Abstract
INTRODUCTION
Brazilian spotted fever is an infectious disease with a high mortality rate if not treated early. Differential diagnosis is difficult, as the first clinical signs are non-specific and can be confused with other diseases. The aim of the study was to investigate evidence of infection with Rickettsia rickettsii and Rickettsia parkeri in negative sera samples, collected in 2014, from patients with suspected leptospirosis, dengue fever, and meningococcal disease in Atibaia and Bragança Paulista municipalities of the State of São Paulo.
METHODS
The samples stored at the Institute Adolfo Lutz in Campinas were tested using an indirect immunofluorescence assay (IFA) with IgG and IgM against R. rickettsii and R. parkeri. Real-time polymerase chain reaction (PCR) testing was performed for the sera samples of patients who died (n = 3), those with initial suspicion of meningococcal disease (n = 6), and those with positive IFA results.
RESULTS
Of 258 samples from Bragança Paulista, 4 (1.6%) were positive, with IgG titers of 1:64 and 1:128 against R. rickettsii and R. parkeri, respectively. Of 155 samples from Atibaia, 2 (1.3%) were positive, with IgG titers of 1:64 and 1:128 against R. rickettsii and R. parkeri, respectively. No sample showed positive PCR results.
CONCLUSIONS
This serological investigation suggests there is evidence of exposure to Rickettsia spp. in residents of areas that have environmental conditions favorable to the spread of bacteria, in which Brazilian spotted fever incidence was not previously confirmed.
Keywords:
Brazilian spotted fever; Rickettsia rickettsii; Rickettsia parkeri; Indirect immunofluorescence test; Differential diagnosis
INTRODUCTION
Brazilian spotted fever (BSF) is an infectious disease, marked by acute fever, with a high mortality rate when not diagnosed and treated early. In Brazil, it is considered a re-emerging zoonosis of great importance to public health because of the difficulty in establishing a clinical diagnosis, the high rate of mortality, and the involvement of an economically active population11. Ministério da Saúde. Secretaria de Vigilância em Saúde. Guia de Vigilância epidemiológica/Ministério da Saúde. 6 edition. Brasília: Ministério da Saúde; 2005. 816 p..
Brazilian spotted fever is caused by Rickettsia rickettsii, a Gram-negative pleomorphic bacterium22. Walker DH. Rocky Mountain spotted fever: a disease in need of microbiological concern. Clin Microbiol Rev 1989; 2:227-240., and transmitted in Brazil by ticks of the genus Amblyomma, notably Amblyomma sculptum and Amblyomma aureolatum33. Krawczak FS, Nieri-Bastos FA, Nunes FP, Soares JF, Moraes-Filho J, Labruna MB. Rickettsial infection in Amblyomma cajennenense ticks and capybaras (Hydrochoerus hydrochaeris) in a Brazilian spotted fever- endemic area. Parasit Vectors 2014; 7:7.) (44. Pinter A, Labruna MB. Isolation of Rickettsia rickettsii and Rickettsia belii in cell culture from the tick Amblyomma aureolatum in Brazil. Ann N Y AcadSci 2006; 1078:523-529.. Rickettsia parkeri also belongs to Rickettsia spp. that cause BSF and has been associated with milder forms of the disease55. Angerami RN, Silva AMR, Nascimento EMM, Colombo S, Wada MY, Santos FCP, et al. Brazilian spotted fever: two faces of a same disease? A comparative study of clinical aspects between an old and a new endemic area in Brazil. Clin Microbiol Infect 2009; 15 (suppl 2):207-208.) (66. Spolidorio MG, Labruna MB, Mantovani E, Brandão PE, Richtzenhain LJ, Yoshinari NH. Novel spotted fever group rickettsiosis, Brazil. Emerg Infect Dis 2010; 16:521-523..
According to data from the Brazilian epidemiological surveillance system, between 2000 and June 2015, 1,387 cases of BSF were recorded in the country, with 417 deaths, representing a lethality rate of 30%. Of the 1,387 total cases, 1,009 (72.7%) were confirmed in the Southeast region, where 414 (99.3%) of a total of 417 BSF-related deaths were recorded.
Both domestic and wild animals play an important role as vertebrate hosts in the epidemiology of BSF by temporarily developing rickettsemia (over the course of days or weeks), favoring the infection of new ticks by R. rickettsii. Hosts are also important as they transport vectors to environments in and around places where humans live. They are called amplifier hosts and include animals such as the capybara (Hydrochoerus hydrochaeris), opossum (Didelphis spp.), and domestic dog (Canis familiaris)77. Souza CE, Moraes-Filho J, Orgzewalska M, Uchoa FC, Horta MC, Souza SS, et al. Experimental infection of capybaras (Hydrochoerus hydrochaeris) by Rickettsia rickettsii and evaluation of the transmission of the infection to ticks Amblyomma cajennense. Vet Parasit 2009; 161:116-121.) (88. Horta MC, Moraes-Filho J, Casagrande RA, Saito TB, Rosa SC, Ogrzewalska M, et al. Experimental infection of opossums Didelphis aurita by Rickettsia rickettsii and evaluation of the transmission of the infection to ticks Amblyomma cajennense. Vector Borne Zoonotic Dis 2009; 9:109-118.) (99. Piranda EM, Faccini JLH, Pinter A, Pacheco RC, Cançado PHD, Labruna MB. Experimental infection of Rhipicephalus sanguineus ticks with the bacterium Rickettsia rickettsii, using experimentally infected dogs. Vector Borne Zoonotic Dis 2011; 11:29-36.. Horses and dogs with positive serology are often found in endemic regions. These animals have been considered sentinels and are important in epidemiological studies of BSF, as positive serology is related to the circulation of the bacteria, particularly if the animals are autochthonous1010. Sangioni LA, Horta MC, Vianna MCB, Gennari SM, Soares RM, Galvão MAM, et al. Rickettsial infection in animals and Brazilian spotted fever endemicity. Emerg Infect Dis 2005; 11:265-270.) (1111. Pinter A, Horta MC, Pacheco RC, Moraes-Filho J, Labruna MB. Serosurvey of Rickettsia spp. in dogs and humans from an endemic area for Brazilian spotted fever in the State of São Paulo, Brazil. Cad Saude Publica 2009; 24:247-252..
In the State of São Paulo, Campinas is the region with the highest number of cases from 1998 to 2012 (294, or 53% of the total number of cases in the country), followed by metropolitan São Paulo (98, or 17.5%), the region of Piracicaba (59, or 10.6%), and the coastal region (28, or 5%). According to the Epidemiological Surveillance Center, most of the confirmed cases occurred in municipalities belonging to the basins of the Piracicaba, Capivari, and Jundiaí (PCJ) rivers.
Atibaia and Bragança Paulista are located in the PCJ Basin in the Northeast region of the State of São Paulo and had no reports of confirmed human cases of BSF in the last 25 years, even though there is evidence of R. rickettsii circulation among horses and the presence of vectors and capybaras in these areas1212. Souza CE, Camargo LB, Pinter A, Donalisio MR. High seroprevalence for Rickettsia rickettsii in equines suggests risk of human infection in silent areas for the Brazilian spotted fever. PloSOne 2016; 11:e0153303..
In endemic areas, BSF in the initial clinical phase is part of the differential diagnosis of acute febrile exanthematic syndromes. Due to non-specific symptoms, the diagnosis is difficult, particularly in the early days of the disease55. Angerami RN, Silva AMR, Nascimento EMM, Colombo S, Wada MY, Santos FCP, et al. Brazilian spotted fever: two faces of a same disease? A comparative study of clinical aspects between an old and a new endemic area in Brazil. Clin Microbiol Infect 2009; 15 (suppl 2):207-208.. Thus, given the similarity of the clinical picture with endemic diseases such as leptospirosis, meningococcal disease, and dengue fever, the present study aimed to investigate evidence of infection by Rickettsia spp. (R. rickettsii and R. parkeri) in patients with negative serology and no confirmation for such diseases in residents of Atibaia and Bragança Paulista.
METHODS
This is a descriptive study of the prevalence of antibodies against R. rickettsii and R. parkeri in negative serum samples for dengue virus (DV), leptospirosis, and meningococcal disease, stored in a public health reference laboratory, Adolfo Lutz Institute in Campinas, State of São Paulo.
The inclusion criteria of the samples were as follows: collected 1) from residents of the municipalities of Atibaia and Bragança Paulista, in 2014 and in the period potentially associated with the increase of antibodies after the first symptoms, and 2) from patients with non-confirmed initial suspicion of dengue fever and/or leptospirosis and meningococcal disease.
Bragança Paulista and Atibaia were chosen because they are considered non-endemic for BSF, because there is evidence of R. rickettsii transmission in horses, and because they are located in the PCJ Basin, the most endemic region of BSF in the State of São Paulo1212. Souza CE, Camargo LB, Pinter A, Donalisio MR. High seroprevalence for Rickettsia rickettsii in equines suggests risk of human infection in silent areas for the Brazilian spotted fever. PloSOne 2016; 11:e0153303..
The samples were tested by indirect immunofluorescence assay (IFA), taking into consideration that the period in which antibody titers increased varied approximately between 7 and 10 days after the onset of BSF symptoms. However, the only samples processed were those in which the period between the onset of symptoms and sample collection was equal or superior to 6 days.
For Atibaia, we identified 155 samples that could be subjected to analysis by IFA, of which 10 were negative for leptospirosis, 143 for dengue virus, and 2 for meningococcal disease in counter-immunoelectrophoresis. For Bragança Paulista, we identified 258 samples, of which 20 were negative for leptospirosis, 234 for dengue virus, and 4 for meningococcal disease in counter-immunoelectrophoresis.
Indirect immunofluorescence assay was conducted by using 12 hole blades sensitized with R. rickettsii and R. parkeri antigens and stored at -20° C. Sera were diluted in phosphate-buffered saline, starting from a 1:64 dilution (315µL of phosphate-buffered saline and 5µL of serum). A positive and a negative control were included in each slide. The slides were incubated in humid chambers in bacteriological incubators at 37°C for 30 minutes. The slides were then washed with a buffer and distilled water and left to dry naturally.
Conjugates of human immunoglobulin G (IgG) antibody, diluted to a ratio of 1:80 (15µL of conjugate in 1,185µL of Evans blue) were then used to perform the indirect IFA as described by Moares Filho et al.1313. Moraes-Filho J, Pinter A, Pacheco RC, Gutmann TB, Barbosa SO, Gonzáles MAR, et al. New epidemiological data on Brazilian spotted fever in endemic area of the State of São Paulo, Brazil. Vector Borne Zoonotic Dis 2009; 9 (supl I):73-78.. Sera that reacted to the 1:64 dilution were titrated to a dilution of 1:512. A real-time polymerase chain reaction (PCR) was performed for samples from patients that died (n = 3) and those with initial suspicion of meningococcal disease (n = 6), since qPCR is primarily indicated for severe cases.
Immunofluorescence-positive samples (IgG) from the 1:64 dilution were also processed for titration of immunoglobulin M (IgM) with the use of PCR. The clinical symptoms of positive patients were obtained from reporting forms of epidemiological surveillance databases of the municipalities.
Ethicals considerations
This project was approved by the Research Ethics Committee of the Faculdade de Ciências Médicas under the process reference: CAAE 35540114.3.0000.5404 and by the Ethics Committee of the Institute Adolfo Lutz of São Paulo under the process reference: CAAE 35540114.3.3001.0059.
RESULTS
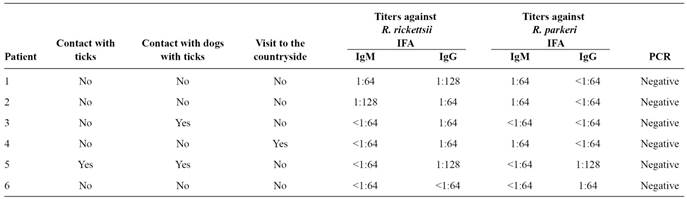
A total of 413 samples were taken from patients from the municipalities of Atibaia (n = 155) and Bragança Paulista (n = 258). Using IFA, we obtained six positive samples, 1.3% (2/155) in Atibaia and 1.6% (4/258) in Bragança Paulista (Table 1).
Among the reactive samples in Atibaia, most initial suspicions were for dengue fever (5/6), with one suspected case of leptospirosis. The average age of the patients was 34.3 years, including four men and two women. The collection period ranged from 6 to 13 days after the onset of symptoms.
Samples from deceased patients (n = 3) and those with early suspicion of meningococcal disease (n = 6) returned negative results using qPCR. The six positive IFA samples were also processed by PCR and tested negative (Table 1).
Samples with positive IFA results were collected at different times of the year: March, April, May, June, September, and November. Most (4, or 80%), were in the first semester in both municipalities, a period in which the highest occurrence of dengue feverand leptospirosis were observed.
Signs and symptoms presented by patients whoseserum was reactive to IFA are described in Table 2. We did not detect the appearance of petechiae, skin rash, or hemorrhagic manifestations in any of the six patients. Patient 6 reported antibiotic usage (penicillin) during symptoms.Patients 3 and 5 reported contact with dogs with ticks during the illness period, and patient 4 had been in the countryside (Table 1).
DISCUSSION
The seroprevalence (IFA) for Rickettsia spp. in negative samples for dengue virus, leptospirosis, and meningococcal disease in Atibaia and Bragança Paulista were 1.3% and 1.6%, respectively. Six samples titrated against R. rickettsii and R. parkeri showed detectable titers (≥ 1:64) and were considered positive for rickettsial infections. We identified two patients in Atibaia with titers of IgM and IgG reactive against R. rickettsii, one presenting an IgG titer of 1:128 and the other an IgM titer of 1:128. In a patient from Bragança Paulista, we observed IgG titers of 1:128 (R. rickettsii and R. parkeri), suggesting exposure to and possible infection with Rickettsia. However, when considering that the clinical picture resulted from an infection by Rickettsia spp., the occurrence of titers considered borderline (1:64 in patients 3, 4, and 6) can be explained by the early collection.
We chose to test for more than one antigen owing to the suspicion of a possible association of R. parkeri to milder clinical cases, similar to what has been observed in the States of Santa Catarina55. Angerami RN, Silva AMR, Nascimento EMM, Colombo S, Wada MY, Santos FCP, et al. Brazilian spotted fever: two faces of a same disease? A comparative study of clinical aspects between an old and a new endemic area in Brazil. Clin Microbiol Infect 2009; 15 (suppl 2):207-208. and São Paulo66. Spolidorio MG, Labruna MB, Mantovani E, Brandão PE, Richtzenhain LJ, Yoshinari NH. Novel spotted fever group rickettsiosis, Brazil. Emerg Infect Dis 2010; 16:521-523.. In addition, we found individuals with prior contact with Rickettsia, although we were unable to confirm whether the contact occurred in the PCJ Basin or elsewhere. Previously published data suggest circulationof Rickettsiain other municipalities in the same area1414. Lemos ERS, Melles HHB, Colombo S, Machado RD, Coura JR, Guimarães MAA, et al. Primary isolation of spotted fever group rickettsiae from Amblyomma cooperi collected from Hydrochaeris hydrochaeris in Brazil. Mem Inst Oswaldo Cruz 1996; 91:273-275.) (1515. Labruna MB, Whitworth T, Horta MC, Bouyer DH, McBride JW, Pintes A, et al. Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of São Paulo, Brazil, where Brazilian spotted fever is endemic. J ClinMicrobiol 2004; 42:90-98..Cross-reaction between Rickettsia spp. is possible, especially among R. rickettsii and R. parkeri; this may frequently hinder accurate identification of the etiologic agent in an infection1616. Paddock CD. Rickettsia parkerias a paradigm for multiple causes of tick-borne spotted fever in the Western Hemisphere. Ann N Y Acad Sci 2005; 1063:315-326.. Thus, serological test using only R. rickettsii antigen can generate not only false negative but also false positive results of infection caused by other bacteria of spotted fever group, thus limiting the clinical diagnosis and understanding of Rickettsia distribution. In a serological study of patients presenting with spotted fever in North Carolina, USA, cases of infection with R. parkeri and R. amblyommii were identified in patients with an initial suspicion of R. rickettsii1717. Vaughn MF, Delisle J, Johnson J, Daves G, Williams C, Reber J, et al. Soroepidemiologic study of human infections with Spotted Fever Group Rickettsiae in North Carolina. J Clin Microbiol 2014; 52:3960-3966..
While the observed titers in patients are not necessarily associated with the clinical picture (in general, flu-like or dengue-like), in three patients (3, 4, and 5) there was an epidemiological history that could explain possible exposure to the vector and subsequent Rickettsia infection.
Contact with dogs parasitized by A. aureolatum or Rhipicephalus sanguineus and environments where these dogs present are potentially relevant epidemiological factors to BSF transmission, since these species of ticks may also parasitize humans1818. Serra-Freire NM, Sena LMM, Borsoi ABP. Parasitismo Humano por carrapatos na Mata Atlântica, Rio de Janeiro, Brasil. Entomol Brasilis 2011; 4:67-72.. Amblyomma aureolatum is considered the main vector of R. rickettsii in the São Paulo region1919. Pinter A, Dias RA, Gennari SM, Labruna MB. Study of the seasonal dynamics, life cycle and host specificity of Amblyomma aureolatum (Acari: Ixodidae). J Med Entomol 2004; 41:324-332.) and has been reported to frequently parasitize dogs in counties (Nazaré Paulista and Mairiporã) close to the study region2020. Ogrzewalska M, Saraiva DG, Moraes-Filho J, Martins TF, Costa FB, Pinter A, et al. Epidemiology of Brazilian spotted fever in the Atlantic Forest, state of São Paulo, Brazil. Parasitol 2012; 139:1283-1300.. Moreover, based on the molecular characterization of R. rickettsii in R. sanguineus in Brazil, the possible risk of an active, or even an indirect, participation of R. sanguineusin the transmission of R. rickettsii to humans cannot be excluded2121. Cunha NC, Fonseca AH, Rezende J, Rozental T, Favacho ARM, Barreira JD, et al. First identification of natural infection of Rickettsia rickettsii in the Rhipicephalus sanguineus tick, in the state of Rio de Janeiro. Pesq Vet Bras 2009; 29:105-108.. In addition, a potential risk of transmission of BSF or other rickettsial infectionsviaparasitized dogs cannot be ruled out. In this respect, there is a recent report regarding a Rocky Mountain spotted fever outbreak among employees of an animal shelter, where the authors, despite the inability to confirm the involved tick species, point out the risk of infection to humans when in close contact with dogs2222. Rozental T, Ferreira MS, Gomes R, Costa CM, Barbosa PR, Bezerra IO, et al. A cluster of Rickettsia rickettsii infection at an animal shelter in an urban area of Brazil. Epidemiol Infect 2015; 143:2446-2450..
Another justification is that false positive results would be possible due to other acute infections. Such a situation could explain the titers observed in subjects 1, 2, and 6, who had no epidemiological history. A titer of 1:128 in patient 1 calls attention to the limitation of IFA as an isolated result, without considering the presence or absence of infection risk factors. Moreover, it reinforces the need to consider clinical, laboratorial, and epidemiological evidence in medical care and epidemiological surveillance.
In the patients evaluated, the clinical picture led to suspicion of dengue fever, leptospirosis, and meningococcal disease. This situation has been previously observed in a 2001 report of an investigation carried out in Paulínia, a municipality of the State of São Paulo, where it was observed that these diseases were among the initial diagnostic hypotheses associated with an outbreak of BSF2323. Santos ED, Hironi ST, Sabioni CM, Ascenzi ED, Buzzar MR, Alves MJCP, et al. Surto de rickettsiose do grupo febre maculosa entre pescadores, Paulínia, São Paulo, 2001. Boletim Eletrônico Epidemiológico FUNASA 2002; 2:7.. There is evident difficulty in distinguishing between rickettsial diseases and other pathologies, including dengue fever, especially in endemic periods2424. Kemp B. Os casos que não se confirmaram como dengue durante a epidemia de dengue no município de Campinas/SP, 2002. Tese de Doutorado. Campinas: Faculdade de Ciências Médicas da Universidade Estadual de Campinas; 2005. 202p.; leptospirosis; and other febrile diseases, including exanthematic and hemorrhagic fever. Thus, cases of rickettsial diseases may exist, but are not recognized in the municipalities. Indeed, these are considered silent areas for BSF, in which there was intense circulation of dengue virus in 2014.
Moreover, there was not an asymptomatic infection by R. rickettsii in Brazil, since R. rickettsiiis usually associated with severe and highly lethal outcomes; nevertheless, the presence of reactive titers higher than 1:64 may suggest infection. The infection may be due to contact with Rickettsia spp. or other Spotted Fever Group rickettsia, including R. parkeri infection, as shown in the current study.
The period of increased incidence of transmission by A. sculptum is between late April to early May to late September and early October2525. Labruna MB, Kasai N, Ferreira F, Faccini JLH, Gennari SM. Seasonal dynamics of ticks (Acari: Ixodidae) on horses in the state of São Paulo, Brazil. Vet Parasitol 2002; 105:65-77.. Confirmed cases were also observed during months in which epidemic periods of dengue fever have been reported1313. Moraes-Filho J, Pinter A, Pacheco RC, Gutmann TB, Barbosa SO, Gonzáles MAR, et al. New epidemiological data on Brazilian spotted fever in endemic area of the State of São Paulo, Brazil. Vector Borne Zoonotic Dis 2009; 9 (supl I):73-78.. However, in the current study, the IFA-positive samples were collected in different months, mostly in the first semester.
Several study limitations could be highlighted, including serological testing of a single sample, which does not allow for a diagnostic confirmation of BSF according to current criteria. It is noteworthy, however, that just prior to 2004, single samples with IgG titers equal to 1:64 met criteria for laboratory confirmation, which would confirm cases of BSF. Also remarkable is that positive titers of IFA suggested a possible acute infection in the early phase or a previous history of rickettsial diseases in six patients with negative serology for important differential diagnoses. Such information is supported by the fact that they are resident of municipalities where seropositivity in horses for R. rickettsii was detected and where the primary host (capybara) and the vector A. sculptum were present1212. Souza CE, Camargo LB, Pinter A, Donalisio MR. High seroprevalence for Rickettsia rickettsii in equines suggests risk of human infection in silent areas for the Brazilian spotted fever. PloSOne 2016; 11:e0153303.. In the same study, in municipalities considered endemic to BSF, such as Piracicaba, 54% of investigated horses tested positive. Notably, horses are considered sentinels for the occurrence of human cases, even when tested in a single sample, and they are considered an early indicator of exposure to infected vectors1010. Sangioni LA, Horta MC, Vianna MCB, Gennari SM, Soares RM, Galvão MAM, et al. Rickettsial infection in animals and Brazilian spotted fever endemicity. Emerg Infect Dis 2005; 11:265-270.. A study carried out in Pedreira, an endemic municipality in the State of São Paulo, showed 77.3% IFA-positivity in investigated horses2626. Horta MC, Labruna MB, Sangioni LA, Vianna MC, Gennari SM, Galvão MA, et al. Prevalence of antibodies to spotted fever group rickettsiae in humans and domestic animals in a Brazilian spotted fever- endemic area in the state of São Paulo, Brazil: serologic evidence for infection by Rickettsia rickettsii and another spotted fever group Rickettsia. Am J Trop Med Hyg 2004; 71:93-97.. In three regions considered not endemic, there were no horses with reactive IFA sera, further suggesting the role of horses as a sentinel for Rickettsia circulation1010. Sangioni LA, Horta MC, Vianna MCB, Gennari SM, Soares RM, Galvão MAM, et al. Rickettsial infection in animals and Brazilian spotted fever endemicity. Emerg Infect Dis 2005; 11:265-270.. Nevertheless, some authors suggest that different species of Rickettsia can circulate naturally in non-endemic regions without the occurrence of human cases; these would be considered accidental hosts. The method and frequency by which individuals are exposed to transmission foci could justify the absence or low prevalence of the infection in humans2727. Lemos ERS, Machado RD, Coura JR, Guimarães MAAM, Chagas N. Epidemiological aspects of the Brazilian spotted fever: serological survey of dogs and horses in a endemic area in the state of São Paulo, Brazil. Rev Inst Med Trop São Paulo 1996; 38:427-430..
Bragança Paulista, and Atibaia are municipalities that are part of the PCJ river basin. This region presents environmental and ecological characteristics that are propitious for Rickettsia circulation and the presence of hosts and vectorsthat favor the occurrence of the disease in humans. Knowing the epidemiological determinants of risk (areas of transmission, type of vegetation, exposure to vectors, and contact with host animals) is essential to increase clinical suspicion and guide surveillance activities. Besides detecting infection in sentinel animals, the evidence of individuals exposed to risk factors with positive serology for Rickettsia may ultimately contribute to calling attention to the disease and improving the ability to detect suspected cases.
Expanding the area of serological testing of sentinel animals may increase the awareness and suspicion of BSF in patients suspected of dengue fever, leptospirosis, and meningococcal disease. Suspicion is essential for early, timely, and appropriate laboratory testing (with collection of paired samples) for early diagnostic in endemic and non-endemic areas. Furthermore, other suspected tick-borne diseases should be considered, for which there is no structured surveillance.
In conclusion, based on serological results and reported clinical and epidemiological antecedents, there is evidence of circulation of Rickettsia spp. in the studied municipalities, which were not previously classified as being endemic to BSF. Additionally, with consideration of IgM detection among acute febrile patients, the circulation of potentially pathogenic rickettsial species cannot be excluded.
Although a definitive confirmation of human spotted fever cases is not possible with currently established surveillance system criteria, the results presented here may serve as a warning to epidemiological surveillance and health services to improve epidemiological surveillance strategies and increase clinical suspicion for BSF and other rickettsial diseases. In particular, this would ensure the proper case detection and laboratory investigation of patients with possible rickettsial infections that, eventually, may have not been routinely identified.
Acknowledgments
The author acknowledge Valmir Roberto Andrade, Superintendência de Controle de Endemias- Campinas and João Fred, Centro de Vigilância Epidemiológica, Campinas.
References
-
1Ministério da Saúde. Secretaria de Vigilância em Saúde. Guia de Vigilância epidemiológica/Ministério da Saúde. 6 edition. Brasília: Ministério da Saúde; 2005. 816 p.
-
2Walker DH. Rocky Mountain spotted fever: a disease in need of microbiological concern. Clin Microbiol Rev 1989; 2:227-240.
-
3Krawczak FS, Nieri-Bastos FA, Nunes FP, Soares JF, Moraes-Filho J, Labruna MB. Rickettsial infection in Amblyomma cajennenense ticks and capybaras (Hydrochoerus hydrochaeris) in a Brazilian spotted fever- endemic area. Parasit Vectors 2014; 7:7.
-
4Pinter A, Labruna MB. Isolation of Rickettsia rickettsii and Rickettsia belii in cell culture from the tick Amblyomma aureolatum in Brazil. Ann N Y AcadSci 2006; 1078:523-529.
-
5Angerami RN, Silva AMR, Nascimento EMM, Colombo S, Wada MY, Santos FCP, et al. Brazilian spotted fever: two faces of a same disease? A comparative study of clinical aspects between an old and a new endemic area in Brazil. Clin Microbiol Infect 2009; 15 (suppl 2):207-208.
-
6Spolidorio MG, Labruna MB, Mantovani E, Brandão PE, Richtzenhain LJ, Yoshinari NH. Novel spotted fever group rickettsiosis, Brazil. Emerg Infect Dis 2010; 16:521-523.
-
7Souza CE, Moraes-Filho J, Orgzewalska M, Uchoa FC, Horta MC, Souza SS, et al. Experimental infection of capybaras (Hydrochoerus hydrochaeris) by Rickettsia rickettsii and evaluation of the transmission of the infection to ticks Amblyomma cajennense Vet Parasit 2009; 161:116-121.
-
8Horta MC, Moraes-Filho J, Casagrande RA, Saito TB, Rosa SC, Ogrzewalska M, et al. Experimental infection of opossums Didelphis aurita by Rickettsia rickettsii and evaluation of the transmission of the infection to ticks Amblyomma cajennense Vector Borne Zoonotic Dis 2009; 9:109-118.
-
9Piranda EM, Faccini JLH, Pinter A, Pacheco RC, Cançado PHD, Labruna MB. Experimental infection of Rhipicephalus sanguineus ticks with the bacterium Rickettsia rickettsii, using experimentally infected dogs. Vector Borne Zoonotic Dis 2011; 11:29-36.
-
10Sangioni LA, Horta MC, Vianna MCB, Gennari SM, Soares RM, Galvão MAM, et al. Rickettsial infection in animals and Brazilian spotted fever endemicity. Emerg Infect Dis 2005; 11:265-270.
-
11Pinter A, Horta MC, Pacheco RC, Moraes-Filho J, Labruna MB. Serosurvey of Rickettsia spp. in dogs and humans from an endemic area for Brazilian spotted fever in the State of São Paulo, Brazil. Cad Saude Publica 2009; 24:247-252.
-
12Souza CE, Camargo LB, Pinter A, Donalisio MR. High seroprevalence for Rickettsia rickettsii in equines suggests risk of human infection in silent areas for the Brazilian spotted fever. PloSOne 2016; 11:e0153303.
-
13Moraes-Filho J, Pinter A, Pacheco RC, Gutmann TB, Barbosa SO, Gonzáles MAR, et al. New epidemiological data on Brazilian spotted fever in endemic area of the State of São Paulo, Brazil. Vector Borne Zoonotic Dis 2009; 9 (supl I):73-78.
-
14Lemos ERS, Melles HHB, Colombo S, Machado RD, Coura JR, Guimarães MAA, et al. Primary isolation of spotted fever group rickettsiae from Amblyomma cooperi collected from Hydrochaeris hydrochaeris in Brazil. Mem Inst Oswaldo Cruz 1996; 91:273-275.
-
15Labruna MB, Whitworth T, Horta MC, Bouyer DH, McBride JW, Pintes A, et al. Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of São Paulo, Brazil, where Brazilian spotted fever is endemic. J ClinMicrobiol 2004; 42:90-98.
-
16Paddock CD. Rickettsia parkerias a paradigm for multiple causes of tick-borne spotted fever in the Western Hemisphere. Ann N Y Acad Sci 2005; 1063:315-326.
-
17Vaughn MF, Delisle J, Johnson J, Daves G, Williams C, Reber J, et al. Soroepidemiologic study of human infections with Spotted Fever Group Rickettsiae in North Carolina. J Clin Microbiol 2014; 52:3960-3966.
-
18Serra-Freire NM, Sena LMM, Borsoi ABP. Parasitismo Humano por carrapatos na Mata Atlântica, Rio de Janeiro, Brasil. Entomol Brasilis 2011; 4:67-72.
-
19Pinter A, Dias RA, Gennari SM, Labruna MB. Study of the seasonal dynamics, life cycle and host specificity of Amblyomma aureolatum (Acari: Ixodidae). J Med Entomol 2004; 41:324-332.
-
20Ogrzewalska M, Saraiva DG, Moraes-Filho J, Martins TF, Costa FB, Pinter A, et al. Epidemiology of Brazilian spotted fever in the Atlantic Forest, state of São Paulo, Brazil. Parasitol 2012; 139:1283-1300.
-
21Cunha NC, Fonseca AH, Rezende J, Rozental T, Favacho ARM, Barreira JD, et al. First identification of natural infection of Rickettsia rickettsii in the Rhipicephalus sanguineus tick, in the state of Rio de Janeiro. Pesq Vet Bras 2009; 29:105-108.
-
22Rozental T, Ferreira MS, Gomes R, Costa CM, Barbosa PR, Bezerra IO, et al. A cluster of Rickettsia rickettsii infection at an animal shelter in an urban area of Brazil. Epidemiol Infect 2015; 143:2446-2450.
-
23Santos ED, Hironi ST, Sabioni CM, Ascenzi ED, Buzzar MR, Alves MJCP, et al. Surto de rickettsiose do grupo febre maculosa entre pescadores, Paulínia, São Paulo, 2001. Boletim Eletrônico Epidemiológico FUNASA 2002; 2:7.
-
24Kemp B. Os casos que não se confirmaram como dengue durante a epidemia de dengue no município de Campinas/SP, 2002. Tese de Doutorado. Campinas: Faculdade de Ciências Médicas da Universidade Estadual de Campinas; 2005. 202p.
-
25Labruna MB, Kasai N, Ferreira F, Faccini JLH, Gennari SM. Seasonal dynamics of ticks (Acari: Ixodidae) on horses in the state of São Paulo, Brazil. Vet Parasitol 2002; 105:65-77.
-
26Horta MC, Labruna MB, Sangioni LA, Vianna MC, Gennari SM, Galvão MA, et al. Prevalence of antibodies to spotted fever group rickettsiae in humans and domestic animals in a Brazilian spotted fever- endemic area in the state of São Paulo, Brazil: serologic evidence for infection by Rickettsia rickettsii and another spotted fever group Rickettsia Am J Trop Med Hyg 2004; 71:93-97.
-
27Lemos ERS, Machado RD, Coura JR, Guimarães MAAM, Chagas N. Epidemiological aspects of the Brazilian spotted fever: serological survey of dogs and horses in a endemic area in the state of São Paulo, Brazil. Rev Inst Med Trop São Paulo 1996; 38:427-430.
Publication Dates
-
Publication in this collection
Sep-Oct 2016
History
-
Received
09 June 2016 -
Accepted
14 Sept 2016