Abstracts
The effects of high and low-protein diets on the structure of the jejunal mucosa were studied in Schistosoma mansoni infected mice (morphology and histomorphometry). Weaning male albino mice were infected with 80 cercariae, fed with high (20%) or low-protein (5%) diets and compared to uninfected controls under the same conditions. Mice were sacrificed 12 weeks after infection. Animals submitted to a low-protein diet showed lower weight curves, mainly when infected. In the jejunal mucosa, finger-like villi were the predominant pattern among uninfected high-protein fed animals, while the infected ones showed leaf-shaped and flattened villi in most cases. Undernourished infected mice had 65.7% leaf-shaped villi. A significant increase in the number of goblet cells was seen in infected mice. A decrease in the number of absorptive cells was detected in undernourished mice, particularly in infected ones.
Schistosoma mansoni; Undernutrition; Mice; Intestinal mucosa; Histomorphometry
Os efeitos de dietas hiper e hipoprotéica sobre a estrutura da mucosa jejunal de camundongos infectados com Schistosoma mansoni foram estudados por métodos histológicos e histomorfométricos. Camundongos albinos, machos, recém-desmamados, foram infectados com 80 cercárias e alimentados com dietas hiper (20%) ou hipoproteica (5%) e comparados com controles não-infectados, nas mesmas condições, sendo sacrificados após 12 semanas de infecção. Animais em dieta hipoproteica tiveram curvas ponderais menos elevadas, sobretudo quando infectados. A mucosa do jejuno mostrou predominância de vilosidades digitiformes nos camundongos não infectados, recebendo dieta hiperprotéica; vilosidades foliáceas foram mais numerosas nos desnutridos infectados (65,7%). Nas vilosidades dos animais infectados, ocorreu significativo aumento no número de células caliciformes. Entre os desnutridos foi detectada redução do número de enterócitos, condição agravada pela infecção.
Schistosoma mansoni; Desnutrição; Camundongo; Mucosa intestinal; Histomorfometria
ARTIGO
Structural changes in the jejunal mucosa of mice infected with Schistosoma mansoni, fed low or high protein diets
Alterações estruturais na mucosa jejunal de camundongos infectados com Schistosoma mansoni, alimentados com dietas hipo ou hiperprotéicas
Janira Lúcia Assumpção CoutoI; Haroldo da Silva FerreiraII; Dinalva Bezerra da RochaIII; Maria Eugênia Leite DuarteIV; Monica Lopes AssunçãoII; Eridan de Medeiros CoutinhoV
IDepartamento de Parasitologia do Centro de Ciências Biológicas da Universidade Federal de Alagoas, Maceió, AL
IIDepartamento de Nutrição do Centro de Ciências da Saúde da Universidade Federal de Alagoas. Maceió, AL
IIIEscola de Ciências Médicas de Alagoas, Maceió, AL
IVDepartamento de Patologia da Universidade Federal Fluminense. Niterói, RJ
VCentro de Pesquisas Aggeu Magalhães da Fundação Oswaldo Cruz, Recife, PE, Brasil
Address to correspondence Address to correspondence Dr. Haroldo S. Ferreira R. Des. Almeida Guimarães 37, Pajuçara 57030-160 Maceió, AL, Brasil Tel: 55 82 214-1165; 337-4481/9381-2731; Fax: -55-82 214-1665 e-mail: haroldo@fapeal.br
ABSTRACT
The effects of high and low-protein diets on the structure of the jejunal mucosa were studied in Schistosoma mansoni infected mice (morphology and histomorphometry). Weaning male albino mice were infected with 80 cercariae, fed with high (20%) or low-protein (5%) diets and compared to uninfected controls under the same conditions. Mice were sacrificed 12 weeks after infection. Animals submitted to a low-protein diet showed lower weight curves, mainly when infected. In the jejunal mucosa, finger-like villi were the predominant pattern among uninfected high-protein fed animals, while the infected ones showed leaf-shaped and flattened villi in most cases. Undernourished infected mice had 65.7% leaf-shaped villi. A significant increase in the number of goblet cells was seen in infected mice. A decrease in the number of absorptive cells was detected in undernourished mice, particularly in infected ones.
Key-words: Schistosoma mansoni. Undernutrition. Mice. Intestinal mucosa. Histomorphometry.
RESUMO
Os efeitos de dietas hiper e hipoprotéica sobre a estrutura da mucosa jejunal de camundongos infectados com Schistosoma mansoni foram estudados por métodos histológicos e histomorfométricos. Camundongos albinos, machos, recém-desmamados, foram infectados com 80 cercárias e alimentados com dietas hiper (20%) ou hipoproteica (5%) e comparados com controles não-infectados, nas mesmas condições, sendo sacrificados após 12 semanas de infecção. Animais em dieta hipoproteica tiveram curvas ponderais menos elevadas, sobretudo quando infectados. A mucosa do jejuno mostrou predominância de vilosidades digitiformes nos camundongos não infectados, recebendo dieta hiperprotéica; vilosidades foliáceas foram mais numerosas nos desnutridos infectados (65,7%). Nas vilosidades dos animais infectados, ocorreu significativo aumento no número de células caliciformes. Entre os desnutridos foi detectada redução do número de enterócitos, condição agravada pela infecção.
Palavras-chaves: Schistosoma mansoni. Desnutrição. Camundongo. Mucosa intestinal. Histomorfometria.
Schistosomiasis and malnutrition are public health problems of major concern in underdeveloped countries and usually overlap in many endemic areas of the world7 8 9.
Previous papers have shown the importance and frequency of intestinal lesions in schistosomiasis mansoni, not only in man15 19 31 but also in experimental animals13 30. An intestinal malabsorption syndrome has been detected by some authors in the hepato-splenic clinical form of the disease in humans26 28 and in infected mice12 16.
Structural changes of the small intestine have been reported in human4 5 22 24 26 and experimental schistosomiasis9, as well as in undernutrition2 23 35 36 37. However, the relationships between schistosomiasis infection and the nutritional status of the host are not quite clear at the present time, mainly regarding the jejunal mucosal changes and their probable effects on the outcome of a malabsorption syndrome in infected undernourished hosts.
This study was undertaken aiming primarily to detect, through histomorphometry of the jejunal mucosa, the role of ingested high and low-protein diets on the structure of the intestinal mucosa of mice experimentally infected with S. mansoni and the relative importance of each of the variables, infection and nutrition, on the mucosal histological and cytological disturbances. Weight curves were also investigated in all the groups.
MATERIAL AND METHODS
Animals. Male Swiss weaning (21 to 25 days old) albino mice, weighing 10 to 15g, were kept in individual wireless steel cages under standardized conditions of temperature and light.
Diets. A high protein diet having 20% casein as its main protein source was prepared to supply all the nutritional needs of mice (Table 1), according to Reeves et al32. An experimental, low protein diet (5% casein) was also prepared with similar composition, but using corn starch to replace partially the dietary protein. The diets were isocaloric, prepared under pellet form and given ad libitum to the animals during 12 consecutive weeks.
Infection. Mice were infected with 80 cercariae of the SL Brazilian strain (São Lourenço da Mata, Pernambuco State, Northeast Brazil) recently shed from Biomphalaria glabrata raised in laboratory. Infection was made by percutaneous route, for 60 minutes and confirmed by stool examination21 eight or nine weeks later.
Experimental protocol. A total of 74 mice were divided into the following groups: a. high protein, uninfected mice (18); b. low protein, uninfected mice (24) c. high protein, infected mice (17) c. low protein, infected mice (15).
The animals were weighed at the beginning and then weekly throughout the experiment. At the time of sacrifice (12 weeks of infection), mice were exsanguinated by severing of the abdominal aorta under ether anesthesia and then necropsied and inspected for gross lesions, particularly on the liver, spleen and intestines. Cross sections were taken from the midle portion of the total length of the small intestine which was assumed to correspond to the jejunal segment in mice. The rings of intestine were immediately dropped into saline and then into phosphate buffered 10% formalin pH 7.4 (Millonig) for paraffin embedding and sectioning. Sections were stained with hematoxylin-eosin and Masson trichrome stain.
Morphological studies. The mean percentage of jejunal villi in 10 microscopic fields were determined according to the following villous mucosal nomenclature: finger-like, leaf or spade forms, ridged forms, convoluted forms, flattened mucosa27.
The inflammatory infiltrate in the mucosa was quantitfied as zero (absent), 1+ (slight), 2+ (moderate) and 3+ (severe).
Morphological studies also included the comparison of periovular granulomas in the acute or chronic phase and their distribution through the different histological layers of the jejunal wall.
Morphometric studies. A quantitative image analysis for selected parameters was performed on eight cross sections of the jejunal wall for each animal in each group. For each mouse, ten microscopical fields were studied. The arithmetic mean of these fields was considered as representative of the animal and the values for the groups were assumed to be the arithmetic mean of their component mice. The numerical profile densities (Nv) were analyzed at x10 magnification with a Leica DMLS microscope coupled to a video camera, a video monitor Sony HR Trinitron and a computer.
A point counting method (square test grid) according to a standardized technique39 was used to study the following morphometric parameters: a) linear counting of the number of jejunal villi per microscopic field; b) mean villous height per microscopic field (distance between the top of the villi and the opening of the glandular crypts); c) number of goblet cells on the villi per microscopic field; d) number of goblet cells per villus; e) mean height of the jejunal mucosa (distance between the top of the villus and the upper edge of the muscularis mucosae layer). Measurements for variables b and e were performed with a ruler (scale) and equivalence from centimeters to micra was obtained with a Neubauer chamber; f) number of absorptive cells (enterocytes) on the villous surface.
Variable f was calculated using the Merz grid modified by Rocha34. In a square area there are five senoidal curves and 30 points. This grid was adapted to the objective of the microscope. Microscopic measurements were made a x40 objective magnification.
Statistical analysis. Analysis of variance (ANOVA) and Student's t test were used to check the statistical significance of the data. The limit of significance was set at p<0.05.
RESULTS
Weight curves. Infected and non-infected animals fed a high-protein diet had a similar weight gain until the 5th week of infection (29.1g ± 6.9 v. 28.4g ± 5.5), weight values decreasing for the infected group afterwards. Infected and non-infected mice fed the low-protein diet also had similar weights until the sixth week after infection, the infected ones showing lower weight values onward (Figure 1). However, mice fed low-protein diet in both infected and non-infected groups always displayed lower values regarding body weight as compared to high-protein diet fed animals (37.1g ± 5.7). At the end of the experiment (12th week) the body weight of infected mice ingesting high-protein diet (34.4g ± 8.6) showed a trend to be closer to the body weight values of non-infected mice fed a low-protein diet (33.8g ± 5.7).
Morphological studies. A diffuse inflammatory infiltrate of eosinophils and mononuclear cells, mainly lymphocytes, was seen at the mucosa and submucosa layers, in all the groups, but eosinophils were much more abundant in the intestinal wall of infected animals.
Egg granulomas were most frequently seen in the submucosa, muscular and adventitial layers, in all the groups.
Granulomas around eggs containing well preserved miracidia showed an acute inflammatory reaction, with predominance of polymorphonuclear cells, including eosinophils, and sometimes, macrophages. In granulomas around empty egg-shells or eggs containing degenerated miracidia, the inflammatory reaction was of the chronic type, with macrophages, fibroblasts and collagen deposition in varying amounts.
No remarkable differences were detected regarding size and composition of the intestinal granulomas when high and low protein infected mice were compared to each other.
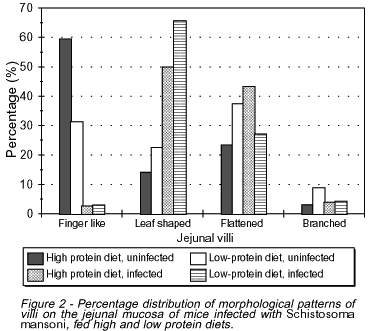
In uninfected mice most villi were of the finger-like type (59.4%) in high-protein fed animals and flattened (37.5%) in low-protein fed ones (Figure 2). In infected mice, however, both high-protein and low-protein fed groups showed a predominance of leaf-shaped villi (50% and 65.7%, respectively).
Morphometric studies. Morphometric studies were focused on the structural changes of the mucosal layer (jejunal villi), since quantitative studies on the egg granulomas were not considered for the purposes of this investigation.
In spite of different villous patterns detected among the various groups of mice, the mean values for number and height of the jejunal villi were not statistically significant.
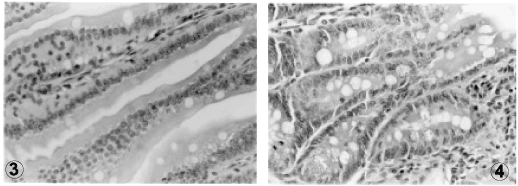
In infected mice, a significant increase in the number of goblet cells per villus and microscopic field was noticed, as compared to the uninfected groups (p<0.0005). However, no difference could be detected between groups ingesting high or low protein diets (Figures 3 and 4).
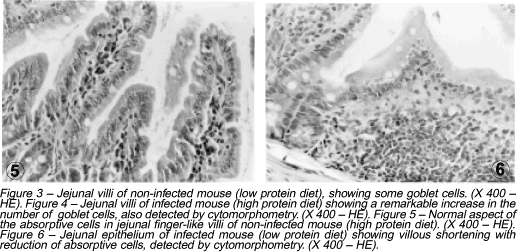
Uninfected mice fed a low protein diet showed a decrease in the number of absorptive cells (enterocytes) as compared to uninfected high protein fed animals (Figures 5 and 6). This finding was even more remarkable in infected mice ingesting either high or low protein diet when compared to uninfected high protein fed animals (p<0.0005).
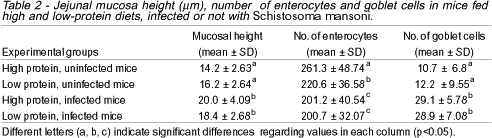
Infected mice showed a significant increase in the mucosa height, although they did not differ regarding the protein content of their diets (Table 2).
DISCUSSION
Animals fed a low-protein diet showed lower weight curves mainly in the infected group, as compared to high-protein fed mice.
Infection with S. mansoni had some effect on animals' growth only after the 5th week in high-protein fed mice, and after the 6th week in undernourished hosts. This probably bears some relationship to the increase of oviposition by female worms at that time, with a slight delay in egg-laying among undernourished mice1 25.
On the other hand, mice infected with S. mansoni show a reduction of food intake and some metabolic disturbances such as decrease in dietary protein intestinal absorption and utilization11 16.
Finger-like villi were the predominant morphological pattern among control (uninfected) high-protein fed animals; infected high-protein fed mice showing leaf-shaped and flattened villi in most cases. Among undernourished uninfected animals, however, most villi were flattened, sometimes finger-like or leaf-shaped. The latter type was the most prominent among undernourished infected animals (65.7%).
The occurrence of intestinal villi different from the finger-like pattern has been reported in human malnutrition2 3 35 36 37 38. A tendency to jejunal mucosa flattening was also detected in the hepato-splenic clinical form of schistosomiasis mansoni by Nigro et al28, Penna and Brasileiro Filho29 and Machado et al26 in some patients and by Coutinho et al9 in mice. This finding, due to the edema and inflammatory infiltration of the lamina propria according to Machado et al26, has led to the speculation that a malabsorption syndrome may eventually occur in severe schistosomiasis. So far it is not clear whether these morphological changes are the origin or the effect of such eventual dysfunction. Clinical observations by El-Rooby et al14, Fikry17, Fikry et al18, Pucci et al30 also point to the existence of a malabsorption syndrome in man. Using mice as an experimental model, Coutinho et al12 and Ferreira et al16 described similar findings.
In infected animals, a widespread inflammatory infiltration by lymphocytes, plasma cells and polymorphonuclear cells (mainly eosinophils) was always detected in the mucosa and submucosal layers. This reaction was usually of moderate or severe degree in infected mice. Sometimes a hyperplasia of lymphatic nodules was also noticed. In control uninfected animals, the inflammation was only slight or even absent.
Egg-granulomas, most of them of the productive type, were composed by macrophages, a few eosinophils and giant cells, showing some degree of fibrosis. These were observed in all the intestinal layers, but most abundant in the submucosa and serosa.
Although functional studies were not undertaken in the present investigation, some findings in the cytoarchitecture of the jejunal villi are worth discussing.
A significant increase in the number of goblet cells was detected in the epithelium of the jejunal villi in schistosome infected mice. This is in accordance with the development of a severe catarrhal enteritis that is seen in both acute and chronic stages of schistosomiasis mansoni4 5, though with no relationship to the type of diet. Intestinal mucosal changes including a goblet cell hyperplasia mediated by Th cells have been previously reported in experimental schistosomiasis33 and also in some nematode infections20 22 24.
Data obtained in this investigation confirm that cytoarchitectural changes occur on the jejunal mucosa in schistosomiasis and undernutrition as isolated conditions and should call attention to the role played by a superimposed undernutrition on the deterioration of the morphology and subsequent function of the host's intestinal tract in endemic areas where both health problems coexist.
A decrease in the number of absorptive cells (enterocytes) was detected in both undernutrition and schistosomiasis. This is probably related to the generalized mucosal atrophy, to the presence of flattened villi seen in undernutrition and to the low mitotic index of enterocytes at the jejunal crypts6 38.
Morphometric studies also showed that the jejunal mucosa was higher in infected mice due to inflammation and formation of granulomas, diets having but little influence on this.
Recebido para publicação em 27/09/2001.
Financial support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de Alagoas (FAPEAL).
- 1. Akpom CA, Warren KS. Calorie and protein malnutrition in chronic murine schistosomiasis mansoni: effect on the parasite and the host. The Journal of Infectious Diseases 132:6-14, 1975.
- 2. Brunser O, Castillo C, Arraya M. Fine structure of small intestinal mucosa in infantile marasmic malnutrition. Gastroenterology 70: 495-507, 1976.
- 3. Burman D. The jejunal mucosa in kwashiorkor. Archives of Disease in Chilhood 40:526-553, 1965.
- 4. Campos JVM. Repercussões morfológicas e funcionais da esquistossomose mansônica (EM) no intestino delgado. Arquivos de Gastroenterologia 25: 185-187, 1988.
- 5. Castro LP, Dani R, Alvarenga RJ, Chamone DAF, Oliveira CAA. Peroral biopsy study of the jejunum in human schistosomiasis mansoni. Revista do Instituto de Medicina Tropical de São Paulo 13:103-109, 1971.
- 6. Cotran RS, Kumar V, Robbins SL. Doenças Nutricionais e Ambientais. ln: Robbins SL (ed) Patologia Estrutural e Funcional. Guanabara-Koogan, Rio de Janeiro, p. 336, 1996.
- 7. Coutinho EM. Relações hospedeiro-parasito na esquistossomose mansônica em função da Dieta Básica Regional: Estudo epidemiológico e anátomo-patológico. Tese de Livre-Docência, Universidade Federal de Pernambuco, Recife, PE, 1976.
- 8. Coutinho EM. Estado nutricional e esquistossomose. Revista da Sociedade Brasileira de Medicina Tropical 13: 91-96, 1980.
- 9. Coutinho EM, Abath FGC, Barbosa CS, Domingues ALC, Melo MCV, Montenegro SML, Lucena MAF, Romani SAM, Souza WV, Coutinho AD Factors involved in Schistosoma mansoni infection in rural areas of Northeast Brazil. Memórias do Instituto Oswaldo Cruz 92:707-715, 1997.
- 10. Coutinho EM, Amorim FMS, Lacerda CAM, Souza WVS, Melo FL, Cavalcanti CL. Stereological study and pathomorphology of the jejunum in undernourished mice infected with Schistosoma mansoni In: Resumos of 5th International Symposium on Schistosomiasis, Salvador, 1995.
- 11. Coutinho EM, Ferreira HS, Freitas LP, Silva MR, Cavalcante CL, Samico MJA. Nutrition and acute schistosomiasis. Memórias do Instituto Oswaldo Cruz 87 (supl IV): 297-301, 1992.
- 12. Coutinho EM, Freitas LP, Abath FGC. The influence of the Regional Basic Diet from Northeast Brazil on health and nutritional conditions of mice infected with Schistosoma mansoni Revista da Sociedade Brasileira de Medicina Tropical 25:13-20, 1992.
- 13. Domingo EO, Warren KS. Pathology and pathophysiology of the small intestine in murine schistosomiasis mansoni, including a review of the literature. Gastroenterology 56:231-240, 1969.
- 14. El-Rooby A, Gad MN, Galil N, Abdalla A, Shakir M. Studies on the malabsorption syndrome among Egyptians. 11 - Malabsorption in bilharzial hepatic fibrosis. Journal of Egypt Medical Association 46: 777-782, 1963.
- 15. Fedail SS, Gadir AFMA. The pathology of the small intestine in human schistosomiasis mansoni in the Sudan. Tropical Medicine and Parasitology 36:94-96, 1985.
- 16. Ferreira HS, Coutinho EM, Teodósio NR, Cavalcanti CL, Samico MJA. Intestinal protein absorption in malnourished mice with acute schistosomiasis mansoni. Memórias do Instituto Oswaldo Cruz 88: 581-587, 1993.
- 17. Fikry, ME. Disturbances of digestion and absorption in bilharzial hepatic fibrosis. Journal of Tropical Medicine and Hygiene 66:213-215, 1963.
- 18. Fikry ME, Hanno MG, El-Sayed M, Dorry K. The iodine 131 triolein intestinal absorption test in schistosomal (bilharzial) hepatic fibrosis patients. Acta Gastroenterology 29: 1966.
- 19. Gelfand M. A clinical study of intestinal bilharziasis (Schistosoma mansoni) in Africa. Edward Arnold Publishers Ltd. London, 1967.
- 20. Garside P, Grencis RK, Mcwat A. T-lymphocyte dependent enteropathy in murine Trichinella spiralis infection. Parasite Immunology 14: 217-225, 1992.
- 21. Hoffman WA, Pons JA, Janer JL. The sedimentation - concentration method in Schistosomiasis mansoni. Puerto Rico Journal of Public Health and Tropical Medicine 9: 283-291, 1934.
- 22. Ishikawa N, Wakelin D, Mahida Y. Role of the T helper 2 cells in intestinal goblet cell hyperplasia in mice infected with Trichinella spiralis Gastroenterology 113:542-549, 1997.
- 23. James WPT. Intestinal absorption in protein-calorie malnutrition. Lancet 17:333-335, 1968.
- 24. Khan W, Blennerhasset P, Ma C, Matthaei KI, Collins SM. Stat 6 dependent goblet cell hyperplasia during intestinal nematode infection. Parasite Immunology 23:39-42, 2001.
- 25. Knauft RF, Warren KS. The effect of calorie and protein malnutrition on both the parasite and the host in acute murine schistosomiasis mansoni. The Journal of Infectious Diseases 120: 560-575, 1969.
- 26. Machado RJC, Amorim AG, Lira V, Machado AC, Análise morfométrica do intestino de esquistossomóticos. Anais da Faculdade de Medicina da Universidade Federal de Pernambuco 41: 34-42, 1996.
- 27. Morson BC, Dawson IMP. Gastrointestinal Pathology. 2nd Edition Blackwell Scientific Publications. Oxford, 1979.
- 28. Nigro SP, Miszputen S, Hayashi H, Saad FA. Estudo morfométrico da mucosa jejunal na esquistossomose mansônica humana. Revista da Associação Médica Brasileira 30: 61-63, 1984.
- 29. Penna FS, Brasileiro Filho G. Lack of correlation between jejunal mucosal morphology and intestinal absorption in the hepatosplenic form of schistosomiasis mansoni. Arquivos de Gastroenterologia 25: 224-228, 1988.
- 30. Pucci H, Vilela MP, Miszputen SJ. Estudo da absorção intestinal de gorduras na esquistossomose mansônica humana. Revista da Associação Médica Brasileira 24:341-344, 1978.
- 31. Raso P, Pedroso ERP. Esquistossomose mansônica. In: Bogliolo L (ed) Patologia, Guanabara Koogan, Rio de Janeiro, 1987.
- 32. Reeves PG, Nulsen FH, Fahey Jr GC. AIN-93, Purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc Writing Committee on the reformulation of the AIN-76, a rodent diet. Journal. Nutrition 123: 1939-1951, 1993.
- 33. Robinson KL, Crompton DWT. Nutritional disturbance during experimental schistosomiasis mansoni. Transactions of the Royal Society of Tropical Medicine and Hygiene 87: 123, 1993.
- 34. Rocha JES. Alterações estruturais das placentas de gestantes fumantes: estudo morfométrico. Tese de Doutorado, Faculdade de Medicina da Universidade de São Paulo, Ribeirão Preto, SP, 1995.
- 35. Shinner M, Redmond AOB, Hansen JDL. The jejunal mucosa in protein-energy malnutrition. A clinical, histological and ultrastructural study. Experimental and Molecular Pathology 19: 61- 78, 1973.
- 36. Tandon BN, Magotra ML, Saraya AK, Ramalingaswami V. Small intestine in protein malnutrition. The American Journal of Clinical Nutrition 21: 813-819, 1968.
- 37. Vannuchi H, Franco MF, Campana AO. Mucosa jejunal em pacientes com desnutrição protéica, anemia e parasitoses. Arquivos de Gastroenterologia 8: 61-64, 1974.
- 38. Waterlow JC, Tomkins A, Grantham-Mcgregor SM. Malnutritión Proteico-Energética. Organización Panamericana de la Salud. Publicación Científica n. 555. Washington, 1996.
- 39. Weibel ER. Principles and methods for the morphometric study of the lung and other organs. Laboratory Investigation 12:131-135, 1963.
Publication Dates
-
Publication in this collection
26 Feb 2003 -
Date of issue
Dec 2002