Abstracts
SUMMARY
The molluscicidal activity of the leaf powder of Moringa oleifera and lyophilized fruit powder of Momordica charantia against the snail Lymnaea acuminata was time and concentration dependent. M. oleifera leaf powder (96 h LC50: 197.59 ppm) was more toxic than M. charantia lyophilized fruit powder (96 h LC50: 318.29 ppm). The ethanolic extracts of M. oleifera leaf powder and Momordica charantia lyophilized fruit powder were more toxic than other organic solvent extracts. The 96 h LC50 of the column purified fraction of M. oleifera leaf powder was 22.52 ppm, while that of M. charantia lyophilized fruit powder was 6.21 ppm. Column, thin layer and high performance liquid chromatography analysis show that the active molluscicidal components in M. oleifera leaf powder and lyophilized fruit of M. charantia are benzylamine (96 h LC50: 2.3 ppm) and momordicine (96 h LC50: 1.2 ppm), respectively. Benzylamine and momordicine significantly inhibited, in vivo and in vitro, the acetylcholinesterase (AChE), acid and alkaline phosphatase (ACP/ALP) activities in the nervous tissues of L. acuminata. Inhibition of AChE, ACP and ALP activity in the nervous tissues of L. acuminata by benzylamine and momordicine may be responsible for the molluscicidal activity of M. oleifera and M. charantia fruits, respectively.
Acetylcholinesterase; Lymnaea acuminata; Momordica charantia; Moringa oleifera; Phosphatases
RESUMO
A atividade moluscicida do pó das folhas de Moringa oleifera e do pó liofilizado das frutas da Momordica charantia contra o caramujo Lymnaea acuminata é dependente do tempo e da sua concentração. O pó da folha da M. oleifera (96 h LC50: 197.59 ppm) foi mais tóxico do que o pó liofilizado da fruta da M. charantia (96 h LC50: 318.29 ppm). Os extratos etanólicos do pó de folha da M. oleifera e do pó liofilizado da fruta da M. charantia foram mais tóxicos do que outros extratos orgânicos solventes. O 96 h LC50 da fração purificada por coluna do pó das folhas da M. oleifera foi 22.52 ppm enquanto que o pó liofilizado do fruto da M. charantia foi 6.21 ppm. Coluna, camada fina e a alta performance da análise da cromatografia líquida mostram que os componentes ativos moluscicidas do pó da folha da M. oleifera e do liofiliizado da fruta da M. charantia são a benzilamina (96 h LC50: 22.3 ppm) e a momordicina (96 h LC50: 1.2 ppm), respectivamente. A benzilamina e a momordicina inibiram de maneira significante in vivo e in vitro a acetilcolinesterase (AChE), as atividades das fosfatases alcalina e ácida (ACP/ALP) nos tecidos nervosos da L. acuminata. A inibição da atividade da AChE, ACP e ALP nos tecidos nervosos da L. acuminata pela benzilamina e momordicina podem ser responsáveis pela atividade moluscicida da M. oleifera e dos frutos da M. charantia, respectivamente.
INTRODUCTION
Fascioliasis caused by Fasciola species is of considerable medical and veterinarian importance2020. Mas-Coma S, Valero MA, Bargues MD. Fasciola, Lymnaeids and human fascioliasis with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. In: Rollinson D, Hay SI, editors. Advances in Parasitology. Burlington: Academic Press; 2009. p. 41-146.,2626. Singh A, Singh DK, Mishra TN, Agarwal RA. Molluscicide of plant origin. Biol Agric Hortic. 1996;13:205-52.. It severely affects breeding in cattle, goats, horses, ovines and swine, resulting in serious losses which ultimately affect the economy of live-stock keepers in many countries1212. Hammami H, Hamed N, Ayadi A. Epidemiological studies on Fasciola hepatica in Gafsa oases (South West of Tunisia). Parasite. 2007;14:261-4.,1919. Mas-Coma S, Funatsu IR, Bargues MD. Fasciola hepatica and lymnaeid snails occurring at very high altitude in South America. Parasitology. 2001;123(Suppl):S115-27.. The control of the snail population with the help of molluscicide is one of the major tools to reduce the incidence of fascioliasis in cattle as well as in human beings2020. Mas-Coma S, Valero MA, Bargues MD. Fasciola, Lymnaeids and human fascioliasis with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. In: Rollinson D, Hay SI, editors. Advances in Parasitology. Burlington: Academic Press; 2009. p. 41-146.,2626. Singh A, Singh DK, Mishra TN, Agarwal RA. Molluscicide of plant origin. Biol Agric Hortic. 1996;13:205-52.. The development of plant molluscicides, as possible substitutes for synthetic molluscicides, is gaining more attention because they are effective, less expensive and eco-friendly1818. Marston A, Hostettmann K. Plant molluscicides. Phytochemistry. 1985;24:639-52.. Many plant products have been found to have a high molluscicidal potential2525. Silva TM, Camara CA, Agra MF, de Carvalho MG, Frana MT, Brandoline SV, et al. Molluscicidal activity of Solanum species of the Northeast of Brazil on Biomphalaria glabrata. Fitoterapia. 2006;77:449-52.,3131. Singh S, Singh VK, Singh DK. Molluscicidal activity of some common spice plants. Biol Agric Hortic. 1997;14:237-49.,3434. Srivastava P, Kumar P, Singh DK. Control of harmful snails: Tejpat (Cinnamomum tamala) a potential molluscicide. J Appl Biosci. 2005;31:128-32.,3636. Tripathi SM, Singh DK. Molluscicidal activity of Punica granatum bark and Canna indica root. Braz J Med Biol Res. 2000;33:1351-5..
In the present study, the molluscicidal activities of the leaf powder of Moringa oleifera Lam. (Moringaceae) and lyophilized fruit powder of Momordica charantia Linn. (Cucurbitaceae) against the target snail Lymnaea acuminata were evaluated. Active molluscicidal components responsible for snail death were isolated, characterized and their effects on acetylcholinesterase (AChE) and acid / alkaline phosphatase (ACP/ALP) activity in the nervous tissue of L. acuminata were evaluated.
MATERIALS AND METHODS
One afternoon in Gorakhpur (Latitude 26° 46′ N, Longitude 83° 22′ E), U.P. India, fresh M. oleifera leaves from the University campus and fresh M. charantia fruits from the local agricultural fields were obtained. The specimens of M. oleifera leaf (voucher specimen number M-5132) and M. charantia fruit (voucher specimen number M-3431) were identified and authenticated by Prof. R.P. Shukla, Taxonomist, Department of Botany, DDU Gorakhpur University, Gorakhpur, U.P. India.
1. Preparation of crude powder: Leaves of M. oleifera were dried in an incubator at 37 °C for 24 h and then pulverized in an electric grinder (Twister mixer grinder, 410025). The crude powder, thus obtained, was used for the toxicity experiments. The fresh paste of M. charantia was obtained by grinding small pieces of fruit with water. This aqueous paste was lyophilized at -40 °C. The lyophilized fruit powder was stored in airtight desiccators and used for toxicity experiments.
2. Preparation of organic solvent extracts: Five grams each of crude powder of M. oleifera leaf and M. charantia fruit were extracted separately with 100 mL of each solvent viz. chloroform, ether, acetone and ethanol at room temperature for 24 h. Each preparation was filtered separately through sterilized Whatman no.1 filter paper2222. Ogundiya MO, Okunade MB, Kolapo AL. Antimicrobial activities of some Nigerian chewing sticks. Ethnobotanical Leaflets. 2006;10:265-71. and the filtered extracts were subsequently evaporated under vacuum at 24 °C. The residues, thus obtained, were used for the determination of molluscicidal activity. The leaf powder of M. oleifera yielded 230 mg of chloroform extract, 355 mg of ether extract, 442 mg of acetone extract and 460 mg of ethanol extract. The lyophilized fruit powder of M. charantia yielded 50 mg of chloroform extract, 62 mg of ether extract, 260 mg of acetone extract and 1000 mg of ethanol extract.
3. Column chromatography: Twenty five mL each of the ethanolic extracts of M. oleifera leaf powder and M. charantia lyophilized fruit powder were subjected to silica gel (60-120 mesh, Qualigens glass, Precious Electrochemical industry, Pvt. Ltd. Mumbai, India) chromatography in a 5 cm × 45 cm column. Five mL fractions of 90 and 60 eluates were eluted with 95% ethanol for each column preparation of M. oleifera leaf powder and lyophilized fruit powder of M. charantia, respectively. Eluate nos. 20-30 (M. oleifera leaf powder) and 30-40 (M. charantia lyophilized fruit powder) were used for toxicity studies. Ethanol was evaporated under vacuum at 24 °C and the residues were used for the determination of molluscicidal activity.
4. Pure compounds: Benzylamine (1-Phenylmethanamine) was purchased from Sigma Chemical Co., USA. and momordicine (3,7,23-trihydroxycucurbita-5,24-dien-19-al) from Yian Khonest Bio-Tech Co. Ltd., China.
5. Thin layer chromatography: Thin layer chromatography (TLC) was performed according to the method of JAISWAL & SINGH1515. Jaiswal P, Singh DK. Molluscicidal activity of Carica papaya and Areca catechu against the freshwater snail Lymnaea acuminata. Vet Parasitol. 2008;152:264-70. to identify the active molluscicidal components in M. oleifera leaf powder and M. charantia lyophilized fruit powder. Thin layer chromatography was carried out on 20 cm × 20 cm precoated silica gel (Precious Electrochemical industry, Pvt. Ltd. Mumbai, India) using benzene/ethyl acetate (9:1, v:v) as the mobile phase. The loading of column purified fractions of M. oleifera leaf powder and M. charantia lyophilized fruit powder along with their respective active components were applied on TLC plates with a micropipette. TLC plates were developed with I2 vapor. Copies of chromatogram were made by tracing the plates immediately and Rf values were calculated.
6. High performance liquid chromatography: Identification of active components present in M. oleifera leaf and M. charantia fruit were done by HPLC.
6.1. Sample preparation: M. oleifera leaf and M. charantia fruit samples were prepared by dissolving separately 50 mg each of their column chromatographed extracts in 20 mL of acetonitrile. The samples were properly vortexed to ensure dissolution. Prior to sample injection, the solutions were passed through a Millipore filter (ultra filter disc 3K 43 mm 10 pk, Cole Parmer, Germany) to remove any undissolved particles.
6.2. Preparation of standard solution: Pure standard solutions of benzylamine (0.01 M) and momordicine (0.001 M) were prepared by diluting 0.01 mL of benzylamine in 20 mL of acetonitrile and dissolving 10 mg of momordicine in 20 mL of acetonitrile. The mixtures were vortexed to ensure proper dissolution of pure compounds. The solutions, thus obtained, were passed through Millipore filter (ultra filter disc 3K 43 mm 10 pk, Cole Parmer, Germany).
6.3. Instrumentation: The HPLC system was equipped with two LC-10 AT VP pumps, a Cecil CE 4201 UV-variable detector and a Microliter® #702 (Hamilton-Bonaduz, Schweiz) syringe with a loop size of 20 µL. Reverse-phase chromatographic analysis was carried out under isocratic conditions using a reverse-phase Luna 5 µ C18 Phenomenex column (250 mm × 4.6 mm) at 27 °C. Acetonitrile (HPLC grade) was used as the mobile phase solvent under a pressure of 260-270 Kgf/cm22. Aruna P, Chetty CS, Naidu RC, Swami KS. Acid phosphatase activity in Indian apple snail, Pila globosa (Swainson), during aestivation and starvation stress. Proc Indian Acad Sci. 1979;88:363-5. and run time of 15 min. The analysis was carried out at a flow rate of one mL/min., the column effluent being monitored at 260 nm. Data acquisition were done with Power StreamTM software.
7. Collection of snails: Adult freshwater snails (L. acuminata, 2.25 cm ± 0.20 cm in shell length) were collected locally from different ponds in Gorakhpur, India. They were acclimatized for 72 h to laboratory conditions in a glass aquarium containing dechlorinated tap water (Temp., 22-24 °C; pH, 7.1-7.3; dissolved oxygen, 6.5-7.2 ppm; free carbon dioxide, 5.2-6.3 ppm; bicarbonate alkalinity, 102-105 ppm).
8. Treatment protocol for concentration-response relationship: The toxicity experiments were performed according to the method of SINGH & AGARWAL2929. Singh DK, Agarwal RA. Correlation of the anticholinesterase and molluscicidal activity of the latex of Euphorbia royleana on the snail Lymnaea acuminata. J Nat Prod. 1984;47:702-5.. Ten experimental snails were kept in a glass aquarium containing 3 L of dechlorinated tap water. Snails were continuously exposed for 96 h to different concentrations of plant products, separately. Six aquaria were set up for each concentration. Control snails were kept in the equal volumes of dechlorinated tap water under similar conditions without treatment. Snail mortality was assessed at 24 hourly intervals up to 96 h. Dead animals were promptly removed to avoid contamination of aquarium water. Mortality was indicated by the contraction of body within the shell; lack of response to a needle probe was taken as evidence of death. The LC values, lower and upper confidence limits (LCL and UCL), slope values, t-ratio, g-values and heterogeneity factors were calculated using the POLO computer software of ROBERTSON et al. 2424. Robertson JL, Russell RM, Priesler HK, Savin NE. Bioassay with arthropods. POLO computer programme for analysis of bioassay data. 2nd ed. Boca Raton: CRC Press; 2007. p. 1-224.. The regression coefficient between exposure time and different LC50 values was determined by the method of SOKAL & ROHLF3232. Sokal RR, Rohlf FJ. Introduction to biostatistics. San Francisco: W.H. Freeman; 1973. p. 171-3..
9. Bioassays: Each set of experimental snails were exposed to sublethal concentrations; 40% and 80% of 24 h LC50 and 40% and 80% of 96 h LC50 of different molluscicides for 24 and 96 h, respectively. The sublethal concentrations were based on 24 and 96 h LC50 values obtained from section 8. After 24 and 96 h of exposure, snails were removed from aquaria and rinsed with water. The nervous tissues of snails in experimental and control groups were taken out for the measurement of acetylcholinesterase (AChE), acid phosphatase (ACP) and alkaline phosphatase (ALP) activities.
The in vitro experiments were performed by dissolving benzylamine (0.3, 0.5, 0.7 and 0.8 mM) and momordicine (0.06, 0.11, 0.15 and 0.19 mM) in ether, and 4 mL of each was added separately to 10 mm path length cuvette. Ether was then allowed to evaporate. Molluscicides were pre-incubated with an enzyme source for 15 min at 25 °C, following which enzyme activity was determined. The control cuvette contained ether only. The Michaelis-Menten constant (Km) and maximum velocity (Vmax) were calculated by plotting Lineweaver-Burk plots for the hydrolysis of different concentrations of substrate by treated (0.7 mM of benzylamine and 0.15 mM of momordicine) and untreated enzyme2828. Singh DK, Agarwal RA. Inhibition kinetics of certain organophosphorous and carbamate pesticides on acetylcholinesterase from the snail Lymnaea acuminata. Toxicol Lett. 1983;19:313-9..
10. Enzyme assay
10.1. Acetylcholinesterase: Acetylcholinesterase activity was measured according to the method of ELLMAN et al.99. Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88-95. as modified by SINGH & AGARWAL2727. Singh DK, Agarwal RA. In vivo and in vitro studies on synergism with anticholinesterase pesticides in the snail Lymnaea acuminata. Arch Environ Contam Toxicol. 1983;12:483-7.. Fifty milligrams of the nervous tissue of L. acuminata taken around the buccal mass was homogenized in 1.0 mL of 0.1 M phosphate buffer pH 8.0 for five min in an ice bath and centrifuged at 1000g for 30 min at 4 °C. The supernatant was used as the enzyme source. Enzyme activity was measured in a 10 mm path-length cuvette using an incubation mixture consisting of 0.1 mL of enzyme source, 2.9 mL of 0.1 M phosphate buffer pH 8, 0.1 mL of chromogenic agent DTNB (5,5′-dithio-bis-2-nitrobenzoic acid) and 0.02 mL of freshly prepared ATChI (acetylthiocholine iodide) solution in distilled water. The change in optical density at 412 nm was recorded every 30 secs for three min at 25 °C. Enzyme activity was expressed as µmol ‘SH’ hydrolyzed/min/mg protein.
For the estimation of the kinetic constants of AChE, in vitro inhibition of the enzyme was carried out at different concentrations (3.0 × 10−4, 5.0 × 10−4, 7.0 × 10−4 and 1.0 × 10−3 M) of the substrate acetylthiocholine iodide.
10.2. Acid and alkaline phosphatase: Acid and alkaline phosphatase activities in the nervous tissue of L. acuminata were measured according to the method of BERGMEYER33. Bergmeyer UH. Methods of enzymatic analysis. New York: Academic Press; 1967. p. 1129. as modified by SINGH & AGARWAL3030. Singh DK, Agarwal RA. Toxicity of piperonyl butoxide-carbaryl synergism on the snail Lymnaea acuminata. Int Revue Ges Hydrobiol. 1989;74:689-99. using p-Nitrophenyl phosphate as the substrate. Tissue homogenate (2%, w/v) was prepared in ice cold 0.9% NaCl and centrifuged at 5000g for 15 min at 4 °C. The supernatant was used as the enzyme source. Standard curves were drawn with p-nitrophenol. The yellow color developed due to the formation of p-nitrophenol was determined colorimetrically at 420 nm. Enzyme activities of acid and alkaline phosphatases (ACP/ALP) were expressed as µmol substrate hydrolyzed/30 min/mg protein.
For the determination of the kinetic constants of acid and alkaline phosphatases, in vitro inhibition of the enzymes was carried out at different concentrations (1.25 × 10−5, 1.8 × 10−5, 3.0 × 10−5 and 5.4 × 10−5 M) of the substrate p-nitrophenyl phosphate.
11. Protein: Protein estimation was carried out according to the method of LOWRY et al.1717. Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:265-75. using bovine serum as a standard. 1.0 mL of enzyme supernatant was mixed in 5.0 mL of 5% trichloroacetic acid TCA and centrifuged at 6000 g for 20 min. The precipitate was washed with 5.0 mL of 5% TCA and again centrifuged at the same speed for 20 min. The precipitate was dissolved in 4.0 mL of 1 N NaOH. One mL of dissolved solution was mixed with 5.0 mL of reagent C { 50 mL of 2% sodium carbonate in 0.1 N NaOH (reagent A) mixed with 1 mL of copper sulphate in 1% sodium potassium tartarate (reagent B)}, and the mixture was left standing for 10 min at room temperature. In the reaction mixture, 0.5 mL of reagent D (freshly prepared phenol reagent and distilled water in 1:2 ratio) was added and mixed thoroughly. Resulting blue color was monitored in absorbance at 600 nm after 10 min. Standard curves were prepared with different concentrations of bovine serum albumin.
12. Statistical analysis: Each experiment was replicated at least six times and the results expressed as mean ± SE of six replicates. The Student's t-test was used to test for any significant variation (p < 0.05) between control and treated groups3232. Sokal RR, Rohlf FJ. Introduction to biostatistics. San Francisco: W.H. Freeman; 1973. p. 171-3..
RESULTS
1. Molluscicidal activity: The toxicity of different extracts of the leaf powder of M. oleifera and lyophilized fruit powder of M. charantia were time and concentration dependent. The 24 h and 96 h LC50 values of the leaf powder of M. oleifera were 602.75 ppm and 197.6 ppm, respectively (Fig. 3) while the corresponding values for lyophilized M. charantia fruit powder were 1249.12 and 318.29 ppm, respectively (Fig. 4). Maximum toxicities were recorded with the ethanolic extracts ( Fig. 3-4). The column-purified fractions of M. oleifera and M. charantia were highly toxic. The 24 h and 96 h LC50 values of the column- purified fraction of M. oleifera leaf powder were 53.16 and 22.52 ppm, respectively while those of the column-purified fraction of lyophilized fruit powder of M. charantia were 12.33 and 6.21 ppm, respectively. The 24 h LC50 values of benzylamine and momordicine were 14.4 and 10.0 ppm, respectively ( Fig. 5-6).
The slope values were steep and separate estimation of LC, based on each of the six replicates, were found to be within 95% confidence limits of LC50. The t-ratio was higher than 1.96 and heterogeneity factor was less than 1.0. The g-value was less than 0.5 at all the probability levels i.e. 90, 95, 99. There was significant negative regression (p < 0.05) between exposure time and LC50 values ( Fig. 1-2).
Regression analysis in between LC50 of different preparations of Moringa oleifera and exposure time.
Regression analysis in between LC50 of different preparations of Momordica chaiarant and exposure time.
The thin layer chromatography analysis showed that the Rf values of benzylamine (0.06) and momordicine (0.13) were equivalent to the Rf values of column-purified fractions of M. oleifera (0.06) and M. charantia (0.13).
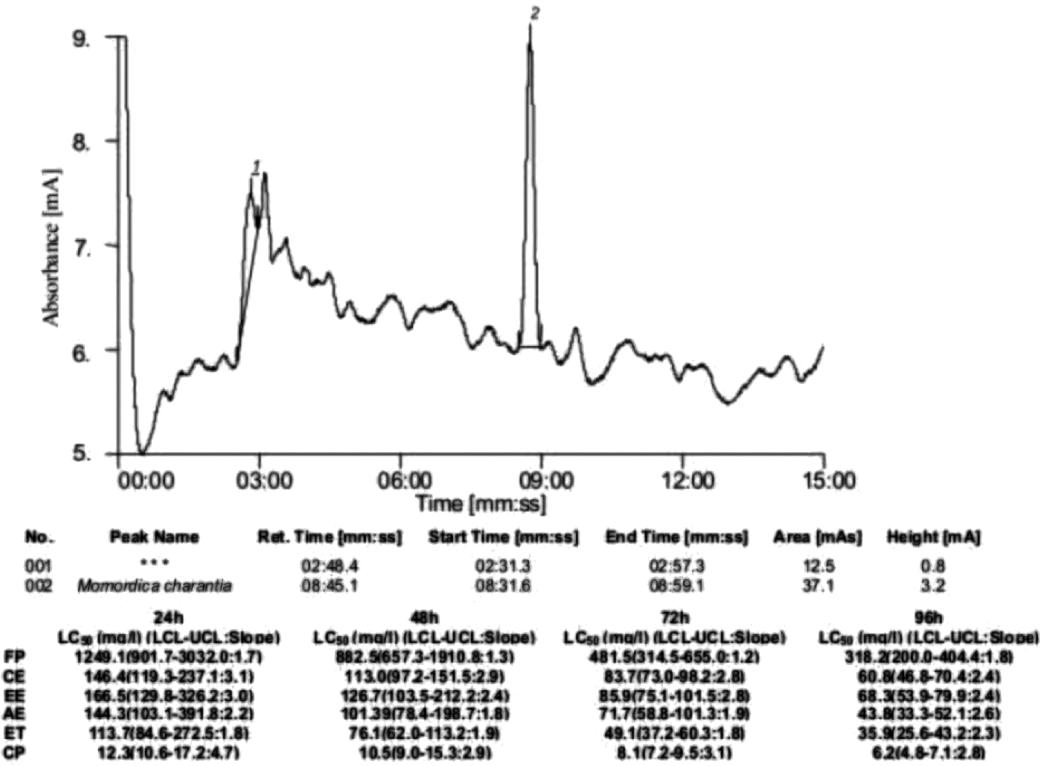
Identification of active components was done by comparing the retention time (Rt) and chromatographic peaks of M. oleifera leaf and M. charantia fruit samples with their respective active components, benzylamine and momordicine ( Fig. 3-6). The HPLC fingerprint profiles of M. oleifera leaf and M. charantia fruit samples showed major peaks at the retention time of 2.59 min and 8.45 min, respectively, whereas, the pure standard solutions of benzylamine and momordicine showed major peaks at the retention time of 2.58 min and 8.49 min, respectively.
High performance liquid chromatogram of column purified Moringa oleifera and toxicity of their different preparations viz. Leaf powder (LP), Chloroform extract (CE), Ether extract (EE), Acetone extract (AE), Ethanol extract (ET) and Column purified extract (CP) against the snail Lymnaea acuminata at different exposure period. LCL (Lower confidence limit), UCL (Upper confidence limit).
High performance liquid chromatogram of column purified Momordica charantia and toxicity of their different preparations viz. Fruit powder (FP), Chloroform extract (CE), Ether extract (EE), Acetone extract (AE), Ethanol extract (ET) and Column purified extract (CP) against the snail Lymnaea acuminata at different exposure period. LCL (Lower confidence limit), UCL (Upper confidence limit).
High performance liquid chromatogram of Benzylamine and its toxicity against the snail Lymnaea acuminata at different exposure period. LCL (Lower confidence limit), UCL (Upper confidence limit).
High performance liquid chromatogram of Momordicine and its toxicity against the snail Lymnaea acuminata at different exposure period. LCL (Lower confidence limit), UCL (Upper confidence limit).
2. In vivo inhibition of enzymes
2.1. Acetylcholinesterase: Table 1 shows that acetylcholinesterase activity in the nervous tissue of control L. acuminata was 0.74 µmol ‘SH’ hydrolyzed/min/mg protein. In vivo exposure to 40% and 80% of 24 and 96 h LC50 of benzylamine and momordicine caused significant inhibition of AChE activity in the nervous tissue of L. acuminata. AChE activity decreased to 85.27% and 64.32% of control values after exposure to 80% of the 24 h LC50 of benzylamine and momordicine, respectively. Maximum inhibition of AChE activity was observed when snails were exposed to 80% of the 96 h LC50 values of momordicine (48.91% of control) and benzylamine (57.02% of control) (Table 1).
2.2. Acid phosphatase: Acid phosphatase activity in the nervous tissue of control L. acuminata of control group was 35.44 µmol substrate hydrolyzed /30 min/mg protein (Table 1). In vivo exposure to 40% and 80% respectively, of the 24 and 96 h LC50 values of benzylamine and momordicine caused significant inhibition of ACP activity in the nervous tissue of L. acuminata. ACP activity decreased to 55.30% and 53.52% of control values after exposure to 80% of the 24 h LC50 values of benzylamine and momordicine, respectively. Maximum inhibition of ACP activity was observed when snails were exposed to 80% of the 96 h LC50 of benzylamine (40.74% of control) and momordicine (42.40% of control) (Table 1).
2.3. Alkaline phosphatase: Alkaline phosphatase activity in the nervous tissue of control L. acuminata was 32.44 µmol substrate hydrolyzed /30 min/mg protein (Table 1). In vivo exposure to 40% and 80% respectively of the 24 and 96 h LC50 values of benzylamine and momordicine caused significant inhibition of ALP activity in the nervous tissue of L. acuminata. ALP activity decreased to 75.30% and 59.55% of control values after exposure to 80% of the 24 h LC50 values of benzylamine and momordicine, respectively. Maximum inhibition of ALP activity was observed when snails were exposed to 80% of the 96 h LC50 values of momordicine (53.20% of control) and benzylamine (63.62% of control) (Table 1).
3. In vitro inhibition of enzymes: In vitro pre-incubation with 0.3, 0.5, 0.7 and 0.8 mM benzylamine and 0.06, 0.11, 0.15 and 0.19 mM momordicine caused significant dose dependent inhibition of AChE, ACP and ALP activities (Table 2). In vitro exposure to 0.7 mM of benzylamine and 0.15 mM of momordicine reduced AChE, ACP and ALP activity in the nervous tissue of L. acuminata (Table 2).
Fig. 7 shows Lineweaver-Burk plots of benzylamine and momordicine inhibited and uninhibited AChE activities at different substrate concentrations. This plot shows that the Km and Vmax values of uninhibited AChE were 7.69×10−4 M and 1.67 µ mol ‘SH’ hydrolyzed/min/mg protein, respectively (Table 3). Km of benzylamine (Fig. 7A) and momordicine (Fig. 7B) inhibited AChE were 10×10−4 and 2.85×10−4 M, respectively. Vmax of benzylamine and momordicine-inhibited AChE were 1.67 and 0.48 µ mol ‘SH’ hydrolyzed/min/mg protein, respectively (Table 3). Km and Vmax value of uninhibited ACP were 1.89×10−5 M and 58.82 µmole substrate hydrolyzed/30 min/mg protein, respectively. Km of benzylamine (Fig. 8A) and momordicine (Fig. 8B) inhibited ACP were 1.40×10−5 and 1.85×10−5 M, respectively. Vmax values of benzylamine and momordicine inhibited ACP were 45.45 and 47.61 µmol substrate hydrolyzed/30 min/mg protein, respectively. Fig. 9 shows Lineweaver-Burk plots of benzylamine and momordicine inhibited and uninhibited ALP activity. Km and Vmax value of uninhibited ALP were 2.12×10−5 M and 66.67 µmole substrate hydrolyzed/30 min/mg protein, respectively (Table 3). Km of benzylamine (Fig. 9 A) and momordicine (Fig. 9 B) inhibited ALP were 3.22×10−5 and 2.12×10−5 M, respectively. Vmax values of benzylamine and momordicine inhibited ALP were 62.50 and 52.63 µmole substrate hydrolyzed/30 min/mg protein, respectively (Table 3).
Lineweaver-Burk plots showing the effects of active molluscicidal components benzylamine (0.7 mM) (a) and momordicine (0.15 mM) (b) on the inhibition of acetylcholinesterase (AChE) activity in the nervous tissues of snail Lymnaea acuminata.
Lineweaver-Burk plots showing the effects of active molluscicidal components benzylamine (0.7 mM) (a) and momordicine (0.15 mM) (b) on the inhibition of acid phosphatase (ACP) activity in the nervous tissues of snail Lymnaea acuminata.
Lineweaver-Burk plots showing the effects of active molluscicidal components benzylamine (0.7 mM) (a) and momordicine (0.15 mM) (b) on the inhibition of alkaline phosphatase (ALP) activity in the nervous tissues of snail Lymnaea acuminata.
DISCUSSION
Results of the present study indicate that the leaf powder of M. oleifera and lyophilized fruit powder of M. charantia are potential molluscicides of plant origin. Their toxicities are time and concentration dependent, as evidenced in the negative regression between exposure period and LC50 values of the different treatments. The time-dependent toxic effects of tested plant products may be due to the uptake of active components by snails, which progressively increases in the body with an increase in exposure duration. It is also possible that the active compound(s) could change into more toxic forms in the aquarium water or in the snail's body due to the action of various enzymes. The higher toxicities of ethanolic extracts of M. oleifera leaf powder and M. charantia lyophilized fruit powder compared to other organic solvent extracts indicates that the molluscicidal components in the leaves and fruits are more soluble in ethanol than other organic solvents. Thin layer chromatography studies indicate that benzylamine and momordicine are probably the active components in M. oleifera leaf powder and M. charantia lyophilized fruit powder, respectively. HPLC fingerprinting is the best way for chemical characterization3333. Springfield EP, Eagles PKF, Scott G. Quality assessment of South African herbal medicine by means of HPLC fingerprinting. J Ethnopharmacol. 2005;101:75-83.. In the present study, similar retention times were recorded for column purified M. oleifera leaf extract (2.59 min) and benzylamine (2.58 min). Benzylamine is identical to the alkaloid moringine44. Bose CK. Possible role of Moringa oleifera Lam. root in epithelial ovarian cancer. Med Gen Med. 2007;9:26.. However, there is one major peak at the retention time of 8.42 min which indicates that although benzylamine is the molluscicidal component in M. oleifera leaf, there could be some other chemical components in the column-purified fraction that may be responsible for M. oleifera leaf molluscicidal activity. Chronic administration of benzylamine in the drinking water improves glucose tolerance, reduces body weight gain and circulating cholesterol in high-fat diet-fed mice1414. Iffiú-Soltész Z, Wanecq E, Lomba A, Portillo MP, Pellati F, Szöko E, et al. Chronic benzylamine administration in the drinking water improves glucose tolerance, reduces body weight gain and circulating cholesterol in high-fat diet-fed mice. Pharmacol Res. 2010;61:355-63.. M. oleifera leaf extract has a protective effect against lipid peroxidation77. Diallo A, Eklu-Gadegkeku K, Mobio T, Moukha S, Agbonon A, Aklikokou K, et al. Protective effect of Moringa oleifera Lam. and Lannea kerstingii extracts against cadmium and ethanol-induced lipid peroxidation. J Pharmacol Toxicol. 2009;4:160-6.. Extract of M. oleifera leaf is also reported to have anti-carcinogenic66. Costa-Lotufo LV, Khan MTH, Ather A, Wilke DV, Jimenez PC, Pessoa C, et al. Studies of the anticancer potential of plants used in Bangladeshi folk medicine. J Ethnopharmacol. 2005;99:21-30. and anti-bacterial88. Doughari JH, Pukuma MS, De N. Antibacterial effects of Balanites aegyptiaca L. Drel. and Moringa oleifera Lam. on Salmonella typhi. Afr J Biotechnol. 2007;6:2212-5. activity.
The molluscicidal activity of lyophilized fruit powder of M. charantia is due to the presence of momordicine, as evidenced from individual toxicity, thin layer chromatography, and is confirmed by the HPLC retention value (8.4 min; same with that of pure standard compound). Momordicine, a bitter glucoside, is an alkaloid, concentrated in the fruits of M. charantia 55. Chaturvedi P, Geoge S, Milinganyo M, Tripathi YB. Effect of Momordica charantia on lipid profile and oral glucose tolerance in diabetic rats. Phytother Res. 2004;18:954-6., the extracts of which possess anti-diabetic activity1616. Krawinkel MB, Keding GB. Bitter gourd (Momordica charantia): a dietary approach to hyperglycemia. Nutr Rev. 2008;64:331-7..
A comparison of the molluscicidal activity of column-purified fractions of M. oleifera leaf powder and M. charantia lyophilized fruit powder with synthetic molluscicides clearly demonstrates that the former are more potent. The 96 h LC50 value of the column purified fraction of Momordica charantia lyophilized fruit powder (6.21 ppm) against L. acuminata is lower than those of synthetic molluscicides-carbaryl (14.40 ppm), phorate (15.0 ppm), formothion (8.56 ppm) and niclosamide (11.8 ppm)1414. Iffiú-Soltész Z, Wanecq E, Lomba A, Portillo MP, Pellati F, Szöko E, et al. Chronic benzylamine administration in the drinking water improves glucose tolerance, reduces body weight gain and circulating cholesterol in high-fat diet-fed mice. Pharmacol Res. 2010;61:355-63.. The 96 LC50 values of the crude powder of M. oleifera leaf (197.59 ppm) and M. charantia lyophilized fruit (318.29 ppm) against Lymnaea acuminata are lower than the crude powders of Zingiber officinale rhizome (273.80 ppm), Allium cepa bulb (253.27 ppm)3131. Singh S, Singh VK, Singh DK. Molluscicidal activity of some common spice plants. Biol Agric Hortic. 1997;14:237-49., Canna indica root (359.02 ppm)3636. Tripathi SM, Singh DK. Molluscicidal activity of Punica granatum bark and Canna indica root. Braz J Med Biol Res. 2000;33:1351-5., Cinnamomum tamala leaf powder (830.90 ppm)3434. Srivastava P, Kumar P, Singh DK. Control of harmful snails: Tejpat (Cinnamomum tamala) a potential molluscicide. J Appl Biosci. 2005;31:128-32..
It is evident from the steep slope values that a small increase in the concentration of different treatments causes a marked mortality in snails. A t-ratio value greater than 1.96 indicates that the regression is significant2424. Robertson JL, Russell RM, Priesler HK, Savin NE. Bioassay with arthropods. POLO computer programme for analysis of bioassay data. 2nd ed. Boca Raton: CRC Press; 2007. p. 1-224.. Values of heterogeneity factor less than 1.0 denote that in the replicate tests of random samples, the concentration response lines would fall within 95% confidence limits2424. Robertson JL, Russell RM, Priesler HK, Savin NE. Bioassay with arthropods. POLO computer programme for analysis of bioassay data. 2nd ed. Boca Raton: CRC Press; 2007. p. 1-224., and thus the model fits the data adequately. The index of significance of potency estimation g-values (less than 0.5) indicates that the values of the mean are within the limits at all probability levels (90, 95, 99) as it is less than 0.5.
It is clear from the results that in vivo and in vitro exposure to sublethal concentration of benzylamine and momordicine caused a significant inhibition of AChE, ACP and ALP activity in the nervous tissue of L. acuminata. Inhibition of AChE activity inhibition results in accumulation of acetylcholine at the nerve synapses, such that the postsynaptic membrane is in a state of permanent stimulation, resulting in producing paralysis, ataxia, general lack of coordination in neuromuscular system and eventual death2121. Matsumura F. Toxicology of insecticides. New York: Plenum Press; 1985. p. 161.. The methanolic extract of M. oleifera leaf significantly inhibited AChE activity at a concentration of 100 µg/mL3737. Vinutha B, Prashanth D, Salma K, Sreeja SL, Pratiti D, Padmaja R, et al. Screening of selected Indian medicinal plants for acetylcholinesterase inhibitory activity. J. Ethnopharmacol. 2007;109:359-63. Untreated pulp extract of M. charantia (IC50 - 7.2 mg/mL) similarly inhibited AChE activity1010. Ghous T, Aziz N, Abid A, Rasheed A, Iqubal M. On-line inhibition study of immobilized acetylcholinesterase by aqueous extracts of Momordica charantia (Bitter melon). J Chem Soc Pak. 2010;32:814-8..
Acid phosphatase, a lysosomal enzyme22. Aruna P, Chetty CS, Naidu RC, Swami KS. Acid phosphatase activity in Indian apple snail, Pila globosa (Swainson), during aestivation and starvation stress. Proc Indian Acad Sci. 1979;88:363-5., which plays an important role in catabolism, pathological necrosis, autolysis and phagocytosis11. Abou-Donia MB. Increase acid phosphatase activity in hens following an oral dose of leptophos. Toxicol Lett. 1978;2:199-203., and alkaline phosphatase, which plays a critical role in protein synthesis2323. Pilo B, Asnani MV, Shah RV. Studies on wound healing and repair in pigeon liver. III. Histochemical studies on acid and alkaline phosphatase activity during the process. J Anim Morphol Physiol. 1972;19:205-12., shell formation and other secretory activities1313. Ibrahim AM, Hegazi MG, Demian ES. Histochemical localization of alkaline phosphatase activity in the alimentary tract of the snail Marisa cornuarietis (L.). Bull Zool Soc Egypt. 1974;26:94-105., transport of metabolites3838. Vorbrodt A. The role of phosphate in intracellular metabolism. Postepy Hig Med Dosw. 1959;13:200-6. in gastropods. M. oleifera fruit seeds inhibits ACP and ALP activities in arsenic exposed mice1111. Gupta R, Dubey DK, Kannan GM, Flora SJ. Concomitant administration of Moringa oleifera seed powder in the remediation of arsenic-induced oxidative stress in mouse. Cell Biol Int. 2007;31:44-56., while M. charantia fruit extract decreased ALP levels in ammonium chloride-induced hyperammonemic rats3535. Thenmozhi AJ, Subramanian P. Antioxidant potential of Momordica charantia in ammonium chloride-induced hyperammonemic rats. Evid Based Complement Alternat Med. 2011;1-7..
Results of the kinetic study clearly indicate that inhibition of AChE by benzylamine is competitive as the Km values of uninhibited and inhibited enzymes were different while the Vmax were the same (same intercept (1/Vmax) on the Y axis of LineWeaver-Burk plots). Inhibition of AChE by momordicine is uncompetitive; the slopes of momordicine inhibited and uninhibited AChE were parallel to each other, whereas the intercepts were changed. The Km and Vmax of uninhibited and inhibited enzymes were different. Inhibition of ALP by benzylamine is competitive non-competitive. It is a mixed type of inhibition. In this case, Km and Vmax values of uninhibited and inhibited enzymes were different and slopes were also changed.
Inhibition of ACP and ALP by momordicine is non-competitive, as the Km values of uninhibited and inhibited enzymes were the same while their Vmax were different, as evident from different intercepts (1/Vmax) on the Y axis of Lineweaver-Burk plots. Inhibition of ACP by benzylamine is also uncompetitive.
In conclusion, it can be stated that the molluscicidal activity of the leaf powder of M. oleifera and lyophilized fruit powder of M. charantia is due to benzylamine and momordicine, respectively. Inhibition of AChE, ACP and ALP in the nervous tissue of L. acuminata by benzylamine and momordicine may be responsible for the molluscicidal activity of M. oleifera and M. charantia. Therefore, purified ethanolic extracts can be used as potent molluscicides as they are easily available, eco-friendly and culturally more acceptable.
One of the authors Aparna Upadhyay is thankful to the Department of Science and Technology, New Delhi for financial assistance (Inspire Fellowship number- IF10296).
REFERENCES
-
1Abou-Donia MB. Increase acid phosphatase activity in hens following an oral dose of leptophos. Toxicol Lett. 1978;2:199-203.
-
2Aruna P, Chetty CS, Naidu RC, Swami KS. Acid phosphatase activity in Indian apple snail, Pila globosa (Swainson), during aestivation and starvation stress. Proc Indian Acad Sci. 1979;88:363-5.
-
3Bergmeyer UH. Methods of enzymatic analysis. New York: Academic Press; 1967. p. 1129.
-
4Bose CK. Possible role of Moringa oleifera Lam. root in epithelial ovarian cancer. Med Gen Med. 2007;9:26.
-
5Chaturvedi P, Geoge S, Milinganyo M, Tripathi YB. Effect of Momordica charantia on lipid profile and oral glucose tolerance in diabetic rats. Phytother Res. 2004;18:954-6.
-
6Costa-Lotufo LV, Khan MTH, Ather A, Wilke DV, Jimenez PC, Pessoa C, et al. Studies of the anticancer potential of plants used in Bangladeshi folk medicine. J Ethnopharmacol. 2005;99:21-30.
-
7Diallo A, Eklu-Gadegkeku K, Mobio T, Moukha S, Agbonon A, Aklikokou K, et al. Protective effect of Moringa oleifera Lam. and Lannea kerstingii extracts against cadmium and ethanol-induced lipid peroxidation. J Pharmacol Toxicol. 2009;4:160-6.
-
8Doughari JH, Pukuma MS, De N. Antibacterial effects of Balanites aegyptiaca L. Drel. and Moringa oleifera Lam. on Salmonella typhi. Afr J Biotechnol. 2007;6:2212-5.
-
9Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88-95.
-
10Ghous T, Aziz N, Abid A, Rasheed A, Iqubal M. On-line inhibition study of immobilized acetylcholinesterase by aqueous extracts of Momordica charantia (Bitter melon). J Chem Soc Pak. 2010;32:814-8.
-
11Gupta R, Dubey DK, Kannan GM, Flora SJ. Concomitant administration of Moringa oleifera seed powder in the remediation of arsenic-induced oxidative stress in mouse. Cell Biol Int. 2007;31:44-56.
-
12Hammami H, Hamed N, Ayadi A. Epidemiological studies on Fasciola hepatica in Gafsa oases (South West of Tunisia). Parasite. 2007;14:261-4.
-
13Ibrahim AM, Hegazi MG, Demian ES. Histochemical localization of alkaline phosphatase activity in the alimentary tract of the snail Marisa cornuarietis (L.). Bull Zool Soc Egypt. 1974;26:94-105.
-
14Iffiú-Soltész Z, Wanecq E, Lomba A, Portillo MP, Pellati F, Szöko E, et al. Chronic benzylamine administration in the drinking water improves glucose tolerance, reduces body weight gain and circulating cholesterol in high-fat diet-fed mice. Pharmacol Res. 2010;61:355-63.
-
15Jaiswal P, Singh DK. Molluscicidal activity of Carica papaya and Areca catechu against the freshwater snail Lymnaea acuminata. Vet Parasitol. 2008;152:264-70.
-
16Krawinkel MB, Keding GB. Bitter gourd (Momordica charantia): a dietary approach to hyperglycemia. Nutr Rev. 2008;64:331-7.
-
17Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:265-75.
-
18Marston A, Hostettmann K. Plant molluscicides. Phytochemistry. 1985;24:639-52.
-
19Mas-Coma S, Funatsu IR, Bargues MD. Fasciola hepatica and lymnaeid snails occurring at very high altitude in South America. Parasitology. 2001;123(Suppl):S115-27.
-
20Mas-Coma S, Valero MA, Bargues MD. Fasciola, Lymnaeids and human fascioliasis with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. In: Rollinson D, Hay SI, editors. Advances in Parasitology. Burlington: Academic Press; 2009. p. 41-146.
-
21Matsumura F. Toxicology of insecticides. New York: Plenum Press; 1985. p. 161.
-
22Ogundiya MO, Okunade MB, Kolapo AL. Antimicrobial activities of some Nigerian chewing sticks. Ethnobotanical Leaflets. 2006;10:265-71.
-
23Pilo B, Asnani MV, Shah RV. Studies on wound healing and repair in pigeon liver. III. Histochemical studies on acid and alkaline phosphatase activity during the process. J Anim Morphol Physiol. 1972;19:205-12.
-
24Robertson JL, Russell RM, Priesler HK, Savin NE. Bioassay with arthropods. POLO computer programme for analysis of bioassay data. 2nd ed. Boca Raton: CRC Press; 2007. p. 1-224.
-
25Silva TM, Camara CA, Agra MF, de Carvalho MG, Frana MT, Brandoline SV, et al. Molluscicidal activity of Solanum species of the Northeast of Brazil on Biomphalaria glabrata. Fitoterapia. 2006;77:449-52.
-
26Singh A, Singh DK, Mishra TN, Agarwal RA. Molluscicide of plant origin. Biol Agric Hortic. 1996;13:205-52.
-
27Singh DK, Agarwal RA. In vivo and in vitro studies on synergism with anticholinesterase pesticides in the snail Lymnaea acuminata. Arch Environ Contam Toxicol. 1983;12:483-7.
-
28Singh DK, Agarwal RA. Inhibition kinetics of certain organophosphorous and carbamate pesticides on acetylcholinesterase from the snail Lymnaea acuminata. Toxicol Lett. 1983;19:313-9.
-
29Singh DK, Agarwal RA. Correlation of the anticholinesterase and molluscicidal activity of the latex of Euphorbia royleana on the snail Lymnaea acuminata. J Nat Prod. 1984;47:702-5.
-
30Singh DK, Agarwal RA. Toxicity of piperonyl butoxide-carbaryl synergism on the snail Lymnaea acuminata. Int Revue Ges Hydrobiol. 1989;74:689-99.
-
31Singh S, Singh VK, Singh DK. Molluscicidal activity of some common spice plants. Biol Agric Hortic. 1997;14:237-49.
-
32Sokal RR, Rohlf FJ. Introduction to biostatistics. San Francisco: W.H. Freeman; 1973. p. 171-3.
-
33Springfield EP, Eagles PKF, Scott G. Quality assessment of South African herbal medicine by means of HPLC fingerprinting. J Ethnopharmacol. 2005;101:75-83.
-
34Srivastava P, Kumar P, Singh DK. Control of harmful snails: Tejpat (Cinnamomum tamala) a potential molluscicide. J Appl Biosci. 2005;31:128-32.
-
35Thenmozhi AJ, Subramanian P. Antioxidant potential of Momordica charantia in ammonium chloride-induced hyperammonemic rats. Evid Based Complement Alternat Med. 2011;1-7.
-
36Tripathi SM, Singh DK. Molluscicidal activity of Punica granatum bark and Canna indica root. Braz J Med Biol Res. 2000;33:1351-5.
-
37Vinutha B, Prashanth D, Salma K, Sreeja SL, Pratiti D, Padmaja R, et al. Screening of selected Indian medicinal plants for acetylcholinesterase inhibitory activity. J. Ethnopharmacol. 2007;109:359-63
-
38Vorbrodt A. The role of phosphate in intracellular metabolism. Postepy Hig Med Dosw. 1959;13:200-6.
Publication Dates
-
Publication in this collection
Jul-Aug 2013
History
-
Received
14 Apr 2012 -
Accepted
23 Oct 2012