Abstracts
Twelve strains of Trypanosoma cruzi isolated from wild reservoirs, triatomines, and chronic chagasic patients in the state of Paraná, southern Brazil, and classified as T. cruzi I and II, were used to test the correlation between genetic and biological diversity. The Phagocytic Index (PI) and nitric-oxide (NO) production in vitro were used as biological parameters. The PI of the T. cruzi I and II strains did not differ significantly, nor did the PI of the T. cruzi strains isolated from humans, triatomines, or wild reservoirs. There was a statistical difference in the inhibition of NO production between T. cruzi I and II and between parasites isolated from humans and the strains isolated from triatomines and wild reservoirs, but there was no correlation between genetics and biology when the strains were analyzed independently of the lineages or hosts from which the strains were isolated. There were significant correlations for Randomly Amplified Polymorphic Deoxyribonucleic acid (RAPD) and biological parameters for T. cruzi I and II, and for humans or wild reservoirs when the lineages or hosts were considered individually.
Trypanosoma cruzi; Genetic lineages; Phagocytic index; Infectivity in vitro; Nitric oxide
Doze cepas de Trypanosoma cruzi isoladas de reservatórios silvestres, triatomíneos e de pacientes chagásicos crônicos do Estado do Paraná, Brasil, classificadas como Tc I e II foram usadas para avaliar a correlação entre genética e diversidade biológica. Índice fagocítico (IF) e produção de óxido nítrico (ON) in vitro foram os parâmetros biológicos utilizados. O IF de cepas T. cruzi I e II não diferiram significativamente assim como o IF de cepas isoladas de humanos, triatomíneos ou de reservatórios silvestres. Há diferença estatística na inibição da produção de ON entre T. cruzi I e II e entre parasitos isolados de humanos e de cepas isoladas de triatomíneos e reservatórios silvestres, mas não foi observada correlação entre genética e biologia quando as cepas foram analisadas independentemente da linhagem ou hospedeiros das quais elas foram isoladas. Observou-se correlação significativa para amplificação aleatória do DNA polimórfico e parâmetros biológicos de Tc I ou II e para os seres humanos ou reservatório silvestre quando linhagens ou hospedeiros são consideradas separadamente.
TRYPANOSOMIASIS
Induction of phagocytic activity and nitric-oxide production in natural populations of Trypanosoma Cruzi I and II from the state of Paraná, Brazil
Indução da atividade fagocitária e produção de óxido nítrico numa população natural de Trypanosoma cruzi I e II do Estado do Paraná, Brasil
Leila ZalloumI; Eliane Raquel Peres LalaI; Neide Martins MoreiraI; Thaís Gomes Verzignassi SilveiraII; Márcia Machado de Oliveira DalálioIII; Max Jean de Ornelas ToledoI; Mônica Lúcia GomesI; Silvana Marques de AraújoI
IDepartamento de Ciências Básicas da Saúde, Laboratório de Parasitologia, Bloco I-90, Universidade Estadual de Maringá, PR, Brazil
IIDepartamento de Análises Clínicas, Laboratório de Imunologia Clínica, Universidade Estadual de Maringá, PR, Brazil
IIIDepartamento de Ciências Básicas da Saúde, Laboratório de Imunologia, Universidade Estadual de Maringá, Av. Colombo 5790, C.P. 331, 87020-900 Maringá, Paraná, Brazil
Correspondence Correspondence to: Silvana Marques de Araújo, Departamento de Ciências Básicas da Saúde, Laboratório de Parasitologia Universidade Estadual de Maringá, Av. Colombo 5790, C.P. 331, 87020-900 Maringá, Paraná, Brasil. Phone: +55-21-44-32618986; Fax: +55-21-44-32614860. E-mail: smaraujo@uem.br
SUMMARY
Twelve strains of Trypanosoma cruzi isolated from wild reservoirs, triatomines, and chronic chagasic patients in the state of Paraná, southern Brazil, and classified as T. cruzi I and II, were used to test the correlation between genetic and biological diversity. The Phagocytic Index (PI) and nitric-oxide (NO) production in vitro were used as biological parameters. The PI of the T. cruzi I and II strains did not differ significantly, nor did the PI of the T. cruzi strains isolated from humans, triatomines, or wild reservoirs. There was a statistical difference in the inhibition of NO production between T. cruzi I and II and between parasites isolated from humans and the strains isolated from triatomines and wild reservoirs, but there was no correlation between genetics and biology when the strains were analyzed independently of the lineages or hosts from which the strains were isolated. There were significant correlations for Randomly Amplified Polymorphic Deoxyribonucleic acid (RAPD) and biological parameters for T. cruzi I and II, and for humans or wild reservoirs when the lineages or hosts were considered individually.
KEYWORDS:Trypanosoma cruzi; Genetic lineages; Phagocytic index; Infectivity in vitro; Nitric oxide.
RESUMO
Doze cepas de Trypanosoma cruzi isoladas de reservatórios silvestres, triatomíneos e de pacientes chagásicos crônicos do Estado do Paraná, Brasil, classificadas como Tc I e II foram usadas para avaliar a correlação entre genética e diversidade biológica. Índice fagocítico (IF) e produção de óxido nítrico (ON) in vitro foram os parâmetros biológicos utilizados. O IF de cepas T. cruzi I e II não diferiram significativamente assim como o IF de cepas isoladas de humanos, triatomíneos ou de reservatórios silvestres. Há diferença estatística na inibição da produção de ON entre T. cruzi I e II e entre parasitos isolados de humanos e de cepas isoladas de triatomíneos e reservatórios silvestres, mas não foi observada correlação entre genética e biologia quando as cepas foram analisadas independentemente da linhagem ou hospedeiros das quais elas foram isoladas. Observou-se correlação significativa para amplificação aleatória do DNA polimórfico e parâmetros biológicos de Tc I ou II e para os seres humanos ou reservatório silvestre quando linhagens ou hospedeiros são consideradas separadamente.
doi: 10.1590/S0036-46652011000500002
INTRODUCTION
Chagas' disease, the etiologic agent of which is Trypanosoma cruzi, is a serious public-health problem in Latin America, where around eight million people are infected by the parasite42. In the United States, 50 to 100 thousand people are infected, and in Brazil, 1.9 to 3.5 million35. T. cruzi is a digenetic flagellate protozoan of the order Kinetoplastida, family Trypanosomatidae, that circulates in nature among humans, vectors, and wild and domestic reservoirs. The interaction of the parasite with natural reservoirs and triatomine bugs is known as the wild or sylvatic transmission cycle of the parasite. The colonization of non-natural habitats by triatomine vectors allowed T. cruzi to infect humans and domestic mammals, resulting in a domestic transmission cycle15.
Trypanosoma cruzi is composed of a variety of subpopulations with different characteristics. Natural populations of the parasite display great biological, biochemical, immunological, and genetic heterogeneity5,9,22,26,29,34,44. This heterogeneity, together with the genetic characteristics of the host, may explain the clinical variability of Chagas' disease and the local differences in morbidity14. The relationship between Trypanosoma cruzi and humans, at least to some extent, appears to be a successful adaptation of an infectious agent that survives in the host's body, occasionally causing harm45. In addition to the parasite's genetic lineage, the host's genetic makeup seems to have a marked influence on the biological behavior of T. cruzi8.
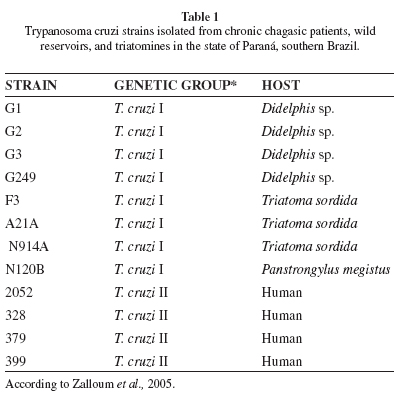
Two main evolutionary lineages, T. cruzi I and T. cruzi II, have been identified by different methods4,21. A third lineage, termed T. cruzi III, also exists. The T. cruzi III lineage is equivalent to the sublineage called TCIIc identified by BRISSE et al.10, who proposed five subdivisions for DTU (discrete typing unit) II, termed IIa-e. Although the present consensus is to refer to six DTUs (TcI-VI) for these strains52, many studies have investigated the genetic-biological relationship in terms of virulence in mice, transmissibility by triatomines, infectivity to cell cultures, and drug sensitivity in vitro and in vivo, using populations belonging to the two lineages that were formerly distinguished as T. cruzi I and II23,24,30,39,46. Populations of T. cruzi from northern and northwestern areas of the state of Paraná, Brazil have been determined to belong to these two major genetic lineages51. Strains isolated from triatomines and wild reservoirs belong to T. cruzi I, and isolates from humans belong to T. cruzi II.
Characteristics of T. cruzi natural populations related to the growth kinetics of epimastigotes, metacyclogenesis, infectivity to mammal cells, and susceptibility to trypanocidal drugs can be considered natural markers of the parasites' heterogeneity as well as of the genetic characteristics of these populations28. Although macrophages play a crucial role in the immune response to flagellate infection, the infectivity of T. cruzi strains may be related to the capacity of the parasite to evade the macrophages' effector mechanisms, and nitric-oxide production is an important mechanism that restricts the replication of intracellular parasites. Together with the phagocytic index, nitric-oxide production is a characteristic of each isolate, and is therefore a useful parameter for comparing strains13,17,18,25.
In this study, we determined the macrophage phagocytic index (PI) and nitric-oxide production of natural populations of T. cruzi isolated from wild reservoirs, triatomines, and humans, belonging to the T. cruzi I and II lineages from Paraná, and evaluated the existence of any correlation between genetic diversity and the PI and NO production.
MATERIALS AND METHODS
Parasite strains: Table 1 shows the T. cruzi strains isolated in Paraná, their genetic lineages, and hosts. The trypomastigote forms used in the macrophage infections were obtained on day 8 of culture in LIT (Liver Infusion Tryptose) medium. The total counts ranged from 6.8 to 9.3 x 107 parasites/mL, and the differential counts ranged from 2.4 to 7.0% trypomastigotes for the strains used. Because the strains of T. cruzi isolated in Paraná show a low rate of metacyclogenesis in LIT medium, with little variation among them4, macrophages were placed in contact together with trypomastigotes and epimastigotes without purification of the trypomastigotes. For the biological characterization, the thawed strains were maintained in LIT medium until they reached the exponential growth stage.
Cells: Peritoneal macrophages were obtained from female BALB/c mice, 60 days old. Mice were inoculated intraperitoneally (i.p.) with 1 mL of sterile thioglycolate broth four days prior to the assay. Ketamine hydrochloride (50 mg/Kg) and xylazine (10 mg/Kg) i.p. were used to euthanize the mice. The peritoneal macrophages were harvested by washing the peritoneal cavity with cold phosphate-buffered saline (PBS).
Macrophage infection and phagocytic index (PI): In 24-well plates, 5 x 105 macrophages were placed on sterile glass coverslips. Non-adherent cells were removed by several washes with Roswell Park Memorial Institute medium (RPMI), and the plates were maintained at 37 °C in a 5% CO2 incubator. The T. cruzi parasites (trypomastigotes and epimastigotes) were incubated with macrophages (in order to have two trypomasigotes/macrophage) for four h in RPMI medium without serum at 37 °C. Non-adherent parasites were removed by washing the monolayers with RPMI medium. Infected macrophages were maintained in RPMI 1640 with 5% fetal calf serum at 37 ºC in a 5% CO2 incubator for 20 h. The macrophages were stained with HEMA 3 Stain (Biochemical Science, Inc., USA). The phagocytic index (PI) was determined by multiplying the percentage of macrophages that had phagocytosed at least one parasite by the mean numbers of parasites per infected macrophage, as described by BUCHI & DE SOUZA11,12. Three hundred cells were examined. The phagocytic index (PI) was determined for the genetic lineages and hosts. The assays were carried out in quadruplicate.
Nitric oxide (NO) determination: For the NO production assays, the macrophages were stimulated with LPS (50 ng/mL) (Sigma L6511, Steinheim, Germany) four h before, during, and four h after the addition of the parasites. Two controls were used: uninfected macrophages and infected macrophages without LPS stimulation. The plates were maintained at 37 °C in a CO2 incubator for 20 h. The assays were carried out in triplicate.
NO production was indirectly evaluated in the supernatant from the macrophage cultures, by the determination of nitrites16. 50 mL of cell-culture supernatant was incubated with the same volume of Griess reagent (1% sulfanilamide, 0.1% naphthyldiamine dihydrochloride, and 2.5% orthophosphoric acid), for 15 min at ambient temperature. The absorbance was determined in a spectrophotometer (Microplate Fluorescence Reader FL-600) at 530 hm. The nitrite concentration was calculated based on a standard curve (5, 10, 30, 60 mM) of sodium nitrite (NaNO2) in culture medium.
Genetic characterization: The genetic characterization was carried out using RAPD and SSR-PCR51.
Statistical analyses: The nonparametric Mann-Whitney test and the means test were used to compare the phagocytic index in relation to the genetic lineage to which the strains belonged. The NO production was compared between the genetic lineages by analysis of variance and Tukey test. The NO production was compared between the lineages and the controls using Student´s t-test. The Statistica 6.0 program was used.
The nonparametric Mantel test (SAS Program - Statistical Analysis System - version 8.02) was used to evaluate the existence of correlations between genetic diversity and the phagocytic index or NO production. Mantel's Test27 estimates the correlation (association) between two squared symmetrical distance matrices obtained from different measurements (type of data, observations) of the same group of elements42. Genetic distance matrices between strains, taken two by two, for RAPD and SSR-PCR data were obtained by the arithmetic complement of Jaccard's similarity coefficient. For biological distance analyses, an equal score was assigned to each biological parameter, with 1 the highest value, and the other values in proportion. To construct the biological data matrix, the values were weighted so that the results were expressed in proportion to the highest value obtained for each parameter. Next, a mean of all the weighted parameters for each strain was calculated. The biological distance between two strains was calculated by the difference of their means. Genetic distance matrices between strains, taken two by two, for RAPD and SSR-PCR data were obtained by the arithmetic complement of Jaccard's similarity coefficient (1 - the coefficient). The significance level was 5% for all the analyses.
Ethics Committee Evaluation: The Ethics Committee for Animal Experiments (CEEA) of the State University of Maringá approved this study.
RESULTS
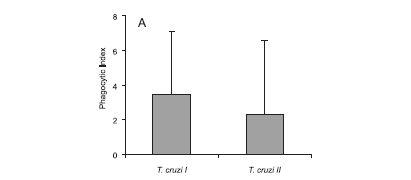
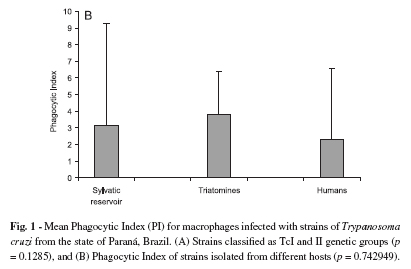
Phagocytic Index (PI) for macrophages infected with T. cruzi I and II strains isolated from different hosts: The PIs for the strains belonging to the T. cruzi I lineage ranged from 1.77 to 15.02, and the PIs for the T. cruzi II lineage strains ranged from 2.10 to 10.46. No significant difference (p = 0.1285) was observed between the mean phagocytic indexes for the genetic lineages of strains T. cruzi I (3.47) and II (2.32) (Fig. 1A). A statistically significant T. cruzi I intra-lineage variation was observed in PI (p = 0.0251), but not for T. cruzi II (p = 0.116). In the T. cruzi I group, 50% of the strains (G1, A21A, N120B and N914A) displayed a PI ranging from 5 to 15.0, and for the other strains the PI ranged from 1.77 to 2.89. In the T. cruzi II group, only one strain (399) displayed a discrepant PI.
The PIs for the strains isolated from humans ranged from 1.84 to 10.48. Among the strains isolated from triatomines, the PIs ranged from 1.77 to 8.02, while the PIs of the T. cruzi strains isolated from wild reservoirs ranged from 2.44 to 15.02. There was no significant difference (p = 0.7429) among the PIs of T. cruzi strains isolated from different hosts (Fig. 1B). The mean PIs of T. cruzi strains isolated from human, triatomines, and wild reservoirs were 2.33, 3.80, and 3.12 respectively. The PIs were not statistically significant for T. cruzi strains isolated from a specific host (humans, p = 0.08; triatomines, p = 0.1116; wild reservoirs, p = 0.0719).
NO production by macrophages infected with T. cruzi I and II strains isolated from different hosts: The parasites from the T. cruzi I genetic lineage were able to inhibit NO production by the macrophages stimulated with LPS prior to (p < 0.000) or during the parasite infection (p < 0.000), but not by macrophages stimulated after the infection. The parasites from the T. cruzi II genetic lineage inhibited NO production in all three periods. The difference in inhibition of NO production between the lineages was significantly greater for T. cruzi I parasites only when the LPS stimulation was carried out prior to the infection (p = 0.013) (Fig. 2A). A statistically significant intra-lineage variation in NO production was observed for T. cruzi I (p = 0.003), but not for T. cruzi II (p = 0.079). Figure 2B shows the NO production by the infected macrophages, according to the different hosts from which the strains were isolated. When the LPS stimulation was carried out prior to or during the addition of the parasite, parasites isolated from humans, triatomines, and sylvatic reservoirs all significantly inhibited NO production compared to the control (p < 0.000).
In the assays where macrophages were stimulated with LPS four h after the parasite infection, the parasites isolated from wild reservoirs did not inhibit NO production compared to the control (p = 0.9463), whereas the parasites isolated from humans and triatomines significantly inhibited NO production (p = 0.00015 for humans; p = 0.0017 for triatomines). The inhibition of NO production was significantly lower for parasites isolated from humans (p < 0.0002) than for those isolated from triatomines.
Correlation between genetics and biology: No correlation between genetics and biology was observed, when all T. cruzi lineages and hosts were analyzed together by the Mantel test, using the genetic distance and matrices from four biological parameters (phagocytic index, mean number of parasites per infected macrophage, percentage of infected macrophages, and nitric oxide production) (r = 0.023 and p = 0.4506).
When the biological parameters and the genetic distance were tested for T. cruzi I or T. cruzi II by RAPD or SSP-PCR, a significant correlation was found only for RAPD when the lineages were considered individually (T. cruzi I: r = 0.430, p = 0.0142; T. cruzi II: r = 0.80834, p = 0.0379). When biological parameters and the genetic distance were tested by type of host and RAPD or SSP-PCR, a significant correlation was found only for RAPD and humans (r = 0.80834, p = 0.0379) or wild reservoirs (r = 0.843, p = 0.0411).
DISCUSSION
Although the PIs were not statistically different for T. cruzi I and II, the values varied significantly among strains of T. cruzi I. Also the values observed for NO production varied significantly among strains of T. cruzi I. This finding may be explained by the source of the strains of T. cruzi I, from wild reservoirs and triatomines. The biological characteristics of strains can be influenced by the parasite-host relationship. BÉRTOLI et al.8 showed that the infectivity of the strains analyzed in their study was related to the host from which the strains were isolated, but was not related to the genetic lineage. This difference in behavior may also be a consequence of the isolated behavior of G1 (wild reservoir) and A21A, N914A and N120B (bugs) strains, which displayed significantly higher PI values than the others in the T.cruzi I group. In the case of strain A21A, it was shown that it is a mixture (Tc I+Tc II), in contrast to samples N914A and N120B, which are pure Tc I36. In another article from our group (in publication2), using the rRNA gene analysis (24Sa) and mitochondrial DNA (subunit 2 of cytochrome oxidase gene - COII) we found that the G1 strain also belongs to the T. cruzi I group. Even though the T. cruzi samples used in this study were randomly selected, they were all obtained from a very limited area in the state of Paraná. Also, no information on whether or which of these strains are composed by more than one clone (aside from strain A21A) is yet available. Thus, further studies to characterize the presence of polyclonal strains as well as the use of other T. cruzi isolates, obtained from other regions and from different hosts will help to elucidate the true significance of this variability. The PI of T. cruzi strains was lower in the strain isolated from humans, than from the wild reservoirs or triatomines, although not significantly. Because the PI for strains isolated from triatomines was higher than the others, we can infer that the host influences the PI. As reported elsewhere, biological characteristics can be determined by factors related to the host from which the strain has been isolated37,41. One report41 clearly showed biological differences between three strains classified as T. cruzi I, with respect to parasitemia, tissue tropism, pathogenicity, and mortality. Other researchers have also discussed the influence that the host can have on the parasite, both with its metabolic versatility and with the wide range of environmental conditions where the host population lives50.
In natural populations, the T. cruzi I strains showed a mean PI higher than that shown by the T. cruzi II strains. Other studies have demonstrated that clones of the T. cruzi II lineage show a lower percentage of infectivity to Vero cells or to BALB/c mice39,47. Although these results are in line with our data, infectivity is a much more complex phenomenon. In other studies with T. cruzi I and II strains, metacyclic trypomastigotes from T. cruzi II were more infective than were those from T. cruzi I33. Conversely, FERNANDES et al.20, using extracellular amastigotes of the same strains, demonstrated that T. cruzi I displayed much higher infectivity than T. cruzi II. Metacyclic trypomastigotes and amastigotes from these lineages depend on different signaling mechanisms to invade various types of host cells19,20,33.
Our data indicated a difference in NO production by natural populations of T. cruzi belonging to the genetic lineages T. cruzi I and T. cruzi II, or by strains isolated from different hosts. T. cruzi I parasites and strains isolated from wild reservoirs or triatomines showed the greatest suppression of NO production. These results are in agreement with reports by other authors36,38 that T. cruzi has developed strategies to evade NO-mediated anti-microbial activity by suppressing its production. The ability of T. cruzi I parasites to escape from NO is in agreement with other biological characteristics of this genetic group, which include its greater infectivity, rate of intracellular replication, and virulence23,39,47.
When LPS is added together with the parasite, the macrophages are so strongly stimulated that NO production is greatly suppressed. The suppression of production of a reagent against an exaggerated stimulus is not a rare event in the physiology of the immune system1. Experimentally, the NO levels were undetectable for two different genetic lineages of parasites. If we consider parasites isolated from different hosts, we find that the inhibition of NO production is higher for parasites isolated from wild reservoirs and humans than from triatomines, although parasites isolated from humans inhibited NO production similarly to the parasites isolated from triatomines. BÉRTOLI et al.8 demonstrated a significantly higher infectivity to mice for strains isolated from wild reservoirs than those from humans and triatomines, illustrating the influence of the host on the virulence of T. cruzi strains. In the present study, the influence of the host on NO production was not as distinct; however, it was stronger than the influence of genetic lineage, because the strains isolated from wild reservoirs and triatomines, from the same genetic lineage (T. cruzi I), suppressed NO production at different rates.
When the infected macrophages are stimulated with LPS, the NO concentration can be understood as a result of the intrinsic capacity of each genetic lineage or strain. This stimulus is not strong and does not induce the down-regulation of NO production, as observed in macrophages stimulated with LPS prior to the infection. Under these conditions, the parasites from T. cruzi I that were isolated from wild reservoirs seemed to be a more efficient inducer of NO production than the T. cruzi II parasites. This could explain the higher infectivity, parasitemia, pathogenicity, and mortality in mice of T. cruzi I parasites41, as NO acts as an inflammatory mediator in the T. cruzi infection.
As mentioned above, macrophages were placed in contact together with trypomastigotes and epimastigotes without purification of the trypomastigotes. The trypomastigote-macrophage ratio was the same for all strains (2 trypomastigotes/macrophage). As far as we could determine, this procedure did not affect the phagocytic index or NO production. It is well established40 that whereas trypomastigotes escape from the phagosome and divide in the cytoplasm, epimastigotes do not escape and will be killed. Therefore, the NO evaluated in this experiment was produced by activated macrophages and is known to be cytotoxic to T. cruzi31,32. Internalization of T. cruzi trypomastigotes by macrophages triggers the assembly of the NADPH oxidase complex, which does not interfere with sustained NO production (~24 h)3.
There was no correlation between the genetic and biological parameters when T. cruzi lineages and hosts were considered together, although a significant correlation with RAPD was observed when data from both lineages or from human or wild reservoirs were tested separately. This result can be understood by considering that although some parameters may be directly related to each genetic marker, this relationship becomes diluted in the whole and the result is then not significant. Each particular parameter can be defined by multiple genes, and the molecular technique or the parameter evaluated may affect the result. The finding of a non-significant association between phylogenetic lineages and susceptibility to benznidazol suggests that different genes may be involved with this biological characteristic48. Similarly, another study related many different genes to metacyclogenesis7. A lack of correlation between genetics and biology has been suggested for T. cruzi strains in Mexico, because the biological characteristics may be determined by different factors from those identified in the genotypic characterization41. Another aspect possibly related to the lack of correlation between genetics and biology in our study is the existence of the third T. cruzi lineage21,52, which was not considered here since it was only reported recently. Additional factors may influence the relationship between these strains. The host may influence the parasite, affecting its metabolic versatility over the wide range of environmental conditions where the hosts live50. Also, another study found that half of the triatomines analyzed in Paraná were co-infected by parasites from both T. cruzi I and II lineages43.
Our data showed that biological parameters, including the Phagocytic Index, did not vary between the T. cruzi I and II genetic lineages, but did vary among T. cruzi strains isolated from a specific host. Nitric oxide production was inhibited by parasites of both lineages, and was related to the host from which the parasites were isolated, both depending on the stimulation status of the macrophage. The analysis also showed a correlation between biological parameters and the genetic distance of the T. cruzi strains.
ACKNOWLEDGEMENTS
This study was supported by grants from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-PROAP) of Brazil. Leila Zalloum holds a scholarship from CAPES.
Received: 22 September 2010
Accepted: 8 September 2011
- 1. Abbas AK, Lichtman AH, Pillai S. Imunologia celular e molecular. 6a ed. Rio de Janeiro: Elsevier; 2008.
- 2. Abolis NG, Araújo SM, Toledo MJO, Fernandez MA, Gomes ML. Trypanosoma cruzi I-III in Southern Brazil causing individual and mixed infections in humans, sylvatic reservoirs and triatomines. Acta Trop. 2011 (in press).
- 3. Alvarez MN, Peluffo G, Piacenza L, Radi R. Intraphagosomal peroxynitrite as a macrophage-derived cytotoxin against internalized Trypanosoma cruzi: consequences for oxidative killing and role of microbial peroxiredoxins in infectivity. J. Biol. Chem., 2011;286:6627-40.
- 4. Anonymous. Recommendations from a Satellite Meeting. Mem Inst Oswaldo Cruz. 1999;94(suppl. 1):429-32.
- 5. Araújo SM, Chiari E. Caracterizaçăo biológica de clones das cepas Y, CL e MR de Trypanosoma cruzi em camundongos C3H isogęnico. Mem Inst Oswaldo Cruz. 1988.83:175-81.
- 6. Araújo SM, Guilherme ALF, Ornelas Toledo MJO, Oliveira PJG, Silva JC, Gomes ML. Biology of Trypanosoma cruzi strains isolated from chagasic patients from different geographic origins residing in northwestern region of the state of Paraná, Brazil. Acta Scientiarum, 1999;21:229-35.
- 7. Avila AR, Dallagiovanna B, Yamada-Ogatta SF, Monteiro-Góes V, Fragoso SP, Krieger MA, et al. Stage-specific gene expression during Trypanosoma cruzi metacyclogenesis. Genet Mol Res. 2003;2:159-68.
- 8. Bértoli M, Andó MH, De Ornelas Toledo MJ, De Araújo SM, Gomes ML. Infectivity for mice of Trypanosoma cruzi I and II strains isolated from different hosts. Parasitol Res. 2006;99:7-13.
- 9. Brener Z. Trypanosoma cruzi: taxonomy, morphology and life cycle. In: Wendel S, Brener Z, Camargo ME, Rassi A, editors. Chagas disease (American Trypanosomiasis): its impact on transfusion and Clinical Medicine. Săo Paulo: ISBT Brazil' 92; 1992. p. 13-29.
- 10. Brisse S, Verhoef J, Tibayrenc M. Characterization of large and small subunit rRNA and mini-exon genes further supports the distinction of six Trypanosoma cruzi lineages. Int J Parasitol. 2001;31:1218-26.
- 11. Buchi DF, De Souza W. Internalization of surface components during ingestion of Saccharomyces cerevisiae by macrophages. J Submicrosc Cytol Pathol. 1992;24 135-41.
- 12. Buchi DF, De Souza W. Internalization of surface components during Fc-receptor mediated phagocytosis by macrophages. Cell Struc Funct. 1993;18:399-407.
- 13. Denkers EY, Butcher BA. Sabotage and exploitation in macrophages parasitized by intracellular protozoans.Trends Parasitol. 2005;21:35-41.
- 14. Devera R, Illarramendi X, Montoya-Araújo R, Pirmez C, Fernandes O, Coura JR. Biodemas de cepas de Trypanosoma cruzi isoladas de humanos de tręs áreas endęmicas de Minas Gerais. Rev Soc Bras Med Trop. 2002;35:323-30.
- 15. Dias JCP. Aspectos socioculturais e econômicos na expansăo e no controle da doença de Chagas humana. Ann Soc Belge Med Trop. 1985;65(suppl 1):119-26.
- 16. Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;7:2407-12.
- 17. Dost CK, Saraiva J, Zentgraf U, Monesi N, Engels W, Albuquerque S. Is nitric oxide involved in the tolerance of Calomys callosus as a reservoir host towards Trypanosoma cruzi infection? J Infect. 2006;52:49-55.
- 18. Escaron CJ, Lees DM, Tewari R, Smith DF, Caron E. A simple, robust and versatile method to characterise intracellular parasitism. Mol Biochem Parasitol. 2007;153:72-6.
- 19. Fernandes AB, Mortara RA. Invasion of MDCK epithelial cells with altered expression of Rho GTPases by Trypanosoma cruzi amastigotes and metacyclic trypomastigotes of strains from the two major phylogenetic lineages. Microbes Infect. 2004;6:460-7.
- 20. Fernandes AB, Neira I, Ferreira AT, Mortara RA. Cell invasion by Trypanosoma cruzi amastigotes of distinct infectivities: studies on signaling pathways. Parasitol Res. 2006;100:59-68.
- 21. Freitas JM, Augusto-Pinto L, Pimenta JR, Bastos-Rodrigues L, Gonçalves VF, Teixeira SM, et al. Ancestral genomes, sex, and the population structure of Trypanosoma cruzi. PLoS Pathog. 2006;2:e24.
- 22. Gomes ML, Macedo AM, Pena SDJ, Chiari E. Genetic relationships between Trypanosoma cruzi strains isolated from chronic chagasic patients in southern Brazil as revealed by RAPD and SSR-PCR analysis. Acta Trop. 1998;69:99-109.
- 23. Lana M, Pinto AS, Barnabé C, Quesney V, Noël S, Tibayrenc M. Trypanosoma cruzi: compared vectorial transmissibility of three major clonal genotypes by Triatoma infestans. Exp Parasitol. 1998;90:20-5.
- 24. Laurent JP, Barnabe C, Quesney V, Noel S, Tibayrenc M. Impact of clonal evolution on the biological diversity of Trypanosoma cruzi. Parasitology. 1997;114:213-8
- 25. Lüder CG, Algner M, Lang C, Bleicher N, Gross U. Reduced expression of the inducible nitric oxide synthase after infection with Toxoplasma gondii facilitates parasite replication in activated murine macrophages. Int J Parasitol. 2003;33:833-44.
- 26. Macedo AM, Machado CR, Oliveira RP, Pena SD. Trypanosoma cruzi: genetic structure of populations and relevance of genetic variability to the pathogenesis of Chagas disease. Mem Inst Oswaldo Cruz. 2004;99:1-12.
- 27. Mantel N. The detection of diasease clustering and generalized regression approach. Cancer Res. 1967;27:209-20.
- 28. Martínez-Díaz RA, Escario JA, Nogal-Ruiz JJ, Gómez-Barrio A. Biological characterization of Trypanosoma cruzi strains. Mem Inst Oswaldo Cruz. 2001;96:53-9.
- 29. Morel C, Chiari E, Camargo EP, Mattei DM, Romanha AJ, Simpson L. Strains and clones of Trypanosoma cruzi can be characterized by pattern of restriction endonuclease products of kinetoplast DNA minicircles. Proc Natl Acad Sci USA.1980;77:6810-4.
- 30. Mortara RA, Andreoli WK, Taniwaki NN, Fernandes AB, Silva CV, Fernandes MC, et al. Mammalian cell invasion and intracellular trafficking by Trypanosoma cruzi infective forms. An Acad Bras Cienc. 2005;77:77-94.
- 31. Muńoz-Fernández MA, Fernández MA, Fresno M. Synergism between tumor necrosis factor-alpha and interferon-gamma on macrophage activation for the killing of intracellular Trypanosoma cruzi through a nitric oxide-dependent mechanism. Eur J Immunol. 1992;22:301-7.
- 32. Muńoz-Fernández MA, Fernández, MA, Fresno M. Activation of human macrophages for the killing of intracellular Trypanosoma cruzi by TNF-alpha and IFN-gamma through a nitric oxide-dependent mechanism. Immunol Lett. 1992;33:35-40.
- 33. Neira I, Ferreira AT, Yoshida N. Activation of distinct signal transduction pathways in Trypanosoma cruzi isolates with differential capacity to invade host cells. Int J Parasitol. 2002;32:405-14.
- 34. Oliveira RP, Macedo AM, Chiari E, Pena SD. An alternative approach to evaluating the intraspecific genetic variability of parasites. Parasitol Today. 1997;13:196-200.
-
35OPAS (Organización Panamericana de la Salud). XIII Reunión de la Comisión Intergubernamental para la eliminación de Triatoma infestans y la interrupción de la tripanosomiasis americana por transfusión, Buenos Aires, Argentina, 29-31 de marzo de 2004. Montevideo: OPAS; 2004.
- 36. Pakianathan DR, Kuhn RE. Trypanosoma cruzi affects nitric oxide production by murine peritoneal macrophages. J Parasitol. 1994;80:432-7.
- 37. Pinho AP, Cupolillo E, Mangia RH, Fernandes O, Jansen AM. Trypanosoma cruzi in the sylvatic environment: distinct transmission cycles involving two sympatric marsupials. Trans R Soc Trop Med Hyg. 2000;94:509-14.
- 38. Proudfoot L, Nikolaev AV, Feng GJ, Wei WQ, Ferguson MA, Brimacombe JS, et al. Regulation of the expression of nitric oxide synthase and leishmanicidal activity by glycoconjugates of Leishmania lipophosphoglycan in murine macrophages. Proc Natl Acad Sci USA, 1996;93:10984-9.
- 39. Revollo S, Oury B, Laurent JP, Barnabé C, Quesney V, Carričre V, et al Trypanosoma cruzi: impact of clonal evolution of the parasite on its biological and medical properties. Exp Parasitol. 1998;89:30-9.
- 40. Roitt I, Brostoff J, Male D, editors. Immunology. 5th ed. London: Mosby, 1998. p.255.
- 41. Sánchez-Guillén MC, Bernabé C, Tibayrenc M, Zavala-Castro J, Totolhua JL, Méndez-López J, et al. Trypanosoma cruzi strains isolated from human, vector, and animal reservoir in the same endemic region in Mexico and typed as T. cruzi I, discrete typing unit 1 exhibit considerable biological diversity. Mem Inst Oswaldo Cruz. 2006;101:585-90.
- 42. Sokal RR. Testing statistical significance of geographic variation patterns. Syst Zool. 1979;28,227-32
- 43. Spitzner FL, Freitas JM, Macedo AM, Toledo MJ, Araújo SM, Priolli AJ, et al. Trypanosoma cruzi-triatomine associations and the presence of mixed infections in single triatomine bugs in Paraná State, Brazil. Acta Parasitol. 2007;52:74-81.
- 44. Steindel M, Dias Neto E, de Menezes CL, Romanha AJ, Simpson AJ. Random amplified polymorphic DNA analysis of Trypanosoma cruzi strains. Mol Biochem Parasitol. 1993;60:71-9.
- 45. Teixeira A, Hecht MM. O agente infeccioso e o hospedeiro. In: Teixeira A. editor. Doença de Chagas e evoluçăo. Brasília: Editora da Universidade de Brasília; 2007. p. 51-7.
- 46. Toledo MJ, Bahia MT, Carneiro CM, Martins-Filho OA, Tibayrenc M, Barnabé C, et al. Chemotherapy with benznidazole and itraconazole for mice infected with different Trypanosoma cruzi clonal genotypes. Antimicrob Agents Chemother. 2003;47:223-30.
- 47. Toledo MJ, de Lana M, Carneiro CM, Bahia MT, Machado-Coelho GL, Veloso VM, et al. Impact of Trypanosoma cruzi clonal evolution on its biological properties in mice. Exp Parasitol. 2002;100:161-72.
- 48. Villarreal D, Nirdé P, Hide M, Barnabé C, Tibayrenc M. Differential gene expression in benznidazole-resistant Trypanosoma cruzi parasites. Antimicrob Agents Chemother. 2005;49:2701-9.
- 49. WHO (World Health Organization). New global effort to eliminate Chagas disease. Wkly Epidemiol Rec. 2007;82:259-60.
- 50. Yeo M, Acosta N, Llewellyn M, Sánchez H, Adamson S, Miles GA, et al. Origins of Chagas disease: Didelphis species are natural hosts of Trypanosoma cruzi I and armadillos hosts of Trypanosoma cruzi II, including hybrids. Int J Parasitol. 2005;35:225-33.
- 51. Zalloum L, Gomes ML, Kinoshita AT, Toledo MJ, Prioli AJ, de Araújo SM. Trypanosoma cruzi: two genetic groups in Paraná State, Southern Brazil. Exp Parasitol. 2005;111:55-8.
- 52. Zingales B, Andrade SG, Briones MR, Campbell DA, Chiari E, Fernandes O, et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz. 2009;104:1051-4.
Publication Dates
-
Publication in this collection
13 Oct 2011 -
Date of issue
Oct 2011
History
-
Accepted
08 Sept 2011 -
Received
22 Sept 2010