Abstracts
Leishmaniasis is a typical parasite infection whose protective immunity depends on macrophage activation. Susceptibility to Leishmania donovani infection was compared in H (high antibody responder) and L (low antibody responder) mice from selection IV-A. H mice infected intravenously with 10(7) amastigotes of L. donovani were more susceptible to infection than their L counterparts. This higher susceptibility was characterized by a higher splenic and hepatic parasite burden. An increased splenic index was observed in both lines after sixty days of infection. This splenomegaly was caused, at least partially, by an increase in the number of splenic cells as determined by direct counts of cells from spleen. The results show that selection IV-A is susceptible to visceral leishmaniasis, with the H line being more susceptible than the L line.
Leishmania donovani; Biozzi mice
A leishmaniose é uma infecção parasitária cuja imunidade protetora envolve a ativação de macrófagos. Neste trabalho avaliamos a susceptibilidade de camundongos H e L (bons e maus produtores de anticorpos, respectivamente) da seleção IV-A, à infecção com o protozoário L. donovani. Camundongos H infectados com 10(7) amastigotas por via intravenosa foram mais suscetíveis, apresentando maior carga parasitária tanto no fígado quanto no baço. Após 60 dias de infecção ambas as linhagens apresentaram um aumento no índice esplênico. Esta esplenomegalia foi conseqüência, pelo menos parcialmente, de um aumento no número de células esplênicas. Os resultados indicam que a seleção IV-A é susceptível à infecção com L. donovani e que dentro desta seleção a linhagem H apresenta maior suscetibilidade do que a linhagem L.
Leishmania donovani; Camundongos Biozzi
ARTIGO
Experimental visceral leishmaniasis in high and low antibody - producer mice (selection IV-A)
Leishmaniose visceral experimental em camundongos bons e maus produtores de anticorpos
Cristiane Jellmayer Fechio, Angela Maria Victoriano de Campos Soares, Silvio Luís de Oliveira and Alexandrina Sartori
Abstract Leishmaniasis is a typical parasite infection whose protective immunity depends on macrophage activation. Susceptibility to Leishmania donovani infection was compared in H (high antibody responder) and L (low antibody responder) mice from selection IV-A. H mice infected intravenously with 107 amastigotes of L. donovani were more susceptible to infection than their L counterparts. This higher susceptibility was characterized by a higher splenic and hepatic parasite burden. An increased splenic index was observed in both lines after sixty days of infection. This splenomegaly was caused, at least partially, by an increase in the number of splenic cells as determined by direct counts of cells from spleen. The results show that selection IV-A is susceptible to visceral leishmaniasis, with the H line being more susceptible than the L line.
Key-words: Leishmania donovani. Biozzi mice.
Resumo A leishmaniose é uma infecção parasitária cuja imunidade protetora envolve a ativação de macrófagos. Neste trabalho avaliamos a susceptibilidade de camundongos H e L (bons e maus produtores de anticorpos, respectivamente) da seleção IV-A, à infecção com o protozoário L. donovani. Camundongos H infectados com 107 amastigotas por via intravenosa foram mais suscetíveis, apresentando maior carga parasitária tanto no fígado quanto no baço. Após 60 dias de infecção ambas as linhagens apresentaram um aumento no índice esplênico. Esta esplenomegalia foi conseqüência, pelo menos parcialmente, de um aumento no número de células esplênicas. Os resultados indicam que a seleção IV-A é susceptível à infecção com L. donovani e que dentro desta seleção a linhagem H apresenta maior suscetibilidade do que a linhagem L.
Palavras-chaves: Leishmania donovani. Camundongos Biozzi.
Leishmaniae are obligate intracellular protozoan parasites of macrophages that cause a spectrum of human diseases, including self- healing skin lesions, diffuse cutaneous and mucosal manifestations, or severe visceral diseases. Important immunological features of visceral leishmaniasis include circulating immune complexes12, polyclonal B-cell activation with elevated IgM and IgG levels15, immunosuppression and absence of detectable cell-mediated immunity during acute infection13.
High (H) and low (L) responder lines of mice provide a useful model to investigate the contribution of humoral and cellular immunity to immune phenomena. H and L antibody responder lines of mice were produced by bidirectional selective breeding of high and low responder lines of mice to natural multideterminant immunogens administered at optimal doses. The different antigens used were sheep erythrocytes (SE) and pigeon erythrocytes (Selection I), SE only (Selection II), flagellar and somatic antigens of Salmonellae (Selections III and IV respectively), bovine serum albumin, and rabbit gamma globulin (Selection V)3. Selection IV-A was derived from F2 interline segregants between HIV and LIV lines in response to SE9. This approach has permitted to establish that quantitative antibody production is a polygenic trait regulated by a group of about 10 independent loci2 14. The alleles separated in each line by selective breeding have a nonspecific effect, operating on the quantitative antibody response to many unrelated immunogens3. Fundamental immunological differences of H and L lines have been described. The level of serum antibody is markedly higher in H than L lines and T cell-mediated immunity is of similar intensity in both lines1 8. It was also demonstrated that humoral and cell-mediated responses to the same antigens are subjected to independent polygenic regulation21. The importance of these immunogenetic characteristics for the general strategy of anti-infectious immunity has been hypothesized5. More recently, differences in T cell proliferative responses and cytokine production by H and L lines have been characterized10 22.
The resistance of H and L lines to various bacterial and parasitic infections has been studied. These studies demonstrated that H mice are more resistant than L mice to infections in which antibodies play a major protective role17 20. In contrast, L mice are more resistant to infections caused by intracellular pathogens11. Low antibody responses in L mice have been attributed to higher catabolism of immunogens in the macrophages and consequent decreased antigen presentation by these cells. These macrophage catabolic differences have been previously demonstrated in selections I, II and IV-A16.
Considering that L. donovani is an intracellular parasite and that the macrophage function is altered in selection IV-A, the purpose of this study was to evaluate the susceptibility of H and L mice from selection IV-A to infection with L. donovani.
MATERIAL AND METHODS
Animals. Twenty-nine H male mice and 29 L male mice from selection IV-A aged 8-10 weeks, developed by the Immunology Section of the Biological Institute of São Paulo and maintained at the Animal Facility of the Department of Microbiology and Immunology, Institute of Biosciences, UNESP, Botucatu, were used.
Parasites. L. donovani strain 1S was maintained in vivo by serial passage in golden hamsters. Amastigotes for inoculation into mice were obtained from the spleens of chronically infected hamsters, washed three times in phosphate buffered saline (PBS, pH 7.2) and adjusted to a concentration of 5 x 107 amastigotes/ml.
Infection. Thirty eight mice (19 H and 19 L) were infected by the intravenous route with 107 amastigotes. Groups of 8 (4 H and 4 L) infected animals were sacrificed at 15, 20, 30 and 60 days postinoculation and their spleens and livers collected to evaluate the different parameters. Six mice (3 H and 3 L) were sacrificed 24 hours after inoculation to establish the acute parasite population growth rate in the liver. Twenty non-infected mice (10 H and 10 L) were used as normal controls.
Hepatic and splenic parasite burden. Impression smears from livers and spleens were stained with Giemsa to evaluate parasite burden. The number of amastigotes per host cell nucleus was determined by counting 1000 host cells as previously described6. The relative and total numbers of parasites per organ, named Leishman-Donovan Units (LDU) and total Leishman-Donovan Units (total LDU). respectively, were calculated according to the formula:
LDU
number of parasites
______________________________
1000 host cell nucleus
Total LDU = LDU x organ weight (mg) x 2.105
Splenic index. This index was determined according to the formula:
Splenic index
=
spleen weight
_________________________
x 100
whole body weight
Splenic cell number. Spleens from normal and infected mice were removed and cell suspensions prepared by teasing the material through a stainless steel sieve in PBS. The total number of cells was counted in a Neubauer chamber.
Statistical analysis. The Kruskall-Wallis non-parametric test was performed to determine the statistical significance of the data (p < 0.05 was considered significant).
RESULTS
Acute growth rate of L. donovani in H and L mice from selection IV-A. Three and four mice from the H and L lines were killed after 1 and 15 days of infection, respectively. Liver parasite counts on the first day showed no difference between H and L mice, with 138 LDU being the mean count for both lines. After 15 days of infection the mean parasite burden was 1803 and 1500 LDU for H and L mice, respectively. These results, regardless of the later course of infection, characterized H and L mice from selection IV-A as intermediate in terms of acute susceptibility when compared with inbred lines of mice experimentally infected with L. donovani6.
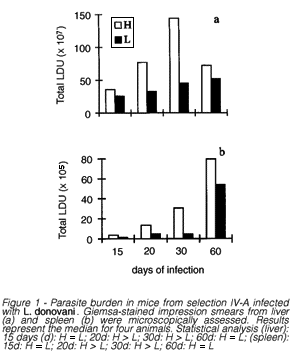
Splenic and hepatic parasite burden. To establish the extent of parasitism in the liver and spleen, imprints from these organs were microscopically assessed after 15, 20, 30 and 60 days of infection. As can be observed in figure 1a for the liver and 1b for the spleen, the parasitic burden was always higher in the H than in the L line. However, the difference between H and L was statistically significant only after 20 and 30 days of infection. After 60 days of infection, the last period evaluated, the parasitic burden was similar in liver and spleen of both lines.
Splenic index. The splenic index in normal H mice was higher than in normal L mice. This difference was preserved in infected animals and was statistically significant during all periods analyzed (figure 2a). When evaluated in each line, the splenic index increased significantly at 30 and 60 days of infection. In the L line the increase in the splenic index was significant only at 60 days of infection.
Quantitation of splenic cells. Spleens from normal and infected H and L mice were removed and cell suspensions prepared and counted in a Neubauer chamber. The results are presented in figure 2b. Control H mice had a significantly higher splenic cell number than control L mice and this difference was unchanged during the first 30 days of infection. Also during this period there was no alteration in total splenic cell number. However, both lines showed a striking increase in total splenic cell number after 60 days of infection. This increase was statistically significant in relation to any of the other periods analyzed in the same line.
DISCUSSION
In this work we used high (H) and low (L) responder lines of mice from selection IV-A as an experimental model for visceral leishmaniasis.
The H mice presented a higher parasite burden in spleen and liver compared to their L counterparts. This result is similar to those obtained when the lines H and L were used as experimental models for other intracellular parasites such as Leishmania tropica4 and Salmonella typhimurium24 and Brucella suis11. In our model we observed that after 20 and 30 days H mice were more susceptible than L mice since H mice showed a significantly higher number of parasites both in spleen and liver. However, after 60 days of infection the parasite burden was equivalent in H and L mice. In addition, after 15 days of infection, the parasite burden in the liver, which has been used as an index to evaluate acute susceptibility in experimental murine leishmaniasis6, was identical in H and L mice. It is possible that this difference observed on the 20th and 30th day of infection was caused by a delay in developing a specific immune response able to partially restrain the parasite growth in H mice. This possibility finds some support in the fact that macrophages from the H line have a lower metabolic and bactericidal activity16. This may consequently delay the ability of these macrophages to present antigens to Th cells or to kill the intracellular leishmania amastigotes.
Another parameter evaluated was the splenic index. Interestingly, normal H mice already presented a significantly higher splenic index compared to normal L mice and this difference was maintained during infection. In each line there was also an increase in the splenic index from the 30th day of infection on. This increase was highly significant after 60 days of infection. Splenic hipertrophy is a classical description both in human and experimental kala-azar18 and has been attributed to both monocyte recruitment23 and polyclonal activation7. In our study we found a very pronounced increase in the splenic cell number at 60 days of infection in both mouse lines. This result shows that both lines from the IV-A selection, experimentally infected with L. donovani, reproduce splenomegaly, which is the hallmark for visceral leishmaniasis.
In view of the significant difference between the H and L lines from selection IV-A in terms of parasite burden after 20 and 30 days of infection, the distinct immunological characteristics already described for these two lines and the fact that they represent extreme phenotypes found in a natural heterogeneous (outbred) population, we believe that this model could be used to study the macrophage-Leishmania interaction and the kinetics of the specific immune response to this parasite.
ACKNOWLEDGMENTS
The authors thank Paulo Robeto Curi for statistical analysis and Paulo Sérgio Ferreira for assistance with the manuscript.
Departamento de Microbiologia e Imunologia, Instituto de Biociências, Universidade Estadual Paulista (UNESP), Campus de Botucatu, Botucatu,SP.
Endereço para correspondência: Profª Alexandrina Sartori. Depto de Microbiologia e Imunologia/IB/UNESP, Campus de Botucatu, Distrito de Rubião Júnior, 18618-000 Botucatu, SP.
Tel: 55 14 820-6240 ou 820-6058; Fax: 55 14 821-3744.
E-mail: microimuno@laser.com.br
Recebido para publicação em 27/2/98.
- 1. Biozzi G, Asofsky R, Lieberman R, Stiffel C, Mouton D, Benacerraf B. Serum concentrations and allotypes of immunoglobulins in two lines of mice genetically selected for "high" and "low" antibody synthesis. Journal of Experimental Medicine 132:752-764, 1970.
- 2. Biozzi G, Mouton D, Heumann AM, Bouthillier Y, Stiffel C, Mevel JC. Genetic analysis of antibody responsiveness to sheep erythrocytes in crosses between lines of mice selected for high or low antibody synthesis. Immunology 36:427-438, 1979.
- 3. Biozzi G, Mouton D, Sant'Anna, Passos HC, Gennari M, Reis MH, Ferreira VCA, Heumann AM, Bouthillier Y, Ibanez OM, Stiffel C, Siqueira M. Genetics of immunoresponsiveness to natural antigens in the mouse. Current Topics in Microbiology and Immunology 85:31-98, 1979.
- 4. Biozzi G, Mouton D, Stiffel C, Bouthillier Y. A major role of the macrophage in the quantitative genetic regulation of immunoresponsiveness and antiinfectious immunity. Advances in Immunology 36:189-233, 1984.
- 5. Biozzi G, Mouton D, Stiffel C, Sant'Anna OA, Bouthillier Y. The genetic regulation of antibody responsiveness to natural immunogens in relation to the protective effect of vaccination. In: Werner GH, Floc'h F (eds) The pharmacology of immunoregulation, Academic Press Inc., New York, p. 123-139, 1978.
- 6. Bradley DJ, Kirkley J. Regulation of Leishmania populations within the host. 1. The variable course of Leishmania donovani infections in mice. Clinical and Experimental Immunology 30:119-129, 1977.
- 7. Bunn-Moreno MM, Madeira ED, Menezes JA, Campos Neto A. Hypergammaglobulinaemia in Leishmania donovani infected hamsters:possible association with a polyclonal activation of B cells and with suppression of T cell function. Clinical and Experimental Immunology 59:427-434, 1895.
- 8. Byfield PE, Howard JG. Equivalent graft-versus host reactivity of spleen cells from two lines of mice genetically selected for high and low humoral antibody fromation. Transplantation 14:133-134, 1972.
- 9. Cabrera WH, Ibanez OM, Oliveira SL, Sant'Anna OA, Siqueira M, Mouton D, Biozzi G. Evidence for distinct polygenic regulation of antibody responses to some unrelated antigens in lines of mice selected for high or low antibody responses to somatic antigens of Salmonella. Immunogenetics 16:583-592, 1982.
- 10. Cabrera WH, Siqueira M, Takahashi NSH, Ribeiro OG, Araujo LMM, Mouton D, Ibańez OM. Specific and non specific T-cell activation in high and low antiboby producing mice (Selection IV-A). Scandinavian Journal of Immunology 41:293-297, 1995.
- 11. Cannat A, Bousquet C, Serre A. Response of high and low antibody producers to Brucella Annals of Immunology 129 C:669-683, 1978.
- 12. Carvalho EM, Andrews BS, Martinelli R, Dutra M, Rocha H. Circulating immune complex and rheumatoid factor in visceral leishmaniasis and schistosomiasis. American Journal of Tropical Medicine and Hygiene 32:61-76, 1983.
- 13. Carvalho EM, Teixeira RS, Johnson WD. Cell-mediated immunity in american visceral leishmaniasis:reversible immunosuppression during acute infection. Infection and Immunity 33:498-502, 1981.
- 14. Feingold N, Feingold J, Mouton D, Bouthillier Y, Stiffel C, Biozzi G. Polygenic regulation of antibody synthesis to sheep erythrocytes in the mouse:a genetic analysis. European Journal of Immunology 6:43-51, 1976.
- 15. Galvăo-Castro B, Sá-Ferreira JA, Marzochi KF, Marzochi MC, Coutinho SG, Lambert PH. Polyclonal B-cell activation, circulating immune complexes and autoimmunity in human american visceral leishmaniasis. Clinical and Experimental Immunology 56:58-66, 1984.
- 16. Gennari M, Bouthillier Y, Ibanez OM, Ferreira VCA, Mevel JC, Reis MH, Piatti RM, Ribeiro OG, Biozzi G. Effect of silica on the genetic regulation of antibody responsiveness. Annales de l' Institut Pasteur Immunology 138:359-370, 1987.
- 17. Heuman AM, Stiffel C, Manjour L, Bucci A, Biozzi G. Correlation between genetic regulation of antibody responsiveness and protective immunity induced by Plasmodium berghei vaccination. Infection and Immunity 24:829-836, 1979.
- 18. Hommel M. The genus of Leishmania: biology of the parasites and clinical aspects. Bulletin de l' Institut Pasteur 75:5-102, 1978.
- 19. Ibanez OM, Mouton D, Oliveira SL, Ribeiro Filho OG, Piatti RM, Sant'anna AO, Massa S, Biozzi G, Siqueira M. Polygenic control of quantitative antibody responsiveness: restriction of the multispecific effect related to the selection antigen. Immunogenetics 28:6-12, 1988.
- 20. Kierzembaum F, Howard JG. Mechanisms of resistance against experimental Trypanosoma cruzi infection:the importance of antibodies and antibody-forming capacity in the Biozzi high and low responder mice. Journal of Immunology 116:1208-1211, 1976.
- 21. Oliveira SL, Ibanez OM, Mouton D, Sant'Anna OA, Siqueira M, Biozzi G. Independent polygenic regulation of quantitative antibody responsiveness and expression of delayed-type hypersensitivity (DTH). Experimental and clinical Immunogenetics 2:223-233, 1985.
- 22. Reis MH, Ibanez OM, Cabrera WH, Ribeiro OG, Mouton D, Siqueira M, Couderc J. T helper functions in lines of mice selected for high or low antibody production (Selection III):Modulation by anti-CD4+ monoclonal antibody. Immunology 75:80-85, 1992.
- 23. Ridley MJ, Ridley, DS. Monocyte recruitment, antigen degradation and localization in cutaneous leishmaniasis. British Journal of Experimental Pathology 67:209-218, 1986.
- 24. Sant'Anna OA, Massa S, Mouton D, Bouthillier Y, Mevel JC, Ibanez OM, Vassao R, Franco M, Bellinati R, Siqueira M, Biozzi G. Salmonella typhimurium infection in high and low antibody responder mice:inverse correlation between antibody responsiveness and resistance to infection. FEMS (Federation of European Microbiological Societies) Microbiology Immunology 47:465-472, 1989.
Publication Dates
-
Publication in this collection
13 June 2000 -
Date of issue
June 1999
History
-
Received
27 Feb 1998