Abstracts
An autochthonous case of visceral leishmaniasis is reported in a dog (Canis familiaris) as an apparently natural infection in a non-endemic area. DNA obtained from spleen and liver samples produced the expected fragment in a Leishmania-specific rDNA-based nested-PCR assay. The PCR product, a 490 bp fragment, was sequenced and the nucleotide sequence was identical to that of Leishmania (Leishmania) infantum chagasi. These results are surprising since no autochthonous human or canine cases of visceral leishmaniasis have ever been reported in this municipality. This case suggests that natural transmission of this disease is occurring in this area.
Visceral leishmaniasis; PCR; Epidemiology; Dogs; São Paulo; Brazil
Caso autóctone de leishmaniose visceral é relatado em cão (Canis familiaris), aparentemente em área não endêmica. DNA obtido a partir de amostras do baço e fígado foram submetidos a nested-PCR baseada no rDNA específico de Leishmania. Os produtos das PCR foram sequenciados e os 490 pares de base foram idênticos a Leishmania (Leishmania) infantum chagasi. Esses resultados são surpreendentes, uma vez que, nenhum caso autóctone canino ou humano de leishmaniose visceral havia sido relatado neste município. Esse caso sugere que a transmissão natural da doença está ocorrendo nesta área.
BRIEF COMMUNICATION
First occurrence of an autochthonous canine case of Leishmania (Leishmania) infantum chagasi in the municipality of Campinas, State of São Paulo, Brazil
Ocorrência do primeiro caso autóctone canino por Leishmania (Leishmania) infantum chagasi no Município de Campinas, Estado de São Paulo, Brasil
Elisa San Martin Mouriz SavaniI; Douglas PresottoII; Thais RobertoIII; Maria Cecília Gibrail de Oliveira CamargoI; Sandra Regina Nicoletti D'auriaI; Débora Veiga SacramentoIV
ICentro de Controle de Zoonoses do Município de São Paulo, São Paulo, SP, Brazil
IICentro de Controle de Zoonoses do Município de Campinas, Campinas, SP, Brazil
IIIHospital Veterinário Taquaral, Campinas, SP, Brazil
IVGenomic Engenharia Molecular, São Paulo, SP, Brazil
Correspondence to Correspondence to: Elisa San Martin Mouriz Savani Centro de Controle de Zoonoses do Município de São Paulo Rua Santa Eulália 86 02031-020 São Paulo, SP, Brasil Fax: +55 11 2251 2249 E-mail: elisa@prefeitura.sp.gov.br
SUMMARY
An autochthonous case of visceral leishmaniasis is reported in a dog (Canis familiaris) as an apparently natural infection in a non-endemic area. DNA obtained from spleen and liver samples produced the expected fragment in a Leishmania-specific rDNA-based nested-PCR assay. The PCR product, a 490 bp fragment, was sequenced and the nucleotide sequence was identical to that of Leishmania (Leishmania) infantum chagasi. These results are surprising since no autochthonous human or canine cases of visceral leishmaniasis have ever been reported in this municipality. This case suggests that natural transmission of this disease is occurring in this area.
Keywords: Visceral leishmaniasis; PCR; Epidemiology; Dogs; São Paulo, Brazil.
RESUMO
Caso autóctone de leishmaniose visceral é relatado em cão (Canis familiaris), aparentemente em área não endêmica. DNA obtido a partir de amostras do baço e fígado foram submetidos a nested-PCR baseada no rDNA específico de Leishmania. Os produtos das PCR foram sequenciados e os 490 pares de base foram idênticos a Leishmania (Leishmania) infantum chagasi. Esses resultados são surpreendentes, uma vez que, nenhum caso autóctone canino ou humano de leishmaniose visceral havia sido relatado neste município. Esse caso sugere que a transmissão natural da doença está ocorrendo nesta área.
INTRODUCTION
Visceral leishmaniasis is a vector-borne disease mainly characterized by spleen and liver enlargement. In the Americas, this zoonosis is caused by Leishmania (Leishmania) infantum chagasi10 and domestic dogs are an important animal reservoir.
American visceral leishmaniasis (AVL) is showing a gradual spread throughout the State of São Paulo. The first autochthonous canine and human cases3 were verified at Araçatuba county region. Other areas of transmission, such as the cities of Cotia and Embu5,6 have been identified. Usually, canine visceral leishmaniasis precedes human cases3, thus the detection of new areas of transmission in animals is crucial for the epidemiological surveillance of leishmaniasis and the control program of AVL in the State of São Paulo.
MATERIALS AND METHODS
In September 2009, a 9 year-old domestic female dog, called Athena, was taken to a local veterinary hospital. The dog had lost both weight and muscle mass, presented a cough, increased lymphatic ganglia, liver and spleen, onychogryphosis and skin lesions.
The dog was born and raised in the metropolitan area of Campinas, State of São Paulo, Brazil. It lived in a residential complex built in an area of environmental protection between Sousas and Joaquim Egidio sub-districts, in the eastern zone of the municipality. The region presents topography and vegetation characteristics that favor the presence of sandflies.
Athena was euthanized at the veterinary hospital, at the request of the owner, and sent to the Campinas Zoonosis Control Center for autopsy.
Spleen and liver samples and bone marrow and lymph node aspirates were sent to the São Paulo Zoonosis Control Center for parasitological and molecular tests.
Tissue samples were inoculated into blood agar base culture medium with brain heart infusion and incubated at 23 °C during one month.
DNA from every sample was extracted1 and submitted to a nested SSU rDNA-based PCR to detect and identify the parasite7. Reactions took place in a final volume of 50 µL containing 1X PCR buffer, 2 mM MgCl2, 0.2 mM dNTPs, 0.2 µM of each primer and 2U of Taq DNA polymerase (Life Technologies). The first PCR was performed using S4 and S12 primers11. DNA (about 20 ng) was first denatured at 94 °C for three minutes and then cycled 35 times at 94 °C for one min, 50 °C for one min and 72 °C for one min. A final extension of seven min was performed at 72 °C. The amplified products were analyzed by electrophoresis in 2% agarose gel stained with ethidium bromide.
The 520 bp fragment produced by S4/S12 PCR was used as the template in a nested-PCR with primers S17 and S188. The reactions were performed under the same conditions as those described above.
The S4/S12 PCR product (1 µL) was denatured at 94 °C for four min and cycled 30 times; each cycle was performed at 94 °C for one min, 55 °C for one min and 72 °C for 30 sec.
The nested-PCR product, a 490 bp fragment, was purified and sequenced. Sequencing reactions were performed using ABI BigDye Terminator v3.1 (Applied Biosystems, Foster City, CA, USA), through 25 cycles (96 ºC/10 sec, 50 ºC/5 sec and 60 ºC/4 min). Direct sequencing of the purified amplicons was performed using an ABI 3130-XL sequencer (Applied Biosystems, Carlsbad, CA, USA). Sequences were analyzed using the BLAST program (http://www.ncbi.nlm.nih.gov/blast.html) to confirm amplification of the specific product. Sequences were imported into BioEdit4 compared with the rDNA sequence of reference strains of Leishmania as described by ULIANA et al. (1991)11.
RESULTS AND DISCUSSION
The serum sample was positive at a 1:40 dilution by indirect fluorescence test for leishmaniasis at the Tecsa Laboratory.
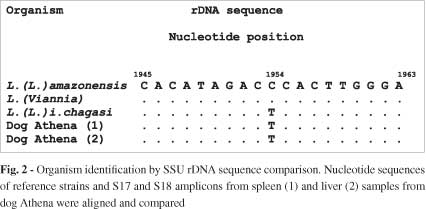
No parasites were detected in any of the samples inoculated into the culture medium. Otherwise, liver and spleen samples and bone marrow and lymph node aspirates were positive for Leishmania when using the nested SSU-rDNA-based PCR strategy (Fig. 1). The fragments produced by S17 and S18 oligonucleotides were sequenced and identified as Leishmania (Leishmania) infantum chagasi parasite (Fig. 2).
The municipality of Campinas was previously classified as silent, nonresponsive and not vulnerable to AVL2. Based on the present results, a research focus was initiated in the same area where the dog Athena lived, involving blood collection from 198 dogs. This led to the identification of three other dogs with AVL and recorded the first evidence of Lutzomyia longipalpis in the city of Campinas9. The Study Committee on Leishmaniosis had already reclassified Campinas as a transmission area for canine AVL, although in a very circumscribed zone.
These findings indicate that entomological and canine surveillance must be maintained in Campinas to detect new cases of AVL in dogs to prevent and control this zoonosis. In this context it is also important to raise awareness in health professionals to the possible occurrence of human cases of AVL in order to indicate early treatment.
ACKNOWLEDGEMENTS
This work received financial support from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) No. 06/60575-3.
Received: 1 September 2010
Accepted: 6 May 2011
- 1. Castilho TM, Shaw JJ, Floeter-Winter LM. New PCR assay using glucose-6-phosphate dehydrogenase for the identification of Leishmania species. J Clin Microbiol. 2003;41:540-6.
- 2. Centro de Controle de Doenças. Grupo de Estudos em Leishmanioses. Atualização da classificação epidemiológica dos municípios para Leishmaniose Visceral Americana. Bol Epidemiol Paul. 2008;5:18-25.
- 3. Galimbertti MZ, Katz G, Camargo-Neves VLF, Rodas LAC, Casanova C, Costa AL et al Leishmaniose visceral americana no Estado de São Paulo. Rev Soc Bras Med Trop. 1999;32(Suppl 1):217.
- 4. Hall T A. BioEdit: a user friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95-8.
- 5. Savani ES, Neves E, D'Auria SR, Zampieri RA, Ishikawa E, Camargo MCGO, et al Autochthonous visceral leishmaniasis in dogs of Embu-das-Artes, São Paulo. Rev Inst Med Trop Sao Paulo. 2003;45(Suppl13):166.
- 6. Savani ES, de Oliveira Camargo MC, de Carvalho MR, Zampieri RA, dos Santos MG, D'Auria SR, et al. The first record in the Americas of an autochthonous case of Leishmania (Leishmania) infantum chagasi in a domestic cat (Felix catus) from Cotia County, Sao Paulo State, Brazil. Vet Parasitol. 2004;120:229-33.
- 7. Savani ES, Nunes VL, Galati EA, Castilho TM, Araujo FS, Ilha IM, et al Occurrence of co-infection by Leishmania (Leishmania) chagasi and Trypanosoma (Trypanozoon) evansi in a dog in the state of Mato Grosso do Sul, Brazil. Mem Inst Oswaldo Cruz. 2005; 100:739-41.
- 8. Savani ES, Nunes VL, Galati EA, Castilho TM, Zampieri RA, Floeter-Winter LM. The finding of Lutzomyia almerioi and Lutzomyia longipalpis naturally infected by Leishmania spp. in a cutaneous and canine visceral leishmaniases focus in Serra da Bodoquena, Brazil. Vet Parasitol. 2009;160:18-24.
- 9. Secretaria de Estado da Saúde. Comitê de Leishmaniose Visceral Americana. Classificação epidemiológica dos municípios segundo o Programa de Vigilância e Controle da Leishmaniose Visceral Americana no Estado de São Paulo, atualizado em maio de 2010. Bol. Epidemiol. Paul. 2010;7(77):21-40.
- 10. Shaw JJ. Further thoughts on the use of the name Leishmania (Leishmania) infantum chagasi for the aetiological agent of American visceral leishmaniasis. Mem Inst Oswaldo Cruz. 2006;101:577-9.
- 11. Uliana, SRB, Affonso, MH, Camargo, EP, Floeter-Winter, LM. Leishmania: genus identification based on a specific sequence of the 18S ribosomal RNA sequence. Exp. Parasitol. 1991;72:157-63.
Publication Dates
-
Publication in this collection
05 Sept 2011 -
Date of issue
Aug 2011
History
-
Received
01 Sept 2010 -
Accepted
06 May 2011