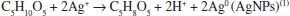Abstracts
Silver nanoparticles (AgNPs) are metal structures at the nanoscale. AgNPs have exhibited antimicrobial activities against fungi and bacteria; however synthesis of AgNPs can generate toxic waste during the reaction process. Accordingly, new routes using non-toxic compounds have been researched. The proposal of the present study was to synthesize AgNPs using ribose as a reducing agent and sodium dodecyl sulfate (SDS) as a stabilizer. The antifungal activity of these particles against C. albicans and C. tropicalis was also evaluated. Stable nanoparticles 12.5 ± 4.9 nm (mean ± SD) in size were obtained, which showed high activity against Candida spp. and could represent an alternative for fungal infection treatment.
Nanopartículas de Prata (AgNPs) são estruturas metálicas em escala nanométrica. AgNPs apresentam atividades antimicrobianas contra fungos e bactérias; no entanto, a síntese de AgNPs pode gerar resíduos tóxicos e devido a isso novas rotas utilizando compostos atóxicos têm sido buscadas. O objetivo desse estudo foi sintetizar AgNPs utilizando a ribose como agente redutor e dodecil sulfato de sódio (SDS) como estabilizante e avaliar a atividade antifúngica dessas partículas contra C. albicans e C. tropicalis. Foram sintetizadas nanopartículas estáveis com 12,5 ± 0,2 nm (média ± DP) que apresentaram elevada atividade contra Candida spp. e podem representar boa alternativa no tratamento de infecções fúngicas.
Silver nanoparticles; Antifungal activity; Candida spp
Candida albicans and Candida tropicalis yeasts are responsible for a number of major diseases as well as recent cases of resistance to the main antifungals. Therefore, new substances should be researched as an alternative to combat such resistance44 Cornistein W, Mora A, Orellana N, Capparelli FJ, Castillo M. Candida: epidemiología y factores de riesgo para especies no albicans. Enferm Infecc Microbiol Clin. 2013;31:380-4. , 1111 Lockhart SR, Iqbal N, Cleveland AA, Farley MM, Harrison LH, Bolden CB, et al. Species identification and antifungal susceptibility testing of Candida bloodstream isolates from population-based surveillance studies in two U.S. cities from 2008 to 2011. J Clin Microbiol. 2012;50:3435-42.. C. albicans and C. tropicalis are the main yeasts isolated from samples of patients admitted to hospitals in the state of Ceará, Brazil1212 Menezes EA, Cunha MCSO, Cunha FA. Identificação preliminar de algumas espécies do gênero Candida spp. em meio cromógeno: resultados de dois anos de um estudo multicêntrico realizado no Ceará. Rev Patol Trop. 2011;40:297-303..
Nanotechnology is responsible for the production and study of metal nanoparticles. These structures present several applications that highlight antimicrobial activity, and this property is an important tool in combating microorganisms resistant to conventional drugs1818 Shenashen MA, El-Safty SA, Elshehy EA. Synthesis, morphological control, and properties of silver nanoparticles in potential applications. Part Part Syst Charact. 2014;31:293-316..
Silver nanoparticles (AgNPs) are a new kind of material with several applications, such as sensors, catalysts, anticancer agents and antimicrobial agents. AgNPs have exhibited activity against bacteria, fungi and viruses88 Kashyap PL, Kumar S, Srivastava AK, Sharma AK. Myconanotechnology in agriculture: a perspective. World J Microbiol Biotechnol. 2013;29:191-207.. However, synthesis of AgNPs produces toxic waste, such as ammonia1414 Panácek A, Kolár M, Vecerová R, Prucek R, Soukupová J, Krystof V, et al. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials. 2009;30:6333-40., which can affect human health and the environment1818 Shenashen MA, El-Safty SA, Elshehy EA. Synthesis, morphological control, and properties of silver nanoparticles in potential applications. Part Part Syst Charact. 2014;31:293-316.. The green synthesis of AgNPs has used various routes: plants, microorganisms and non-toxic substances66 Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011;13:2638-50. , 1515 Quester K, Avalos-Borja M, Castro-Longoria E. Biosynthesis and microscopic study of metallic nanoparticles. Micron. 2013;54-55:1-27. , 1919 Sintubin L, Verstraete W, Boon N. Biologically produced nanosilver: current state and future perspectives. Biotechnol Bioeng. 2012;109:2422-36..
The aim of this study was to synthesize AgNPs using ribose sugars as reducing agents and sodium dodecyl sulfate (SDS) as the capping agent. The antifungal activity of these nanoparticles was evaluated against strains of C. albicans and C. tropicalis.
The synthesis of AgNPs was performed using ribose (Sigma-USA). First, 500 mL of a 5 mM AgNO3 (Merck-Brazil) solution was added to 1.0 g of ribose and 0.5 g of SDS (Sigma-USA) was used as the stabilizer. This solution was stirred and the temperature was raised to 50 ºC. SDS had the function of preventing agglomeration and subsequent precipitation of the AgNPs. The reaction was considered complete when the solution acquired a pale yellow color, characteristic of AgNPs (Fig. 1a)22 Bhaduri GA, Little R, Khomane RB, Lokhande SU, Kulkarni BD, Mendis BG, et al. Green synthesis of silver nanoparticles using sunlight. J Photochem Photobiol A: Chemistry. 2013;258:1-9. , 1414 Panácek A, Kolár M, Vecerová R, Prucek R, Soukupová J, Krystof V, et al. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials. 2009;30:6333-40..
The purification was carried out by centrifugation at 10,000g/10min. Characterization of the synthesized AgNPs was carried out using a UV-Visible spectrophotometer (Thermo Scientific GENESYSTM 10s), by scanning of the absorbance spectra in a 300-700 nm range of wavelength. The size of the AgNPs was analyzed on Zetasizer, NanoZS Malvern® by dynamic light scattering (DLS)55 Gao X, Wei L, Yan H, Xu B. Green synthesis and characteristic of core-shell structure silver/starch nanoparticles. Mater Lett. 2011;65:2963-5..
In this study, 30 strains of Candida spp. were selected (14 C. albicans and 16 C. tropicalis) and isolated from blood samples of patients hospitalized in the state of Ceará, Brazil. C. albicans was purified and identified in a chromogenic medium, with production of chlamydospores in rice extract agar containing Tween-80, and germ tube formation. This identification was carried out by molecular biology with the primer hwp1 (cr-f-5'-GCT ACC ACT TCA GAA TCA TCA TC-3'; cr-r-5' GCA CCT TCA GTC GTA GAG ACG-3') and the PCR conditions were 95 °C for five min, followed by 30 cycles of 94 °C for 45 s, 58 °C for 40 s, and 72 °C for 55 s; extension was performed at 72 °C for 10 min. The DNA fragment size that was produced had 945 bp. The molecular identification of C. tropicalis was performed using the trf4 gene. The following primers were used (trf4 5'-ATT GGC TGA AAC AGA GGT-3 '; trf4-5' CAA CCC TGC TAA GTC ATT AC-3') and the PCR conditions were 95 °C for five min, followed by 30 cycles of 94 °C for one min, 50 °C for one min, and 72 °C for 90 s; extension was performed at 72 °C for 10 min. The DNA fragment size that was produced had 324 bp77 Kang Y, Iida S, Yamamoto S, Kogure T, Tanaka R, Mikami Y. Trf4 is a useful gene for discrimination of Candida tropicalis from other medically important Candida species. Nikon Ishinkin Gakkai Zasshi. 2008;49:39-43. , 1616 Romeo O, Criseo G. First molecular method for discriminating between Candida africana, Candida albicans and Candida dubliniensis by using hwp1 gene. Diagn Microbiol Infect Dis. 2008;62:230-3..
The sensitivity of Candida spp. was evaluated by the well diffusion method on a Mueller-Hinton medium supplemented with 2% glucose and 0.05% methylene blue. In mediums containing Candida spp, wells were made and filled with 80 µg of AgNPs. Discs of amphotericin B 10 µg were used as control. The plates were incubated at 35 °C for 24h, and after this period fungal growth inhibition halos were measured (mm). Each test was conducted three times, according to the protocol of CLSI M44-A233 Clinical and Laboratory Standards Institute. Method for antifungal disk diffusion susceptibility testing of yeasts: approved standard M44-A2. Wayne: Clinical and Laboratory Standards Institute; 2008. , 2020 Vasconcelos Júnior AA, Menezes EA, Cunha FA, Cunha MCSO, Braz BHL, Capelo LG, et al. Comparação entre microdiluição e disco difusão para o teste de susceptibilidade aos antifúngicos contra Candida spp. Semina Ciênc Biol Saúde. 2012;33:135-42..
Production of AgNPs using ribose as a reducing agent and SDS as a capping agent was simple and easy to perform. The entire process was completed in 30 min. The chemical reaction proposed can be represented by the following equation:
These AgNPs showed strong spectrophotometric absorbance, around 420 nm, as shown in Figure 1b. This is typical behavior of these structures. Use of SDS as the capping agent provided prolonged stability for up to four months when stored at room temperature and exposed to ambient light. Some sugars have reducing properties and are used in the production of nanoparticles. The process does not harm the environment because it does not produce toxic waste and requires no accelerator1313 Oluwafemi OS, Lucwaba Y, Gura A, Masabeya M, Ncapayi V, Olujimi OO, et al. A facile completely 'green' size tunable synthesis of maltose-reduced silver nanoparticles without the use of any accelerator. Colloids Surf B Biointerfaces. 2013;102:718-23..
AgNPs produced in this study had a size of 12.5 ± 4.9 nm (mean ± SD), with a narrow particle size distribution, as shown in Figure 1c. This feature gives a high surface area, better for antimicrobial activity and good order. In previous studies using glucose as the reducing agent, the size of AgNPs was around 15 nm1010 Lanje AS, Sharma SJ, Pode RB. Synthesis of silver nanoparticles: a safer alternative to conventional antimicrobial and antibacterial agents. J Chem Pharm Res. 2010;2:478-83..
The AgNPs exhibited high antimicrobial activity, and this property can be very useful, especially against microorganisms resistant to conventional antimicrobials1717 Sharma VK, Yngard RA, Lin Y. Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interface Sci. 2009;145:83-96.. C. albicans and C. tropicalis showed high sensitivity to AgNPs (Fig 1d). The activity of 80 µg of AgNPs can be compared with the activity of amphotericin B, a powerful antifungal (Table 1). Studies highlight this same result with activity of AgNPs against Candida spp99 Kumar P, Selvi SS, Govindaraju M. Seaweed-mediated biosynthesis of silver nanoparticles using Gracilaria corticata for its antifungal activity against Candida spp. Appl Nanosci. 2013;3:495-500. , 1414 Panácek A, Kolár M, Vecerová R, Prucek R, Soukupová J, Krystof V, et al. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials. 2009;30:6333-40.. The statistical analysis of the results, carried out by Student's t-test, showed that C. albicans was more sensitive than C. tropicalis (p = 0.02).
Effect of AgNPs produced by green synthesis and Amphotericin B against C. albicans and C. tropicalis
In conclusion, AgNPs were easily prepared by green synthesis using ribose as a reducing agent and SDS as a stabilizer. Additionally, they showed high activity against C. albicans and C. tropicalis, a similar activity observed by the antifungal amphotericin B, and may represent an alternative for treating fungal infections.
ACKNOWLEDGMENTS
The financial support received from CNPq (Brazilian agency), Process 473417/2007.
REFERENCES
-
1Barie PS. Multidrug-resistant organisms and antibiotic management. Surg Clin North Am. 2012;92:345-91.
-
2Bhaduri GA, Little R, Khomane RB, Lokhande SU, Kulkarni BD, Mendis BG, et al Green synthesis of silver nanoparticles using sunlight. J Photochem Photobiol A: Chemistry. 2013;258:1-9.
-
3Clinical and Laboratory Standards Institute. Method for antifungal disk diffusion susceptibility testing of yeasts: approved standard M44-A2. Wayne: Clinical and Laboratory Standards Institute; 2008.
-
4Cornistein W, Mora A, Orellana N, Capparelli FJ, Castillo M. Candida: epidemiología y factores de riesgo para especies no albicans Enferm Infecc Microbiol Clin. 2013;31:380-4.
-
5Gao X, Wei L, Yan H, Xu B. Green synthesis and characteristic of core-shell structure silver/starch nanoparticles. Mater Lett. 2011;65:2963-5.
-
6Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011;13:2638-50.
-
7Kang Y, Iida S, Yamamoto S, Kogure T, Tanaka R, Mikami Y. Trf4 is a useful gene for discrimination of Candida tropicalis from other medically important Candida species. Nikon Ishinkin Gakkai Zasshi. 2008;49:39-43.
-
8Kashyap PL, Kumar S, Srivastava AK, Sharma AK. Myconanotechnology in agriculture: a perspective. World J Microbiol Biotechnol. 2013;29:191-207.
-
9Kumar P, Selvi SS, Govindaraju M. Seaweed-mediated biosynthesis of silver nanoparticles using Gracilaria corticata for its antifungal activity against Candida spp. Appl Nanosci. 2013;3:495-500.
-
10Lanje AS, Sharma SJ, Pode RB. Synthesis of silver nanoparticles: a safer alternative to conventional antimicrobial and antibacterial agents. J Chem Pharm Res. 2010;2:478-83.
-
11Lockhart SR, Iqbal N, Cleveland AA, Farley MM, Harrison LH, Bolden CB, et al Species identification and antifungal susceptibility testing of Candida bloodstream isolates from population-based surveillance studies in two U.S. cities from 2008 to 2011. J Clin Microbiol. 2012;50:3435-42.
-
12Menezes EA, Cunha MCSO, Cunha FA. Identificação preliminar de algumas espécies do gênero Candida spp. em meio cromógeno: resultados de dois anos de um estudo multicêntrico realizado no Ceará. Rev Patol Trop. 2011;40:297-303.
-
13Oluwafemi OS, Lucwaba Y, Gura A, Masabeya M, Ncapayi V, Olujimi OO, et al A facile completely 'green' size tunable synthesis of maltose-reduced silver nanoparticles without the use of any accelerator. Colloids Surf B Biointerfaces. 2013;102:718-23.
-
14Panácek A, Kolár M, Vecerová R, Prucek R, Soukupová J, Krystof V, et al Antifungal activity of silver nanoparticles against Candida spp. Biomaterials. 2009;30:6333-40.
-
15Quester K, Avalos-Borja M, Castro-Longoria E. Biosynthesis and microscopic study of metallic nanoparticles. Micron. 2013;54-55:1-27.
-
16Romeo O, Criseo G. First molecular method for discriminating between Candida africana, Candida albicans and Candida dubliniensis by using hwp1 gene. Diagn Microbiol Infect Dis. 2008;62:230-3.
-
17Sharma VK, Yngard RA, Lin Y. Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interface Sci. 2009;145:83-96.
-
18Shenashen MA, El-Safty SA, Elshehy EA. Synthesis, morphological control, and properties of silver nanoparticles in potential applications. Part Part Syst Charact. 2014;31:293-316.
-
19Sintubin L, Verstraete W, Boon N. Biologically produced nanosilver: current state and future perspectives. Biotechnol Bioeng. 2012;109:2422-36.
-
20Vasconcelos Júnior AA, Menezes EA, Cunha FA, Cunha MCSO, Braz BHL, Capelo LG, et al Comparação entre microdiluição e disco difusão para o teste de susceptibilidade aos antifúngicos contra Candida spp. Semina Ciênc Biol Saúde. 2012;33:135-42.
Publication Dates
-
Publication in this collection
Mar-Apr 2015
History
-
Received
30 Apr 2014 -
Accepted
14 July 2014