Abstracts
Formalin-killed promastigotes (FKP) of Leishmania major, in combination with Montanide ISA 720 (MISA), BCG or alum were used in vaccination of an inbred murine model against cutaneous leishmaniasis (CL). Significant and specific increases in anti-FKP IgG responses were detected for both alum-FKP and BCG-FKP compared to MISA-FKP (p < 0.001). Significant increases in splenic lymphocyte recall proliferation was obtained in the MISA-FKP vaccinated mice compared to alum-FKP or BCG-FKP vaccinated groups (p < 0.01). The highest interferon-γ responses were observed in the BCG-FKP group followed by the MISA-FKP while the alum-FKP gave the least responses. Significantly reduced lesion sizes were obtained in the MISA-FKP group compared to the BCG/alum adjuvants-FKP vaccinated groups. Although the BCG-FKP group showed the highest IFN-γ responses, it failed to control cutaneous lesions. Significant reductions in parasite numbers were observed in the MISA-FKP and BCG-FKP vaccinated groups (p < 0.001). There was a good correlation between parasite burden and IFN-γ level indicating IFN-γ response as a sensitive parameter of the immune status. In conclusion, MISA-FKP is the most efficacious vaccine formulation against murine cutaneous leishmaniasis.
Cutaneous leishmaniasis; Formalin-killed promastigotes (FKP) promastigotes; Montanide ISA 720 (MISA); BCG; Alum; BALB/c mice; Immune responses
Promastigotos mortos pela formalina (FKP) de Leishmania major combinados com Montanide ISA 720 (MISA), BCG ou alumen foram usados na vacinação de modelo murino cutâneo de leishmaniose (CL). Aumento significante e específico de resposta IgG anti FKP foram detectados tanto no FKP com alumen como naquele com BCG comparados ao MISA-FKP (p < 0,001). Aumento significante da proliferação esplênica de linfócitos de memória foi obtida nos camundongos vacinados com MISA-FKP quando comparados aos grupos vacinados com alumen-FKP ou BCG-FKP (p < 0,01). As maiores respostas por interferon-gama foram observadas no grupo BCG-FKP seguido pelo MISA-FKP enquanto que o alumen-FKP deu a menor resposta. No grupo MISA-FKP foram obtidas reduções significantes do tamanho das lesões quando comparado aos grupos vacinados com BCG/adjuvante de alumen-FKP. Embora o grupo BCG-FKP tenha mostrado a maior resposta por interferon-gama, não houve controle das lesões cutâneas. Redução significante no número de parasitas foi observada tanto no grupo vacinado com MISA-FKP como no BCG-FKP (p < 0,001). Houve boa correlação entre a carga parasitária e o nível de interferon-gama indicando que a resposta do interferon-gama é parâmetro sensível do estado imunológico. Em conclusão, MISA-FKP é a forma mais eficaz de vacina contra a leishmaniose cutânea murina.
LEISHMANIASIS
Subcutaneous immunization against Leishmania major - infection in mice: efficacy of formalin-killed promastigotes combined with adjuvants
Imunização subcutânea na infecção do camundongo pela Leishmania major: eficácia dos promastigotos mortos pela formalina combinada com adjuvantes
Joshua M. MutisoI; John C. MachariaI; Rosemary M. MutisyaII; Evans TarachaI
IDepartment of Tropical and Infectious Diseases, Institute of Primate Research, P.O. Box 24481-00502, Karen, Nairobi, Kenya
IIDepartment of Zoological Sciences, Kenyatta University, Nairobi, Kenya, P.O. Box 43844 -00100, Nairobi, Kenya
Correspondence to Correspondence to: Joshua M. Mutiso P.O. Box 24481-00502 Karen, Nairobi, Kenya Fax: +254 20 882546, Tel: +254 20 2161157 Email: mutisovic@yahoo.co.uk
SUMMARY
Formalin-killed promastigotes (FKP) of Leishmania major, in combination with Montanide ISA 720 (MISA), BCG or alum were used in vaccination of an inbred murine model against cutaneous leishmaniasis (CL). Significant and specific increases in anti-FKP IgG responses were detected for both alum-FKP and BCG-FKP compared to MISA-FKP (p < 0.001). Significant increases in splenic lymphocyte recall proliferation was obtained in the MISA-FKP vaccinated mice compared to alum-FKP or BCG-FKP vaccinated groups (p < 0.01). The highest interferon-γ responses were observed in the BCG-FKP group followed by the MISA-FKP while the alum-FKP gave the least responses. Significantly reduced lesion sizes were obtained in the MISA-FKP group compared to the BCG/alum adjuvants-FKP vaccinated groups. Although the BCG-FKP group showed the highest IFN-γ responses, it failed to control cutaneous lesions. Significant reductions in parasite numbers were observed in the MISA-FKP and BCG-FKP vaccinated groups (p < 0.001). There was a good correlation between parasite burden and IFN-γ level indicating IFN-γ response as a sensitive parameter of the immune status. In conclusion, MISA-FKP is the most efficacious vaccine formulation against murine cutaneous leishmaniasis.
Keywords: Cutaneous leishmaniasis; Formalin-killed promastigotes (FKP) promastigotes; Montanide ISA 720 (MISA); BCG; Alum; BALB/c mice; Immune responses.
RESUMO
Promastigotos mortos pela formalina (FKP) de Leishmania major combinados com Montanide ISA 720 (MISA), BCG ou alumen foram usados na vacinação de modelo murino cutâneo de leishmaniose (CL). Aumento significante e específico de resposta IgG anti FKP foram detectados tanto no FKP com alumen como naquele com BCG comparados ao MISA-FKP (p < 0,001). Aumento significante da proliferação esplênica de linfócitos de memória foi obtida nos camundongos vacinados com MISA-FKP quando comparados aos grupos vacinados com alumen-FKP ou BCG-FKP (p < 0,01). As maiores respostas por interferon-gama foram observadas no grupo BCG-FKP seguido pelo MISA-FKP enquanto que o alumen-FKP deu a menor resposta. No grupo MISA-FKP foram obtidas reduções significantes do tamanho das lesões quando comparado aos grupos vacinados com BCG/adjuvante de alumen-FKP. Embora o grupo BCG-FKP tenha mostrado a maior resposta por interferon-gama, não houve controle das lesões cutâneas. Redução significante no número de parasitas foi observada tanto no grupo vacinado com MISA-FKP como no BCG-FKP (p < 0,001). Houve boa correlação entre a carga parasitária e o nível de interferon-gama indicando que a resposta do interferon-gama é parâmetro sensível do estado imunológico. Em conclusão, MISA-FKP é a forma mais eficaz de vacina contra a leishmaniose cutânea murina.
INTRODUCTION
Leishmaniasis remains a major health problem, due to inefficiency of available control measures. To date, there is no vaccine against any form of leishmaniasis for general human use (KHAMESIPOUR et al., 2006). Current control measures rely on chemotherapy to alleviate disease and on vector control to reduce transmission. Protection from CL has been achieved through artificial infection, a technique called 'leishmanization' (GUIRGES, 1971). However, the use of live vaccines has had many problems including the development of large uncontrolled skin lesions, exacerbation of psoriasis and other skin diseases, and even immunosuppression (SEREBRYAKOV et al., 1972; MODABBER, 1975). Leishmania replicates intracellularly in macrophages, and effective control requires macrophage activation and nitric oxide (NO)-mediated killing in response to Th 1- produced cytokine IFN-γ (SCOTT et al., 1988; STACEY & BLACKWELL, 1999). Killed antigens that could be safer as vaccines generally require an adjuvant for induction of a strong Th1 immune response in murine models (KENNEY et al., 1999).
Formalin-killed promastigotes have been used in several studies as antigens in vaccine candidates against leishmaniasis (HOLBROOK et al., 1981; COOK & HOLBROOK, 1983; HOLBROOK & COOK, 1983). The availability of hundreds of different adjuvants has prompted a need for identifying rational standards for selection of adjuvant formulations based on safety and sound immunological principles for human vaccines (ALVING, 2002). The number of adjuvants currently approved for use in humans is quite limited and of those adjuvants that have been tested in humans, alum has had the greatest clinical use and is relatively nonreactogenic (KASLOW et al., 1994). Alum is currently the only Food and Drug Administration-approved adjuvant in clinical use in humans (TONUI et al., 2004). Aluminium salts are inexpensive, safe and simple to formulate (BOMFORD, 1989). Alum has been previously used in leishmania candidate vaccines alone or in combination with other adjuvants (KENNEY et al., 1999; MISRA et al., 2001; TONUI et al., 2004). Montanide ISA 720 (MISA 720) has been recommended by the manufacturer for clinical trials in humans (GOMEZ et al., 1999). This adjuvant has been used in malaria, HIV and cancer vaccine trials (KENNEY & EDELMAN, 2003) and has showed good responses (MYRIAM et al., 2005; OLIVEIRA et al., 2005; COLLINS et al., 2006).
Montanide ISA 720 adjuvant combined with recombinant histone-1 antigen was demonstrated to generate a durable cellular response that was sufficient to control infection in the majority of immunized Vervet monkeys (MASINA et al., 2003). However, despite the promising results, studies on Montanide ISA 720 as a potential adjuvant for Leishmania vaccines are very limited. The use of Bacille Calmette-Guerin (BCG) as an adjuvant is regarded as an acceptable practice in humans, and at present this adjuvant is routinely used in vaccination and immunotherapy trials against leishmaniasis (CONVIT et al., 1989; BAHAR et al., 1996; SHARIFI et al., 1998; MOMENI et al., 1999; KHALIL et al., 2000). Despite the availability of numerous studies on development and evaluation of candidate vaccines against leishmaniasis, very limited data is available on the use of formalin-killed promastigotes in these studies. Most studies have focused on the use of autoclaved L. major promastigotes with or without adjuvants. It is of common knowledge that heat (autoclaving) denatures protein and this may interfere with the appropriate conformational structure of important antigen epitopes resulting in less efficacious vaccines. This study reports a new protocol that utilizes the subcutaneous route of immunization with formalin-killed promastigotes with alum, BCG or Montanide ISA 720 as adjuvants in a vaccine against murine cutaneous leishmaniasis.
MATERIALS AND METHODS
Mice and parasites: Female BALB/c mice, 6-8 weeks old, were obtained from the breeding stock and maintained in the same rodent facility of the Institute of Primate Research (IPR) throughout the experimental period. The L. major strain, NLB-144, originally isolated from Phlebotomus duboscqui in Baringo District, Kenya, and kept in a virulent state by continuous passage in BALB/c mice, was used in this study. An aspirate from the foot of an infected BALB/c mouse was cultured in complete M199 medium (Sigma, St. Louis, Missouri) and incubated at 25 ºC. Stationary phase promastigotes were harvested by centrifugation at 2500 rpm for 15 minutes at room temperature. The resulting pellet was washed three times by centrifugation in sterile Phosphate Buffered Saline (PBS) and then enumerated in a haemocytometer before the parasites were used.
Preparation of soluble Leishmania antigen (SLA): Antigen was prepared following the method described (GICHERU et al., 1995). Promastigotes were washed briefly and sonicated at 20 kHz for four periods of 40 seconds each on ice, separated by intervals of one minute. The sonicated material was rapidly frozen and thawed three times in liquid nitrogen for extraction of whole soluble protein. The parasite suspension was centrifuged at 10,000g for 30 minutes at 4 °C. The protein concentration from the supernatant was determined using the Bio Rad protein assay kit (Bio Rad) and stored at -70 °C until use. This antigen was used for coating ELISA plates for antibody assay.
Preparation of formalin-killed Leishmania major antigens:Leishmania major promastigotes used for immunization were harvested at stationary phase and washed three times in sterile PBS as described previously. Parasites were killed by suspension in 0.1% formalin overnight and then washed three times in PBS. The dead promastigotes were counted by haemocytometer and resuspended in a concentration of 5 x 108/mL in sterile PBS and stored at -70 ºC until required. The criteria for the determination of promastigote death were the absence of parasite motility and failure of parasites to grow in vitro when inoculated into culture medium.
Adjuvants: Montanide ISA 720 (Seppic France), kindly provided by Dr. Nicolas Fasel of Lausanne University, Switzerland, alum and Bacille Calmette-Guerin (BCG; Pasteur Institute of Iran) were used as adjuvants along with formalin-killed Leishmania major promastigotes.
Immunization: Five groups of twelve male BALB/c mice were immunized as follows: Group 1 received alum plus Leishmania major formalin-killed promastigotes (FKP), group 2 received BCG plus FKP, group 3 was injected with FKP alone, group 4 received Montanide ISA 720 adjuvant (MISA) plus FKP and group 5 was injected with phosphate buffered saline (PBS) only and served as the negative control group. Two booster vaccinations of the same vaccine components and amount were given at four and six weeks following the initial vaccination. In all the vaccinated groups the amount of FKP per vaccine dose was 1 x 107. All the vaccines were reconstituted in PBS and given subcutaneously at the tailbase with a syringe and needle. The adjuvant dosages were 1 mg alum, 10 μL and 50 μL of Montanide ISA 720. In total, 300 μL of vaccine (promastigotes plus adjuvant) were injected to each experimental mouse. Two weeks after the third vaccination, mice from each group were either euthanized for the purpose of immunological assays or challenged with 1 x 106 virulent L. major parasites for vaccine efficacy assessment. The Institutional Animal Care and Use and Scientific Review committees of the Institute of Primate Research approved this experiment. All mice experiments were performed in a biocontainment facility.
Infection of immunized and control mice: Two weeks after the third vaccination six mice from each group were challenged with virulent L. major promastigotes. Stationary phase promastigotes were prepared as described previously and counted. The right hind footpad was swabbed with 70% alcohol and allowed to dry as described (MACHARIA et al., 2004). The footpad was infected by subcutaneously inoculating 50 μL of PBS containing 1 x 106 promastigotes. The thickness of the infected footpads was measured weekly for eight weeks using vernier caliper. Increase in footpad thickness was expressed as the difference between infected and the same noninfected footpad as described (SOLBACH et al., 1986).
Footpad parasitic load: To evaluate the relative efficacy of these vaccination protocols, challenged mice were euthanized and infected footpads removed between the ankle joint and toes and then homogenized with a tissue grinder in 3 mL of complete Schneider's Drosophila insect tissue medium. Under sterile conditions, serial dilutions including 0.5 mL (neat), 0.5 mL (1:2), 0.25 mL (1:4) and 0.125 mL (1:8) of the tissue homogenate were prepared in a total volume of 0.5 mL/well in wells of 48-well culture plates and incubated at 25 °C. Stationary phase (six days of incubation) parasites were counted by hemocytometer under a microscope and the total number of parasites in the original homogenate calculated using parasite counts in each dilution. The one-in-four dilutions were selected as the best dilution for the microtitration assay for data analysis.
T cell proliferation assay: Splenic cells were harvested aseptically and purified with percoll gradients (Sigma, St. Louis, Missouri) to obtain splenic lymphocytes (PBL). The cells were adjusted to 3 x 106/mL in complete RPMI-1640 (RPMI-1640 supplemented with 10% heat inactivated fetal bovine serum (Flow Laboratories, Irvin, UK) 2mM L-glutamine and 100 μg/mL gentamicin (Sigma) medium. One hundred microliters containing 3x106 cells/mL PBL in complete RPMI-1640 were distributed to each well of a 96-well round-bottomed microtiter plate (Nunc, Rosklide, Denmark) followed by the addition of 100 μL of formalin-fixed L. major antigens (5 x 106 /mL) or concanavalin A (con A; 10 μg/mL; Sigma). Control wells received 100 μL of complete RPMI 1640 medium. Cultures were set up in duplicates and incubated at 37 ºC in a humidified atmosphere containing 5% CO2 for five days for Leishmania antigen cultures and for three days for con A cultures. The cells were pulsed with 0.5μci of [Methyl-3H] thymidine (New England, Nuclear Boston, Massachusetts; 1.85 mBg/mL) over 18 hours and harvested on fibre glass filters (Titertek, Microtitration Equipment, UK). Incorporation of the radionuclide into DNA was determined by liquid scintillation spectrometry. Results were expressed as the stimulation index (SI) obtained by dividing the counts per minute of antigen or Con A by the counts per minute of the control cultures.
Determination of cytokines: Interferon gamma (IFN-γ) level was measured by enzyme-linked immunosorbent assay (ELISA). Purified splenic lymphocytes were adjusted to 3 x 106 cells/mL in complete RPMI 1640 medium and stimulated in vitro with either Con A or L. major antigens as described (OLOBO et al., 1992). Culture supernatants pooled from triplicate wells after 72 hours of stimulation were used to determine IFN-γ using mouse IFN-γ ELISA kit (Mabtech, AB, Sweden, Code 3321-1H-6) according to the manufacturer's instructions. Polystyrene micro-ELISA plates (Nunc, Copenhagen, Denmark) were coated overnight with 100 μL of monoclonal antibody, AN18 (1 μg/mL) to capture IFN-γ from supernatants of samples and recombinant mouse IFN-γ standard. Nonspecific binding sites were blocked with 3% bovine serum albumin (BSA) in PBS for one hour at room temperature. The plate was washed six times with washing buffer before the addition of 100 μL of supernatant samples or mouse IFN-γ standards and incubation for two hours at room temperature. One hundred micro liters of monoclonal IFN-γ detecting antibody, R4-6A2-biotin (0.5 μg/mL) were added per well and the plate incubated for one hour at room temperature. Streptavidin-HRP was added to the wells at a dilution of 1:1000 and the plate incubated for one hour at room temperature before the addition of 100 μL/well of Tetramethlebenzidine (TMB) microwell peroxidase substrate (KPL, Maryland). Optical densities were read at 630 nm in a micro-plate reader (Dynatech Laboratories, UK).
Measurement of Leishmania-specific antibody responses: The assay was performed as described (GICHERU et al., 1995). Polystyrene Micro-ELISA plates (Nunc, Copenhagen, Denmark) were coated overnight with 100 μL of Leishmania major soluble antigen at a concentration of 10 μg/mL, diluted in carbonate-bicarbonate buffer (pH 9.6). Nonspecific binding sites were blocked with 3% bovine serum albumin (BSA) in 0.01 M PBS (pH 7.2) for one hour at 37 °C. The plate was washed six times with washing buffer before the addition of 100 μL of the serum samples and incubation for two hours at 37°C. The plate was washed six times as above and 100 μL of 1:4000 horse radish-conjugated sheep anti-mouse IgG (Rockland Immunochemicals) was used as detecting antibody. Tetramethlebenzidine microwell peroxidase substrate (KPL, Maryland) was added to the wells and the plate incubated in the absence of light for 20 minutes before the optical densities were read at 630 nm in a micro-plate reader (Dynatech Laboratories, UK). All sera were tested at a dilution of 1:100 and the optical densities for different groups compared.
Statistical analysis: The differences in the levels of immune responses and protection were determined by one way ANOVA, Spearman's rank correlation and the Tukey-Kramer test. Differences were considered significant when p < 0.05.
RESULTS
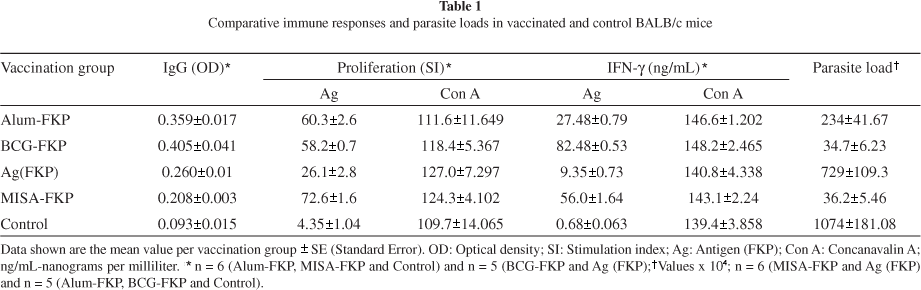
Antileishmanial antibodies: All vaccinated animals were clearly shown to be sero-positive when compared to the unvaccinated controls as indicated by the ELISA results. There was a significant difference in the production of L. major specific IgG between all the experimental and the negative control group with antibody responses being comparable and higher in the BCG-FKP and alum-FKP groups as compared to the MISA 720 vaccinated group (p < 0.001). The MISA-FKP and the FKP-alone vaccinated groups showed similar IgG responses (Table 1).
Lymphoproliferative responses: All the animals responded to Con A with minimal variation. There was no significant difference in response to Con A between the vaccinated and control animals indicating the viability of the cells used. The ability of splenic lymphocytes to respond in vitro to the FKP antigen was significantly higher for the three adjuvant-FKP vaccinated groups than for the FKP vaccinated group (p < 0.001). The MISA-FKP vaccinated group showed significantly higher antigen-specific lymphoproliferative responses as compared to both the alum-KLM (p < 0.01) and the BCG-FKP (p < 0.001) vaccinated groups. Responses in BCG-KLM and alum-FKP were similar (Table 1).
Interferon-γ in cell supernatants: Cell culture supernatants collected from Con A in vitro stimulation for all the groups showed no difference in their IFN-γ response (Table 1). Antigen stimulated culture supernatants from the vaccinated groups produced significantly higher levels of IFN-γ as compared to supernatants from the unvaccinated (negative control) group of mice. As compared to the FKP-alone vaccinated group, the adjuvant-FKP vaccinated groups produced significantly higher IFN-γ responses following stimulation with the FKP antigen (p < 0.0001). The BCG-FKP vaccinated mice produced IFN-γ responses that were significantly higher than that produced by the MISA-FKP vaccinated group (p < 0.001; Table 1) while IFN-γ responses induced by the MISA-FKP vaccinated animals were significantly higher than the levels produced by the alum-FKP vaccinated group (p < 0.001). Among the three adjuvants the BCG-FKP vaccinated mice produced the highest IFN-γ levels, followed by the MISA-FKP group with the alum-FKP recording the least quantities of the cytokine.
Parasite burden in footpads of mice after challenge: Significantly lower levels of parasite numbers were obtained from the vaccinated groups of mice as compared to the negative control animals. Similarly, the parasite loads were found to be significantly lower for all the adjuvant-FKP vaccinated groups when compared with the FKP group (Table 1). The BCG-FKP and MISA 720-FKP vaccinated groups showed no significant difference in parasite numbers with both groups recording significantly lower parasite numbers when compared to all the other groups (p < 0.001). Spearman's rank analysis of the mean parasite numbers and IFN-γ in the corresponding experimental and control groups indicated significant correlation between the two parameters.
Lesion measurements in Leishmania major challenged mice: There was delayed lesion development in the MISA-FKP vaccinated group at least up to the third week post-challenge when the sizes of the footpads of mice in this group gradually started to increase (Fig. 1). This group maintained the lowest footpad sizes throughout the experimental period. The BCG-FKP vaccinated group, unexpectedly, developed higher lesion sizes as compared to all the other vaccinated groups up to the seventh week when the lesion sizes in this group gradually reduced to lower levels when compared only to the FKP antigen group among the vaccinated mice. The alum-FKP and FKP groups showed intermediate lesion development between the BCG-FKP and MISA-FKP at least up to the seventh week post challenge when the FKP vaccinated group showed slightly elevated footpad swellings as compared to all the adjuvant-FKP vaccinated groups. The negative control group showed immediate response to the challenge infection by developing thicker footpads, which increased by 1.2 mm by the end of the first week post infection (Fig. 1). Eight weeks post challenge, the footpad thickness of the negative control mice had increased by an average of 3.5 mm, leading to marked swelling of the infected footpad. Spearman's rank correlation analysis showed no significant correlation between the mean lesion sizes measured at eight weeks post challenge and the mean IFN-γ of the corresponding vaccinated groups.
DISCUSSION
Results of the experiments described in this report demonstrate that injection of formalin-killed promastigotes with BCG induced the highest levels of antibody responses as compared with other adjuvants. This was not unexpected as BCG has been shown to be a strong stimulant of both cellular and humoral responses (SOHRABI et al., 2005). It was interesting to note that high antibody responses were accompanied by large cutaneous lesions. This is consistent with data that antibodies do not play a prominent role in protective immunity in leishmaniasis (OLOBO et al., 1980; HOWARD et al., 1984; GICHERU et al., 1995). Similarly to the BCG-FKP, vaccination using alum combined with FKP induced high antibody responses confirming previous findings that, alum-adjuvanted vaccines induce high and prolonged antibody responses (MISRA et al., 2001; EICKHOFF & MAYERS, 2002). Montanide ISA 720 with FKP failed to increase the antibody responses to the antigen. These results differ from earlier findings in which peak antibody responses and persistence of parasite-specific antibody following human vaccination with Montanide ISA 720 formulated with Plasmodium falciparum antigen were comparable to those obtained following immunization with the same antigen mixed with alum (OLIVEIRA et al., 2005).
Parasite specific recall lymphocyte proliferation was demonstrated in all of the experimental groups. Marked Con A stimulation was demonstrated in all of the animals and there was no significant difference in response to Con A in both the experimental and control animals, indicating that the cells used were viable and active. The adjuvants used here were able to immunopotentiate the FKP as all the adjuvant-FKP vaccinated groups had higher antigen specific recall proliferative responses when compared to the group vaccinated with FKP alone. Cells taken from mice vaccinated with MISA-FKP proliferated more when stimulated in vitro with formalin-fixed L. major promastigotes than cells taken from mice vaccinated with either alum-FKP or BCG-FKP. Our previous experience of the MISA 720 adjuvant + killed L. major vaccine in mice using the intraperitoneal route also gave the highest recall lympho-proliferative response than other adjuvants combined with the same antigen. This may indicate that Montanide ISA 720 adjuvant is more potent in immunopotentiating FKP in priming lymphocytes than both alum and BCG adjuvants.
Leishmania major studies in Vervet monkeys established that high levels of IFN-γ are produced in self cured animals (OLOBO et al., 1992). In the murine model of L. major infection it is now well established that protection depends on a cell-mediated immune response with expansion of a Th 1 subset of lymphocytes (LIEW et al., 1989; MOLL & MARTIN, 1990; SYPEK et al., 1993). The results of the current study clearly showed that high levels of IFN-γ were obtained in the BCG-FKP and MISA-FKP vaccinated groups of mice, with the BCG-FKP group producing significantly higher levels of the cytokine than the MISA-FKP group. Vaccination of mice with alum-FKP failed to produce protective levels of IFN-γ responses. This differs with previous studies where autoclaved L. major-alum vaccines have been shown to produce significant levels of IFN-γ and have been shown to be effective in treating persistent kala-azar dermal leishmaniasis (PKDL; MUSA et al,. 2008), although this may be an effect of the route of vaccination: intramuscular versus intra-dermal. However, the use of alum in combination with another adjuvant such as the BCG has contributed more to the high effectiveness of such vaccines (MISRA et al., 2001; KAMIL et al., 2003).
The good correlation obtained between the parasite burden and IFN-γ responses is an indication that resistance against L. major can only be achieved in vaccination protocols inducing high levels of IFN-γ. However, although BCG appears to be a better adjuvant as shown by the significantly higher IFN-γ responses in mice vaccinated with this adjuvant, it may lead to inflammatory reaction (SMRKOVSKI & LARSON, 1977; ALIMOHAMMADIAN et al., 2002) as evidenced by the largest cutaneous lesions recorded in the BCG group when compared to all other vaccinated groups. This may be the cause of failure to conclude significant correlation between the IFN-γ responses and lesion sizes. For this reason, the use of BCG as an adjuvant in vaccines needs to be discouraged. It would be equally important to discourage the use of lesion sizes as a parameter of evaluating vaccine efficacy in mice.
Another concern may be the route of immunization. In a previous study using the intraperitoneal route (data not shown), the outcome on the lesion size was better as compared to data on the present study. It is interesting to note that the subcutaneous route shows significantly reduced parasite numbers in both the BCG-FKP and MISA-FKP when these groups are compared with data obtained from the intraperitoneal immunization with BCG-FKP and MISA-FKP respectively. In conclusion, Montanide ISA 720 adjuvant appears to be the most appropriate for Leishmania vaccines due to its safety and ability to induce protective Th 1 responses when given by the subcutaneous route. It would be important to carry out further investigations on the potential use of Montanide ISA 720 as an adjuvant for Leishmania vaccine in non-human primate models. Such studies would give a guide in designing vaccination protocols for clinical trials in humans.
ACKNOWLEDGEMENTS
We acknowledge the Institute of Primate Research, Department of Animal Sciences for the provision and care of the mice used for this study.
Received: 5 September 2009
Accepted: 22 January 2010
- 1. Alimohammadian MH, Darabi H, Kariminia A, Rivier D, Bovay P, Mauel J, et al. Adjuvant effect of Leishmania major promastigotes on the immune response of mice to ovalbumin. Iran Biomed J. 2002;6:123-8.
- 2. Alving CR. Design and selection of vaccine adjuvants: animal models and human trials. Vaccine. 2002;20(suppl 3):56-64.
- 3. Bahar K, Dowlati Y, Shidani B, Alimohammadian MH, Khamesipour A, Ehsasi S. et al. Comparative safety and immunogenicity trial of two killed Leishmania major vaccines with or without BCG in human volunteers. Clin Dermatol. 1996;14:489-95.
- 4. Bomford R. Aluminium salts: perspectives in their use as adjuvants. In: Gregoriandis G, Allison AC, Poste G, editors. Immunological adjuvants and vaccines. NATO ASI Series A: Life sciences V. 179. Proceedings of a NATO Advanced Study Institute on Immunological Adjuvants and Vaccines, 24 June-5 July 1988, Cape Sounion Beach. New York: Plenum Press, 1989. p. 35-41.
- 5. Collins WE, Barnwell JW, Sullivan JS, Nace D, Williams T, Boungaseng A, et al. Assessment of transmission-blocking activity of candidate pvs25 vaccine using gametocytes from chimpanzees. Am J Trop Med Hyg. 2006;74:215-21.
- 6. Convit J, Castellanos PI, Ultrich M, Castes M, Rondon A, Pinardi ME, et al. Immunotherapy of localized, intermediate, and diffuse forms of American cutaneous leishmaniasis. J Infect Dis. 1989;160:104-15.
- 7. Cook JA, Holbrook TW. Immunogenicity of soluble and particulate antigens from Leishmania donovani: effect of glucan as an adjuvant. Infect Immun. 1983;40:1038-43.
- 8. Eickhoff TC, Mayers M. Workshop summary: aluminium in vaccines. Vaccine. 2002;20(suppl 3):S1-4.
- 9. Gicheru MM., Olobo JO, Kariuki TM, Adhiambo C. Visceral leishmaniasis in Vervet monkeys: immunological responses during asymptomatic infections. Scand J Immunol. 1995;41:202-8.
- 10. Gomez CE, Navea L, Lobaina L, Dubed M, Exposito N, Soto A, et al. The V3 loop based multi-epitope polypeptide TAB9 adjuvated with montanide ISA720 is highly immunogenic in nonhuman primates and induces neutralizing antibodies against five HIV-1 isolates. Vaccine. 1999;17:2311-9.
- 11. Guirges SY. Natural and experimental re-infection of man with Oriental sore. Ann Trop Med Parasitol. 1971;65:197-205.
- 12. Holbrook TW, Cook JA. Immunization of mice against Leishmania donovani by subcutaneous injections of dead promastigotes. Am J Trop Med Hyg. 1983,32:51-3.
- 13. Holbrook TW, Cook JA, Parker BW. Immunization against Leishmania donovani: Glucan as an adjuvant with killed promastigotes. Am J Trop Med Hyg. 1981;30:762-8.
- 14. Howard JG, Liew FY, Hale C, Nicklin S. Prophylactic immunization against experimental leishmaniasis II. Further characterization of the protective immunity against fatal L. tropica infection induced by irradiated promastigotes. J Immunol. 1984;132:450-5.
- 15. Kamil AA, Khalil EAG, Musa AM, Modabber F, Mukhtar MM, Ibrahim ME, et al. Alum-precipitated autoclaved Leishmania major plus Bacille Calmette-Guerrin, a candidate vaccine for visceral leishmaniasis: safety, skin-delayed type hypersensitivity response and dose finding in healthy volunteers. Trans R Soc Trop Med Hyg. 2003;97:365-8.
- 16. Kaslow DC, Bathurst IC, Lensen T, Ponnudurai T, Barr PJ, Keister DB. Saccharomyces cerevisiae recombinant Pfs25 adsorbed to alum elicits antibodies that block transmission of Plasmodium falciparum. Infect Immun. 1994;62:5576-80.
- 17. Kenney RT, Edelman R. Survey of human-use adjuvants. Expert Rev Vaccines. 2003;2:167-88.
- 18. Kenney RT, Sacks DL, Sypek JP, Vilela L, Gam AA, Evans-Davis K. Protective immunity using recombinant human IL-12 and alum as adjuvants in a primate model of cutaneous leishmaniasis. J Immunol. 1999;163:4481-8.
- 19. Khalil EAG, El Hassan AM, Zijlstra EE, Mukhtar MM, Ghalib HW, Musa B, et al. Autoclaved Leishmania major vaccine for prevention of visceral leishmaniasis: a randomized, double-blind, BCG-controlled trial in Sudan. Lancet. 2000;356:1565-9.
- 20. Khamesipour A, Rafati S, Davoudi N, Maboudi F, Modabber F. Leishmaniasis vaccine candidates for development: a global overview. Indian J Med Res. 2006;123:423-38.
- 21. Liew FY, Millott S, Li Y, Lelchuck R, Chan WL, Ziltener H. Macrophage activation by interferon-γ from host protective T cells is inhibited by IL-3 and IL-4 produced by disease promoting T cells in Leishmaniasis. Eur J Immunol. 1989;19:1227-32.
- 22. Macharia JC, Bourdichon AJ, Gicheru MM. Efficacy of trypan: a diminazene based drug as antileishmanial agent. Acta Trop. 2004;92:267-72.
- 23. Masina S, Gicheru MM, Demotz SO, Fasel NJ. Protection against cutaneous leishmaniasis in outbred Vervet monkeys, using a recombinant histone-1 antigen. J Infect Dis. 2003;188:1250-7.
- 24. Misra A, Dube A, Srivastava B, Sharma P, Srivastava JK, Katiyar JC, et al. Successful vaccination against Leishmania donovani infection in Indian langur using alum-precipitated autoclaved Leishmania major with BCG. Vaccine 2001;19:3485-92.
- 25. Modabber F. Vaccines against leishmaniasis. Ann Trop Med Parasitol. 1995;89(suppl 1):83-8.
- 26. Moll H, Martin R. Resistance to murine cutaneous leishmaniasis is mediated by T H l cells, but disease-promoting CD4+ cells are different from TH2 cells. Eur J Immunol. 1990;20:2067-74.
- 27. Momeni AZ, Jalayer T, Emamjomeh M, Khamesipour A, Zicker F, Ghassemi RL, et al. A randomised, double-blind, controlled trial of a killed L. major vaccine plus BCG against zoonotic cutaneous leishmaniasis in Iran. Vaccine. 1999;17:466-72.
- 28. Musa AM, Khalil EAG, Mahgoub FA, Elgawi SH, Modabber F, Elkadaru AE, et al. Immunochemotherapy of persistent post kala-azar dermal leishmaniasis: a novel approach of treatment. Trans R Soc Trop Med Hyg. 2008;102:58-63.
- 29. Myriam A, Angelica C, Syed SY, Ahmad RS, Chetan ChE, Rosalie D, et al Immunogenicity and protective efficacy of recombinant vaccine based on the receptor-binding domain of the Plasmodium vivax duffy binding protein in Aotus monkeys. Am J Trop Med Hyg. 2005;73(suppl 5):25-31.
- 30. Oliveira GA, Wetzel K, Calvo-Calle JM, Nussenzweig R, Schmidt A, Dubovsky F, et al. Safety and enhanced immunogenicity of a hepatitis B core particle Plasmodium falciparum malaria vaccine formulated in adjuvant montanide ISA 720 in a phase I trial. Infect Immun. 2005;73:3585-97.
- 31. Olobo JO, Handman E, Curtis JM, Mitchell GF. Antibodies to Leishmania tropica promastigotes during infection in mice of various genotypes. Aust J Exp Biol Med Sci. 1980;58:595-601.
- 32. Olobo JO, Reid GDF, Githure JI, Anjili CO. IFN-γ and delayed type hypersensitivity are associated with cutaneous leishmaniasis in vervet monkeys following secondary rechallenge with Leishmania major Scand J Immunol. 1992;36(suppl 11):48-52.
- 33. Scott P, Natovitz P, Coffman RL, Pearce E, Sher A. Immunoregulation of cutaneous leishmaniasis: T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988;168:1675-84.
- 34. Serebriakov VA, Karakhodzbaeva SKH, Dzhumaev MD. Effect of leishmanial vaccinations on the dynamics of immunity to diphteria in conditions of secondary revaccination with adsorbed pertussis-diphteria-tetanus vaccine. Med Parazitol. (Mosk.). 1972;41:303-9.
- 35. Sharifi I, FeKri AR, Aflatonian MR, Khamesipour A, Nadim A, Mousavi MR, et al. Randomised vaccine trial of single dose of killed Leishmania major plus BCG against anthroponotic cutaneous leishmaniasis in Bam, Iran. Lancet. 1998;351:1540-3.
- 36. Smrkovski LL, Larson CL. Effect of treatment with BCG on the course of visceral leishmaniasis in BALB/c mice. Infect Immun. 1977;16:249-57.
- 37. Sohrabi Y, Jaafari MR, Mohammadi AM, Eskandari SE, Khamesipour A. Evaluation of immune response against leishmaniasis in resistance C57 BL/6 mice immunized with liposomes containing autoclaved Leishmania major with BCG. Cell Mol Biol Lett. 2005;10:S98.
- 38. Solbach W, Forberg K, Rollinghoff M. Effect of T-Lymphocyte suppression on the parasite burden in Leishmania major-infected, genetically susceptible BALB/c mice. Infect Immun. 1986;54:909-12.
- 39. Stacey KJ, Blackwell JM. Immunostimulatory DNA as an adjuvant in vaccination against Leishmania major. Infect Immun. 1999;67:3719-26.
- 40. Sypek JP, Chung CL, Mayor SE, Subramanyan JM, Goldman SJ, Sieburth DS, et al Resolution of cutaneous leishmaniasis: Interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797-802.
- 41. Tonui WK, Mejia JS, Hochberg L, Mbow ML, Ryan JR, Chan AST, et al Immunization with Leishmania major exogenous antigens protects susceptible BALB/c mice against challenge infection with Leishmania major Infect Immun. 2004;72:5654-61.
Publication Dates
-
Publication in this collection
23 Apr 2010 -
Date of issue
Apr 2010
History
-
Accepted
22 Jan 2010 -
Received
05 Sept 2009