Abstracts
Foodborne diseases represent operational risks in industrial restaurants. We described an outbreak of nine clustered cases of acute illness resembling acute toxoplasmosis in an industrial plant with 2300 employees. These patients and another 36 similar asymptomatic employees were diagnosed with anti-T. gondii IgG titer and avidity by ELISA. We excluded 14 patients based on high IgG avidity and chronic toxoplasmosis: 13 from controls and one from acute disease other than T. gondii infection. We also identified another three asymptomatic employees with T.gondii acute infection and also anti-T. gondii IgM positive as remaining acute cases. Case control study was conducted by interview in 11 acute infections and 20 negative controls. The ingestion of green vegetables, but not meat or water, was observed to be associated with the incidence of acute disease. These data reinforce the importance of sanitation control in industrial restaurants and also demonstrate the need for improvement in quality control regarding vegetables at risk for T. gondii oocyst contamination. We emphasized the accurate diagnosis of indexed cases and the detection of asymptomatic infections to determine the extent of the toxoplasmosis outbreak.
Toxoplasmosis; Outbreak; Foodborne disease; Green vegetables; Case-control study; ELISA; IgG avidity
Doenças transmitidas por alimentos representam riscos operacionais em restaurantes industriais. Descrevemos surto de nove casos agrupados de doença aguda semelhante à toxoplasmose em indústria de 2300 funcionários. Estes pacientes e outros 36 funcionários assintomáticos foram diagnosticados por ELISA para o título e avidez de IgG anti-T. gondii. Foram excluídos 14 pacientes com toxoplasmose crônica e alta avidez: 13 de controles e um de doença aguda não relacionada à infecção por T. gondii. Também identificamos três empregados assintomáticos com infecção aguda por T.gondii, que como os restantes agudos apresentavam anti-T.gondii IgM ELISA positivo. Conduzimos estudo caso controle por entrevista em 11 infecções agudas e 20 controles negativos. A ingestão de vegetais, mas não de carne ou água, foi associada com a incidência da doença aguda. Esses dados reforçam a importância do controle sanitário em restaurantes industriais e também demonstram a necessidade de melhoria no controle de qualidade sobre vegetais em risco de contaminação por oocistos de T. gondii. Enfatizamos o diagnóstico preciso de casos e a detecção de infecções assintomáticas para determinar a extensão do surto de toxoplasmose.
TOXOPLASMOSIS
Case-control study of an outbreak of acute toxoplasmosis in an industrial plant in the state of São Paulo, Brazil
Estudo caso-controle de surto de toxoplasmose aguda em indústria no estado de São Paulo, Brasil
Claudio Cesar Jaguaribe EkmanI; Maria Fernanda do Valle ChiossiII; Luciana Regina MeirelesI; Heitor Franco de Andrade JúniorI; Walter Manso FigueiredoII; Maria Aparecida Moraes MarcianoI,III; Expedito José de Albuquerque LunaI
IInstituto de Medicina Tropical, Universidade de São Paulo, São Paulo, SP, Brasil
IIServiço Especial de Saúde de Araraquara, Faculdade de Saúde Publica, Universidade de São Paulo, Araraquara, SP, Brasil
IIIInstituto Adolfo Lutz, Secretaria de Estado da Saúde, São Paulo, SP, Brasil
Correspondence to Correspondence to: Luciana Regina Meireles, D.V.M., M.Sc., Ph.D. Laboratório de Protozoologia, IMTSP Av. Dr. Enéas de Carvalho Aguiar 470 05403-000 São Paulo, SP, Brasil. Phone +55.11.3061-7010; Fax +55.11.3088-5237. E-mail: lrmeirel@usp.br
SUMMARY
Foodborne diseases represent operational risks in industrial restaurants. We described an outbreak of nine clustered cases of acute illness resembling acute toxoplasmosis in an industrial plant with 2300 employees. These patients and another 36 similar asymptomatic employees were diagnosed with anti-T. gondii IgG titer and avidity by ELISA. We excluded 14 patients based on high IgG avidity and chronic toxoplasmosis: 13 from controls and one from acute disease other than T. gondii infection. We also identified another three asymptomatic employees with T.gondii acute infection and also anti-T. gondii IgM positive as remaining acute cases. Case control study was conducted by interview in 11 acute infections and 20 negative controls. The ingestion of green vegetables, but not meat or water, was observed to be associated with the incidence of acute disease. These data reinforce the importance of sanitation control in industrial restaurants and also demonstrate the need for improvement in quality control regarding vegetables at risk for T. gondii oocyst contamination. We emphasized the accurate diagnosis of indexed cases and the detection of asymptomatic infections to determine the extent of the toxoplasmosis outbreak.
Keywords: Toxoplasmosis; Outbreak; Foodborne disease; Green vegetables; Case-control study; ELISA; IgG avidity.
RESUMO
Doenças transmitidas por alimentos representam riscos operacionais em restaurantes industriais. Descrevemos surto de nove casos agrupados de doença aguda semelhante à toxoplasmose em indústria de 2300 funcionários. Estes pacientes e outros 36 funcionários assintomáticos foram diagnosticados por ELISA para o título e avidez de IgG anti-T. gondii. Foram excluídos 14 pacientes com toxoplasmose crônica e alta avidez: 13 de controles e um de doença aguda não relacionada à infecção por T. gondii. Também identificamos três empregados assintomáticos com infecção aguda por T.gondii, que como os restantes agudos apresentavam anti-T.gondii IgM ELISA positivo. Conduzimos estudo caso controle por entrevista em 11 infecções agudas e 20 controles negativos. A ingestão de vegetais, mas não de carne ou água, foi associada com a incidência da doença aguda. Esses dados reforçam a importância do controle sanitário em restaurantes industriais e também demonstram a necessidade de melhoria no controle de qualidade sobre vegetais em risco de contaminação por oocistos de T. gondii. Enfatizamos o diagnóstico preciso de casos e a detecção de infecções assintomáticas para determinar a extensão do surto de toxoplasmose.
INTRODUCTION
Infections by the protozoan parasite Toxoplasma gondii are widely prevalent in humans and animals worldwide5,18. Toxoplasmosis is generally asymptomatic, except in immunocompromised adults and congenitally infected children18.
Humans acquire infection postnatally mainly by ingesting food and water contaminated with oocysts shed in the feces of infected cats or by ingesting viable tissue cysts in raw or undercooked meat. The major routes of transmission vary between different human populations and depend on social culture, eating habits and environmental factors20,21. Furthermore, estimates of the rate of infection of meat vary widely depending on the animal species12.
Several reports concerning toxoplasmosis outbreaks have been published in recent decades, mainly regarding outbreaks associated with the consumption of undercooked meat7,10. These outbreaks usually affect a small group of individuals and are associated with exposure to a common source, such as that described by a group of American medical students who had eaten undercooked hamburgers11 and that described in the same year by a community of students from a Brazilian university who ate their meals together at the cafeteria17. Another well-documented Brazilian outbreak occurred in1993, with 17 cases of acute toxoplasmosis transmitted by raw lamb meat2.
Until recently, toxoplasmosis was not often considered a waterborne zoonosis, and the occurrence of outbreaks of Toxoplasma infection involving more than a single family or small group were rarely reported. However, a major outbreak of acute toxoplasmosis in humans occurred in Brazil in 20014, which was associated with the T. gondii contamination of a town's water supply. A similar waterborne outbreak was reported in Canada but with a smaller number of cases3. Oocyst-related Brazilian outbreaks of toxoplasmosis had previously been reported in a specific region of the State of São Paulo, the mesoregion of Araraquara, which featured excellent sanitary conditions. One such incident was a large outbreak that affected 113 students from the one large public university campus at São Carlos near our study area, with numerous cats on campus including inside the cafeteria8, which were linked to the contamination of a water reservoir by cat feces. Here, we describe an outbreak of 11 cases of acute toxoplasmosis in the same mesoregion at an industrial plant with approximately 2300 employees.
MATERIAL AND METHODS
Epidemiologic investigation: In April 2009, the Institute of Tropical Medicine of the University of Sao Paulo (IMTSP - USP) became aware of a cluster of acute toxoplasmosis cases in an industrial plant located in the mesoregion of Araraquara, Sao Paulo State, Brazil, through the Adolfo Lutz Institute, the State of São Paulo's public health laboratory (IAL). In a collaborative research effort with the State's Epidemiologic Surveillance Center (CVE), we developed a case-control study to investigate the outbreak. The investigation was conducted by professionals of the IMTSP - USP, IAL and the Special Health Service of Araraquara, School of Public Health of University of São Paulo (SESA - FSP- USP). Upon the manifestation of symptoms, the patients sought the company's outpatient services, which then referred them to SESA - FSP - USP to confirm the diagnosis. The initial information provided indicated the existence of eight laboratory-confirmed cases of acute toxoplasmosis and one suspected case diagnosed between March and April 2009. The case subjects shared the common characteristic of working in a large industrial plant located in the region, and the temporal distribution of the dates of the onset of symptoms suggested a common source of exposure.
Initially, we conducted a descriptive study aimed at addressing active cases. The study began in July 2009 with an active search of suspected cases through the medical records of the company's outpatient services and the Special Health Service of Araraquara (SESA). A suspected case was defined as "any person assisted in the services mentioned above who had at least two of the following symptoms: lymphadenopathy, fever, headache and fatigue, or who had laboratory results with serologic evidence of toxoplasmosis in the period from March 15 to March 31, 2009." Moreover, an investigation of the risk factors for toxoplasmosis was conducted through an interview with the nutritionist responsible for the company's industrial kitchen, who provided information regarding the origin and suppliers of the ingredients used in preparing meals, as well as the location and storage temperature of meat products. On this occasion, we requested menus with a list of all of the food items used in each meal for the period from February 2 to March 15, 2009, and search for T.gondii cysts in meat samples (50 g) from the alleged suppliers used during the period of employee infection.
To identify risk factors for toxoplasmosis and possible sources of infection, such as food and water, we performed a case-control study. Initially, the study included the eight laboratory-confirmed cases and one suspected case that did not have laboratory confirmation. As controls, we selected 36 asymptomatic employees who worked in the same teams and during the same work shifts as the laboratory-confirmed cases. All symptomatic individuals (cases) were seropositive for IgM and IgG anti-T. gondii, and only one case with clinical suspicion had not been evaluated by laboratory testing.
Because we selected controls based on clinical symptoms, the control group may have included asymptomatic chronic toxoplasmosis individuals who were protected and did not become cases in the investigated outbreak. We carried out the serological testing of all asymptomatic toxoplasmosis controls and all 45 individuals selected for the study underwent laboratory tests for antibodies IgG and IgM anti-T. gondii IgG avidity to confirm whether reagent individuals had acute or chronic toxoplasmosis.
Blood samples used for laboratory tests were obtained from May 19 to May 21, 2009, from the staff of the company's outpatient clinic with the written consent of each participant. All samples were processed at the Laboratory of Protozoology of IMTSP - USP. At the time of blood collection, subjects were interviewed by the IMTSP / USP and SESA team through a standardized questionnaire featuring questions regarding culture, hygiene, food habits and the menu of meals offered by the company during the period from February 2 to March 15, 2009. Only questionnaires from patients with acute toxoplasmosis and susceptible, laboratory-confirmed cases were analyzed, excluding those with chronic toxoplasmosis, for whom serological evidence of previous exposure had already been established.
Serological detection: Sera were assayed by in-house standard ELISA with determination of IgG avidity. Sera were screened at a dilution of 1/100, with a cut-off (0.417), which was previously determined and confirmed by a test set of 16 negative sera. The reaction was performed as described elsewhere22 using T. gondii whole-saline antigen and conjugated anti-human IgG, with subsequent titration of positive samples.
IgG avidity was determined by measuring antibody resistance to washing with 6 M urea chaotropic solution and expressed as the percentage of antibody-resistant urea. IgG avidity was considered low when the percentage of resistant antibodies was less than 30%, it was considered undetermined when this percentage was between 30 and 50% and high (chronic infection of > six months duration) when this percentage was greater than 50%.
IgM antibodies were determined by classic ELISA, with a cut-off of (0.331). Serum samples with high titers of IgG were previously treated for the removal of rheumatoid factor.
Detection of T. gondii cysts in meat samples: T. gondii tissue cysts were detected by molecular biology techniques (PCR) and bioassay in Swiss mice. Briefly, 50 g of meat from each sample was subjected to peptic digestion using commercial pepsin (1:10,000) according to a protocol described elsewhere6. The sediment from the digestion by pepsin containing T. gondii cysts was used to bioassay the samples and was also used for DNA extraction in PCR. The DNA extraction of samples was performed using a commercial extraction kit (Brasílica, LCG®) according to the manufacturer's instructions.
PCR was performed to detect a 115-bp fragment from the T. gondii B1 gene. In each reaction, we included DNA samples extracted from the T. gondii RH strain (positive control) and a sample of sterile ultra-pure water as a control negative. The PCR products were observed by electrophoresis on 2% agarose gel stained with ethidium bromide solution. The bands were observed by transillumination with ultraviolet light and recorded using a digital imaging system (AlphaImager ® EC, Alpha Innotech Corporation).
The bioassay was performed by the subcutaneous inoculation of one mL of digested sample in Swiss mice. The animals were kept in the Laboratory of Protozoology IMTSP. After a period of 30 days, the animals were euthanized for the microscopic study of brain cysts and T. gondii antibodies by ELISA.
Statistical analyses: Risk factors assessed by the questionnaire were analyzed by 2x2 tables comparing the infected and control groups using Fisher's exact test or a χ2 test at a significance level of p < 0.05.
RESULTS
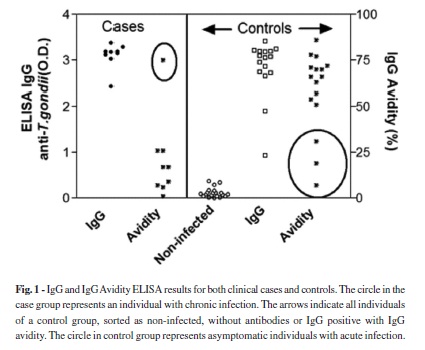
Screening for T. gondii IgG antibodies by ELISA in individuals selected for the case-control study revealed the presence of 25 anti-T. gondii IgG-positive sera, including the nine indexed cases, and 20 IgG-negative samples. All 25 reactive sera were tested for IgG avidity by ELISA via serial dilution using 6 M urea as a chaotrope, 14 samples exhibited high avidity (> 50%), indicating chronic infection from candidate controls and one indexed case, and 11 samples exhibited low avidity (< 30%), including eight indexed cases and three asymptomatic controls, indicating recent infection (Fig. 1). All eleven samples of low avidity were positive for T. gondii IgM antibodies, as determined by ELISA.
Table 1 shows the sorting of the tested population according to the serological classification. Using the clinical classifications of the case-control population, nine cases from among 45 individuals were selected to participate in the study, giving a 20% clinical infection rate. Additionally, three candidates from the control group presented serological evidence of acute asymptomatic toxoplasmosis. The selection of the patients according to the incidence of susceptible and acute infection resulted in an unprotected population of 31 participants. The final classification revealed 11 cases and 20 controls, with an infection rate of 35.5%, excluding chronically infected subjects. In the group of cases with compatible clinical symptoms, 8/9 individuals showed evidence of recent infection, but one case showed evidence of chronic infection. This patient visited the infirmary more than one week after the other acute cases had visited the infirmary and presented chronic toxoplasmosis. The incidence of asymptomatic infections was therefore 9.67% (3/31). According to laboratory results, we reclassified the case and control groups, resulting in 11 cases of acute toxoplasmosis and 20 susceptible controls. These results clearly show that there was a recent outbreak of toxoplasmosis in the population but that the clinical symptoms did not occur in all of the infected patients, with a percentage of at least 27% (3/11) of infected patients without clinical symptoms.
According to the dates during which the study was conducted, the first index case developed symptoms on March 15, 2009. The main reported symptoms were fever (9/11 = 81.81%), night sweats (9/11 = 81.81%), asthenia (9/11 = 81.81%), malaise (9/11 = 81.81%), lymphadenopathy (7/11 = 63.64%), headache (7/11 = 63.64%), appetite loss (6/11 = 54.55%), myalgia (6/11 = 54.55%), arthralgia (4/11 = 36.36%), nausea (3/11 = 27.27%), sore throat (3/11 = 27.27%), diarrhea (2/11 = 18.18%), vision problems (2/11 = 18.18%), dysuria (2/11 = 18.18%), abdominal pain (1/11 = 9.09%), neurological symptoms (1/11 = 9.09%), dizziness (1/11 = 9.09%) and irritability (1/11 = 9.09%). The outbreak peaked between March 17 and March 22, 2009. Some symptoms were reported in interview by alleged previously asymptomatic patients.
The analysis of possible associations between the exposure to risk factors and disease is described in Table 2. Significant events were analyzed in connection with the risk association of habits, environments and food consumption in case-control analysis using the serologically defined acute cases (11 individuals) and controls (20 individuals). As shown, the individual consumption of a vegetable item (escarole) on March 7, 2009, was the single variable significantly associated with the incidence of disease (p < 0.05). The potential impact of this variable is limited as only two infected cases had consumed this recipe, despite the significant association. None of the other variables investigated were significantly associated with the illness. When looking for an association with meals, green vegetable ingestion was strongly associated with infection (p < 0.005). Protection factors were associated with individual habits such as the consumption of filtered water, ingestion of well-cooked meat and good hygiene practices. The DNA extracted by PCR and the mice bioassay were negative in the frozen meat obtained from restaurants two months after the outbreak.
DISCUSSION
This epidemic outbreak of toxoplasmosis revealed an unspecific clinical picture of acute toxoplasmosis in most infected patients; similar to what has been reported in other studies19. This clinical picture overlaps with that of several other acute infections, and we found a reported case that lacked serological evidence of acute toxoplasmosis and so was excluded from our group of cases. Among our candidate controls, we also found three patients who presented asymptomatic disease and were included in our infected group. These data show the importance of accurate serology for diagnosis of acute toxoplasmosis.
Our study did not identify other factors common to the cases outside the workplace or individual behavioral risks. The epidemic curve showed common and isolated exposure because the onset of symptoms in cases occurred over a period of 14 days and peaked within a eight days interval. These results are consistent with the incubation period of 5-23 days described in the literature18.
We searched and detected the absence of cats in the surroundings of the plant, without access to water reservoirs, which could have been an alternative source of oocysts, as elsewhere described4. The clustered and small number of symptomatic cases does not suggest water as a factor of exposure to Toxoplasma gondii because water contaminated with oocysts spreads infection to a large number of people and the number of cases increases over time, as illustrated by the outbreaks in Canada3 and Brazil4, which was not observed for this outbreak. Reported outbreaks featuring water as a mode of transmission have led to high attack rates, due to a high dosage of oocysts10 or a particularly virulent strain1, distinct from our typical self-limited benign disease infected group, with three asymptomatic infections. Our data, as well as those previously described in the literature8, suggest that acute cases of human toxoplasmosis in the Araraquara mesoregion are associated with transmission by oocysts, reinforcing the need for preventive measures in this region, with a focus on maintaining the quality of water and processing of raw foods.
The other exposure factor that could be implicated in the outbreak of toxoplasmosis is food, such as fresh vegetables and raw or undercooked meat20. We investigated the list of all food items provided for each meal in the company's menu over the three-week period corresponding to the exposure period. Except for escarole, no other individual food item was implicated in the outbreak, but ingestion of a meal with green vegetables was intensely associated with the infection. Those vegetables are composed of hard green leaves, which are considered to be difficult to clean, as evidenced by Salmonella assays14. The food supplier of the plant also informed us that aside from mechanical washing, the main method used to wash fresh vegetables is low-concentration chlorine immersion, which does not affect the viability of T. gondii oocysts23. Another problem with cleaning escarole leaves is the fact that this vegetable possesses isolated leaves without an unrolling leaf pattern, such as that of cabbage. In fact, it is more similar to arugula, which has also been reported to be easily contaminated by Salmonella9. This leaf organization allows contaminants to reach all leaves, especially if contaminated fertilizer is dispersed over the vegetables. This relationship between the consumption of raw green vegetables and toxoplasmosis has also been observed in other areas of Brazil15.
The absence of the implication of other possible food items as sources of the outbreak may be due to the small sample size, because the small number of cases and controls reduces the power of the study16. Another factor was the long interval between exposure and the investigation period, which lasted approximately three months. This interval means that participants may have difficulty in accurately reporting the foods they consumed at the time. Difficulty in remembering facts in epidemiologic studies is known as bias, which sometimes leads to mistakes in the classification of exposure to the factors investigated; in this case, the factor is the consumption of food13.
Our study exhibited the same difficulties associated with other Toxoplasma outbreaks. Private organizations usually allow only small studies to be performed, without testing the whole group of employees, which precludes the exact determination of the extent of the outbreak. Food suppliers usually offer biased or outdated samples. Serology without the definition of acute infection leads to an inaccurate estimate of incidence due to imprecise serology and a large proportion of Toxoplasma chronic infection in the population. We performed IgG avidity assays to confirm the incidence of acute infections; this approach must be used together with IgM determination. We also suggest that food restaurant suppliers and nutritionists avoid the use of raw green vegetables with open leafs, such as Brazilian lettuce, escarole, chicory, broccoli and arugula in their salads to prevent exposure to toxoplasmosis caused by viable oocysts after chlorine treatment.
Outbreaks of infectious diseases due to food consumption are difficult to study, and our report dealt with most of these difficulties. We highlight the importance of the accurate diagnosis of indexed cases and also the importance of detecting asymptomatic infections for determining the accurate extent of outbreaks. Outbreaks of toxoplasmosis present a very good opportunity to test this rationale and all of the operative procedures used in outbreak studies. All possible efforts must be made to generate collaborative discussions for the implementation of adequate preventive measures.
Received: 24 February 2012
Accepted: 11 June 2012
- 1. Benenson MW, Takafuji ET, Lemon SM, Greenup RL, Sulzer AJ. Oocyst-transmitted toxoplasmosis associated with ingestion of contaminated water. N Engl J Med. 1982;307:666-9.
- 2. Bonametti AM, Passos JN, da Silva EM, Bortoliero AL. Surto de toxoplasmose aguda transmitido através da ingestão de carne crua de gado ovino. Rev Soc Bras Med Trop. 1997;30:21-5.
- 3. Bowie WR, King AS, Werker DH, Isaac-Renton JL, Bell A, Eng SB, et al. Outbreak of toxoplasmosis associated with municipal drinking water. The BC Toxoplasma Investigation Team. Lancet. 1997;350:173-7.
- 4. De Moura L, Bahia-Oliveira LMG, Wada MY, Jones JL, Tuboi SH, Carmo EH, et al. Waterborne toxoplasmosis, Brazil, from field to gene. Emerg Infect Dis. 2006;12:326-9.
- 5. Dubey JP, Beattie CP. Toxoplasmosis of animals and man. Boca Raton: CRC Press; 1988. p. 220.
- 6. Dubey JP. Refinement of pepsin digestion method for isolation of Toxoplasma gondii from infected tissues. Vet Parasitol. 1998;74:75-7.
- 7. Dubey JP. The history of Toxoplasma gondii--the first 100 years. J Eukaryot Microbiol. 2008;55:467-75.
- 8. Gattás VL, Nunes EM, Soares ALB, Pires MA, Pinto PLS, Andrade Jr HF. Acute toxoplasmose outbreak at campus of the University of Sao Paulo related to food or water oocyst contamination. In: Annals of the International Conference on Emerging Infectious Diseases; 2000; Atlanta, Georgia. p. 135.
- 9. Golberg D, Kroupitski Y, Belausov E, Pinto R, Sela S. Salmonella Typhimurium internalization is variable in leafy vegetables and fresh herbs. Int J Food Microbiol. 2011;145:250-7.
- 10. Jones JL, Dubey JP. Waterborne toxoplasmosis-recent developments. Exp Parasitol. 2010;124:10-25.
- 11. Kean BH, Kimball AC, Christenson WN. An epidemic of acute toxoplasmosis. JAMA. 1969;208:1002-4.
- 12. Kijlstra A, Jongert E. Control of the risk of human toxoplasmosis transmitted by meat. Int J Parasitol. 2008;38:1359-70.
- 13. Kopec JA, Esdaile JM. Bias in case-control studies. A review. J Epidemiol Community Health. 1990;44:179-86.
- 14. Kroupitski Y, Pinto R, Belausov E, Sela S. Distribution of Salmonella typhimurium in romaine lettuce leaves. Food Microbiol. 2011;28:990-7.
- 15. Lopes FM, Mitsuka-Breganó R, Gonçalves DD, Freire RL, Karigyo CJ, Wedy GF, et al. Factors associated with seropositivity for anti-Toxoplasma gondii antibodies in pregnant women of Londrina, Paraná, Brazil. Mem Inst Oswaldo Cruz. 2009;104:378-82.
- 16. Lwanga SK, Lemeshow S. Sample size determination in health studies: a practical manual. Geneva: World Health Organization; 1991.
- 17. Magaldi C, Elkis H, Pattoli D, Coscina AL. Epidemic of toxoplasmosis at a university in São-José-dos Campos, S.P. Brazil. Clinical and serologic data. Rev Latinoam Microbiol Parasitol (Mex). 1969;11:5-13.
- 18. Remington JS, McLeod R, Thulliez P, Desmonts G. Toxoplasmosis. In: Remington JS, Klein JO, Wilson CB, Baker CJ, editors. Infectious diseases of the fetus and newborn infant. 6th ed. Philadelphia: Elsevier Saunders; 2006. p. 947-1091.
- 19. Renoiner EIM, Siqueira AA, Garcia MH, Alves RM, Cardoso ME, Ferreira ABPL, et al. Surto de toxoplasmose adquirida, Anápolis-GO, fevereiro de 2006. Bol Eletrôn Epidemiol. 2007;7(8):1-6. [cited: 2011 Oct 2]. Available from: http://portal.saude.gov.br/portal/arquivos/pdf/ano07_n08_toxopl_adquirida_go.pdf
- 20. Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30:1217-58.
- 21. Tenter AM. Toxoplasma gondii in animals used for human consumption. Mem Inst Oswaldo Cruz. 2009;104:364-9.
- 22. Venkatesan P, Wakelin D. ELISAs for parasitologists: or lies, damned lies and ELISAs. Parasitol Today. 1993;9:228-32.
- 23. Wainwright KE, Miller MA, Barr BC, Gardner IA, Melli AC, Essert T, et al. Chemical inactivation of Toxoplasma gondii oocysts in water. J Parasitol. 2007;93:925-31.
Correspondence to:
Publication Dates
-
Publication in this collection
10 Sept 2012 -
Date of issue
Oct 2012
History
-
Received
24 Feb 2012 -
Accepted
11 June 2012