm against a reagent blank with no homogenate. GSH levels were calculated using standard curve prepared by known amounts of GSH (Aldrich chemical Co. LTD-Germany). The concentration of GSH was expressed as mg/g tissue.
Hepatic lipid peroxidation (LPOX) level was measured by a colorimetric reaction with thiobarbituric acid-positive reactant substances (TBARS) and was expressed in terms of the malondialdehyde (MDA) concentration using 1,1,3,3- tetraethoxy propane as a standard10. The liver samples were homogenized at the tissue concentration of 50 mg/mL in 0.1 M of ice-cold phosphate buffer (pH-7.4). The homogenates were centrifuged at 10,000 xg at 4 ºC for five min. 0.5 mL supernatant was mixed with 0.5 mL of normal saline and 2 mL of TBA-TCA mixture. The mixture was boiled at 100 ºC for 10 min, and then cooled at room temperature. This mixture was centrifuged at 4000 ×g for 10 min. The whole supernatant was transferred in spectrophotometer cuvette and read at 535 nm. The levels of TBARS are expressed as micromoles of MDA per mg of tissue (mmol/mg).
Statistical analysis: Statistical evaluation was conducted with SPSS version 11.0 (SPSS Inc., Chicago, IL, USA). Results were expressed as mean ± S.E. The results were analyzed for statistical significance by one way ANOVA followed by Tukey-Kramer multiple comparison test. Values of p < 0.05 were regarded as significant.
RESULTS
As shown in Table 2, WBCs was significantly higher in NSO group compared to non-infected control (p < 0.001), while Hb was significantly increased in non-infected control group that treated with NSO and AGE in combination. The anemia was evidenced by a significant decrease (p < 0.001) in the levels of Hb content of infected-untreated mice (6.7 ± 0.5) as compared to non-infected control group (10.4 ± 0.6) and decrease in the number of RBCs. Data revealed that treatment of the infected mice with AGE or NSOseparately caused significant increase in Hb level better than infected-treated mice with both compounds in combination. Hematocrit value (PCV) was decreased significantly (p < 0.001) in the infected mice as compared to control uninfected group. PCV enhanced back after the treatment of control mice with AGE and there was significant difference between NSO and AGE treated-infected groups (Table 2).
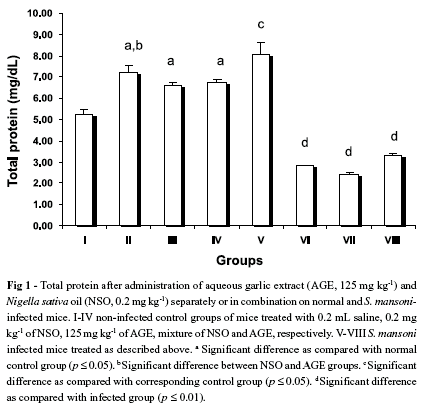
Total protein content of control uninfected mice that were treated with NSO, AGE and NSO + AGE were found to be significantly higher than that of untreated control group (Fig. 1), while there was significant difference between them. Total protein content of infected mice (8.1 ± 0.5 mg/dL) was found to be significantly higher than that of control saline group (5.3 ± 0.2 mg/dL). AGE and NSO treatment significantly reduced this increase to 2.4 ± 0.1 and 2.8 ± 0.03 mg/dL, respectively. However, the treatment with both compounds decreased the total protein content to 3.3 ± 0.1 mg/dL which was higher than the treatment of each compound separately (Fig. 1). Level of globulin increased significantly in the infected group (5.1 ± 0.2) compared to the control group (1.9 ± 0.1). This elevation in the globulin level induced by infection was reversed back to the control level with AGE, NSO and AGE + NSO treatments (4.7 ± 0.2, 3.02 ± 0.03 and 1.2 ± 0.1, respectively). However, there was no significant difference in albumin content among the non-infected control groups. Therefore, the significant decrease in the A/G ratio was related to the change in globulin content not to the albumin content (Table 3). There was significant difference between the infected-group treated with NSO only and treated with NSO + AGE.
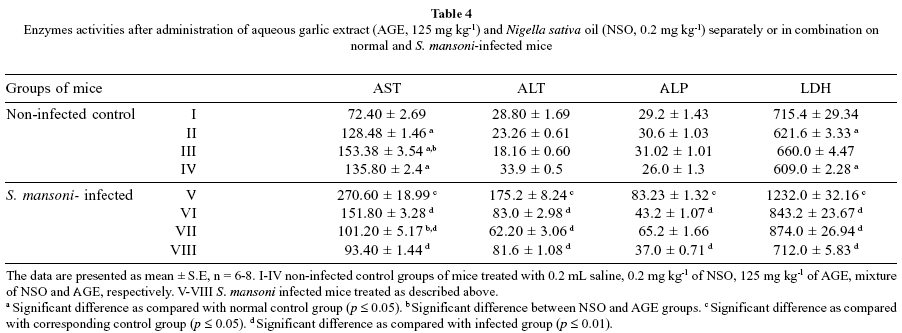
Serum AST was significantly higher in the groups treated with NSO or AGE separately or in combination compared to the non-infected control mice. The elevation of AST activity to approximately 4-fold in infected untreated mice was observed as compared to negative control group. There was no significant difference in ALP activity between non-infected control groups, however LDH activity was significantly increased in NSO (p < 0.04) and mixed treated (p < 0.01) groups compared to the non-infected control mice (Table 4). Serum AST, ALT, ALP and LDH activities were significantly higher in infected-untreated mice compared to the non-infected control group, while NSO or AGE administration separately or in combination reduced the LDH activity of infected group in infected group (p < 0.001). Treatment of the infected mice with mixture of AGE and NSO decreased the level of AST significantly (p < 0.001) as compared to the infected untreated group.
Serum urea and creatinine of infected mice by S. mansoni, were significantly increased (p < 0.001). Results revealed that the level of urea and creatinine of infected mice has been reduced with NSO and AGE separately or in combination (Fig. 2).
There was no significant difference in serum cholesterol level among the non-infected control groups (Fig. 3), except in group IV that received a mixture of AGE and NSO. However infected group had significantly increase in cholesterol level as compared to its respective control mice (p < 0.001). NSO in combination with AGE treatment reversed this effect significantly (p < 0.0001), while AGE treatment only did not show any effect on the cholesterol level compared to the infected untreated mice. Moreover, there was a significantly difference between NSO and AGE groups (p < 0.035).
Administration of the non-infected control group with combination of NSO and AGE enhanced the hepatic GSH levels. Hepatic GSH declined significantly in the infected group (0.11 mg/g) as compared to the control group (1.1 mg/g), while AGE treatment significantly reversed the GSH level reduction (0.38 mg/g). NSO treatment separately or in combination with AGE significantly increased the GSH levels to 0.23 and 0.7 mg/g, respectively (Fig. 4).
The liver MDA increased significantly in the infected group (6.7 ± 0.5 nmol/g) than that measured in the control group (3.2 ± 0.4 nmol/g). Treatment with AGE or NSO decreased the elevated MDA level significantly to 2.0 ± 0.2 and 4.4 ± 0.7 nmol/g, respectively. MDA decreased significantly to 1.6 ± 0.4 in the mice treated with AGE and NSO in combination (Fig. 4).
Analysis of the parasite at day 49 pi showed differences in the total number of the recovered worms in all the infected-treated mice compared to the infected-untreated one, although the differences were not significant (Fig. 5) and the only exception was for NSO where a significant difference (p < 0.04) was recorded compared to the infected untreated group. A significant reduction was recorded in number of eggs/g liver of all the treated groups (1086.7 ± 30.9, p < 0.003; 1251 ± 92.6, p < 0.002; 888.8 ± 140.3, p < 0.0001 for NSO, AGE and NSO+AGE-treated mice, respectively) as compared to the infected untreated group (2348 ± 262).
The treatment regimens significantly affected the oogram patterns as compared to the untreated infected mice (p < 0.0001) (Fig. 6). Treatment of the infected mice with either NSO or AGE resulted in a significant reduction in the percentage of mature eggs compared to the untreated mice (p < 0.001), while treatment with the mixture resulted in a significant increase in percentage of the dead eggs (p < 0.001).
DISCUSSION
Schistosomiasis causes a reduction in the levels of protective endogenous antioxidants and increases generation of free radicals14,15. Most investigations of the response of schistosomiasis to antioxidant substance have concerned on single compound and little is known of possible interactive effects of different antioxidants. As these latter seldom occur in nature, it is important to understand any interaction (synergistic or antagonistic) which may occur. Therefore, the present investigation dealt with important points concerning two aspects. The first one was the possible antioxidant and anti-schistosomiasis properties of AGE and compared with the effect of NSO. The second aspect was the interaction between AGE and NSO extract on infected mice. Hematological parameters, liver functions, kidney functions, redox state, biochemical parameters and the parasite load markers were used to evaluate their effects.
Data obtained in the present work revealed significant decrement in the mean values of RBC count, PCV and Hb content in infected animals. The severity of anemia was observed where the values of the three parameters were recorded being lower than the control. From an etiological point of view, anemia induced by S. mansoni infection may be attributed to blood loss and enhanced rate of hemolysis and shortened life span of RBCs. They may be lost by two ways; from the bleeding induced by extrusion of egg through intestinal wall or due to consumption by adult schistosomes36. As the infected animals were treated with NSO the Hb content and PCV were remarkably improved. This improvement could indicate the stoppage of intestinal bleeding and loss of red cells after eradication of parasite. This suggestion seems coincident with the parasitological results previously reported14. NSO had the ability to increase Hb and PCV levels significantly in the normal mice and this could be the reason to improve the Hb and PCV levels of the infected mice in the present investigation42. It is clear that the AGE had more significant effect on Hb and PCV of the infected mice than NSO. However the interaction between the two antioxidants enhanced theses parameters less than the action of each compound separately.
The present work exhibited remarkable increments in total leukocytes of S. mansoni-infected mice as compared with control animals which could be attributed to the powerful defense reaction and allergic manifestation against the schistosomes and/or their egg35.
On the other hand, the observed increase in globulin fraction in case of infected and mice may represent responsive mechanism enhancing the immunity of the host as described before13. The interaction between the AGE and NSO decreased significantly the level of globulin more than each compound separately.
Serum AST, ALT, ALP and LDH levels were elevated in the infected-untreated group as compared to control group, while this increase was significantly decreased by AGE and NSO treatment. These enzymes are commonly employed as biological markers for hepatic cell damage and impaired cell membrane permeability or due to heavy Schistosoma egg deposition14. There was significant difference (p < 0.001) between the effect of AGE and NSO on AST, ALT and ALP. Moreover, there were significant differences between their effects separately or in combination in AST and ALP. Due to the aforementioned, it is clear that the combination of NSO and AGE had more significant effect on AST and ALP of the infected mice. Treatment of control and infected mice with the combination of AGE and NSO had more potent effect on urea nitrogen and creatinine than the treatment with each compound separately.
Furthermore, increasing hepatic LPOX and decreasing GSH levels following infection with S. mansoni were reversed by AGE or NSO treatment. It appeared that, the effect of AGE on GSH and LPOX could be synergistically enhanced by its combination with NSO.
GSH, a key antioxidant, is an important constituent of intracellular protective mechanisms against oxidative stress33. Because of their exposed sulfhydryl groups, non-protein sulfhydryls bind a variety of electrophilic radicals and metabolites that may be damaging cells37. In different models of experimental liver fibrosis, decreases in the antioxidant levels indicate an increase in free radical level and thereby cellular damage is increased22,41. In accordance with the previous reports, our results also support the notion of depletion of tissue GSH, as observed in the infected-induced hepatic injury13. Since administrations of AGE prevent the hepatic GSH depletion, it appears that the protective effect of AGE involves the maintenance of antioxidant capacity in protecting the hepatic tissue against oxidative stress.
In the present study, it was observed that the infection caused significant increases in the hepatic MDA levels, end products of LPOX. AGE treatment prevented the increase in MDA, probably in part by scavenging the very reactive hydroxyl and peroxyl radicals. Aged garlic extract and diallyl polysulfides inhibit the formation of thiobarbituric acid-reactive and fluorescent substances induced by iron-ascorbic acid in isolated liver microsomal membranes17, indicating the protective effect of garlic against lipid peroxidation. It has been reported that chronic garlic intake significantly decreased lipid peroxidation and increased endogenous antioxidants, such as catalase and GSH5. Oxidative stress-induced tissue damage can be prevented or ameliorated by favoring the balance towards a lower oxidative status.
The present study showed that NSO achieved a considerable reduction in the total number of the recovered worms. The possible explanation of the mechanisms of action of N. sativa oil can be interpreted on the basis of the direct lethal effect on the worms due to its content of the alkaloid nigellicine3. However, The antischistosomal action of NSO was previously reported14,25,35, taking into consideration the difference in duration and the dose of treatment. Interestingly, that combination of NSO with AGE did not significantly affect the number of recovered worm. With regard to combination therapy, the partner drugs should have different mechanisms of action compared to single treatment37. On the other hand, NSO or AGE clearly impaired the development and maturity of Schistosoma eggs which could be attributed to a possible toxic effect of NSO or AGE when used separately. The later may be confirmed by the high numbers of dead eggs in the combined treatment. In contrast, combined treatment resulted in high percentage of mature ova indicating a clear enhancement action of the combination (NSO+AGE) on maturation of Schistosoma ova.
There is a clearly documented link between schistosomiasis complications and the antioxidant- oxidative system. The anti-schistosomiasis activity of NSO has a remarkable association with an enhancement in the antioxidant capacity. The present study demonstrated for the first time that aqueous garlic extract, with its potent free radical scavenging and antioxidant properties, seems to be a highly promising agent in protecting hepatic tissue against oxidative damage due to S. mansoni infection.
Received: 2 March 2007
Accepted: 11 September 2007
- 1. AGARWAL, R.; KHARYA, M.D. & SHRIVASTAVA, R. - Antimicrobial and antihelminthic activities of the essential oil of Nigella sativa L Indian J. exp. Biol., 17: 1264-1275, 1979.
- 2. AKHTAR, M.S. & RIFFAT, S. - Field trial of saussured lappa roots against nematodes, and Nigella sativa seeds against cestodes in children. J. Pak. med. Ass.,41: 185-187, 1991.
- 3. ATTA, R.; MALIK, S.; SOHAIL, M. et al. - Nigelliciene N-oxide a new isoquinoline alkaloid from the seed of N. sativa L Heterocycles, 23: 953-961, 1985.
- 4. AWAD, H.A. & HERRIK, M.K. - The effects of Nigella sativa L extraction experimental giardiasis. New Egypt J. Med., 7: 602-608, 2000.
- 5. BANERJEE, S.K.; MAULIK. M.; MANCAHANDA, S.C. et al. - Dose-dependent induction of endogenous antioxidants in rat heart by chronic administration of garlic. Life Sci., 70: 1509-1518, 2002.
- 6. ELLMAN, G.L. - Tissue sulfhydryl groups. Arch. Biochem. Biophys., 82: 70-77, 1959.
- 7. BONNEFONT-ROUSSELOT, D.; BASTARD, J.P.; JAUDON, M.C. & DELATTRE, J. - Consequences of the diabetic status on the oxidant/antioxidant balance. Diabet. Metab., 26: 163-176, 2000.
- 8. BURITS, M. & BUCAR, F. - Antioxidant activity of Nigella sativa essential oil. Phytother. Res., 14: 323-328, 2000.
- 9. CHUNG, L.Y. - The antioxidant properties of garlic compounds: allyl cysteine, alliin, allicin, and allyl disulfide. J. med. Food, 9: 205-213, 2006.
- 10. DRAPER, H.H. & HADLEY, M. - Malonaldehyde determination as index of lipid peroxidation. In: PARCKER, L. & GLAZER, A., ed. Methods in Enzymology New York, Academic Press, 1991. v. 186 (B), p. 421-443.
- 11. EL BAZ, M.A; MORSY, T.A.; EL BANDARY, M.M. & MOTAWEA, S.M. - Clinical and parasitological studies on the efficacy of Mirazid in treatment of schistosomiasis haematobium in Tatoon, Etsa Center, El-Fayoum Governorate. J. Egypt. Soc. Parasit, 33: 761-776, 2003.
- 12. EL-DAKHAKHNY, M.; MADY, N.I. & HALIM, M.A. - Nigella sativa oil protects against induced hepatotoxicity and improves serum lipid profile in rats. Arzneimit. Forsch., 50: 832-836, 2000.
- 13. EL-SHENAWY, N.S. & SOLIMAN, M.F.M. - On the interaction between induced Diabetes Mellitus and Schistosomiasis: mechanism and protection. Egypt. J. Hosp. Med., 8: 18-31, 2002.
- 14. EL-SHENAWY, N.S. & SOLIMAN, M.F.M. - Evaluation of the protective effect of two antioxidative agents in mice experimentally induced with Schistosoma mansoni: biochemical and parasitological aspects. J. Egypt. Ger. Zool., 40(A): 201-216, 2003.
- 15. EL-SOKKARY, G.H.; OMAR, H.M.; HASSANEIN, A.F.; CUZZOCREA, S. & REITER, R.J. - Melatonin reduces oxidative damage and increases survival of mice infected with Schistosoma mansoni Free rad. Biol. Med., 32: 319-332, 2002.
- 16. FENWICK, A. & WEBSTER, J.P. - Schistosomiasis: challenges for control, treatment and drug resistance. Curr. Opin. infect. Dis.,19: 577-582, 2006.
- 17. HORIE, T.; AWAZU S.; ITAKURA, Y. & FUWA, T. - Identified diallyl polysulfides from an aged garlic extract which protects the membranes from lipid peroxidation. Planta Med., 58: 468-469, 1992.
- 18. HOUGHTON, P.J.; ZARKA, R.; DE LAS HERAS, B. & HOULT, J.R. - Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med, 61: 33-36, 1995.
- 19. IQBAL, M. & ATHAR, M. - Attenuation of iron-nitrilotriacetate (Fe-NTA)-mediated renal oxidative stress, toxicity and hyperproliferative response by the prophylactic treatment of rats with garlic oil. Food Chem. Toxicol., 36: 485-495, 1998.
- 20. KLEIJNEN, J.; KNIPSCHILD, P. & TER, R.G. - Garlic, onions and cardiovascular risk factors. A review of the evidence from human experiments with emphasis on commercially available preparations. Brit. J. clin. Pharmacol., 28: 535-544, 1989.
- 21. KLOETZEL, K. - Egg and pigment production in Schistosoma mansoni infections of the white mouse. Amer. J. trop. Med. Hyg., 16: 293-299, 1967.
- 22. LEE, E.; LEE, H.E.; SHIN, J.Y.; YOON, S. & MOON, J.O. - The flavonoid quercetin inhibits dimethylnitrosamine-induced liver damage in rats. J. Pharm. Pharmacol., 55: 1169-1174, 2003.
- 23. MADY, N.I.; ABDEL-AZIEM, T. & MATTA, M. - Effect of combination of Nigella sativa oil and glibenclamide on some metabolic parameters and oxidative changes in streptozocin-induced diabetic rats (in vivo and in vitro study). J. Egypt. Pharmacol. exp. Ther.,20: 359-383, 2001.
- 24. MADY, N.I.; ALLAM, A.F. & SALEM, A.I. - Evaluation of the addition of Nigella sativa oil triclabendazole therapy in the treatment of human fascioliasis. J. Egypt. Pharmacol. exp. Ther, 20: 807-827, 2001.
- 25. MAHMOUD, M.R.; EL-ABHAR, H.S. & SALEH, S. - The effect of Nigella sativa oil against the liver damage induced by Schistosoma mansoni infection in mice. J. Ethnopharmacol, 79: 1-11, 2002.
- 26. MORIGUCHI, T.; TAKASHINA, K.; CHU, P.; SAITO, H. & NISHIYAMA, N. - Prolongation of life span and improved learning in the senescence accelerated mouse produced by aged garlic extract. Biol. Pharm. Bull., 17: 1589-1594, 1994.
- 27. NAKAGAWA, S.; KASUGA, S. & MATSUURA H. - Prevention of liver damage by aged garlic extract. Phytother. Res., 3: 50-53, 1989.
- 28. NUMAGAMI, Y.; SATO, S. & OHNISHI, S.T. - Attenuation of rat ischemic brain damage by aged garlic extracts: a possible protecting mechanism as antioxidants. Neurochem. Int., 29: 135-143, 1996.
- 29. PELLEGRINO, J.; OLIVEIRA, C.A.; FARIA, J. & CUNHA, A.S. - New approach to the screening of drugs in experimental schistosomiasis mansoni in mice. Amer. J. trop. Med. Hyg.,11: 201-215, 1962.
- 30. QUACK, T.; BECKMANN, S. & GREVELDING, C.G. - Schistosomiasis and the molecular biology of the male-female interaction of S. mansoni. Berl. Munch. Tierarztl. Wochenschr., 119: 365-372, 2006.
- 31. RABINKOV, A.; MIRON, T.; KONSTANTINOVSKI, L. et al. - The mode of action of allicin: trapping of radicals and interaction with thiol containing proteins. Biochim. Biophys. Acta, 1379: 233-244, 1998.
- 32. RANKIN, S.M.; PARTHASARATHY, S. & STEINBERG, D. - Evidence for a dominant role of lipoxygenase (s) in the oxidation of LDL by mouse peritoneal macrophages. J. Lipid Res., 32: 449-456, 1991.
- 33. ROSS, D. - Glutathione, free radicals and chemotherapeutic agents. Mechanisms of free-radical induced toxicity and glutathione-dependent protection. Pharmacol. Therap., 37: 231-249, 1988.
- 34. SENER, G.; SATYROGLU, H.; OZER-SEHIRLI, A. & KACMAZ, A. - Protective effect of aqueous garlic extract against oxidative organ damage in a rat model of thermal injury. Life Sci., 73: 81-91, 2003.
- 35. SOLIMAN, M.F.M. & EL-SHENAWY, N.S. - Evaluation of the protective effect of two antioxidative agents in mice experimentally infected with Schistosoma mansoni: haematological and histopathological aspects. Pak. J. biol. Sci., 6: 887-897, 2003.
- 36. STURROCK, R.F.; KARIUKI, H.C.; THIONGO, F.W. et al. - Schistosomiasis mansoni in Kenya: relationship between infection and anemia in schoolchildren at the community level. Trans. roy. Soc. trop. Med. Hyg., 90: 48-54, 1996.
- 37. SZABO, S.; NAGY, L. & PLEBANI, M. - Glutathione, protein sulfhydryls and cysteine proteases in gastric mucosal injury and protection. Clin. Chim. Acta, 206: 95-105, 1992.
- 38. UTZINGER, J.; KEISER, J.; SHUHUA, X.; TANNER, M. & SINGER, B.H. - Combination chemotherapy of schistosomiasis in laboratory studies and clinical trials. Antimicrob. Agents Chemother., 47: 1487-1495, 2003.
- 39. WANG, Y.; HOLMES, E.; NICHOLSON, J.K. et al. - Metabonomic investigations in mice infected with Schistosoma mansoni: an approach for biomarker identification. Proc. nat. Acad. Sci. (Wash.), 24: 12676-12681, 2004.
- 40. XIAO, H.; SHEN, B.; UTZINGER, J.; CHOLLET, J. & TANNER, M. - Ultrastructural alterations in adult Schistosoma mansoni caused by artemether. Mem. Inst. Oswaldo Cruz,97: 717-724, 2002.
- 41. YANG, S.; TAN, T.M.; WEE, A. & LEOW, C.K. - Mitochondrial respiratory function and antioxidant capacity in normal and cirrhotic livers following partial hepatectomy. Cell Molec. Life Sci., 61: 220-229, 2004.
- 42. ZAOUI, A.; CHERRAH, Y.; MAHASSINI, N. et al. - Acute and chronic toxicity of Nigella sativa fixed oil. Phytomedicine, 9: 69-74, 2002.
Publication Dates
-
Publication in this collection
29 Feb 2008 -
Date of issue
Feb 2008
History
-
Accepted
11 Sept 2007 -
Received
02 Mar 2007