ABSTRACT
In experimental infection with Strongyloides venezuelensis, the acute and recovery phases can be distinguished, unlike human infections caused by Strongyloides stercoralis. The objective of this study was to evaluate the production of anti-Strongyloides IgG antibodies and the recognition of immunogenic protein bands during the acute and the recovery phases in rats experimentally infected with S. venezuelensis. Rats were infected subcutaneously with 400 or 4,000 S. venezuelensis infective larvae. The acute phase was characterized by elimination of a large number of eggs in the faeces on days 6-14 post infection; the recovery phase was characterized by the resolution of the infection between days 30 and 35 post infection. Differences in IgG levels were observed in the acute and the recovery phases. Different antigenic fractions were recognized in both phases of infection. It is concluded that proteins within the 30-40 kDa range are immunoreactive markers for both the acute and the recovery phases in rats experimentally infected with S. venezuelensis, particularly using membrane antigen.
Strongyloides venezuelensis; Acute and recovery phases; 30-40 kDa, IgG
Strongyloides venezuelensis has been used in rodent models of Strongyloides infection, particularly in immunological studies11. Viney M, Kikuchi T. Strongyloides ratti and S. venezuelensis: rodent models of Strongyloides infection. Parasitology. 2017;144:285-94. related to the production of heterologous antigens in the serological diagnosis of human strongyloidiasis22. Levenhagen MA, Costa-Cruz JM. Update on immunologic and molecular diagnosis of human strongyloidiasis. Acta Trop. 2014;135:33-43.. In experimental infections, it is possible to define the acute and the recovery phases33. Chiuso-Minicucci F, Marra NM, Zornella-Pezavento SF, França TG, Ishikawa LL, Amarante MR, et al. Recovery from Strongyloides venezuelensis infection in Lewis rats is associated with a strong Th2 response. Parasite Immunol. 2010;32:74-8., unlike human infections caused by Strongyloides stercoralis. In this context, antibody production and recognition of immunogenic bands during the acute and the recovery phases have not been well explored. Thus, the objective of this study was to evaluate the production of anti-Strongyloides IgG antibodies and the recognition of immunogenic bands produced during the acute and the recovery phases in rats experimentally infected with S. venezuelensis.
Male Wistar rats (Rattus norvegicus), with four weeks old, were obtained from the Bioterio de Producao de Ratos, Instituto de Ciencias Biomedicas, Universidade de Sao Paulo, Brazil and were kept in the Bioterio do Instituto de Medicina Tropical de Sao Paulo (IMT-SP). Rats received sterilized food and water ad libitum and were handled in compliance with the animal ethics guidelines adopted by the Comite de Etica em Experimentacao Animal, IMT (CEUA IMT 317A).
S. venezuelensis infective larvae (iL3) were obtained by charcoal culture of infected rats faeces (CEUA protocol IMT 0356A). The experimental infections were established in 35 rats divided into three groups: infected subcutaneously with 400 S. venezuelensis iL3 (n = 15, 400iL3), infected with 4,000 S. venezuelensis iL3 (n = 15, 4000iL3) and uninfected rats (n = 5, negative control, NC).
The number of eggs per gram of faeces (EPG) was obtained daily until day 35 post infection (pi), according to the Gordon and Whitlock method44. Gordon HM, Whitlock HV. New technique for counting nematode eggs in sheep faeces. J Counc Sci Ind Res. 1939;12:50-2.. EPG was performed in 5 samples of 1 gram of faeces randomly collected on each day post infection in each infected group (400iL3 and 4000iL3). The results were determined after five counts (mean ± standard error). Blood samples (five animals) were collected by cardiac puncture on days 2, 7 and 35 pi after anaesthesia with ketamine/xylazine, and the animals were subsequently euthanized. Blood samples were centrifuged and the serum samples obtained were used in ELISA and Western blotting.
Two antigenic fractions were prepared using approximately 200,000 S. venezuelensis iL3. Briefly, iL3 were resuspended in 1 mL of Tris-HCl (25 mM [pH 7.5]) containing protease inhibitors (Sigma-Aldrich, St. Louis, MO, USA) and sonicated on ice (5 cycles of 20 s). The suspensions were centrifuged at 12,400 × g for 30 min at 4 °C, and the supernatant was collected (soluble fraction, SAg). Pellets were resuspended in 5 M urea, 2 M thiourea and 4% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) in an ice bath for 30 min, and the supernatant was collected after centrifugation at 12,400 × g for 30 min at 4 °C (membrane fraction, MAg).
ELISA was performed as described previously55. Marques PD, Malta FM, Meisel DM, Corral MA, Pinho JR, Costa-Cruz JM, et al. Diagnosis of the strongyloid nematode Strongyloides venezuelensis in experimentally infected rats. J Helminthol. 2016;90:422-7., with some modifications. Microplates were coated overnight at 4 °C with 10 µg/mL (to a final volume 50 µL/well) of each S. venezuelensis antigenic fraction in 0.06 M carbonate-bicarbonate buffer (pH 9.6). Plates were incubated with serum samples (1:20) for 45 min at 37 °C and then with the secondary antibody consisting of peroxidase-labelled goat anti-rat IgG (Sigma-Aldrich) at a dilution of 1:2,000 for 45 min at 37 °C. The assay was developed by adding TMB chromogen solution (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) for 15 min and was stopped by addition of 2 NH2SO4. The optical density (OD) was determined at 450 nm in a plate reader (Thermo Fisher Scientific). Statistical analyses were performed using the GraphPad Prism software version 8.0 (GraphPad Software. San Diego, CA, USA). Statistical significance was determined by ANOVA, followed by Tukey’s multiple comparison test (p < 0.05).
Electrophoresis and Western blotting were performed as previously described66. Corral MA, Paula FM, Meisel DM, Castilho VL, Gonçalves EM, Levy D, et al. Potential immunological markers for diagnosis of human strongyloidiasis using heterologous antigens. Parasitology. 2017;144:124-30.. Briefly, approximately 140 µg (2 µg/mm of gel) of the antigenic fractions (SAg and MAg) underwent electrophoresis in 12% polyacrylamide gel (SDS-PAGE) for 2 h (20 mA). A molecular mass standard (10-260 kDa; Bio-Rad Laboratories, Hercules, CA, USA) was used to quantitate the relative protein bands. After electrophoresis, the proteins on the gel were transferred to a polyvinylidene difluoride (PVDF) membrane (0.2 µm) (Bio-Rad Laboratories). In the Western blotting, after blocking (50 mM Tris-HCl [pH 7.5]; 3% Tween 20, and 3% milk), the membranes were incubated with sera diluted 1:50 in T buffer (50 mM Tris-HCl [pH 7.5], 100 mM NaCl, 0.1% Tween 20 and 5% milk). The secondary antibody (anti-rat IgG conjugated with peroxidase; Sigma-Aldrich) was then diluted 1:2,000 in T buffer and added to the membrane. Binding was detected using ECL Prime Western Blotting detection reagents (GE Healthcare Life Sciences, Little Chalfont, UK). The antigenic components were visualized in a Luminescent Image Analyzer (Fujifilm, Minato, Tokyo, Japan) and analysed by the VisionWorks LS Analysis Software (Analytik Jena, Jena, Germany).
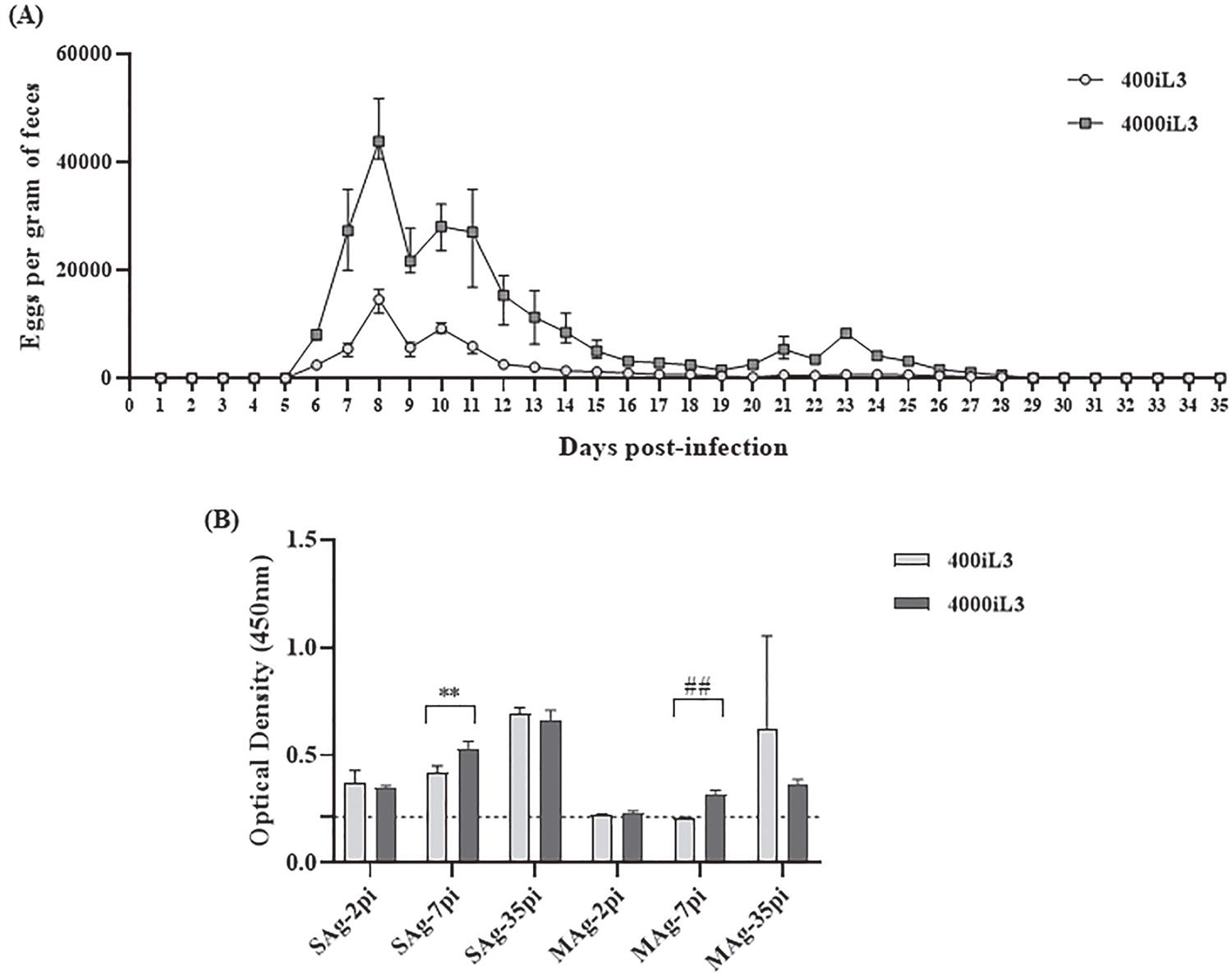
The egg count showed the development of the experimental infection (Figure 1A). The acute and the recovery phases in the experimental infection with S. venezuelensis were well characterized by Chiuso-Minicucci et al.33. Chiuso-Minicucci F, Marra NM, Zornella-Pezavento SF, França TG, Ishikawa LL, Amarante MR, et al. Recovery from Strongyloides venezuelensis infection in Lewis rats is associated with a strong Th2 response. Parasite Immunol. 2010;32:74-8. The acute phase was identified by elimination of a large number of eggs in the faeces on days 6-14 pi (Figure 1A). On day 8 pi, the egg excretion peaked with 14,600 ± 1,702.9 and 43,870 ± 801.5 EPG in the rats infected with 400iL3 and 4000iL3, respectively. The recovery phase was defined by a reduction in egg excretion and resolution of infection between days 30 to 35 pi (Figure 1A). The increase in the EPG on day 8 pi and the reduction after day 13 pi have been reported in the literature in rats experimentally infected with S. venezuelensis33. Chiuso-Minicucci F, Marra NM, Zornella-Pezavento SF, França TG, Ishikawa LL, Amarante MR, et al. Recovery from Strongyloides venezuelensis infection in Lewis rats is associated with a strong Th2 response. Parasite Immunol. 2010;32:74-8.,55. Marques PD, Malta FM, Meisel DM, Corral MA, Pinho JR, Costa-Cruz JM, et al. Diagnosis of the strongyloid nematode Strongyloides venezuelensis in experimentally infected rats. J Helminthol. 2016;90:422-7.,77. Gonçalves AL, Silva CV, Ueta MT, Costa-Cruz JM. Antigen, antibody and immune complex detection in serum samples from rats experimentally infected with Strongyloides venezuelensis. Exp Parasitol. 2012;130:205-8.. Although infection with S. venezuelensis is well tolerated by rats,11. Viney M, Kikuchi T. Strongyloides ratti and S. venezuelensis: rodent models of Strongyloides infection. Parasitology. 2017;144:285-94. infections with 400iL3 and 4000iL3 can be considered moderate and severe, respectively88. Marra NM, Chiuso-Minicucci F, Machado GC, Zorzella-Pezavento SF, França TG, Ishikawa LL, et al. Faecal examination and PCR to detect Strongyloides venezuelensis in experimentally infected Lewis rats. Mem Inst Oswaldo Cruz. 2010;105:57-61.,99. Nakai ES, Amarante AF. Infecção experimental de camundongos (Mus musculus) e ratos (Rattus norvegicus) com Strongyloides venezuelensis. Rev Bras Parasitol Vet. 2001;10:1-6..
Eggs per gram of faeces (A) and IgG anti-S. venezuelensis in serum samples (B) from rats experimentally infected with 400iL3 and 4000iL3 S. venezuelensis. (A) Results are expressed as means ± SEM of five rats per group. (B) ELISA test using soluble (SAg) and membrane (MAg) antigens from S. venezuelensis filariform larvae on days 2, 7 and 35 post infection (pi). Data are expressed as means ± SEM (n = 5), **p = 0.0055, ## p = 0.0024. Dashed lines indicate the mean optical density of the negative control ± SEM (n = 5).
The immune response during the acute and the recovery phases in experimental infection with S. venezuelensis was shown by Chiuso-Minicucci et al.33. Chiuso-Minicucci F, Marra NM, Zornella-Pezavento SF, França TG, Ishikawa LL, Amarante MR, et al. Recovery from Strongyloides venezuelensis infection in Lewis rats is associated with a strong Th2 response. Parasite Immunol. 2010;32:74-8. In this study, the specific IgG for S. venezuelensis was lower in the acute phase than in the recovery phase. Our results showed differences in IgG levels in the acute and the recovery phases (Figure 1B). There was a considerable increase in the OD values for the SAg during the experimental infection, suggesting the differentiation of the acute and the recovery phases, independent of the inoculum. Increased production of antibodies in the recovery phase in rats infected with 2000 iL3 has been observed previously55. Marques PD, Malta FM, Meisel DM, Corral MA, Pinho JR, Costa-Cruz JM, et al. Diagnosis of the strongyloid nematode Strongyloides venezuelensis in experimentally infected rats. J Helminthol. 2016;90:422-7.. On the other hand, previous studies have shown higher OD values on day 8 pi compared with other days pi in animals infected with S. venezuelensis77. Gonçalves AL, Silva CV, Ueta MT, Costa-Cruz JM. Antigen, antibody and immune complex detection in serum samples from rats experimentally infected with Strongyloides venezuelensis. Exp Parasitol. 2012;130:205-8. and S. ratti1010. Rodrigues RM, Cardoso CR, Gonçalves AL, Silva NM, Massa V, Alves R, et al. Increased susceptibility to Strongyloides venezuelensis infection is related to the parasite load and absence of major histocompatibility complex (MHC) class II molecules. Exp Parasitol. 2013;135:580-6..
Comparing the infections with 400iL3 and 4000iL3, a statistical difference (p < 0.05) was observed for OD values only on day 7 pi, considering the two antigens (Figure 1B). The results showed a significant increase in OD values in rats infected with 400iL3 on day 2 pi versus day 7 pi independent of antigens; and on day 35 pi versus 2 pi and 7 pi, only the SAg showed a difference. In addition, for rats infected with 4000iL3, there were statistically significant differences on days 2, 7 and 35 pi for the two antigens and on day 2 pi versus 7 pi for each antigen. Analysing the OD values, we can observe that the 4000iL3 group showed lower values in relation to 400iL3 by MAg. These results could be related to variations in the group, because S. venezuelensis infection is well tolerated by rats11. Viney M, Kikuchi T. Strongyloides ratti and S. venezuelensis: rodent models of Strongyloides infection. Parasitology. 2017;144:285-94..
Few studies have evaluated immunogenic bands during experimental infection1010. Rodrigues RM, Cardoso CR, Gonçalves AL, Silva NM, Massa V, Alves R, et al. Increased susceptibility to Strongyloides venezuelensis infection is related to the parasite load and absence of major histocompatibility complex (MHC) class II molecules. Exp Parasitol. 2013;135:580-6.
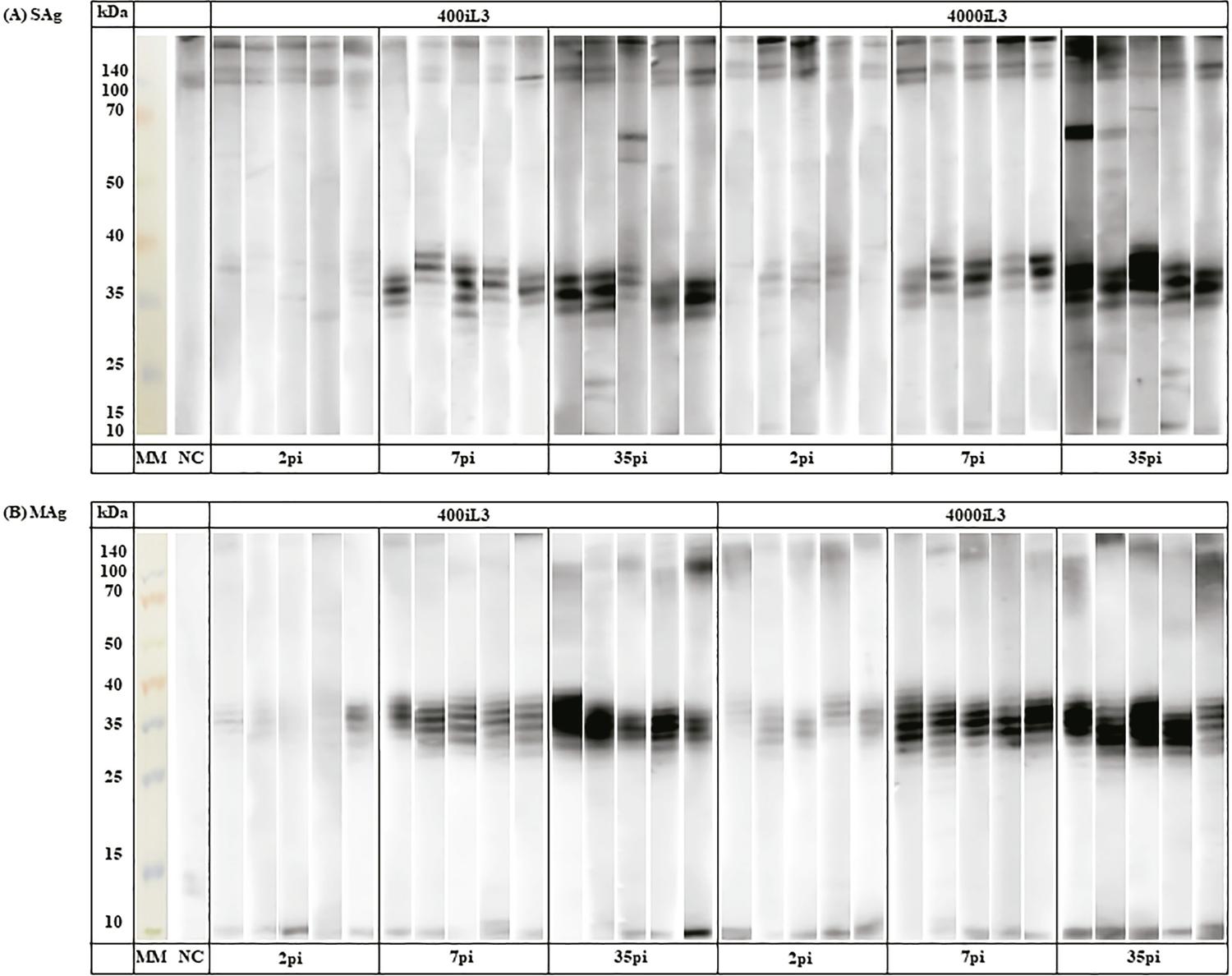
11. Goulart de Carvalho EF, Neto de Sousa JE, Gonçalves AL, Cunha-Junior JP, Costa-Cruz JM. Immunoblotting using Strongyloides venezuelensis larvae, partenogenetic females or eggs extracts for the diagnosis of experimentally infected immunosuppressed rats. Exp Parasitol. 2015;157:117-23.-1212. Sousa JE, Carvalho EF, Levenhagen MA, Faria LS, Gonçalves-Pires MR, Costa-Cruz JM. Serological cross-reactivity between Strongyloides venezuelensis and Shyphacia muris in Wistar rats (Rattus norvegicus). Parasitol Int. 2016;65:137-45.. In the present study, immunogenic bands were recognized (Figure 2), independent of the antigenic fraction and the phase of infection. Bands of ~100-140 kDa were recognized in two groups independently, in the infection phase by SAg and in the recovery phase by MAg. We observed the ~65-70 kDa bands in the recovery phase in the 400iL3 and 4000iL3 groups, mainly using SAg. In addition, there was greater staining intensity of protein bands visualized in MAg. In a recent study of experimental strongyloidiasis1111. Goulart de Carvalho EF, Neto de Sousa JE, Gonçalves AL, Cunha-Junior JP, Costa-Cruz JM. Immunoblotting using Strongyloides venezuelensis larvae, partenogenetic females or eggs extracts for the diagnosis of experimentally infected immunosuppressed rats. Exp Parasitol. 2015;157:117-23., immunoreactive bands at 17, 38 and 50 kDa were demonstrated using S. venezuelensis iL3 alkaline extract. In addition, 36, 68, 76, 83 and 102 kDa bands for a saline extract were considered antigenic in another experimental investigation with S. venezuelensis1212. Sousa JE, Carvalho EF, Levenhagen MA, Faria LS, Gonçalves-Pires MR, Costa-Cruz JM. Serological cross-reactivity between Strongyloides venezuelensis and Shyphacia muris in Wistar rats (Rattus norvegicus). Parasitol Int. 2016;65:137-45..
- Recognition of antigenic bands (soluble fraction [SAg, A] and membrane fraction [Mag, B]) in serum samples from rats experimentally infected with S. venezuelensis (400iL3 and 4000iL3) on days 2, 7 and 35 post infection (pi) (n=5).
The 30-40 kDa band was recognized in the acute and recovery phases of infection in both groups. In agreement with previous reports1010. Rodrigues RM, Cardoso CR, Gonçalves AL, Silva NM, Massa V, Alves R, et al. Increased susceptibility to Strongyloides venezuelensis infection is related to the parasite load and absence of major histocompatibility complex (MHC) class II molecules. Exp Parasitol. 2013;135:580-6.
11. Goulart de Carvalho EF, Neto de Sousa JE, Gonçalves AL, Cunha-Junior JP, Costa-Cruz JM. Immunoblotting using Strongyloides venezuelensis larvae, partenogenetic females or eggs extracts for the diagnosis of experimentally infected immunosuppressed rats. Exp Parasitol. 2015;157:117-23.-1212. Sousa JE, Carvalho EF, Levenhagen MA, Faria LS, Gonçalves-Pires MR, Costa-Cruz JM. Serological cross-reactivity between Strongyloides venezuelensis and Shyphacia muris in Wistar rats (Rattus norvegicus). Parasitol Int. 2016;65:137-45., this mass range has been considered an immunogenic band in experimental strongyloidiasis. In addition, 30-40 kDa bands are considered important for the diagnosis of S. stercoralis infections66. Corral MA, Paula FM, Meisel DM, Castilho VL, Gonçalves EM, Levy D, et al. Potential immunological markers for diagnosis of human strongyloidiasis using heterologous antigens. Parasitology. 2017;144:124-30.,1313. Sudré AP, Siqueira RC, Barreto MG, Peralta RH, Macedo HW, Peralta JM. Identification of a 26-kDa protein fraction as an important antigen for application in the immunodiagnosis of strongyloidiasis. Parasitol Res. 2007;101:1117-23.,1414. Rodpai R, Intapan PM, Thanchomnang T, Sanpool O, Janwan P, Laummaunwai P, et al. Strongyloides stercoralis diagnostic polypeptides for human strongyloidiasis and their proteomic analysis. Parasitol Res. 2016;115:4007-12.. Recognition of this protein range by IgG antibodies from the serum of rats infected with 400iL3 may reinforce its potential diagnostic value. In addition, it is difficult to define the phases in S. stercoralis infection, which makes recognition of the 30-40 kDa band, mainly by MAg, an important diagnostic tool.
Considering the difference in protein extracts and the intrinsic variability of the reaction, it can be suggested that the OD values and the recognition of immunogenic bands occurred according to the phases of the infection. Based on the literature1515. Rodrigues RM, Sopelete MC, Silva DA, Cunha-Júnior JP, Taketomi EA, Costa-Cruz JM. Strongyloides ratti antigenic components recognized by IgE antibodies in immunoblotting as an additional too for improving the immunodiagnosis in human strongyloidiasis. Mem Inst Oswaldo Cruz. 2004;99:89-93., the increase in OD values was related to the increase in the number of immunogenic bands recognized.
Therefore, experimental S. venezuelensis infection is an important tool for diagnostic evaluations for use in human strongyloidiasis. Thus, the present study points to the use of SAg in ELISA and highlights the proteins within the 30-40 kDa range in MAg as a tool for the diagnosis of the acute and the recovery phases in experimental infection by S. venezuelensis.
REFERENCES
-
1Viney M, Kikuchi T. Strongyloides ratti and S. venezuelensis: rodent models of Strongyloides infection. Parasitology. 2017;144:285-94.
-
2Levenhagen MA, Costa-Cruz JM. Update on immunologic and molecular diagnosis of human strongyloidiasis. Acta Trop. 2014;135:33-43.
-
3Chiuso-Minicucci F, Marra NM, Zornella-Pezavento SF, França TG, Ishikawa LL, Amarante MR, et al. Recovery from Strongyloides venezuelensis infection in Lewis rats is associated with a strong Th2 response. Parasite Immunol. 2010;32:74-8.
-
4Gordon HM, Whitlock HV. New technique for counting nematode eggs in sheep faeces. J Counc Sci Ind Res. 1939;12:50-2.
-
5Marques PD, Malta FM, Meisel DM, Corral MA, Pinho JR, Costa-Cruz JM, et al. Diagnosis of the strongyloid nematode Strongyloides venezuelensis in experimentally infected rats. J Helminthol. 2016;90:422-7.
-
6Corral MA, Paula FM, Meisel DM, Castilho VL, Gonçalves EM, Levy D, et al. Potential immunological markers for diagnosis of human strongyloidiasis using heterologous antigens. Parasitology. 2017;144:124-30.
-
7Gonçalves AL, Silva CV, Ueta MT, Costa-Cruz JM. Antigen, antibody and immune complex detection in serum samples from rats experimentally infected with Strongyloides venezuelensis. Exp Parasitol. 2012;130:205-8.
-
8Marra NM, Chiuso-Minicucci F, Machado GC, Zorzella-Pezavento SF, França TG, Ishikawa LL, et al. Faecal examination and PCR to detect Strongyloides venezuelensis in experimentally infected Lewis rats. Mem Inst Oswaldo Cruz. 2010;105:57-61.
-
9Nakai ES, Amarante AF. Infecção experimental de camundongos (Mus musculus) e ratos (Rattus norvegicus) com Strongyloides venezuelensis. Rev Bras Parasitol Vet. 2001;10:1-6.
-
10Rodrigues RM, Cardoso CR, Gonçalves AL, Silva NM, Massa V, Alves R, et al. Increased susceptibility to Strongyloides venezuelensis infection is related to the parasite load and absence of major histocompatibility complex (MHC) class II molecules. Exp Parasitol. 2013;135:580-6.
-
11Goulart de Carvalho EF, Neto de Sousa JE, Gonçalves AL, Cunha-Junior JP, Costa-Cruz JM. Immunoblotting using Strongyloides venezuelensis larvae, partenogenetic females or eggs extracts for the diagnosis of experimentally infected immunosuppressed rats. Exp Parasitol. 2015;157:117-23.
-
12Sousa JE, Carvalho EF, Levenhagen MA, Faria LS, Gonçalves-Pires MR, Costa-Cruz JM. Serological cross-reactivity between Strongyloides venezuelensis and Shyphacia muris in Wistar rats (Rattus norvegicus). Parasitol Int. 2016;65:137-45.
-
13Sudré AP, Siqueira RC, Barreto MG, Peralta RH, Macedo HW, Peralta JM. Identification of a 26-kDa protein fraction as an important antigen for application in the immunodiagnosis of strongyloidiasis. Parasitol Res. 2007;101:1117-23.
-
14Rodpai R, Intapan PM, Thanchomnang T, Sanpool O, Janwan P, Laummaunwai P, et al. Strongyloides stercoralis diagnostic polypeptides for human strongyloidiasis and their proteomic analysis. Parasitol Res. 2016;115:4007-12.
-
15Rodrigues RM, Sopelete MC, Silva DA, Cunha-Júnior JP, Taketomi EA, Costa-Cruz JM. Strongyloides ratti antigenic components recognized by IgE antibodies in immunoblotting as an additional too for improving the immunodiagnosis in human strongyloidiasis. Mem Inst Oswaldo Cruz. 2004;99:89-93.
-
FUNDINGThis research was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (grant Nº FAPESP 2016/06185-0, to FMP), and Coordenação de Aperfeiçoamento Pessoal de Nível Superior (grant Nº CAPES 0001, to PDMF), Brazil.
Publication Dates
-
Publication in this collection
11 May 2020 -
Date of issue
2020
History
-
Received
24 Oct 2019 -
Accepted
30 Mar 2020