Abstract:
INTRODUCTION
: This study evaluated the clinical forms and manifestation severities of Chagas disease among serologically reactive individuals from Western Rio Grande do Norte (Northeastern Brazil).
METHODS
: This cross-sectional study included 186 adults who were evaluated using electrocardiography, echocardiography, chest radiography, and contrast radiography of the esophagus and colon. A clinical-epidemiological questionnaire was also used.
RESULTS
: The indeterminate, cardiac, digestive, and cardiodigestive clinical forms of Chagas disease were diagnosed in 51.6% (96/186), 32.2% (60/186), 8.1% (15/186) and 8.1% (15/186) of the participants, respectively. Heart failure (functional classes I-IV) was detected in 7.5% (14/186) of the participants, and 36.4% (24/66), 30.3% (20/66), 15.2% (10/66), 13.6% (9/66), and 4.5% (3/66) of the patients were at stage A, B1, B2, C, and D, respectively. Dilated cardiomyopathy and electrocardiographic changes were detected in 10.2% (19/186) and 48.1% (91/186) of the participants, respectively. Apical aneurysm was diagnosed in 10.8% (20/186) of the participants, and other changes in the segmental myocardial contractility of the left ventricle were diagnosed in 33.9% (63/186) of the participants. Megaesophagus (groups I-IV) was observed in 7% (13/186) of the participants, megacolon (grades 1-3) was detected in12.9% (24/186) of the participants, and both organs were affected in 29.2% (7/24) of the megacolon cases.
CONCLUSIONS
: We detected various clinical forms of Chagas disease (including the digestive form). Our findings indicate that clinical symptoms alone may not be sufficient to exclude or confirm cardiac and/or digestive damage, and the number of patients with symptomatic clinical forms may be underestimated.
Keywords:
Trypanosoma cruzi; Chagas disease; Chagasic cardiomyopathy; Megaesophagus. Megacolon.
INTRODUCTION
Chagas disease (ChD) remains a major public health challenge in Latin America, where it currently affects 5.7 million individuals, with the largest numbers observed in Argentina, Brazil, and Mexico11. World Health Organization (WHO). Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Weekly Epidemiological Record 2015; 90:33-44..The clinical manifestations of ChD exhibit regional variations that are associated with different factors and are related to the parasite and/or host as the main cause of the chronic phase of the disease22. Macedo AM, Machado CR, Oliveira RP, Pena SD. Trypanosoma cruzi: genetic structure of populations and relevance of genetic variability to the pathogenesis of Chagas disease. Mem Inst Oswaldo Cruz 2004; 99:1-12..The digestive form is considered common in central Brazil and Chile, although it is practically non-existent in Venezuela and Central America33. Luquetti AO, Miles MA, Rassi A, Rezende JM, Souza A, Povoa M et al. Trypanosoma cruzi; zymodeme associated with acute and chronic Chagas' disease in central Brazil. Trans R Soc Trop Med Hyg 1986; 80:462- 470..
The forms of ChD are usually estimated based on clinical signs and symptoms, and without performing esophagus and colon contrast radiography, due to the difficulty of performing these exams. Therefore, in northeastern Brazil, an initial diagnosis of the digestive form has typically been based on the signs and symptoms of intestinal constipation and/or evidence of fecalomas44. Macêdo V. Influência da exposição à reinfecção na evolução da doença de Chagas (estudo longitudinal de cinco anos). Rev Patol Trop 1976; 5:33-116.. In contrast, the indeterminate form is characterized by the absence of symptoms that are compatible with ChD, and common diagnostic testing is not normally performed, such as conventional serology,12-lead electrocardiography, and radiography of the chest, esophagus, and colon55. Revista da Sociedade Brasileira de Medicina Tropical. Primeira Reunião de Pesquisa Aplicada em Doença de Chagas. Validade do conceito de forma indeterminada da doença de Chagas. Rev Soc Bras Med Trop 1985; 18:46.. In endemic areas, 50-70% of patients present with the indeterminate clinical form and 2-5% of these individuals evolve to a known clinical form, with approximately 20-30% of patients developing the cardiac form, 5-8% developing the esophageal form, and 4-6% developing the intestinal form66. Raia A, Campos OM. Megacólon - contribuição ao estudo de sua patogenia e tratamento. Rev Med Cirurg São Paulo 1955; 15:392-560. 77. Macêdo VO. Forma indeterminada da doença de Chagas. J Bras Med 1980; 38:34-40. 88. Dias JCP. Doença de Chagas em Bambuí, Minas Gerais, Brasil. Estudo clínico- epidemiológico a partir da fase aguda, entre 1940 e 1982, 1982, 401p. (Doctor's Thesis). Universidade Federal de Minas Gerais, 1982, Belo Horizonte.. The high mortality rateis due to chronic ChD cardiomyopathy (CCC), which is characterized by manifestations that include complex arrhythmias, heart failure, and thromboembolic events99. Dias JCP. Natural history of Chagas' disease. Arq Bras Cardiol 1995; 65:359-366.. The digestive form is characterized by digestive symptoms of megaesophagus and/or megacolon, which are due to inflammation and fibrosis of the esophagus and/or colon that results in destruction of the autonomic nervous system and subsequent organ dysfunction1010. Rezende JM. Forma digestiva da moléstia de Chagas. Rev Goiana Med 1959; 5:193-227..
The first scientific description of Trypanosoma cruzi infection in the State of Rio Grande do Norte (RN) reported a prevalence of 15.5%1111. Lucena DT, Lima ET. Epidemiologia da doença de Chagas no Rio Grande do Norte, III - A infecção humana determinada pela reação de Guerreiro Machado. Rev Bras Malariol D Trop 1962; 15:361-366.. More recent data support an estimated seroprevalence of 6.5% for chagasic infection in the Western mesoregion of RN, although that study did not examine the clinical forms of ChD1212. Brito CRN, Sampaio GHF, Câmara ACJ, Nunes DF, Azevedo PRM, Chiari E et al. Seroepidemiology of Trypanosoma cruzi infection in the semiarid rural zone of the State of Rio Grande do Norte, Brazil. Rev Soc Bras Med Trop2012; 45:346-352.. Therefore, the present study aimed to characterize the different clinical forms of ChD, its evolution among seroreactive individuals from different municipalities in western RN, and its association with disease severity.
METHODS
Study area
The State of RN is located in Northeastern Brazil, and consists of 167 municipalities that are distributed throughout East, Agreste, Central, and West mesoregions. The present study was performed in the West mesoregion, which covers >40% of RN's surface area (53,077.3km²) (Figure 1).This area was selected because a previous seroepidemiological survey was performed in this region during 2007-2009, and reported an estimated ChD prevalence of 6.5%1212. Brito CRN, Sampaio GHF, Câmara ACJ, Nunes DF, Azevedo PRM, Chiari E et al. Seroepidemiology of Trypanosoma cruzi infection in the semiarid rural zone of the State of Rio Grande do Norte, Brazil. Rev Soc Bras Med Trop2012; 45:346-352..
A map of Brazil with the State of Rio Grande do Norte, the Western mesoregion (in gray), and the municipalities that were evaluated.
Study population and protocol
A total of 215 individuals from 15 municipalities were invited to participate in this cross-sectional study between 2011 and 2013. However, only 186 individuals (23-78 years old) were ultimately included, as various patients were excluded due to disabilities, unwillingness to be examined, and other heart pathologies (e.g., ischemic, valvular, and hypertensive heart disease). Among the 186 included individuals, 83 individuals (38 men and 45 women) had participated in the previous seroepidemiological survey1212. Brito CRN, Sampaio GHF, Câmara ACJ, Nunes DF, Azevedo PRM, Chiari E et al. Seroepidemiology of Trypanosoma cruzi infection in the semiarid rural zone of the State of Rio Grande do Norte, Brazil. Rev Soc Bras Med Trop2012; 45:346-352., and 103 individuals (54 men and 49 women) had spontaneously demanded testing at the ChD outpatient clinic of the State University of Rio Grande do Norte. The patients who spontaneously demanded testing generally presented with two or more previous reactive serological tests for T. cruzi (e.g., at blood banks or primary health centers), and had been instructed to seek treatment at our outpatient clinic. Very few patients spontaneously demanded testing due to clinical symptoms.
All patients completed the same clinical-epidemiological questionnaire, which evaluated their lifestyle and concomitant diseases, and underwent a physical examination. Heart failure was classified according to the New York Heart Association criteria1313. The Criteria Committee of the New York Heart Association. New York Heart Association Functional Classification. : Nomenclature and criteria for diagnosis of diseases of the heart and great vessels, editor. 9th ed. Boston: Little, Brown and Company; 1994. p. 253-256.and categorized according to the American College of Cardiology/American Heart Association classifications that were adapted for ChD1414. Ministério da Saúde. Brazilian Consensus on Chagas disease. Rev Soc Bras Med Trop2005; 38:7-29. 1515. Xavier SS, Sousa AS, Hasslocher-Moreno A. Application of the New Classification of Cardiac Insufficiency (ACC/AHA) in Chronic Chagas Cardiopathy: A critical analysis of the survival curves. Rev SOCERJ 2005; 18:227-232..
Plain posteroanterior and lateral chest radiography were performed to evaluate the cardiac silhouette, signs of pulmonary congestion, and cardiothoracic ratio. The patient's clinical form was classified based on the assumption that a cardiothoracic ratio of >0.5was abnormal1616. Danzer CS. The cardiothoracic ratio: an index of cardiac enlargement. Am J Med Sci 1919; 157:513-521.. Contrast radiography of the esophagus was performed to classify the esophagus into four groups1717. Rezende JM, Lauar KM, Oliveira A. Clinical and radiological aspects of aperistalsis of the esophagus. Rev Bras Gastroenterol 1960; 12:247-262.,and contrast radiography of the colon was performed as previously described1818. Ximenes CA, Rezende JM, Moreira H, Vaz MGM. Técnica simplificada para diagnóstico radiológico do megacólon chagásico. Rev Soc Bras Med Trop1984; 17:23.. The diameter of the rectum was measured in the right lateral decubitus position, and the sigmoid colon was measured in the dorsal decubitus position, or in the ventral decubitus position if necessary1919. Castro C, Hernandez EB, Rezende JM, Prata A. Radiological study on megacolon cases in an endemic area for Chagas disease. Rev Soc Bras Med Trop2010; 43:562-566..The sigmoid colon was classified into four grades (0-3)2020. Silva AL, Giacomin RT, Quirino VA, Miranda ES. Proposta de Classificação do megacólon chagásico através do enema opaco. Rev Col Bras Cir 2003; 30:4-10., with some modifications.
A portable electrocardiography device (EP3 2008; Dixtal(r), Brazil) was used for 12-lead electrocardiography. All electrocardiographic recordings were evaluated by the same examiner, and scored based on the Minnesota Code that was modified and adapted for ChD2121. Maguire JH, Mott KE, Souza, JA, Almeida EC, Ramos NB, Guimarães AC. Electrocardiographic classification and abbreviated lead system for population-based studies of Chagas' disease. Bull Pan Am Health Org 1982; 16:47-58..
All Doppler echocardiography examinations were performed by the same examiner, using conventional views and their variations (Vivid e(r); General Electric, Milwaukee, WI). Linear measurements of the cardiac chambers and left ventricle ejection fraction were visually estimated and confirmed. The cardiac chambers were evaluated according to the recommendations of the American Society of Echocardiography2222. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005; 18:1440-1463.. Simpson's modified method2323. Ciampi Q, Villari B. Role of echocardiography in diagnosis and risk stratification in heart failure with left ventricular systolic dysfunction. Cardiovasc Ultrasound 2007; 5:1-12. was used when changes in segmental contractility were observed, and the Teichholz formula2424. Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol 1976; 37:7-11. was used in the absence of any changes.
Statistical analysis
This study used a convenience sample. Data were reported as mean ± standard deviation (SD).The proportions of the ChD forms were compared before and after the completion of contrast radiography. The chi-square test was used to analyze the associations between the clinical forms of ChD and the patients' age; testing type (seroepidemiological survey vs. spontaneous demand); symptoms of palpitations, dysphagia, and constipation; and the associations between dysphagia and megaesophagus and constipation and megacolon, after the completion of contrast radiography. The prevalence ratios (PRs) and their respective confidence intervals (CIs) were calculated for the associated data. The parametric Student t test was used to evaluate the differences between the mean values of the sigmoid colon and rectum measurements for men and women. Differences were considered significant at a p-value of ≤0.05, and all analyses were performed using Statistical Package for the Social Sciences (SPSS) software (version 20.0; SPSS Inc. Chicago, IL, USA).
Ethical considerations
Informed consent for this study was obtained from all participants, and the study's design was approved by the Research Ethics Committee of the State University of Rio Grande do Norte (protocol no. 027.2011). All experiments were performed in accordance with the human experimental guidelines of the Brazilian Ministry of Health and the Declaration of Helsinki.
RESULTS
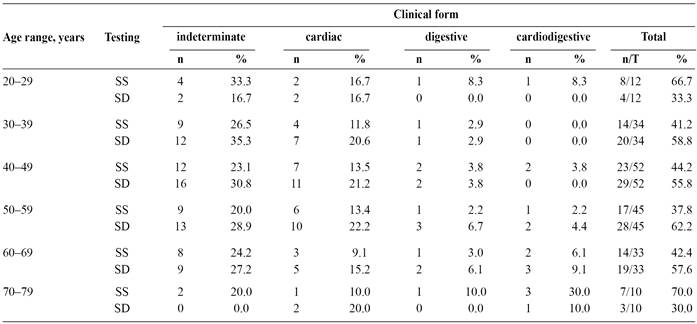
All participants were from rural areas and were currently residing or had previously resided in packed-earth houses; agricultural work was the predominant occupation. Approximately 50.5% of the participants were women, the mean age was 49 ± 12 years, and >70% of patients were illiterate or only had access to elementary/middle school education (which was not always completed) (Table 1). The indeterminate, cardiac, digestive, and cardiodigestive forms of ChD were observed in 51.6% (96/186), 32.2% (60/186), 8.1% (15/186), and 8.1% (15/186) of the participants, respectively. Based on only digestive symptoms (no contrast radiography),the proportions were 59.1%, 33.3%, 3.3%, and 4.3% for the indeterminate, cardiac, digestive, and cardiodigestive forms of ChD, respectively.
Epidemiological variables from 186 individuals with reactive conventional serology results for Trypanosoma cruzi.
When we compared the prevalence ratios of the radiography-confirmed and estimated clinical forms, significant differences for the digestive form (PR: 2.45; 95% CI: 2.18-2.73) and the cardiodigestive form (PR: 1.88; 95% CI: 1.61-2.16). There were no significant differences for the indeterminate form (PR: 0.87; 95% CI: 0.60-1.15) or the cardiac form (PR: 0.97; 95% CI: 0.69-1.24). We also did not observe any significant differences when we compared the testing types (seroepidemiological survey vs. spontaneous demand) among the various age groups (p = 0.6385) (Table 2).
Chest radiography revealed cardiomegaly in 10.2% (19/186) of the participants, which provided a sensitivity of 25.3% (19/75) for identifying heart involvement in the cardiac and cardiodigestive clinical forms.
Contrast radiography revealed megaesophagus in 7% (13/186) of the participants, with group I observed in 7 (53.8%) patients, group II observed in 4 (30.8%) patients, group III observed in 1 (7.7%) patient, and group IV observed in 1 (7.7%) patient. No significant sex-related differences between the megaesophagus groups were observed (p = 0.27) (Figure 2A). Dysphagia was diagnosed in 6.5% (12/186) of the participants, and patients with megaesophagus II and III reported that dysphagia limited their quality of life. However, this symptom did not discriminate between patients that had normal esophageal radiography findings with dysphagia and patients in the megaesophagus group I who did not have dysphagia (p = 0.07). Approximately 5% of the participants complained of occasional dysphagia, despite exhibiting normal esophageal radiography findings. However, patients with the digestive and cardiodigestive forms exhibited the greatest probability of having dysphagia (PR: 3.2; 95% CI: 2.07-4.33; p=0.006), compared to patients who had the indeterminate form. Esophageal radiography revealed three characteristic features: significant dilatation and elongation of the esophagus, food debris due to achalasia of the lower esophagus (the rat's tail sign; even in patients who had fasted for 12h), and folding over the diaphragm (Figure 2B).
Colon contrast radiography revealed megacolon in 12.9% (24/186) of the participants, with grade 1 observed in 17 (70.8%) patients, grade 2 observed in 6 (25%) patients, and grade 3 observed in 1 (4.2%) patient. Both sexes exhibited similar distributions (p = 0.42) (Figure 2C). Constipation was detected in 8.6% (16/186) of the participants, although this symptom did not discriminate between patients with grade 0 or grade 1 sigmoid colon (p = 0.23). Approximately 7% of the participants with grade 0 sigmoid colon also experienced constipation. In contrast, patients with the digestive/cardiodigestive forms exhibited a high probability of constipation (PR: 2.56; 95% CI: 1.24-3.87; p = 0.001), compared to patients who had the indeterminate form. The smallest sigmoid colon diameter was 2.5cm, the largest diameter was 19.0cm, and the mean diameter was 4.9 ± 1.8cm. The smallest rectum diameter was 3.0cm, the largest diameter was 15.0cm, and the mean diameter was 5.7 ± 1.4cm. Among the patients with different megacolon grades, 7 patients also exhibited megaesophagus. Colon contrast radiography revealed significant dilatation of the sigmoid colon and rectum in patients with chronic constipation, and abdominal palpation revealed the presence of fecal impaction (Figure 2D).
Heart failure functional classification revealed that 7.5% (14/186) of the participants had dyspnea, with 8 (57.1%) of these patients being class I, 2 (14.3%) patients being class II, 1 (7.1%) patient being class III, and 3 (21.5%) patients being class IV. The stage classification that was adapted for ChD revealed that 36.4% (24/66) of patients were in stage A, 30.3% (20/66) of patients were in stage B1, 15.2% (10/66) of patients were in stage B2, 13.6% (9/66) of patients were in stage C, and 4.5% (3/66) of patients were in stage D (Figure 2E). Symptoms of palpitation at rest were identified in 15% (28/186) of the participants, and echocardiography in the modified apical view revealed left ventricular aneurysm in patients with chagasic cardiomyopathy and stage C heart failure (Figure 2F).
The clinical evolution of Chagas disease. A. Patients were categorized into different groups of megaesophagus, and B. A contrast radiography image of the characteristic esophagus appearance is shown. C. Patients were categorized into different grades of megacolon, and D. A contrast radiography image of the characteristic colon appearance is shown. E. Patients were categorized according to heart failure stage, and F. Echocardiography reveals a characteristic left ventricle apical aneurysm. LV: left ventricle; RV: right ventricle.
Although electrocardiographic changes are not always suggestive of cardiac ChD, these changes were detected in 48.9% (91/186) of the participants, although they were not associated with any specific cardiac symptoms. Right bundle branch block (RBBB) was present in 30.8% (28/91) of the patients with electrocardiographic changes. Low-amplitude signals in the QRS complex were identified in 27.5% (21/91) of these patients, and the association of RBBB with left anterior fascicular block (LAFB) was observed in 17.6% (16/91) of these patients (Figure 3).
Distribution of the ten most frequent electrocardiographic changes among 91chagasic patients. Note: more than one change was possible in the same patient. RBBB: right bundle branch block; LAFB: left anterior fascicular block; PVCs: premature ventricular complexes; LBBB: left bundle-branch block; VT: ventricular tachycardia; 2n: second; AV: atrioventricular.
Echocardiography revealed left and right ventricle systolic dysfunction in 14% (26/186) and 7% (13/186) of the participants, respectively. Among the 26 patients with left ventricle systolic dysfunction, the dysfunction was discrete in 42.3% (11/26) of the patients, moderate in 50% (13/26) of the patients, and severe in 7.7% (2/26) of the patients. Changes in the left ventricle segment contractility were observed in 33.9% (63/186) of the participants, with 46.7% (28/60) of patients having the cardiac form, 66.7% (10/15) of patients having the cardiodigestive form, 22.9% (22/96) of patients having the indeterminate form, and 20% (3/15) of patients having the digestive form. The most frequently affected left ventricle segment was the apex (31/63; 49.2%), which was followed by the posteroinferior segment (21/63; 33.3%) and global involvement (4/63; 6.3%). Left ventricular apical aneurysm was detected in 10.8% (20/186) of the participants, and 55% (11/20) of these patients had the cardiac form, 20% (4/20) of these patients had the cardiodigestive form, 20% (4/20) of these patients had the indeterminate form, and 5% (1/20) of these patients had the digestive form. Right ventricular apical aneurysm was diagnosed in 5.4% (10/186) of the participants.
DISCUSSION
Studies regarding chronic ChD in endemic areas have typically been conducted by stratifying the clinical forms using the patients' clinical symptoms, and without the use of contrast radiography. This is the first study to characterize the clinical forms of ChD and the severity of its clinical manifestations among patients with chronic infection in Western RN. Our findings revealed that very few participants were <30 years old, which is likely due to recent improvements in rural dwellings, vector control programs2525. Dias JCP, Machado EMM, Fernandes AL, Vinhaes MC. General situation and perspectives of Chagas disease in Northeastern Region, Brazil. Cad Saude Publica 2000; 16:13-34., and population movement into more urban areas. The small number of participants who were >60 years old may be explained by the evolution of the indeterminate form in to a known clinical form (e.g., the cardiac form), which would increase the risk of fatal complications2626. Prata A, Lopes ER, Chapadeiro E. Characteristics of unexpected sudden death in Chagas disease. Rev Soc Bras Med Trop1986; 19:9-12..
We did not observe any significant difference in the clinical forms according to the testing type for each age range. However, the indeterminate form of ChD was predominant (>50% of the participants), which confirms the findings of other studies that examined outpatients2727. Gontijo ED, Rocha MOC, Oliveira UT. Perfil clínico-epidemiológico de chagásicos atendidos em ambulatório de referência e proposição de modelo de atenção ao chagásico na perspectiva do SUS. Rev Soc Bras Med Trop1996; 2:101-108. 2828. Souza LRMF, Guariento ME. Evolução de pacientes chagásicos acompanhados em um serviço de referência. Rev Soc Bras Med Trop1998; 31:54-55. 2929. Bozelli CE, Araújo SM, Guilherme ALFG, Gomes ML. Perfil clínico-epidemiológico de pacientes com doença de Chagas no Hospital Universitário de Maringá, Paraná, Brasil. Cad Saude Publica2006; 22:1027-1034.. Nevertheless, the characterization of ChD is difficult in rural populations, as many small Brazilian towns do not have the equipment for classic barium enema radiography1919. Castro C, Hernandez EB, Rezende JM, Prata A. Radiological study on megacolon cases in an endemic area for Chagas disease. Rev Soc Bras Med Trop2010; 43:562-566.. Therefore, most authors have not radiographically examined the colon, and have assumed that treatment of the digestive manifestations of ChD should be directed to the symptomatic and more advanced forms3030. Ribeiro ALP, Rocha MOC. Indeterminate form of Chagas disease: considerations about diagnosis and prognosis. Rev Soc Bras Med Trop1998; 31:301-314.. This approach likely results in underestimation of the prevalences for the digestive and cardiodigestive forms. Thus, clinical symptoms (e.g., dysphagia and constipation) are insufficient to identify patients with early-stage digestive and cardiodigestive forms. In this context, early identification of digestive lesions can motivate lifestyle changes (e.g., in hygiene and diet) and indicate multidisciplinary care, which might prevent progression to a more severe form of ChD.
Megaesophagus was detected at different stages in the present study, which supports the data from other Brazilian regions3131. Coura JR, Borges-Pereira J, Alves-Filho FI, Castro JAF, Cunha RV, Costa W, et al. Morbidity of Chagas disease in areas of Sertão da Paraíba and Caatinga do Piauí. Rev Soc Bras Med Trop1996; 29:197-205. and studies that reported high prevalences of the digestive form in central Brazil and other South American countries3232. Castro C, Prata A, Macêdo V. A 13-year clinical study on 190 chronic chagasic patients from Mambaí, Goiás, Brazil. Rev Soc Bras Med Trop2001; 34:309-318.. Although cases of grade 2-3 megacolon were diagnosed in the present study, the majority of cases were grade 1 with a sigmoid colon diameter of >5.0cm. Using the same technique, but only considering megacolon with a sigmoid colon diameter of ≥7.0cm, resulted in a lower prevalence3333. Hernandez EBR, Rezende JM, Macêdo V, Castro C. Estudo radiológico do cólon em indivíduos de área endêmica de doença de Chagas através da técnica simplificada de Ximenes. Rev Soc Bras Med Trop2002; 35:188.. Thus, all patients with grade 2-3 megacolon had ChD, although patients with grade 1 megacolon may or may not exhibit intestinal involvement of ChD. Various factors may be involved in the genesis of the digestive form, which include intrinsic denervation that is triggered by T. cruzi along the nerve plexus of the esophagus and intestine, which and which can result in denervation of the autonomic parasympathetic system3434. Köeberle F. Enteromegaly and cardiomegaly in Chagas disease. Gut 1963; 4:399-405.. This denervation affects these organs' functions, and especially those of the esophagus and sigmoid colon, which require coordination to propel their contents through their distal sphincters. The climax of megaesophagus and megacolon is the decompensated stage of the disease3535. Meneghelli UG. Chagasic enteropathy. Rev Soc Bras Med Trop2004; 37:252-260., which can be triggered by various parasite strains3636. Prata A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis 2001; 1:92-100. or a cross-reaction between T. cruzi antigens and the host tissues3737. Brener Z. Pathogenesis and immunopathology of chronic Chagas disease. Mem Inst Oswaldo Cruz1987; 82:205-213..
The most important clinical presentation of chronic ChD is CCC, which has been detected in patients with similar morbidity rates through different endemic areas of Brazil and Colombia3838. Borges-Pereira J, Sarquis O, Zauza PL, Britto C, Lima MM. Epidemiologia da doença de Chagas em quatro localidades rurais de Jaguaruana, Estado do Ceará. Soroprevalência da infecção, parasitemia e aspectos clínicos. Rev Soc Bras Med Trop2008; 41:345-351. 3939. Rosas AF. Enfermedad de Chagas. Rev Colombiana Cardiol 2011; 18:241-244.. However, lower morbidity rates are observed in other endemic regions of Latin America4040. Borges-Pereira J, Zauza PL, Galhardo MC, Nogueira JS, Pereira GROL, Cunha RV. Doença de Chagas na população urbana do distrito sanitário de Rio Verde, Mato Grosso do Sul, Brasil. Rev Soc Bras Med Trop2001; 34:459-466. 4141. Aguilar VHM, Abad-Franch F, Racines VJ, Paucar CA. Epidemiology of Chagas disease in Ecuador. A brief review. Mem Inst Oswaldo Cruz1999; 94:387-393.. In the present study, cardiomegaly was exclusively present with the cardiac and cardiodigestive forms,which is in agreement with previously reported data4242. Coura JR, Abreu LL, Dubois LEG, Lima FC, Arruda-Junior E, Willcox HPF et al. Morbidade da doença de Chagas. II- Estudos seccionais em quarto áreas de campo no Brasil. Mem Inst Oswaldo Cruz1984; 79:101-124. 4343. Pompilio MA, Dorval MEMC, Cunha RV, Britto C, Borges-Pereira J. Epidemiological, clinical and parasitological aspects of Chagas'disease in Mato Grosso do Sul State. Rev Soc Bras Med Trop2005; 38:73-478.. However, the low sensitivity of chest radiography for identifying cardiac involvement is related to the right ventricle being one of the cardiac chambers that is most affected by CCC4444. Rocha MO, Ribeiro AL, Teixeira MM. Clinical management of chronic Chagas cardiomyopathy. Front Biosci 2003; 8:e44-54.. As this is the most anterior chamber of the heart, its enlargement may not noticeably increase the cardiothoracic ratio, which precludes the identification of the cardiomegaly.
The participants with ChD exhibited all functional classifications and stages of heart failure, although the patients were predominantly functional class I and stage A, which is characteristic of an outpatient sample. However, the presence of patients in functional classes III-IV and stages C-D indicates that ChD in this region is more severe than findings from northern Brazil4545. Brum-Soares LM, Xavier SS, Sousa AS, Borges-Pereira J, Ferreira JMBB, Costa IR et al. Morbidity of Chagas disease among autochthonous patients from the Rio Negro microregion, State of Amazonas. Rev Soc Bras Med Trop2010; 43:170-177.. In this context, CCC is associated with a worse prognosis and a survival rate that is one-half of the rates for cardiomyopathies of non-inflammatory etiology (e.g., dilated cardiomyopathy and ischemic heart disease)4646. Mady C, Cardoso RHA, Barretto ACP, Luz PL, Bellotti G, Pileggi F. Survival and predictors of survival in patients with congestive heart failure due to Chagas' cardiomyopathy. Circulation 1994; 90:3098-3102.. Studies in Brazil and Venezuela have also reported that higher functional classes are associated with higher mortality rates among patients with CCC4747. Coura JR, Abreu LL, Borges-Pereira J, Willcox HP. Morbidity in Chagas' disease. IV. Longitudinal study of 10 years in Pains and Iguatama, Minas Gerais, Brazil. Mem Inst Oswaldo Cruz1985; 80:73-80. 4848. Acquatella H, Catalioti F, Gomez-Mancebo JR, Davalos V, Villalobos L. Long-term control of Chagas disease in Venezuela: effects on serologic findings, electrocardiographic abnormalities, and clinical outcome. Circulation1987; 76:556-562., with sudden death, heart failure, and ischemic stroke being the main mechanisms of death in patients with ChD4949. Xavier SS, Sousa AS, Carvalho-Filho HA, Holanda MT, Hasslocher-Moreno A. Mecanismos de morte e função ventricular na fase crônica da doença de Chagas. Rev SOCERJ2006; 19:239-246. 5050. Ayub-Ferreira SM, Mangini S, Issa VS, Cruz FD, Bacal F, Guimarães GV et al. Mode of death on Chagas heart disease: comparison with other etiologies. A subanalysis of the REMADHE prospective trial. PLoS Negl Trop Dis 2013; 7:1-10..
Our findings regarding electrocardiographic changes, such as RBBB and LAFB, are in agreement with the previous studies and are the most characteristic changes of the disease in endemic areas5151. Prata SP, Cunha DF, Cunha SF, Prata SC, Nogueira N. Prevalence of electrocardiographic abnormalities in 2,000 aged and non-aged chagasic patients. Arq Bras Cardiol1993; 60:369-372.. The higher prevalence of RBBB, compared to left bundle branch block, is likely related to the anatomy of the heart's conduction system, as in the classical description5252. Rosenbaum MB, Alvarez AJ. The electrocardiogram in chronic chagasic myocarditis. Am Heart J 1955; 50:492-527.. The right branch of the His-Purkinje system is long, slender, and presents a large intramyocardial course, while the left branch has many sub-branches and takes a subendocardial course along its entire length. Ventricular arrhythmia has been previously described5353. Garzon SAC, Lorga AM, Nicolau JC. Eletrocardiografia na cardiopatia chagásica. Rev Soc Cardiol Estado de São Paulo 1994; 2:133-143., and may be caused by cardiac conduction system lesions that are related to the degree of ventricular dysfunction and to the increased risk of cardiovascular death5454. Rassi-Junior A, Rassi SG, Rassi A. Sudden death in Chagas' disease. Arq Bras Cardiol2001; 76:75-96..
Changes in segmental myocardial contractility were predominantly in the left ventricular apex and posterior basal segment, even in patients with the indeterminate form, which confirms the findings of previous studies5555. Ortiz J, Barreto ACP, Matsumoto AY, Mônaco CAF, Ianni BM, Marotta RHQ et al. Alteração contrátil segmentar na forma indeterminada da doença de Chagas. Estudo ecocardiográfico. Arq Bras Cardiol1987; 49:217-220. 5656. Barros ML, Ribeiro AL, Nunes MC, Rocha MOC. Association between left ventricular wall motion abnormalities and ventricular arrhythmia in the indeterminate form of Chagas disease. Rev Soc Bras Med Trop2011; 44:213-216.. Interestingly, the indeterminate form was assigned to patients who fulfilled the related criteria in the present study, even if they exhibited contractility segmental changes during echocardiography. However, we believe that these patients should be classified as having the cardiac form, as this classification may facilitate the use of more effective prophylactic and/or therapeutic measures. Furthermore, left ventricular apical aneurysm was more common in this study than the reported frequency in central Brazil4343. Pompilio MA, Dorval MEMC, Cunha RV, Britto C, Borges-Pereira J. Epidemiological, clinical and parasitological aspects of Chagas'disease in Mato Grosso do Sul State. Rev Soc Bras Med Trop2005; 38:73-478., and was less common than the reported frequency in southeastern Brazil5757. Borges-Pereira J, Xavier SS, Pirmez C, Coura JR. Chagas disease in Virgem da Lapa, Minas Gerais, Brazil. IV. Clinical and epidemiological aspects of left ventricular aneurism. Rev Soc Bras Med Trop1998; 31:457-463.. Although left ventricular apical aneurysm is not characteristic of the cardiac form, it was more common among patients with this clinical form, which may be related to its severity and the increased risk of ischemic stroke and/or death among these patients5858. Rassi-Junior A, Rassi A, Little WC, Xavier SS, Rassi SG, Rassi AG et al. Development and validation of a risk score for predicting death in Chagas' heart disease. New Engl J Med 2006; 355:799-808. 5959. Sousa AS, Xavier SS, Freitas GR, Hasslocher-Moreno A. Prevention Strategies of Cardioembolic Ischemic Stroke in Chagas Disease. Arq Bras Cardiol2008; 91:306-310..
Unfortunately, the factors that determine the variability of the clinical forms of ChD, pathogenesis, and patterns of development remain unclear. It is possible that these changes may be associated with the wide geographical dispersion of homogeneous T. cruzi genotypes from different parasite sources and groups that have been identified throughout the municipalities of RN6060. Câmara ACJ, Lages-Silva E, Sampaio GHF, D'Ávila DA, Chiari E, Galvão LMC. Homogeneity of Trypanosoma cruzi I, II, and III populations and the overlap of wild and domestic transmission cycles by Triatoma brasiliensis in northeastern Brazil. Parasitol Res 2013; 112:1543-1550.. As a result, the forms of chronic ChD seem to be directly associated with discrete typing units of T. cruzi, which might explain the observed differences in the expression of anti-inflammatory and pro-inflammatory cytokines, and the specific immune responses6161. Poveda C, Fresno M, Gironès N, Martins-Filho AO, Ramírez JD, Santi-Rocca J et al. Cytokine Profiling in Chagas Disease: Towards Understanding the Association with Infecting Trypanosoma cruzi Discrete Typing Units (A BENEFIT TRIAL Sub-Study). PloS One 2014; 9:1-8.. Furthermore, this variability may depend on tissue-specific responses and the intensities of the parasitism, inflammatory response, and immune response6262. Coura JR. Epidemiologic determinants of Chagas' disease in Brazil: the infection, the disease and its morbidity/mortality. Mem Inst Oswaldo Cruz1988; 83:392-402. 6363. Guedes PMM, Gutierrez FRS, Silva GK, Dellalibera-Joviliano R, Rodrigues GJ, Bendhack LM et al. Deficient regulatory T cell activity and low frequency of IL-17-producing T cells correlate with the extent of cardiomyopathy in human Chagas' disease. PLoS Negl Trop Dis2012; 6:e1630.. Moreover, familial aggregation of CCC cases has been well documented, which suggests that genetic components are involved in patients' susceptibility to cardiomyopathy6464. Zicker F, Smith PG, Almeida-Netto JC, Oliveira RM, Zicker EM. Physical activity, opportunity for reinfection, and sibling history of heart disease as risk factors for Chagas' cardiopathy. Am J Trop Med Hyg 1990; 43:498-505..
In conclusion, among patients from Western RN, we believe that approximately one-quarter of patients who are diagnosed with the indeterminate form of ChD should be considered to potentially have the cardiac form or silent heart disease. This approach would decrease the prevalence of the indeterminate form and alter the therapeutic management of these patients, which might subsequently reduce the prevalence of more severe complications. Furthermore, patients' clinical symptoms may not be sufficient to confirm or rule out a diagnosis of cardiac and/or digestive involvement in chronic ChD. Moreover, isolated digestive and cardiodigestive forms were detected in both symptomatic and asymptomatic patients, with different groupings and grades of megaesophagus and megacolon. Thus, while ignoring any potential underdiagnosis, approximately one-third of patients were diagnosed with the cardiac form of ChD, which involved different types and degrees of atrioventricular and ventricular block, several segmental contractility alterations, and varying degrees of heart failure. These findings indicate that the cardiac form may be the most relevant form of chronic ChD, as it was responsible for more severe clinical outcomes. Nevertheless, prospective cohort studies are needed to confirm our findings in this endemic area, especially among patients with the indeterminate form. Furthermore, studies are needed to evaluate the potential immunological, clinical, and/or genetic markers from the host and/or parasite, which may contribute to improving the prognosis of these clinical manifestations. Moreover, interventional studies are needed to examine the etiology of, and damage due to ChD- related manifestations in the cardiovascular and digestive systems.
ACKNOWLEDGMENTS
The authors are grateful to the participants involved in this study, to the health authorities and agents of the Municipal Health Secretariats of the west of the State of Rio Grande do Norte for their indispensable support for the field activities during the development of this survey. We would also like to express to thank to the Clinical Center West Clio for their unconditional support during this study, to Galttieri Tavares Ferreira for his statistical analysis and to Philip S.P. Badiz for his critical reading and revision of the manuscript.
-
1World Health Organization (WHO). Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Weekly Epidemiological Record 2015; 90:33-44.
-
2Macedo AM, Machado CR, Oliveira RP, Pena SD. Trypanosoma cruzi: genetic structure of populations and relevance of genetic variability to the pathogenesis of Chagas disease. Mem Inst Oswaldo Cruz 2004; 99:1-12.
-
3Luquetti AO, Miles MA, Rassi A, Rezende JM, Souza A, Povoa M et al. Trypanosoma cruzi; zymodeme associated with acute and chronic Chagas' disease in central Brazil. Trans R Soc Trop Med Hyg 1986; 80:462- 470.
-
4Macêdo V. Influência da exposição à reinfecção na evolução da doença de Chagas (estudo longitudinal de cinco anos). Rev Patol Trop 1976; 5:33-116.
-
5Revista da Sociedade Brasileira de Medicina Tropical. Primeira Reunião de Pesquisa Aplicada em Doença de Chagas. Validade do conceito de forma indeterminada da doença de Chagas. Rev Soc Bras Med Trop 1985; 18:46.
-
6Raia A, Campos OM. Megacólon - contribuição ao estudo de sua patogenia e tratamento. Rev Med Cirurg São Paulo 1955; 15:392-560.
-
7Macêdo VO. Forma indeterminada da doença de Chagas. J Bras Med 1980; 38:34-40.
-
8Dias JCP. Doença de Chagas em Bambuí, Minas Gerais, Brasil. Estudo clínico- epidemiológico a partir da fase aguda, entre 1940 e 1982, 1982, 401p. (Doctor's Thesis). Universidade Federal de Minas Gerais, 1982, Belo Horizonte.
-
9Dias JCP. Natural history of Chagas' disease. Arq Bras Cardiol 1995; 65:359-366.
-
10Rezende JM. Forma digestiva da moléstia de Chagas. Rev Goiana Med 1959; 5:193-227.
-
11Lucena DT, Lima ET. Epidemiologia da doença de Chagas no Rio Grande do Norte, III - A infecção humana determinada pela reação de Guerreiro Machado. Rev Bras Malariol D Trop 1962; 15:361-366.
-
12Brito CRN, Sampaio GHF, Câmara ACJ, Nunes DF, Azevedo PRM, Chiari E et al. Seroepidemiology of Trypanosoma cruzi infection in the semiarid rural zone of the State of Rio Grande do Norte, Brazil. Rev Soc Bras Med Trop2012; 45:346-352.
-
13The Criteria Committee of the New York Heart Association. New York Heart Association Functional Classification. : Nomenclature and criteria for diagnosis of diseases of the heart and great vessels, editor. 9th ed. Boston: Little, Brown and Company; 1994. p. 253-256.
-
14Ministério da Saúde. Brazilian Consensus on Chagas disease. Rev Soc Bras Med Trop2005; 38:7-29.
-
15Xavier SS, Sousa AS, Hasslocher-Moreno A. Application of the New Classification of Cardiac Insufficiency (ACC/AHA) in Chronic Chagas Cardiopathy: A critical analysis of the survival curves. Rev SOCERJ 2005; 18:227-232.
-
16Danzer CS. The cardiothoracic ratio: an index of cardiac enlargement. Am J Med Sci 1919; 157:513-521.
-
17Rezende JM, Lauar KM, Oliveira A. Clinical and radiological aspects of aperistalsis of the esophagus. Rev Bras Gastroenterol 1960; 12:247-262.
-
18Ximenes CA, Rezende JM, Moreira H, Vaz MGM. Técnica simplificada para diagnóstico radiológico do megacólon chagásico. Rev Soc Bras Med Trop1984; 17:23.
-
19Castro C, Hernandez EB, Rezende JM, Prata A. Radiological study on megacolon cases in an endemic area for Chagas disease. Rev Soc Bras Med Trop2010; 43:562-566.
-
20Silva AL, Giacomin RT, Quirino VA, Miranda ES. Proposta de Classificação do megacólon chagásico através do enema opaco. Rev Col Bras Cir 2003; 30:4-10.
-
21Maguire JH, Mott KE, Souza, JA, Almeida EC, Ramos NB, Guimarães AC. Electrocardiographic classification and abbreviated lead system for population-based studies of Chagas' disease. Bull Pan Am Health Org 1982; 16:47-58.
-
22Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005; 18:1440-1463.
-
23Ciampi Q, Villari B. Role of echocardiography in diagnosis and risk stratification in heart failure with left ventricular systolic dysfunction. Cardiovasc Ultrasound 2007; 5:1-12.
-
24Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol 1976; 37:7-11.
-
25Dias JCP, Machado EMM, Fernandes AL, Vinhaes MC. General situation and perspectives of Chagas disease in Northeastern Region, Brazil. Cad Saude Publica 2000; 16:13-34.
-
26Prata A, Lopes ER, Chapadeiro E. Characteristics of unexpected sudden death in Chagas disease. Rev Soc Bras Med Trop1986; 19:9-12.
-
27Gontijo ED, Rocha MOC, Oliveira UT. Perfil clínico-epidemiológico de chagásicos atendidos em ambulatório de referência e proposição de modelo de atenção ao chagásico na perspectiva do SUS. Rev Soc Bras Med Trop1996; 2:101-108.
-
28Souza LRMF, Guariento ME. Evolução de pacientes chagásicos acompanhados em um serviço de referência. Rev Soc Bras Med Trop1998; 31:54-55.
-
29Bozelli CE, Araújo SM, Guilherme ALFG, Gomes ML. Perfil clínico-epidemiológico de pacientes com doença de Chagas no Hospital Universitário de Maringá, Paraná, Brasil. Cad Saude Publica2006; 22:1027-1034.
-
30Ribeiro ALP, Rocha MOC. Indeterminate form of Chagas disease: considerations about diagnosis and prognosis. Rev Soc Bras Med Trop1998; 31:301-314.
-
31Coura JR, Borges-Pereira J, Alves-Filho FI, Castro JAF, Cunha RV, Costa W, et al. Morbidity of Chagas disease in areas of Sertão da Paraíba and Caatinga do Piauí. Rev Soc Bras Med Trop1996; 29:197-205.
-
32Castro C, Prata A, Macêdo V. A 13-year clinical study on 190 chronic chagasic patients from Mambaí, Goiás, Brazil. Rev Soc Bras Med Trop2001; 34:309-318.
-
33Hernandez EBR, Rezende JM, Macêdo V, Castro C. Estudo radiológico do cólon em indivíduos de área endêmica de doença de Chagas através da técnica simplificada de Ximenes. Rev Soc Bras Med Trop2002; 35:188.
-
34Köeberle F. Enteromegaly and cardiomegaly in Chagas disease. Gut 1963; 4:399-405.
-
35Meneghelli UG. Chagasic enteropathy. Rev Soc Bras Med Trop2004; 37:252-260.
-
36Prata A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis 2001; 1:92-100.
-
37Brener Z. Pathogenesis and immunopathology of chronic Chagas disease. Mem Inst Oswaldo Cruz1987; 82:205-213.
-
38Borges-Pereira J, Sarquis O, Zauza PL, Britto C, Lima MM. Epidemiologia da doença de Chagas em quatro localidades rurais de Jaguaruana, Estado do Ceará. Soroprevalência da infecção, parasitemia e aspectos clínicos. Rev Soc Bras Med Trop2008; 41:345-351.
-
39Rosas AF. Enfermedad de Chagas. Rev Colombiana Cardiol 2011; 18:241-244.
-
40Borges-Pereira J, Zauza PL, Galhardo MC, Nogueira JS, Pereira GROL, Cunha RV. Doença de Chagas na população urbana do distrito sanitário de Rio Verde, Mato Grosso do Sul, Brasil. Rev Soc Bras Med Trop2001; 34:459-466.
-
41Aguilar VHM, Abad-Franch F, Racines VJ, Paucar CA. Epidemiology of Chagas disease in Ecuador. A brief review. Mem Inst Oswaldo Cruz1999; 94:387-393.
-
42Coura JR, Abreu LL, Dubois LEG, Lima FC, Arruda-Junior E, Willcox HPF et al. Morbidade da doença de Chagas. II- Estudos seccionais em quarto áreas de campo no Brasil. Mem Inst Oswaldo Cruz1984; 79:101-124.
-
43Pompilio MA, Dorval MEMC, Cunha RV, Britto C, Borges-Pereira J. Epidemiological, clinical and parasitological aspects of Chagas'disease in Mato Grosso do Sul State. Rev Soc Bras Med Trop2005; 38:73-478.
-
44Rocha MO, Ribeiro AL, Teixeira MM. Clinical management of chronic Chagas cardiomyopathy. Front Biosci 2003; 8:e44-54.
-
45Brum-Soares LM, Xavier SS, Sousa AS, Borges-Pereira J, Ferreira JMBB, Costa IR et al. Morbidity of Chagas disease among autochthonous patients from the Rio Negro microregion, State of Amazonas. Rev Soc Bras Med Trop2010; 43:170-177.
-
46Mady C, Cardoso RHA, Barretto ACP, Luz PL, Bellotti G, Pileggi F. Survival and predictors of survival in patients with congestive heart failure due to Chagas' cardiomyopathy. Circulation 1994; 90:3098-3102.
-
47Coura JR, Abreu LL, Borges-Pereira J, Willcox HP. Morbidity in Chagas' disease. IV. Longitudinal study of 10 years in Pains and Iguatama, Minas Gerais, Brazil. Mem Inst Oswaldo Cruz1985; 80:73-80.
-
48Acquatella H, Catalioti F, Gomez-Mancebo JR, Davalos V, Villalobos L. Long-term control of Chagas disease in Venezuela: effects on serologic findings, electrocardiographic abnormalities, and clinical outcome. Circulation1987; 76:556-562.
-
49Xavier SS, Sousa AS, Carvalho-Filho HA, Holanda MT, Hasslocher-Moreno A. Mecanismos de morte e função ventricular na fase crônica da doença de Chagas. Rev SOCERJ2006; 19:239-246.
-
50Ayub-Ferreira SM, Mangini S, Issa VS, Cruz FD, Bacal F, Guimarães GV et al. Mode of death on Chagas heart disease: comparison with other etiologies. A subanalysis of the REMADHE prospective trial. PLoS Negl Trop Dis 2013; 7:1-10.
-
51Prata SP, Cunha DF, Cunha SF, Prata SC, Nogueira N. Prevalence of electrocardiographic abnormalities in 2,000 aged and non-aged chagasic patients. Arq Bras Cardiol1993; 60:369-372.
-
52Rosenbaum MB, Alvarez AJ. The electrocardiogram in chronic chagasic myocarditis. Am Heart J 1955; 50:492-527.
-
53Garzon SAC, Lorga AM, Nicolau JC. Eletrocardiografia na cardiopatia chagásica. Rev Soc Cardiol Estado de São Paulo 1994; 2:133-143.
-
54Rassi-Junior A, Rassi SG, Rassi A. Sudden death in Chagas' disease. Arq Bras Cardiol2001; 76:75-96.
-
55Ortiz J, Barreto ACP, Matsumoto AY, Mônaco CAF, Ianni BM, Marotta RHQ et al. Alteração contrátil segmentar na forma indeterminada da doença de Chagas. Estudo ecocardiográfico. Arq Bras Cardiol1987; 49:217-220.
-
56Barros ML, Ribeiro AL, Nunes MC, Rocha MOC. Association between left ventricular wall motion abnormalities and ventricular arrhythmia in the indeterminate form of Chagas disease. Rev Soc Bras Med Trop2011; 44:213-216.
-
57Borges-Pereira J, Xavier SS, Pirmez C, Coura JR. Chagas disease in Virgem da Lapa, Minas Gerais, Brazil. IV. Clinical and epidemiological aspects of left ventricular aneurism. Rev Soc Bras Med Trop1998; 31:457-463.
-
58Rassi-Junior A, Rassi A, Little WC, Xavier SS, Rassi SG, Rassi AG et al. Development and validation of a risk score for predicting death in Chagas' heart disease. New Engl J Med 2006; 355:799-808.
-
59Sousa AS, Xavier SS, Freitas GR, Hasslocher-Moreno A. Prevention Strategies of Cardioembolic Ischemic Stroke in Chagas Disease. Arq Bras Cardiol2008; 91:306-310.
-
60Câmara ACJ, Lages-Silva E, Sampaio GHF, D'Ávila DA, Chiari E, Galvão LMC. Homogeneity of Trypanosoma cruzi I, II, and III populations and the overlap of wild and domestic transmission cycles by Triatoma brasiliensis in northeastern Brazil. Parasitol Res 2013; 112:1543-1550.
-
61Poveda C, Fresno M, Gironès N, Martins-Filho AO, Ramírez JD, Santi-Rocca J et al. Cytokine Profiling in Chagas Disease: Towards Understanding the Association with Infecting Trypanosoma cruzi Discrete Typing Units (A BENEFIT TRIAL Sub-Study). PloS One 2014; 9:1-8.
-
62Coura JR. Epidemiologic determinants of Chagas' disease in Brazil: the infection, the disease and its morbidity/mortality. Mem Inst Oswaldo Cruz1988; 83:392-402.
-
63Guedes PMM, Gutierrez FRS, Silva GK, Dellalibera-Joviliano R, Rodrigues GJ, Bendhack LM et al. Deficient regulatory T cell activity and low frequency of IL-17-producing T cells correlate with the extent of cardiomyopathy in human Chagas' disease. PLoS Negl Trop Dis2012; 6:e1630.
-
64Zicker F, Smith PG, Almeida-Netto JC, Oliveira RM, Zicker EM. Physical activity, opportunity for reinfection, and sibling history of heart disease as risk factors for Chagas' cardiopathy. Am J Trop Med Hyg 1990; 43:498-505.
-
This work was supported by research grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico/Ministério de Ciência, Tecnologia (MCT/CNPq) number 472251/2010-4 (EC); MCTI/CNPq/MS-SCTIE-Decit number 404056/2012-1 (LMCG); Programa Nacional de Incentivo à Parasitologia Básica/Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) grant number 23038.005288/2011-48(ACJC); research fellowships from the CNPq (LMCG, EC and PMMG) and scholarship from the CAPES (CMA).
Publication Dates
-
Publication in this collection
Dec 2015
History
-
Received
11 July 2015 -
Accepted
05 Oct 2015