Abstract
INTRODUCTION
In order to detect Trypanosoma cruzi and determine the genetic profiles of the parasite during the chronic phase of Chagas disease (ChD), parasitological and molecular diagnostic methods were used to assess the blood of 91 patients without specific prior treatment.
METHODS
Blood samples were collected from 68 patients with cardiac ChD and 23 patients with an indeterminate form of ChD, followed by evaluation using blood culture and polymerase chain reaction. T . cruzi isolates were genotyped using three different genetic markers.
RESULTS:
Blood culture was positive in 54.9% of all patients, among which 60.3% had the cardiac form of ChD, and 39.1% the indeterminate form of ChD. There were no significant differences in blood culture positivity among patients with cardiac and indeterminate forms. Additionally, patient age and clinical forms did not influence blood culture results. Polymerase chain reaction (PCR) was positive in 98.9% of patients, although comparisons between blood culture and PCR results showed that the two techniques did not agree. Forty-two T . cruzi stocks were isolated, and TcII was detected in 95.2% of isolates. Additionally, one isolate corresponded to TcIII or TcIV, and another corresponded to TcV or TcVI.
CONCLUSIONS
Blood culture and PCR were both effective for identifying T. cruzi using a single blood sample, and their association did not improve parasite detection. However, we were not able to establish an association between the clinical form of ChD and the genetic profile of the parasite.
Keywords:
Trypanosoma cruzi; Chagas disease; Blood culture; Polymerase chain reaction; Genetic diversity
INTRODUCTION
Chagas disease (ChD) is primarily a disease of poverty, disproportionately affecting the rural poor and immigrant populations predominantly in developing countries and is therefore considered a neglected disease 11. Tarleton RL, Curran JW. Is Chagas Disease Really the ''New HIV/AIDS of the Americas''? PLoS Negl Trop Dis. 2012;6(10):e1861. . Clinically, ChD exhibits a primary acute phase characterized by variable signs and symptoms ranging from mild to severe, and patent parasitemia is detected in direct blood tests. This phase progresses to the chronic phase, which persists throughout the patient’s life 22. Laranja FS, Dias E, Nobrega G, Miranda A. Chagas' disease: a clinical, epidemiologic, and pathologic study. Circulation. 1956;14(6):1035-60.,33. Dias JCP. Acute Chagas' disease. Mem Inst Oswaldo Cruz. 1984;79:(Suppl) 85-91. . In this phase, detection of the parasite is rare due to subpatent and transient parasitemia. Approximately 60-70% of individuals will never develop apparent disease and are characterized as indeterminate; these patients present positive serologic reactivity for T. cruzi , which can sometimes be identified by hemoculture, xenodiagnoses, and polymerase chain reaction (PCR) 44. Chiari E, Dias JCP, Lana M, Chiari CA. Hemocultures for the parasitological diagnostic of human chronic Chagas' disease. Rev Soc Bras Med Trop 1989;22(1):19-23.
5.Ávila HA, Pereira JB, Thiemann O, Paiva E, Degrave W, Morel CM, et al. Detection of Trypanosoma cruzi in blood specimens of chronic chagasic patients by polymerase chain reaction amplification of kinetoplast minicircle DNA: comparison with serology and xenodiagnosis. J Clin Microbiol. 1993;31(9):2421-6.
6. Britto C, Cardoso MA, Ravel C, Santoro A, Borges-Pereira J, Coura JR, et al. Trypanosoma cruzi: parasite detection and strain discrimination in chronic chagasic patients from Northeastern Brazil using PCR amplification of kinetoplast DNA and nonradioactive hybridization. Exp Parasitol. 1995;81 (4):462-71.-77. Gomes ML, Macedo AM, Vago AR, Pena SDJ, Galvão LMC, Chiari E. Trypanosoma cruzi: optimization of polymerase chain reaction for detection in human blood. Exp Parasitol. 1998;88(1):28-33. . Patients within this clinical form present normal 12-lead electrocardiogram (ECG) results, as well as normal radiological examinations of the chest, esophagus, and colon 88. Andrade SG. Morphological and behavioral characterization of Trypanosoma cruzi strains. Rev Soc Bras Med Trop. 1985;18(supl):39-46.
9. Ribeiro ALP, Rocha MOC. Forma indeterminada da doença de Chagas: considerações acerca do diagnóstico e do prognóstico. Rev Soc Bras Med Trop. 1998;31(3):301-14.
10. Macêdo V. Indeterminate form of Chagas disease. Mem Inst Oswaldo Cruz. 1999;94(1):311-6.-1111. Rassi Jr A, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375(9723):1388-402. . Moreover, 10-30 years after initial infection, some patients may develop the cardiac, digestive, and/or cardiodigestive clinical forms 1212. Dias JCP. Natural history of Chagas' disease. Arq Bras Cardiol. 1995;65(4):359-66. . There are major variations in clinical manifestations and morbidities among patients with ChD 1313. Coura JR, Anunziato N, Willcox HPF. Chagas' disease morbidity. I - Study of cases originating in various states of Brazil, observed in Rio de Janeiro. Mem Inst Oswaldo Cruz. 1983;78(3):363-72.,1414. Coura JR, Abreu LL, Pereira JB, Willcox H. Morbidity in Chagas' disease. IV. Longitudinal study of 10 years in Pains and Iguatama, Minas Gerais, Brazil]. Mem Inst Oswaldo Cruz. 1985;80(1):73-80. ; these differences can be attributed in part to the effectiveness of the immune response, genetic aspects of infected individuals, and the complex structure of the T. cruzi population 1515. Miles M, Cedillos R, Povoa M, De Souza A, Prata A, Macedo V. Do radically dissimilar Trypanosoma cruzi strains (zymodemes) cause Venezuelan and Brazilian forms of Chagas disease? Lancet 1981;317(8234):1338-40.,1616. Tibayrenc M, Ayala FJ. Evolutionary genetics of Trypanosoma cruzi and Leishmania. Microbes Infect. 1999;1(6):465-72. .
Trypanosoma cruzi exhibits a high degree of intraspecific variability, as detected by biological, biochemical, immunological, and genetic markers 1717. Macedo AM, Pimenta JR, Aguiar RS, Melo AIR, Chiari E, Zingales B, et al. Usefulness of microsatellite typing in population genetic studies of Trypanosoma cruzi. Mem Inst Oswaldo Cruz. 2001;96(3):407-13. . Based on different molecular markers, the Second Satellite Meeting recommended that T. cruzi should be classified into six discrete typing units (DTUs; T. cruzi I-VI) 1818. Zingales B, Andrade SG, Briones MRS, Campbell DA, Chiari E, Fernandes O, et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz. 2009;104(7):1051-4. . The biological characteristics of T. cruzi strains and clones, and particularly their tissue tropism, may play important roles as determinants of ChD and its clinical forms 1919. Coura JR. Chagas disease: what is known and what is needed-A background article. Mem Inst Oswaldo Cruz. 2007;102(Suppl 1):113-22. . The diagnosis of T . cruzi infection should be directed based on the phase of infection. During the acute phase, parasitological methods should be prioritized due to the high number of trypomastigotes in peripheral circulation. In the chronic phase, T. cruzi can only be detected from a limited number of patients by parasitological methods, such as xenodiagnosis or blood culture 44. Chiari E, Dias JCP, Lana M, Chiari CA. Hemocultures for the parasitological diagnostic of human chronic Chagas' disease. Rev Soc Bras Med Trop 1989;22(1):19-23.,2020. Bronfen E, Rocha FSA, Machado GBN, Perillo MM, Romanha AJ, Chiari E. Isolation of Trypanosoma cruzi samples by xenodiagnosis and hemoculture from patients with chronic Chagas' disease. Mem Inst Oswaldo Cruz. 1989;84(2):237-40.
21. Galvão LMC, Nunes RMB, Cançado JR, Brener Z, Krettli AU. Lytic antibody titre as a means of assessing cure after treatment of Chagas disease: a 10 years follow-up study. Trans R SocTrop Med Hyg.1993;87(2):220-3.
22. Castro AM, Luquetti AO, Rassi A, Rassi GG, Chiari E, Galvão LMC. Blood culture and polymerase chain reaction for the diagnosis of the chronic phase of human infection with Trypanosoma cruzi. Parasitol Res. 2002;88(10):894-900.-2323. Meira WSF, Galvão LMC, Gontijo ED, Machado-Coelho GL, Norris KA, Chiari E. Trypanosoma cruzi recombinant complement regulatory protein: a novel antigen for use in an enzyme-linked immunosorbent assay for diagnosis of Chagas' disease. J Clin Microbiol. 2002;40(10):3735-40. . Therefore, the development of additional molecular methods is necessary for parasite detection in patients with unclear serology and for better evaluation of the roles of specific trypanocidal treatments in patients with established Chagas’ cardiomyopathy 2424. Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi Jr A, Rosas F, et al. Randomized trial of benznidazole for chronic Chagas' cardiomyopathy. N Engl J Med. 2015;373(14):1295-306.
25. Marin-Neto JA, Rassi Jr A, Avezum Jr A, Mattos AC, Rassi A. The BENEFIT trial: testing the hypothesis that trypanocidal therapy is beneficial for patients with chronic Chagas heart disease. Mem Inst Oswaldo Cruz. 2009;104(Suppl. I):319-24.-2626. Rassi Jr A, Marin-Neto JA, Rassi A. Chronic Chagas cardiomyopathy: a review of the main pathogenic mechanisms and the efficacy of aetiological treatment following the BENznidazole Evaluation for Interrupting Trypanosomiasis (BENEFIT) trial. Mem Inst Oswaldo Cruz. 2017;112(3):224-35. . In addition, these tools are important for studies involving biological and genetic characterization of the parasite.
Accordingly, the aim of this study was to detect circulating parasites by conventional PCR and blood culture (BC) in samples from patients with two defined and polar clinical forms of ChD, followed by evaluation of the genetic profiles of freshly isolated T. cruzi .
METHODS
Patients
This study included 91 patients in the chronic phase of ChD from endemic areas within the state of Minas Gerais, Brazil, who were identified and selected at the Referral Outpatient Center for Chagas Disease at the Clinical Hospital of the Universidade Federal de Minas Gerais (UFMG), Brazil, by Prof. Dr Manoel Otávio da Costa Rocha. All patients had at least two different reactive serological tests for T. cruzi and fulfilled eligibility criteria. Patients were subjected to a standard screening protocol that included medical history, physical examination, ECG, laboratory and chest X-ray examinations, and echocardiography evolution and characterized according the clinical classification of chronic Chagas cardiomyopathy, as previously reported 2727. Rocha MO, Ribeiro AL, Teixeira MM. Clinical management of chronic Chagas cardiomyopathy. Front Biosci. 2003;8:e44-54. . None of the patients were undergoing etiological treatment nor had been previously treated for T. cruzi infection.
Ethical considerations
The study protocol was approved by the Research Ethic Committee of the Universidade Federal de Minas Gerais (protocol COEP-ETHIC 0559.0.203.000-11/2012/UFMG), and all participants provided written informed consent.
Blood culture
BC was performed with 30mL venous blood collected in heparinized vacuum tubes, and red cells were separated from the plasma by centrifugation at 300 × g for 10 min at 4°C 44. Chiari E, Dias JCP, Lana M, Chiari CA. Hemocultures for the parasitological diagnostic of human chronic Chagas' disease. Rev Soc Bras Med Trop 1989;22(1):19-23. . The packed red blood cells were washed once, resuspended in 6mL liver infusion tryptose (LIT) medium, mixed, and distributed into six plastic tubes (Falcon, USA) containing 3mL LIT. The plasma supernatant was centrifuged at 900 × g for 20 min at 4°C, and 5mL LIT was added to the pellet. All tubes were maintained at 28°C, mixed gently twice a week, and examined monthly for up to 120 days. Microscopic examination was carried out in 10-µL aliquots of each preparation under 22-mm 2 coverslips at a magnification of 400×.
Polymerase chain reaction assays
PCR assays were performed according to previously optimized protocols 77. Gomes ML, Macedo AM, Vago AR, Pena SDJ, Galvão LMC, Chiari E. Trypanosoma cruzi: optimization of polymerase chain reaction for detection in human blood. Exp Parasitol. 1998;88(1):28-33. that were standardized and validated for the diagnosis of chronic ChD 2828. Schijman AG, Bisio M, Orellana L, Sued M, Duffy T, Jaramillo AMM, et al. International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. PLoS Negl Trop Dis. 2011;5(1):e931. . Blood samples (5mL) were collected from each patient and immediately transferred to sterile tubes containing guanidine/ethylenediaminetetraacetic acid buffer (pH 8.0, 5mL) 2929. Ávila HA, Sigman DS, Cohen LM, Millikan RC, Simpson L. Polymerase chain reaction amplification of Trypanosoma cruzi kinetoplast minicircle DNA isolation from whole blood lysates: diagnosis of chronic Chagas' disease. Mol Biochem Parasitol. 1991;48(2):211-21. . The samples were stored for 5-7 days at room temperature, boiled at 100°C for 15 min, and stored at 4°C, after which 200-µL aliquots were collected for deoxyribonucleic acid (DNA) extraction 77. Gomes ML, Macedo AM, Vago AR, Pena SDJ, Galvão LMC, Chiari E. Trypanosoma cruzi: optimization of polymerase chain reaction for detection in human blood. Exp Parasitol. 1998;88(1):28-33.,3030. Britto C, Cardoso MA, Wincker P, Morel CM. A simple protocol for the physical cleavage of Trypanosoma cruzi kinetoplast DNA present in blood samples and its use in polymerase chain reaction (PCR)-based diagnosis of chronic Chagas' disease. Mem Inst Oswaldo Cruz. 1993;88(1):171-2. . Positive controls (ChD patients), negative controls (patients without ChD from nonendemic areas), T. cruzi DNA corresponding to TcI-VI, and reagents without DNA were included.
Trypanonoma cruzi isolation
Positive BCs in LIT medium were maintained in individual tubes for a short period of time without passage, and LIT medium was added every 10-15 days for a maximum of 8 weeks. To obtain epimastigote masses (1 × 10 6 /mL), the cultures were washed twice in Krebs-Ringer-Tris buffer (pH 7.2). Cultures were then concentrated by centrifugation at 900 × g and 4°C for 10 min, and wet masses were stored at -20°C for DNA extraction 3131. Macedo AM, Martins MS, Chiari E, Pena SDJ. DNA fingerprinting of Trypanosoma cruzi: a new tool for characterization of strains and clones. Mol Biochem Parasitol. 1992;55(1-2):147-53. .
Analysis of discrete typing units of Trypanonoma cruzi isolates
Trypanonoma cruzi isolates were typed using three different parasite genomic sequences according to proposed protocols 3232. D'Ávila DA, Macedo AM, Valadares HMS, Gontijo ED, Castro AM, Machado CR, et al. Probing population dynamics of Trypanosoma cruzi during progression of the chronic phase in chagasic patients. J Clin Microbiol. 2009;47(6):1718-25. , as follows: 24Sα ribosomal (rRNA) gene D7 domain 3333. Souto RP, Zingales B. Sensitive detection and strain classification of Trypanosoma cruzi by amplification of a ribosomal RNA sequence. Mol Biochem Parasitol. 1993;62(1):45-52. , mitochondrial cytochrome oxidase subunit 2 gene (COII) 3434. Freitas JM, Augusto-Pinto L, Pimenta JR, Bastos-Rodrigues L, Gonçalves VF, Teixeira SMR, et al. Ancestral genomes, sex, and the population structure of Trypanosoma cruzi. PloS Pathog. 2006;2(3):e24. , and the spliced leader genes intergenic region 3535. Burgos JM, Altcheh J, Bisio M, Duffy T, Valadares HMS, Seidenstein ME, et al. Direct molecular profiling of minicircle signatures and lineages of Trypanosoma cruzi bloodstream populations causing congenital Chagas disease. Int J Parasitol. 2007;37(12):1319-27. , as markers of six DTUs 1818. Zingales B, Andrade SG, Briones MRS, Campbell DA, Chiari E, Fernandes O, et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz. 2009;104(7):1051-4. , TcI (Col1.7G2 Colombiana clone), TcII (JG), TcIII (222), TcIV (CAN III clone), TcV (3253 Lages-Silva et al.: unpublished data), and TcVI (CL) were used as reference strains and DTU controls 1818. Zingales B, Andrade SG, Briones MRS, Campbell DA, Chiari E, Fernandes O, et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz. 2009;104(7):1051-4.,3434. Freitas JM, Augusto-Pinto L, Pimenta JR, Bastos-Rodrigues L, Gonçalves VF, Teixeira SMR, et al. Ancestral genomes, sex, and the population structure of Trypanosoma cruzi. PloS Pathog. 2006;2(3):e24. .
Statistical analysis
Fisher’s exact tests were used to determine the associations among BC, clinical classification, and the degree of cardiac involvement. Mann-Whitney-Wilcoxon tests were used to compare the numbers of positive tubes according to the BC results, patient age, and clinical form 3636. Hollander M, Wolfe DA, Chicken E. Nonparametric Statistical Methods. 3rd edition. Hoboken New Jersey, USA: John Wiley & Sons; 2014. 848p. . Kappa coefficient concordance and 95% confidence intervals were used to quantify the degree of agreement between the results of BCs and PCR 3737. Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions. 3rd edition Hoboken New Jersey, USA: John Wiley & Sons; 2003. 800p.,3838. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-74. . To confirm or refute the evidence found by tests mentioned above, we used a 5% significance level.
RESULTS
Patient demographics
Approximately, 25.3% (23/91) of patients had the indeterminate clinical form, and 74.7% (68/91) of patients showed different degrees of cardiac involvement 2727. Rocha MO, Ribeiro AL, Teixeira MM. Clinical management of chronic Chagas cardiomyopathy. Front Biosci. 2003;8:e44-54. . Among patients with the indeterminate form of ChD, 34.8% (8/23) were men, and 65.2% (15/23) were women [ages 33-70 years; 44 ± 10.3 years (mean ± standard deviation)]. For the cardiac form, 66.2% (45/68) of patients were men, and 33.8% (23/68) were women (ages 25-81; 54 ± 10.3 years).
Detection of Trypanonoma cruzi by blood culture
Fifty of the 91 (54.9%) patients with chronic ChD presented positive BCs. Patients with cardiac and indeterminate clinical forms showed 60.3% and 39.1% BC positivity, respectively. No significant difference in BC positivity could be verified between patients with cardiac and indeterminate forms of ChD ( Figure 1 a). Similarly, there was no consistent evidence of a relationship between BC positivity and cardiac morbidity ( Figure 1 b).
Distribution of blood culture results in patients with chronic Chagas disease versus clinical form (a) and blood culture versus chronic Chagasic cardiomyopathy levels (b) . CCC: chronic Chagasic cardiomyopathy vs: versus.
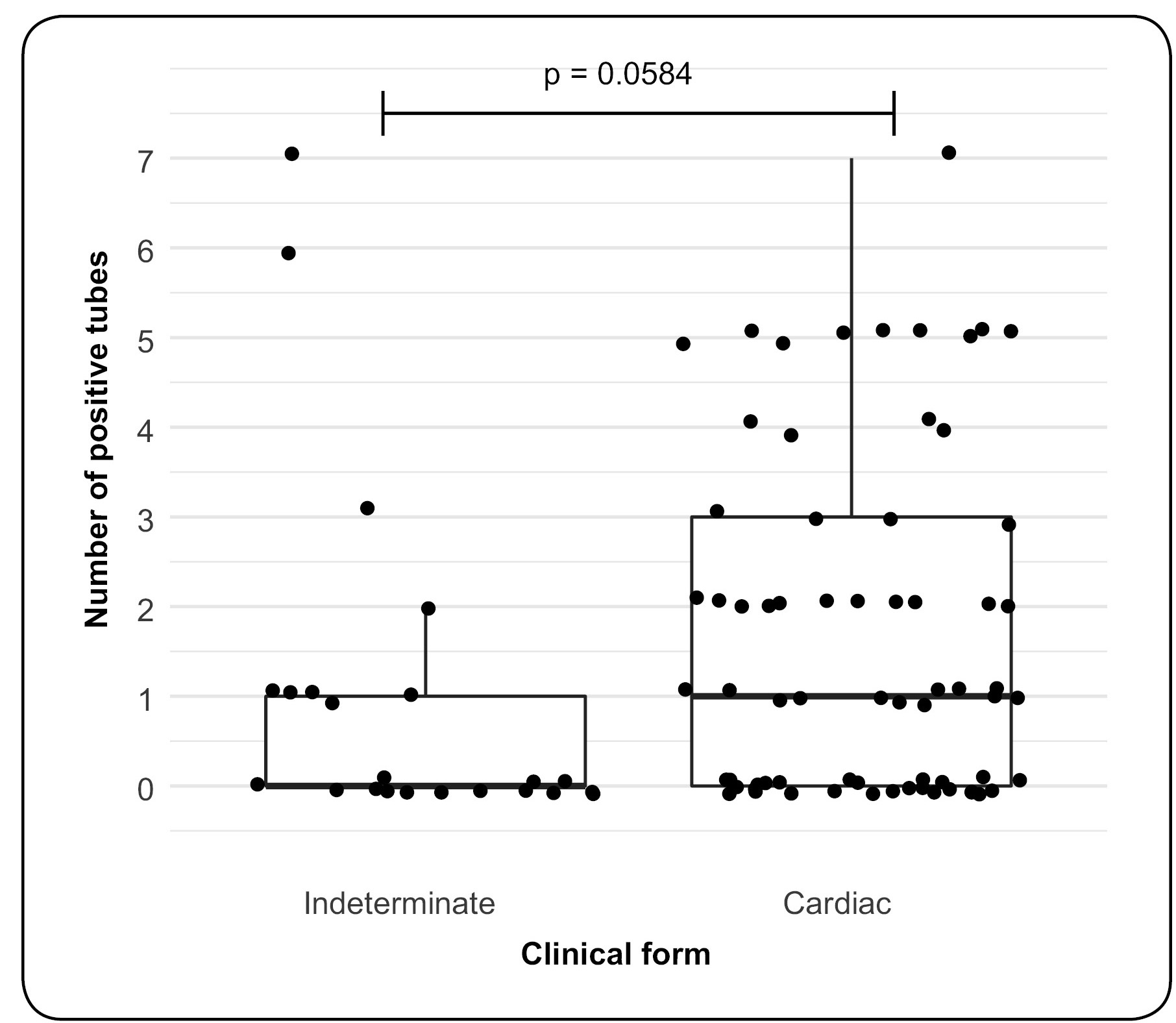
No difference was observed between results obtained for BC in patients of different ages, regardless of the clinical form ( Figure 2 a). Moreover, there were no correlations between age and BC results after adjustment for clinical form ( Figure 2 b and Figure 2 c), i.e., there was no evidence that the age associated with the clinical form may influence BC results. The number of positive tubes for BC was compared in patients with indeterminate and cardiac clinical forms. There was no evidence that patients with the indeterminate form showed a greater frequency of positive tubes than those with the cardiac form. Moreover, in samples from both clinical forms, a high frequency of negative tubes was observed ( Figure 3 ).
Boxplots for the age of patients with chronic Chagas disease according to clinical form and blood culture results, and p values of Mann-Whitney-Wilcoxon tests. (a) Patients with indeterminate or cardiac Chagas disease; (b) patients with the indeterminate form; (c) patients with the cardiac form. From bottom to top in the boxplot: lower quartile-1.5 IQlower quartile (25%), median (50%), upper quartile (75%), and upper quartile-1.5 IQ. IQ (interquartile range): difference between the upper and lower quartile.
Boxplots of the number of positive tubes in blood culture according to the clinical form in patients with chronic Chagas disease.
Associations between blood cultures and PCR methods
Trypanosoma cruzi was amplified by PCR in 98.9% (90/91) of samples from patients with chronic ChD. Of these, 54.9% (50/91) were positive by both PCR and BC; 43.9% (40/91) were only positive by PCR, and 1.1% (1/91) of samples was negative for both methods. All concordance coefficients were negative, indicating no evidence of agreement between BC and PCR results. In addition, all 95% confidence intervals possessed negative lower and upper limits or contained zero ( Table 1 ).
Genotyping of Trypanonoma cruzi isolates
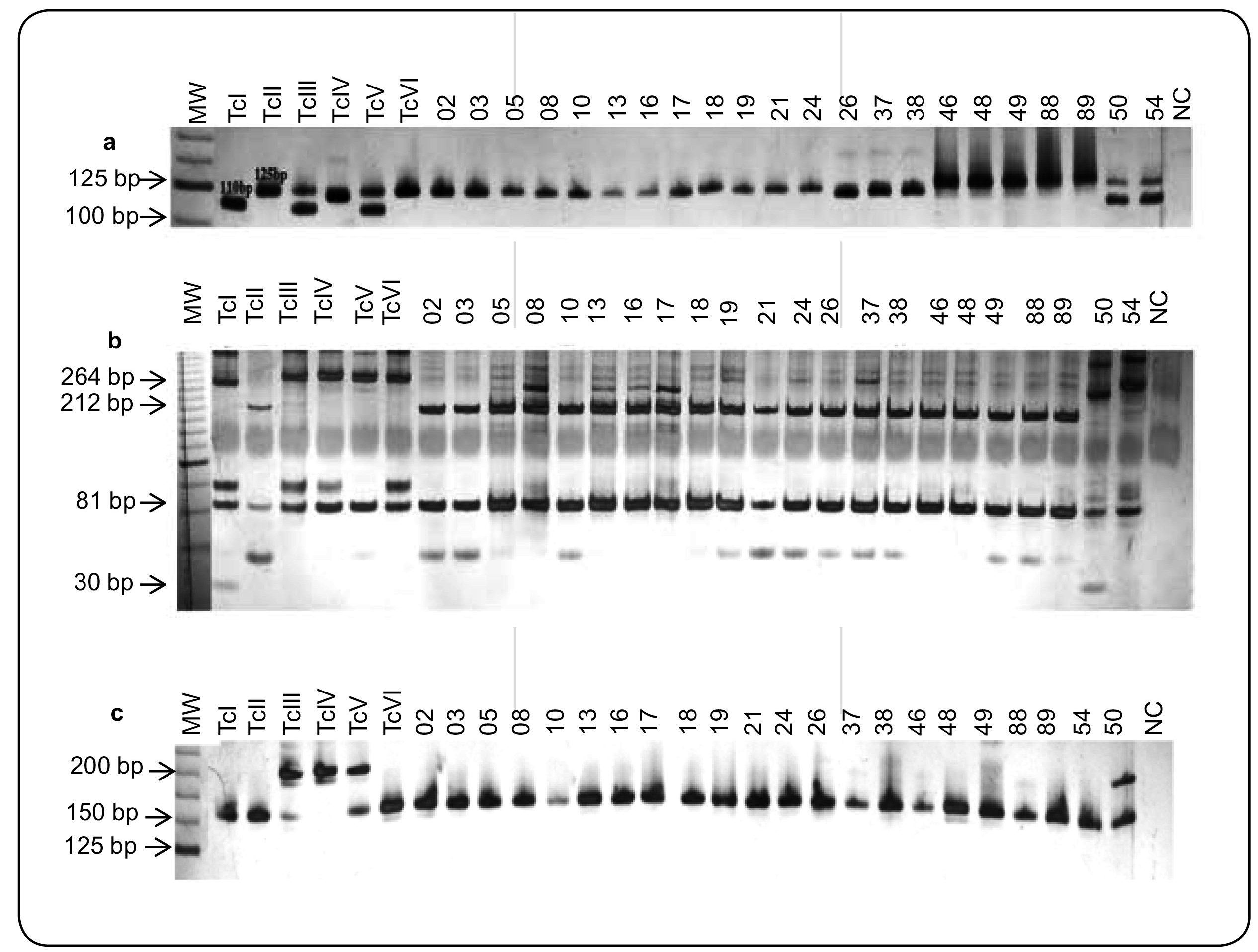
A total of 42 T. cruzi stocks were isolated from 91 patients with ChD using the BC technique and were analyzed by 24Sα rRNA and mitochondrial COII genes. Approximately, 95.2% (40/42) of the analyzed T . cruzi isolates belonged to rDNA group 1 [fragments of 125 base pairs (bp)] and COII haplotype C (fragments of 212 and 81bp), corresponding to TcII. Among these patients, 34 had the cardiac form of ChD, and eight had the indeterminate form of the disease. However, two isolates (50 and 54) from patients with cardiac and indeterminate clinical forms, respectively, were classified as rDNA½ amplified fragments of 110 and 125bp ( Figure 4 a). Genotyping of the mitochondrial COII genes of these isolates amplified restriction fragments of 264, 81, and 30bp for isolate 50 (mitochondrial haplotype A, T. cruzi I) and restriction fragments of 294 and 81bp for isolate 54 (mitochondrial haplotype B), which suggests of TcIII, TcIV, TcV, or TcVI ( Figure 4 b). In order to distinguish populations belonging to TcIII and TcIV (amplicons of 200bp) from those with TcI, TcII, TcV, or TcVI, which present fragments of approximately 150-157bp, we used the SL-IR gene marker. Most of the analyzed T. cruzi populations (40/42) showed amplification of 150-/157-bp fragments corresponding to TcII, as previously identified by rDNA and COII markers. In contrast, analysis of the SL-IR gene showed that isolate 50 corresponded to TcIII or TcIV (amplicons of 200bp), whereas isolate 54 was associated with TcV or TcVI (amplicons of 150-157bp; Figure 4 c).
A: Representative profiles of 24Sα rRNA of T. cruzi isolates from patients with chronic Chagas disease and controls obtained by polyacrylamide gel electrophoresis. Lane 1: MW marker; Lane 2: Col1.7G2 clone (~110bp), TcI; Lane 3: JG (~125bp), TcII; Lane 4: 222 strain (~110bp), TcIII; Lane 5: CAN III clone (~117/119bp), Tc IV; Lane 6: 3253 (~110 and 125bp), TcV; Lane 7: CL strain (~125bp); Lanes 8-29: DNA samples of patients; Lane 30: NC: negative PCR control (reagents without DNA). B: RFLP analysis of the mitochondrial COII gene in the T. cruzi isolates belonging to different haplotypes, obtained by polyacrylamide gel electrophoresis. Digestion of the DNA with AluI generated three RFLP patterns for the T. cruzi strains: restriction fragments of 264, 81, and 30bp are classified as TcI (mitochondrial haplotype A; control, Col1.7G2 clone); restriction fragments of 212 and 81bp are classified as TcII (mitochondrial haplotype C; control, JG strain); restriction fragments of 294 and 81bp are classified as TcIII, TcIV, TcV, and TcVI (mitochondrial haplotype B; control, 222, CAN III, 3253, and CL strains). Lanes 2-7: TcI-VI controls; Lanes 8-29: DNA samples of patients; Lane 30: NC, negative PCR control (reagents without DNA). C: Genetic profiles of T. cruzi isolates obtained by SL-IR genes used to separate T. cruzi III from other DTUs. Lanes 2-7: TcI - VI controls, Col1.7G2 clone, and JG strain (fragments of 150 - 157bp corresponding to TcI and TcII), 222 and CAN III strains (fragment of 200bp, associated with strains TcIII and TcIV), 3253 and CL strains (fragments of 150/157bp, belonging to TcV and TcVI). Lanes 8-29: DNA samples of patients; Lane 30: NC, negative PCR control (reagents without DNA). 24Sα rRNA: 24Sα ribosomal; T.: Trypanosoma; MW: molecular weight; bp: base pairs; Tc: T. cruzi ; DNA: deoxyribonucleic acid; NC: negative control; PCR: polymerase chain reaction; RFLP: restriction fragment length polymorphism; CO: cytochrome oxidase; SL-IR: spliced leader ingergenic regions; DTUs: discrete typing units.
DISCUSSION
In this study, we evaluated the detection of circulating parasites by conventional PCR and BC in samples from patients with ChD. Notably, our findings showed that more than half of the samples from patients with chronic ChD with different clinical forms and not subjected to specific treatment were positive by BC analysis. This positivity could be related to the high number of patients with the cardiac form, most of whom were 50 or more years old; however, there was no evidence of the degree of cardiac involvement and BC and/or PCR positivity. Despite these findings, we cannot rule out the possibility that BC variability may be directly related to the rate of T. cruzi infection in our specific patient group 2222. Castro AM, Luquetti AO, Rassi A, Rassi GG, Chiari E, Galvão LMC. Blood culture and polymerase chain reaction for the diagnosis of the chronic phase of human infection with Trypanosoma cruzi. Parasitol Res. 2002;88(10):894-900.,3939. Minter-Goedbloed E. Hemoculture compared with xenodiagnosis for the detection of Trypanosoma cruzi infection in man and in animal. Proceedings of an International Symposium, Belo Horizonte, MG 1975. In: New approaches in American Tripanosomiasis Research. Washington: Pan American Health Organization, Scientific Publication n. 318; 1976. p. 245-50. . Higher positivity by BC has been shown in seroreactive patients between 4 and 20 years old when submitted to blood culture 4040. Fernandes CD, Tiecher FM, Fernandes DD, Henriques NMP, Steindel M. High rates of positive hemocultures in children and teenagers seropositive for Trypanosoma cruzi in the State of Rio Grande do Sul, Brazil. Mem Inst Oswaldo Cruz. 1999;94(1):7-8. . The period of cultivation also affects BC positivity; in previous studies, BC positivity was increased at 6 months after inoculation 4141. Minter-Goedbloed E, Minter DM, Marshall TFC. Quantitative comparison between xenodiagnosis and hemoculture in the detection of Trypanosoma (Schizotrypanum) cruzi in experimental and natural chronic infections. Trans R Soc Trop Med Hyg. 1978;72(3):217-25.,2121. Galvão LMC, Nunes RMB, Cançado JR, Brener Z, Krettli AU. Lytic antibody titre as a means of assessing cure after treatment of Chagas disease: a 10 years follow-up study. Trans R SocTrop Med Hyg.1993;87(2):220-3. . Additionally, increased positivity when BCs were repeated in the same patient using a larger blood volume and centrifugation at 4°C 2121. Galvão LMC, Nunes RMB, Cançado JR, Brener Z, Krettli AU. Lytic antibody titre as a means of assessing cure after treatment of Chagas disease: a 10 years follow-up study. Trans R SocTrop Med Hyg.1993;87(2):220-3.,2222. Castro AM, Luquetti AO, Rassi A, Rassi GG, Chiari E, Galvão LMC. Blood culture and polymerase chain reaction for the diagnosis of the chronic phase of human infection with Trypanosoma cruzi. Parasitol Res. 2002;88(10):894-900. .
In our study, no correlation was observed when we compared the number of BC-positive tubes with the clinical form of ChD, indicating that this parameter may be useful for assessing parasitemia in patients with chronic ChD; the number of positive tubes from a single BC could be an indicator of parasitemia. T. cruzi parasitemia in patients with chronic ChD varies considerably in different endemic regions. Moreover, clinical forms of the disease, morbidity, and mortality can also vary from one endemic area to another, and the intensity of infection and the immune response of patients also differs 1919. Coura JR. Chagas disease: what is known and what is needed-A background article. Mem Inst Oswaldo Cruz. 2007;102(Suppl 1):113-22. . ChD progression may result from changes in the patient’s immune response during the course of T. cruzi infection 4242. Pereira JB, Willcox HP, Coura JR. Morbidity in Chagas' disease. III. Longitudinal study of 6 years, in Virgem da Lapa, MG, Brazil. Mem Inst Oswaldo Cruz. 1985;80(1):63-71. . Moreover, age influences the degree of parasitemia in patients 4343. Silva IG, Silva HHG, Ostermayer AL, Rezende JM. Positividade do xenodiagnóstico de acordo com a faixa etária, o sexo e a forma clínica da doença de Chagas. Rev Pat Trop. 1995;24(2):193-7.,4444. Castro C, Macêdo V, Prata A. The behavior of Trypanosoma cruzi parasitemia in chronic chagasics over 13 years. Rev Soc Bras Med Trop. 1999;32(2):157-65. , and the decline in trypomastigote circulating forms is related to age 4545. Castro C, Prata A, Macêdo V. Influência da parasitemia na evolução da doença de Chagas crônica. Rev Soc Bras Med Trop. 2005;38(1):1-6. . However, we did not observe significant differences among BC results, patient age, and clinical form.
BC is considered positive if at least one blood sample contains T. cruzi trypomastigote forms that can differentiate into epimastigotes and multiply in culture medium. Interestingly, regardless of the clinical form, most cases were positive between 30 and 60 days, corroborating with previous data 44. Chiari E, Dias JCP, Lana M, Chiari CA. Hemocultures for the parasitological diagnostic of human chronic Chagas' disease. Rev Soc Bras Med Trop 1989;22(1):19-23.,2121. Galvão LMC, Nunes RMB, Cançado JR, Brener Z, Krettli AU. Lytic antibody titre as a means of assessing cure after treatment of Chagas disease: a 10 years follow-up study. Trans R SocTrop Med Hyg.1993;87(2):220-3.,2222. Castro AM, Luquetti AO, Rassi A, Rassi GG, Chiari E, Galvão LMC. Blood culture and polymerase chain reaction for the diagnosis of the chronic phase of human infection with Trypanosoma cruzi. Parasitol Res. 2002;88(10):894-900.,4040. Fernandes CD, Tiecher FM, Fernandes DD, Henriques NMP, Steindel M. High rates of positive hemocultures in children and teenagers seropositive for Trypanosoma cruzi in the State of Rio Grande do Sul, Brazil. Mem Inst Oswaldo Cruz. 1999;94(1):7-8.. BC may be wrongly considered positive due to in vitro production of motionless amastigotes for extended periods until the detection of moving forms, since a low number of amastigotes may be unnoticed 4646. Brener Z, Chiari E. Aspects of early growth of different Trypanosoma cruzi strains in culture medium. J Parasitol. 1965;51(6):922-6.,4747. Minter-Goedbloed E. The primary isolation by haemoculture of Trypanosoma (Schizotrypanum) cruzi from animals and from man. Trans R Soc Trop Med Hyg. 1978;72(1):22-30.. Variabilities in BC positivity have been reported and can be explained by various factors, such as higher volume of collected blood, which increases the chance to obtain intact forms of the parasite; maintenance of blood samples on an ice bath or at 4°C after collection; immediate removal of the plasma; quickness in the processing of samples; quality of culture medium; and correctness of the technique 44. Chiari E, Dias JCP, Lana M, Chiari CA. Hemocultures for the parasitological diagnostic of human chronic Chagas' disease. Rev Soc Bras Med Trop 1989;22(1):19-23.,2121. Galvão LMC, Nunes RMB, Cançado JR, Brener Z, Krettli AU. Lytic antibody titre as a means of assessing cure after treatment of Chagas disease: a 10 years follow-up study. Trans R SocTrop Med Hyg.1993;87(2):220-3.,4848. Chiari E, Brener Z. Contribution to the parasitological diagnosis of human Chagas' disease in its chronic phase. Rev Inst Med Trop São Paulo. 1966;8(3):134-8.. Another important factor to be considered is the technical expertise to examine BCs, since T. cruzi can grow in different morphologies 4646. Brener Z, Chiari E. Aspects of early growth of different Trypanosoma cruzi strains in culture medium. J Parasitol. 1965;51(6):922-6.,4747. Minter-Goedbloed E. The primary isolation by haemoculture of Trypanosoma (Schizotrypanum) cruzi from animals and from man. Trans R Soc Trop Med Hyg. 1978;72(1):22-30..
Trypanosoma cruzi k-DNA was detected by conventional PCR in most patients with ChD. Regardless of the protocol used, PCR is considered superior to BC and/or xenodiagnoses 55.Ávila HA, Pereira JB, Thiemann O, Paiva E, Degrave W, Morel CM, et al. Detection of Trypanosoma cruzi in blood specimens of chronic chagasic patients by polymerase chain reaction amplification of kinetoplast minicircle DNA: comparison with serology and xenodiagnosis. J Clin Microbiol. 1993;31(9):2421-6.,77. Gomes ML, Macedo AM, Vago AR, Pena SDJ, Galvão LMC, Chiari E. Trypanosoma cruzi: optimization of polymerase chain reaction for detection in human blood. Exp Parasitol. 1998;88(1):28-33.,2222. Castro AM, Luquetti AO, Rassi A, Rassi GG, Chiari E, Galvão LMC. Blood culture and polymerase chain reaction for the diagnosis of the chronic phase of human infection with Trypanosoma cruzi. Parasitol Res. 2002;88(10):894-900.,2323. Meira WSF, Galvão LMC, Gontijo ED, Machado-Coelho GL, Norris KA, Chiari E. Trypanosoma cruzi recombinant complement regulatory protein: a novel antigen for use in an enzyme-linked immunosorbent assay for diagnosis of Chagas' disease. J Clin Microbiol. 2002;40(10):3735-40.,4949. Junqueira ACV, Chiari E, Wincker P. Comparison of the polymerase chain reaction with two classical parasitological methods for the diagnosis of Chagas disease in an endemic region of northeastern Brazil. Trans R Soc Trop Med Hyg. 1996;90(2):129-32.,5050. Lages-Silva E, Crema E, Ramirez LE, Macedo AM, Pena SDJ, Chiari E. Relationship between Trypanosoma cruzi and human chagasic megaesophagus: blood and tissue parasitism. Am J Trop Med Hyg. 2001;65(5):435-41.. This can be explained by parasitemia levels in infected individuals living in different endemic areas and may be related to the complexity of the T. cruzi life cycle. Patients living in Virgem da Lapa, Minas Gerais showed high levels of parasitemia based on positive xenodiagnosis when compared with individuals from other areas 5151. Borges-Pereira J, Junqueira ACV, Santos LC, Castro JAF, Araujo IB, Coura JR. Xenodiagnosis in chronic Chagas' disease. I. The sensitivity of Panstrongylus megistus and Triatoma infestans. Rev Soc Bras Med Trop. 1996;29(4):341-7.. The presence of T. cruzi in the peripheral circulation at any given time of blood collection depends on the parasite’s life cycle, the immune balance between parasite and host 5252. Castro C, Prata A. Absence of both circadian rhythm and Trypanosoma cruzi periodicity with xenodiagnosis in chronic chagasic individuals. Rev Soc Bras Med Trop. 2000;33(5):427-30., and the time between the blood collection and sample processing 44. Chiari E, Dias JCP, Lana M, Chiari CA. Hemocultures for the parasitological diagnostic of human chronic Chagas' disease. Rev Soc Bras Med Trop 1989;22(1):19-23.,2121. Galvão LMC, Nunes RMB, Cançado JR, Brener Z, Krettli AU. Lytic antibody titre as a means of assessing cure after treatment of Chagas disease: a 10 years follow-up study. Trans R SocTrop Med Hyg.1993;87(2):220-3.. In this study, the volume of blood collected did not influence the positivity of PCR, consistent with a previous study of patients from the Triângulo Mineiro 5050. Lages-Silva E, Crema E, Ramirez LE, Macedo AM, Pena SDJ, Chiari E. Relationship between Trypanosoma cruzi and human chagasic megaesophagus: blood and tissue parasitism. Am J Trop Med Hyg. 2001;65(5):435-41.. Another hypothesis is that the infection may have been caused by Trypanosoma rangeli ; however, this is unlikely because infection by this protozoan has been detected only in animals in the State of Minas Gerais 5353. Ramirez LE, Lages-Silva E, Alvarenga-Franco F, Matos A, Vargas N, Fernandes O, et al. High prevalence of Trypanosoma rangeli and Trypanosoma cruzi in opossums and triatomids in a formely-endemic area of Chagas disease in Southeast Brazil. Acta Trop. 2002;84(3):189-98..
Studies in different endemic areas have shown that genetic differences between parasite strains can influence parasitemia and PCR positivity 5454. Britto CC. Usefulness of PCR-based assays to assess drug efficacy in Chagas disease chemotherapy: value and limitations. Mem Inst Oswaldo Cruz. 2009;104(Suppl. I):122-35.. This positivity may still be related to several factors, such as the number of transient trypomastigotes in the peripheral circulation at the time of blood collection, appropriate storage conditions of the samples, DNA extraction procedures, amplification targets, the use of the same thermal cycler, and the ability to detect minimal quantities of parasite DNA 77. Gomes ML, Macedo AM, Vago AR, Pena SDJ, Galvão LMC, Chiari E. Trypanosoma cruzi: optimization of polymerase chain reaction for detection in human blood. Exp Parasitol. 1998;88(1):28-33.,2222. Castro AM, Luquetti AO, Rassi A, Rassi GG, Chiari E, Galvão LMC. Blood culture and polymerase chain reaction for the diagnosis of the chronic phase of human infection with Trypanosoma cruzi. Parasitol Res. 2002;88(10):894-900.,2828. Schijman AG, Bisio M, Orellana L, Sued M, Duffy T, Jaramillo AMM, et al. International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. PLoS Negl Trop Dis. 2011;5(1):e931.. PCR is more convenient than BC since it requires the collection of a lower blood volume, has a shorter processing time, and allows analysis of multiple samples simultaneously 5555. Chiaramonte MG, Frank FM, Furer GM, Taranto NJ, Margni RA, Malchiodi EL. Polymerase chain reaction reveals Trypanosoma cruzi suspected by serology in cutaneous and mucocutaneous leishmaniasis patients. Acta Trop. 1999;72(3):295-308.. Nevertheless, it is not possible to use PCR for T. cruzi isolation and subsequent biological, biochemical, and/or molecular studies. Thus, BC is the most efficient, particularly if repeated, because it allows the isolation and multiplication of the parasite in culture medium 2222. Castro AM, Luquetti AO, Rassi A, Rassi GG, Chiari E, Galvão LMC. Blood culture and polymerase chain reaction for the diagnosis of the chronic phase of human infection with Trypanosoma cruzi. Parasitol Res. 2002;88(10):894-900.. BC and PCR will not achieve higher sensitivity because they are under the influence of various intrinsic factors of vertebrate hosts and the heterogeneity of parasite populations circulating in different endemic regions. However, PCR suitability for diagnostic purposes in laboratory routine is important for the detection of chagasic infection in patients with low levels of parasitemia or those with inconclusive serology 2222. Castro AM, Luquetti AO, Rassi A, Rassi GG, Chiari E, Galvão LMC. Blood culture and polymerase chain reaction for the diagnosis of the chronic phase of human infection with Trypanosoma cruzi. Parasitol Res. 2002;88(10):894-900.. Importantly, recent data have demonstrated the standardization and validation of PCR for the diagnosis of chronic ChD. Despite this, the absence of a reliable method to detect and quantify parasitemia is still an obstacle for improving our understanding of the impact of persistent parasitemia in the natural history of ChD and to characterize the parasite load in order to evaluate prognosis and therapy 2828. Schijman AG, Bisio M, Orellana L, Sued M, Duffy T, Jaramillo AMM, et al. International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. PLoS Negl Trop Dis. 2011;5(1):e931.. Recent findings have demonstrated a reliable protocol based on real-time PCR for validation and quantification of T. cruzi DNA in human blood samples, aiming to provide an accurate surrogate biomarker for diagnosis and treatment in patients with ChD 5656. Ramírez JD, Cura CI, Moreira OC, Lages-Silva E, Juiz N, Velázquez E, et al. Analytical validation of quantitative real-time PCR methods for quantification of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. J Mol Diagn. 2015;17(5):605-15..
Our findings showed that association of BC and PCR did not increase T. cruzi detection since PCR was positive in 40/91 patients with negative BC results. These results showed that the combination of the two methods improved the chance of detecting the parasite and/or its genomic fragments, corroborating with previous studies 2222. Castro AM, Luquetti AO, Rassi A, Rassi GG, Chiari E, Galvão LMC. Blood culture and polymerase chain reaction for the diagnosis of the chronic phase of human infection with Trypanosoma cruzi. Parasitol Res. 2002;88(10):894-900.,5050. Lages-Silva E, Crema E, Ramirez LE, Macedo AM, Pena SDJ, Chiari E. Relationship between Trypanosoma cruzi and human chagasic megaesophagus: blood and tissue parasitism. Am J Trop Med Hyg. 2001;65(5):435-41..
Understanding structure of the T. cruzi population is essential due to the links between parasite transmission cycles and the infection/disease. Herein, 42 T. cruzi isolates from untreated patients with chronic ChD and with well-defined clinical forms of ChD were identified. Most isolates from these patients were analyzed by rDNA 24Sα, COII, and SL-IR molecular markers and were found to be associated with DTU II. We also identified one isolate (50) associated with DTU III or IV from a patient with the cardiac form of the disease, and another (54) corresponding to DTU V or VI from a patient with the indeterminate form of the disease. We believe that isolate 50 is an excellent candidate for microsatellite analysis, a technique sensitive enough to detect small differences between clones within a single isolate.
Importantly, our data were consistent with previous studies showing that DTU II was associated with human infection in the State of Minas Gerais 3232. D'Ávila DA, Macedo AM, Valadares HMS, Gontijo ED, Castro AM, Machado CR, et al. Probing population dynamics of Trypanosoma cruzi during progression of the chronic phase in chagasic patients. J Clin Microbiol. 2009;47(6):1718-25.,5757. Freitas JM, Lages-Silva E, Crema E, Pena SDJ, Macedo AM. Real time PCR strategy for the identification of major lineages of Trypanosoma cruzi directly in chronically infected human tissues. Int J Parasitol. 2005;35(4):411-7.,5858. Lages-Silva E, Ramirez LE, Pedrosa AL, Crema E, Galvão LMC, Pena SDJ. Variability of kinetoplast DNA gene signatures of Trypanosoma cruzi II strains from patients with different clinical forms of Chagas' disease in Brazil. J Clin Microbiol. 2006;44(6):2167-71.. The methodologies applied for genetic characterization of isolates of different clinical forms may be a limiting factor for typing T. cruzi populations since the analysis was based on a single marker, leading to potential misinterpretations 5959. Brisse S, Verhoef J, Tibayrenc M. Characterisation of large and small subunit rRNA and mini-exon genes further supports the distinction of six Trypanosoma cruzi lineages. Int J Parasitol. 2001;31(11):1218-26.. Our results showed that using two molecular markers was not sufficient for typing of T. cruzi isolates. These limitations were also observed by other authors 3232. D'Ávila DA, Macedo AM, Valadares HMS, Gontijo ED, Castro AM, Machado CR, et al. Probing population dynamics of Trypanosoma cruzi during progression of the chronic phase in chagasic patients. J Clin Microbiol. 2009;47(6):1718-25.,6060. Steindel M, Pacheco LK, Scholl D, Soares M, Moraes MH, Eger I, et al. Characterization of Trypanosoma cruzi isolated from humans, vectors, and animal reservoirs following an outbreak of acute human Chagas disease in Santa Catarina State, Brasil. Diagn Microbiol Infect Dis. 2008;60(1):25-32.
61. Zafra G, Mantilla JC, Valadares HM, Macedo AM, González CI. Evidence of Trypanosoma cruzi II infection in Colombian Chagasic patients. Parasitol Res. 2008;103(3):731-4.
62. Câmara ACJ, Varela-Freire AA, Valadares HMS, Macedo AM, D'Ávila DA, Machado CR, et al. Genetic analyses of Trypanosoma cruzi isolates from naturally infected triatomines and humans in northeastern Brazil. Acta Trop. 2010;115(3):205-11.-6363. Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM, Teixeira MMG, et al. The revised Trypanosoma cruzi subspecific nomenclature: Rationale, epidemiological relevance and research applications. Infect Genet Evol. 2012;12(2):240-53.. However, the use of three genetic markers with different evolutionary rates was able to ensure the correct typing of T. cruzi isolates. In contrast, it was not possible to correlate the parasite genotype with the clinical form. Findings using low-stringency single specific primer PCR and rDNA 24Sα markers showed TcII in all isolates from patients with indeterminate, cardiac, digestive, and cardiodigestive forms 5858. Lages-Silva E, Ramirez LE, Pedrosa AL, Crema E, Galvão LMC, Pena SDJ. Variability of kinetoplast DNA gene signatures of Trypanosoma cruzi II strains from patients with different clinical forms of Chagas' disease in Brazil. J Clin Microbiol. 2006;44(6):2167-71.. Although TcII was identified in most T. cruzi isolates, no correlation with clinical forms was observed using rDNA 24Sα, COII, and SL-IR markers and polymorphisms in microsatellite analysis 3232. D'Ávila DA, Macedo AM, Valadares HMS, Gontijo ED, Castro AM, Machado CR, et al. Probing population dynamics of Trypanosoma cruzi during progression of the chronic phase in chagasic patients. J Clin Microbiol. 2009;47(6):1718-25.. TcII has been reported as the primary cause of acute ChD and is the typical genotype detected in patients with cardiac and digestive forms of ChD, confirming the association of this DTU with human infection in Brazil and Argentina 5757. Freitas JM, Lages-Silva E, Crema E, Pena SDJ, Macedo AM. Real time PCR strategy for the identification of major lineages of Trypanosoma cruzi directly in chronically infected human tissues. Int J Parasitol. 2005;35(4):411-7.,6363. Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM, Teixeira MMG, et al. The revised Trypanosoma cruzi subspecific nomenclature: Rationale, epidemiological relevance and research applications. Infect Genet Evol. 2012;12(2):240-53.. TcII has also been detected using the same markers with high homology among different hosts and locations in northeastern semiarid 6262. Câmara ACJ, Varela-Freire AA, Valadares HMS, Macedo AM, D'Ávila DA, Machado CR, et al. Genetic analyses of Trypanosoma cruzi isolates from naturally infected triatomines and humans in northeastern Brazil. Acta Trop. 2010;115(3):205-11. . TcI is associated with human infection in the Amazon, the Andean region, Central America, Mexico, and endemic areas of Southeastern and Northeastern Brazil 6363. Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM, Teixeira MMG, et al. The revised Trypanosoma cruzi subspecific nomenclature: Rationale, epidemiological relevance and research applications. Infect Genet Evol. 2012;12(2):240-53.
64. Bosseno MF, Barnabè, C, Gastélum EM, Kasten FL, Ramsey J, Espinoza B, et al. Predominance of Trypanosoma cruzi lineage I in Mexico. J Clin Microbiol. 2002;40(2):627-32.
65. Añez M, Crisante G, Silva FM, Rojas A, Carrasco H, Umezawa ES, et al. Predominance of lineage I among Trypanosoma cruzi isolates from Venezuelan patients with different clinical profiles of acute Chagas' disease. Trop Med Int Health. 2004;9(12):1319-26.
66. Sanchez-Guillén MC, Bernabé C, Tibayrenc M, Zavala-Castro J, Totolhua JL, Méndez-Lopez J, et al. Trypanosoma cruzi strains isolated from human, vector and animal reservoir in the same endemic region in 67.Mexico and typed as T. cruzi I, discrete typing unit 1 exhibit considerable biological diversity. Mem Inst Oswaldo Cruz. 2006;101(6):585-90.-6768. Teixeira MMG, Silva FM, Marcili A, Umezawa ES, Shikanai-Yasuda MA, Cunha-Neto E, et al. Short communication: Trypanosoma cruzi lineage I in endomyocardial biopsy from a north-eastern Brazilian patient at end-stage chronic chagasic cardiomyopathy. Trop Med Int Health. 2006;11(3):294-8.. Few cases of human infection caused by TcI have been described in Brazil 6363. Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM, Teixeira MMG, et al. The revised Trypanosoma cruzi subspecific nomenclature: Rationale, epidemiological relevance and research applications. Infect Genet Evol. 2012;12(2):240-53.,6869. Abolis NG, Araujo SM, Toledo MJO, Fernandez MA, Gomes ML. Trypanosoma cruzi I-III in southern Brazil causing individual and mixed infections in human, sylvatic reservoirs and triatomines. Acta Trop. 2011;120(3):167-72.. However, recent findings have detected TcI in patients with the indeterminate form of ChD in the northern region of Minas Gerais State 3232. D'Ávila DA, Macedo AM, Valadares HMS, Gontijo ED, Castro AM, Machado CR, et al. Probing population dynamics of Trypanosoma cruzi during progression of the chronic phase in chagasic patients. J Clin Microbiol. 2009;47(6):1718-25. and in patients with cardiac, digestive, and indeterminate forms of ChD from Rio Grande do Norte State 6970. Martins K, Andrade CM, Barbosa-Silva AN, do Nascimento GB, Chiari E, Galvão LMC, et al. Trypanosoma cruzi III causing the indeterminate form of Chagas disease in a semi-arid region of Brazil. Int J Infect Dis. 2015;39:68-75.. Interestingly, TcIII (formerly known as TcIIc) is associated with terrestrial ecotopes from different reservoirs and vectors 7071. Yeo M, Acosta N, Llewellyn M, Sánchez H, Adamson S, Miles GAJ, et al. Origins of Chagas disease: Didelphis species are natural host of Trypanosoma cruzi I and armadillos hosts of Trypanosoma cruzi II, including hybrids. Int J Parasitol. 2005;35(2):225-33.,7172. Marcili A, Lima L, Valente VC, Valente SA, Batista JS, Junqueira ACV, et al. Comparative phylogeography of Trypanosoma cruzi TCIIc: New hosts, association with terrestrial ecotopes, and spatial clustering. Infect Genet Evol. 2009;9(6):1265-74. and is able to coexist with other DTUs in the environment, supporting an overlap between sylvatic and peridomestic transmission cycles of T. cruzi6970. Martins K, Andrade CM, Barbosa-Silva AN, do Nascimento GB, Chiari E, Galvão LMC, et al. Trypanosoma cruzi III causing the indeterminate form of Chagas disease in a semi-arid region of Brazil. Int J Infect Dis. 2015;39:68-75.. In Colombia, TcIII has been detected in patients with chronic ChD in whom mixed infection with TcI and TcII was identified 7273. Ramírez JD, Guhl F, Rendón LM, Rosas F, Marin-Neto JA, Morillo CA. Chagas cardiomyopathy manifestations and Trypanosoma cruzi genotypes circulating in chronic chagasic patients. PLoS Negl Trop Dis. 2010;4(11):e899.. Therefore, previous studies have shown how difficult it is to distinguish the role of each DTU in the disease. DTUs may be under-reported from domiciliary and sylvatic transmission cycles because some genotyping methodologies fail to distinguish between TcIV (TcIIa) and TcIII 7374. Fernandes O, Souto RP, Castro JA, Borges-Pereira J, Fernandes NC, Junqueira AC, et al. Brazilian isolates of Trypanosoma cruzi from humans and triatomines classified into two lineages using mini-exon and ribosomal RNA sequences. Am J Trop Med Hyg. 1998;58(6):807-11.. TcIII was identified in patients with chronic ChD from Minas Gerais State 3232. D'Ávila DA, Macedo AM, Valadares HMS, Gontijo ED, Castro AM, Machado CR, et al. Probing population dynamics of Trypanosoma cruzi during progression of the chronic phase in chagasic patients. J Clin Microbiol. 2009;47(6):1718-25. and in patients with the indeterminate form of ChD from Rio Grande do Norte State 6970. Martins K, Andrade CM, Barbosa-Silva AN, do Nascimento GB, Chiari E, Galvão LMC, et al. Trypanosoma cruzi III causing the indeterminate form of Chagas disease in a semi-arid region of Brazil. Int J Infect Dis. 2015;39:68-75.. Therefore, we cannot rule out the possibility of other parasite strains triggering the disease in humans in Minas Gerais. Further studies are needed to fully characterize the epidemiological and clinical aspects of TcI and TcIII in Brazil.
In summary, in this study, we demonstrated that BC detected the parasite in approximately half of the patients, whereas PCR was more effective for detecting T. cruzi in almost all samples, with the exception of one patient. Therefore, PCR and BC were efficient for detecting T . cruzi following collection of only a single blood sample, and the combined use of both methods did not increase positivity in the diagnosis of chronic Chagas disease. PCR does not allow the isolation of T. cruzi , emphasizing the importance of BC for further biological, biochemical, immunological, and genetic studies of parasite populations. Considering genotyping data, it was not possible to establish a correlation between the clinical form of ChD and the genetic profile of T. cruzi isolates.
Acknowledgements
We thank Prof. Manoel Otávio da Costa Rocha for his contribution and support in the clinical analysis of patients. We thank Afonso da Costa Viana and Orlando Carlos Magno from Departamento de Parasitologia for their technical assistance and Walderez Ornelas Dutra for the critical reading and revision of the manuscript.
REFERENCES
-
1Tarleton RL, Curran JW. Is Chagas Disease Really the ''New HIV/AIDS of the Americas''? PLoS Negl Trop Dis. 2012;6(10):e1861.
-
2Laranja FS, Dias E, Nobrega G, Miranda A. Chagas' disease: a clinical, epidemiologic, and pathologic study. Circulation. 1956;14(6):1035-60.
-
3Dias JCP. Acute Chagas' disease. Mem Inst Oswaldo Cruz. 1984;79:(Suppl) 85-91.
-
4Chiari E, Dias JCP, Lana M, Chiari CA. Hemocultures for the parasitological diagnostic of human chronic Chagas' disease. Rev Soc Bras Med Trop 1989;22(1):19-23.
-
5Ávila HA, Pereira JB, Thiemann O, Paiva E, Degrave W, Morel CM, et al. Detection of Trypanosoma cruzi in blood specimens of chronic chagasic patients by polymerase chain reaction amplification of kinetoplast minicircle DNA: comparison with serology and xenodiagnosis. J Clin Microbiol. 1993;31(9):2421-6.
-
6Britto C, Cardoso MA, Ravel C, Santoro A, Borges-Pereira J, Coura JR, et al. Trypanosoma cruzi: parasite detection and strain discrimination in chronic chagasic patients from Northeastern Brazil using PCR amplification of kinetoplast DNA and nonradioactive hybridization. Exp Parasitol. 1995;81 (4):462-71.
-
7Gomes ML, Macedo AM, Vago AR, Pena SDJ, Galvão LMC, Chiari E. Trypanosoma cruzi: optimization of polymerase chain reaction for detection in human blood. Exp Parasitol. 1998;88(1):28-33.
-
8Andrade SG. Morphological and behavioral characterization of Trypanosoma cruzi strains. Rev Soc Bras Med Trop. 1985;18(supl):39-46.
-
9Ribeiro ALP, Rocha MOC. Forma indeterminada da doença de Chagas: considerações acerca do diagnóstico e do prognóstico. Rev Soc Bras Med Trop. 1998;31(3):301-14.
-
10Macêdo V. Indeterminate form of Chagas disease. Mem Inst Oswaldo Cruz. 1999;94(1):311-6.
-
11Rassi Jr A, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375(9723):1388-402.
-
12Dias JCP. Natural history of Chagas' disease. Arq Bras Cardiol. 1995;65(4):359-66.
-
13Coura JR, Anunziato N, Willcox HPF. Chagas' disease morbidity. I - Study of cases originating in various states of Brazil, observed in Rio de Janeiro. Mem Inst Oswaldo Cruz. 1983;78(3):363-72.
-
14Coura JR, Abreu LL, Pereira JB, Willcox H. Morbidity in Chagas' disease. IV. Longitudinal study of 10 years in Pains and Iguatama, Minas Gerais, Brazil]. Mem Inst Oswaldo Cruz. 1985;80(1):73-80.
-
15Miles M, Cedillos R, Povoa M, De Souza A, Prata A, Macedo V. Do radically dissimilar Trypanosoma cruzi strains (zymodemes) cause Venezuelan and Brazilian forms of Chagas disease? Lancet 1981;317(8234):1338-40.
-
16Tibayrenc M, Ayala FJ. Evolutionary genetics of Trypanosoma cruzi and Leishmania. Microbes Infect. 1999;1(6):465-72.
-
17Macedo AM, Pimenta JR, Aguiar RS, Melo AIR, Chiari E, Zingales B, et al. Usefulness of microsatellite typing in population genetic studies of Trypanosoma cruzi Mem Inst Oswaldo Cruz. 2001;96(3):407-13.
-
18Zingales B, Andrade SG, Briones MRS, Campbell DA, Chiari E, Fernandes O, et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz. 2009;104(7):1051-4.
-
19Coura JR. Chagas disease: what is known and what is needed-A background article. Mem Inst Oswaldo Cruz. 2007;102(Suppl 1):113-22.
-
20Bronfen E, Rocha FSA, Machado GBN, Perillo MM, Romanha AJ, Chiari E. Isolation of Trypanosoma cruzi samples by xenodiagnosis and hemoculture from patients with chronic Chagas' disease. Mem Inst Oswaldo Cruz. 1989;84(2):237-40.
-
21Galvão LMC, Nunes RMB, Cançado JR, Brener Z, Krettli AU. Lytic antibody titre as a means of assessing cure after treatment of Chagas disease: a 10 years follow-up study. Trans R SocTrop Med Hyg.1993;87(2):220-3.
-
22Castro AM, Luquetti AO, Rassi A, Rassi GG, Chiari E, Galvão LMC. Blood culture and polymerase chain reaction for the diagnosis of the chronic phase of human infection with Trypanosoma cruzi Parasitol Res. 2002;88(10):894-900.
-
23Meira WSF, Galvão LMC, Gontijo ED, Machado-Coelho GL, Norris KA, Chiari E. Trypanosoma cruzi recombinant complement regulatory protein: a novel antigen for use in an enzyme-linked immunosorbent assay for diagnosis of Chagas' disease. J Clin Microbiol. 2002;40(10):3735-40.
-
24Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi Jr A, Rosas F, et al. Randomized trial of benznidazole for chronic Chagas' cardiomyopathy. N Engl J Med. 2015;373(14):1295-306.
-
25Marin-Neto JA, Rassi Jr A, Avezum Jr A, Mattos AC, Rassi A. The BENEFIT trial: testing the hypothesis that trypanocidal therapy is beneficial for patients with chronic Chagas heart disease. Mem Inst Oswaldo Cruz. 2009;104(Suppl. I):319-24.
-
26Rassi Jr A, Marin-Neto JA, Rassi A. Chronic Chagas cardiomyopathy: a review of the main pathogenic mechanisms and the efficacy of aetiological treatment following the BENznidazole Evaluation for Interrupting Trypanosomiasis (BENEFIT) trial. Mem Inst Oswaldo Cruz. 2017;112(3):224-35.
-
27Rocha MO, Ribeiro AL, Teixeira MM. Clinical management of chronic Chagas cardiomyopathy. Front Biosci. 2003;8:e44-54.
-
28Schijman AG, Bisio M, Orellana L, Sued M, Duffy T, Jaramillo AMM, et al. International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. PLoS Negl Trop Dis. 2011;5(1):e931.
-
29Ávila HA, Sigman DS, Cohen LM, Millikan RC, Simpson L. Polymerase chain reaction amplification of Trypanosoma cruzi kinetoplast minicircle DNA isolation from whole blood lysates: diagnosis of chronic Chagas' disease. Mol Biochem Parasitol. 1991;48(2):211-21.
-
30Britto C, Cardoso MA, Wincker P, Morel CM. A simple protocol for the physical cleavage of Trypanosoma cruzi kinetoplast DNA present in blood samples and its use in polymerase chain reaction (PCR)-based diagnosis of chronic Chagas' disease. Mem Inst Oswaldo Cruz. 1993;88(1):171-2.
-
31Macedo AM, Martins MS, Chiari E, Pena SDJ. DNA fingerprinting of Trypanosoma cruzi: a new tool for characterization of strains and clones. Mol Biochem Parasitol. 1992;55(1-2):147-53.
-
32D'Ávila DA, Macedo AM, Valadares HMS, Gontijo ED, Castro AM, Machado CR, et al. Probing population dynamics of Trypanosoma cruzi during progression of the chronic phase in chagasic patients. J Clin Microbiol. 2009;47(6):1718-25.
-
33Souto RP, Zingales B. Sensitive detection and strain classification of Trypanosoma cruzi by amplification of a ribosomal RNA sequence. Mol Biochem Parasitol. 1993;62(1):45-52.
-
34Freitas JM, Augusto-Pinto L, Pimenta JR, Bastos-Rodrigues L, Gonçalves VF, Teixeira SMR, et al. Ancestral genomes, sex, and the population structure of Trypanosoma cruzi PloS Pathog. 2006;2(3):e24.
-
35Burgos JM, Altcheh J, Bisio M, Duffy T, Valadares HMS, Seidenstein ME, et al. Direct molecular profiling of minicircle signatures and lineages of Trypanosoma cruzi bloodstream populations causing congenital Chagas disease. Int J Parasitol. 2007;37(12):1319-27.
-
36Hollander M, Wolfe DA, Chicken E. Nonparametric Statistical Methods. 3rd edition. Hoboken New Jersey, USA: John Wiley & Sons; 2014. 848p.
-
37Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions. 3rd edition Hoboken New Jersey, USA: John Wiley & Sons; 2003. 800p.
-
38Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-74.
-
39Minter-Goedbloed E. Hemoculture compared with xenodiagnosis for the detection of Trypanosoma cruzi infection in man and in animal. Proceedings of an International Symposium, Belo Horizonte, MG 1975. In: New approaches in American Tripanosomiasis Research. Washington: Pan American Health Organization, Scientific Publication n. 318; 1976. p. 245-50.
-
40Fernandes CD, Tiecher FM, Fernandes DD, Henriques NMP, Steindel M. High rates of positive hemocultures in children and teenagers seropositive for Trypanosoma cruzi in the State of Rio Grande do Sul, Brazil. Mem Inst Oswaldo Cruz. 1999;94(1):7-8.
-
41Minter-Goedbloed E, Minter DM, Marshall TFC. Quantitative comparison between xenodiagnosis and hemoculture in the detection of Trypanosoma (Schizotrypanum) cruzi in experimental and natural chronic infections. Trans R Soc Trop Med Hyg. 1978;72(3):217-25.
-
42Pereira JB, Willcox HP, Coura JR. Morbidity in Chagas' disease. III. Longitudinal study of 6 years, in Virgem da Lapa, MG, Brazil. Mem Inst Oswaldo Cruz. 1985;80(1):63-71.
-
43Silva IG, Silva HHG, Ostermayer AL, Rezende JM. Positividade do xenodiagnóstico de acordo com a faixa etária, o sexo e a forma clínica da doença de Chagas. Rev Pat Trop. 1995;24(2):193-7.
-
44Castro C, Macêdo V, Prata A. The behavior of Trypanosoma cruzi parasitemia in chronic chagasics over 13 years. Rev Soc Bras Med Trop. 1999;32(2):157-65.
-
45Castro C, Prata A, Macêdo V. Influência da parasitemia na evolução da doença de Chagas crônica. Rev Soc Bras Med Trop. 2005;38(1):1-6.
-
46Brener Z, Chiari E. Aspects of early growth of different Trypanosoma cruzi strains in culture medium. J Parasitol. 1965;51(6):922-6.
-
47Minter-Goedbloed E. The primary isolation by haemoculture of Trypanosoma (Schizotrypanum) cruzi from animals and from man. Trans R Soc Trop Med Hyg. 1978;72(1):22-30.
-
48Chiari E, Brener Z. Contribution to the parasitological diagnosis of human Chagas' disease in its chronic phase. Rev Inst Med Trop São Paulo. 1966;8(3):134-8.
-
49Junqueira ACV, Chiari E, Wincker P. Comparison of the polymerase chain reaction with two classical parasitological methods for the diagnosis of Chagas disease in an endemic region of northeastern Brazil. Trans R Soc Trop Med Hyg. 1996;90(2):129-32.
-
50Lages-Silva E, Crema E, Ramirez LE, Macedo AM, Pena SDJ, Chiari E. Relationship between Trypanosoma cruzi and human chagasic megaesophagus: blood and tissue parasitism. Am J Trop Med Hyg. 2001;65(5):435-41.
-
51Borges-Pereira J, Junqueira ACV, Santos LC, Castro JAF, Araujo IB, Coura JR. Xenodiagnosis in chronic Chagas' disease. I. The sensitivity of Panstrongylus megistus and Triatoma infestans. Rev Soc Bras Med Trop. 1996;29(4):341-7.
-
52Castro C, Prata A. Absence of both circadian rhythm and Trypanosoma cruzi periodicity with xenodiagnosis in chronic chagasic individuals. Rev Soc Bras Med Trop. 2000;33(5):427-30.
-
53Ramirez LE, Lages-Silva E, Alvarenga-Franco F, Matos A, Vargas N, Fernandes O, et al. High prevalence of Trypanosoma rangeli and Trypanosoma cruzi in opossums and triatomids in a formely-endemic area of Chagas disease in Southeast Brazil. Acta Trop. 2002;84(3):189-98.
-
54Britto CC. Usefulness of PCR-based assays to assess drug efficacy in Chagas disease chemotherapy: value and limitations. Mem Inst Oswaldo Cruz. 2009;104(Suppl. I):122-35.
-
55Chiaramonte MG, Frank FM, Furer GM, Taranto NJ, Margni RA, Malchiodi EL. Polymerase chain reaction reveals Trypanosoma cruzi suspected by serology in cutaneous and mucocutaneous leishmaniasis patients. Acta Trop. 1999;72(3):295-308.
-
56Ramírez JD, Cura CI, Moreira OC, Lages-Silva E, Juiz N, Velázquez E, et al. Analytical validation of quantitative real-time PCR methods for quantification of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. J Mol Diagn. 2015;17(5):605-15.
-
57Freitas JM, Lages-Silva E, Crema E, Pena SDJ, Macedo AM. Real time PCR strategy for the identification of major lineages of Trypanosoma cruzi directly in chronically infected human tissues. Int J Parasitol. 2005;35(4):411-7.
-
58Lages-Silva E, Ramirez LE, Pedrosa AL, Crema E, Galvão LMC, Pena SDJ. Variability of kinetoplast DNA gene signatures of Trypanosoma cruzi II strains from patients with different clinical forms of Chagas' disease in Brazil. J Clin Microbiol. 2006;44(6):2167-71.
-
59Brisse S, Verhoef J, Tibayrenc M. Characterisation of large and small subunit rRNA and mini-exon genes further supports the distinction of six Trypanosoma cruzi lineages. Int J Parasitol. 2001;31(11):1218-26.
-
60Steindel M, Pacheco LK, Scholl D, Soares M, Moraes MH, Eger I, et al. Characterization of Trypanosoma cruzi isolated from humans, vectors, and animal reservoirs following an outbreak of acute human Chagas disease in Santa Catarina State, Brasil. Diagn Microbiol Infect Dis. 2008;60(1):25-32.
-
61Zafra G, Mantilla JC, Valadares HM, Macedo AM, González CI. Evidence of Trypanosoma cruzi II infection in Colombian Chagasic patients. Parasitol Res. 2008;103(3):731-4.
-
62Câmara ACJ, Varela-Freire AA, Valadares HMS, Macedo AM, D'Ávila DA, Machado CR, et al. Genetic analyses of Trypanosoma cruzi isolates from naturally infected triatomines and humans in northeastern Brazil. Acta Trop. 2010;115(3):205-11.
-
63Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM, Teixeira MMG, et al. The revised Trypanosoma cruzi subspecific nomenclature: Rationale, epidemiological relevance and research applications. Infect Genet Evol. 2012;12(2):240-53.
-
64Bosseno MF, Barnabè, C, Gastélum EM, Kasten FL, Ramsey J, Espinoza B, et al. Predominance of Trypanosoma cruzi lineage I in Mexico. J Clin Microbiol. 2002;40(2):627-32.
-
65Añez M, Crisante G, Silva FM, Rojas A, Carrasco H, Umezawa ES, et al. Predominance of lineage I among Trypanosoma cruzi isolates from Venezuelan patients with different clinical profiles of acute Chagas' disease. Trop Med Int Health. 2004;9(12):1319-26.
-
66Sanchez-Guillén MC, Bernabé C, Tibayrenc M, Zavala-Castro J, Totolhua JL, Méndez-Lopez J, et al. Trypanosoma cruzi strains isolated from human, vector and animal reservoir in the same endemic region in 67.Mexico and typed as T. cruzi I, discrete typing unit 1 exhibit considerable biological diversity. Mem Inst Oswaldo Cruz. 2006;101(6):585-90.
-
68Teixeira MMG, Silva FM, Marcili A, Umezawa ES, Shikanai-Yasuda MA, Cunha-Neto E, et al. Short communication: Trypanosoma cruzi lineage I in endomyocardial biopsy from a north-eastern Brazilian patient at end-stage chronic chagasic cardiomyopathy. Trop Med Int Health. 2006;11(3):294-8.
-
69Abolis NG, Araujo SM, Toledo MJO, Fernandez MA, Gomes ML. Trypanosoma cruzi I-III in southern Brazil causing individual and mixed infections in human, sylvatic reservoirs and triatomines. Acta Trop. 2011;120(3):167-72.
-
70Martins K, Andrade CM, Barbosa-Silva AN, do Nascimento GB, Chiari E, Galvão LMC, et al. Trypanosoma cruzi III causing the indeterminate form of Chagas disease in a semi-arid region of Brazil. Int J Infect Dis. 2015;39:68-75.
-
71Yeo M, Acosta N, Llewellyn M, Sánchez H, Adamson S, Miles GAJ, et al. Origins of Chagas disease: Didelphis species are natural host of Trypanosoma cruzi I and armadillos hosts of Trypanosoma cruzi II, including hybrids. Int J Parasitol. 2005;35(2):225-33.
-
72Marcili A, Lima L, Valente VC, Valente SA, Batista JS, Junqueira ACV, et al. Comparative phylogeography of Trypanosoma cruzi TCIIc: New hosts, association with terrestrial ecotopes, and spatial clustering. Infect Genet Evol. 2009;9(6):1265-74.
-
73Ramírez JD, Guhl F, Rendón LM, Rosas F, Marin-Neto JA, Morillo CA. Chagas cardiomyopathy manifestations and Trypanosoma cruzi genotypes circulating in chronic chagasic patients. PLoS Negl Trop Dis. 2010;4(11):e899.
-
74Fernandes O, Souto RP, Castro JA, Borges-Pereira J, Fernandes NC, Junqueira AC, et al. Brazilian isolates of Trypanosoma cruzi from humans and triatomines classified into two lineages using mini-exon and ribosomal RNA sequences. Am J Trop Med Hyg. 1998;58(6):807-11.
-
Financial support: This work was supported by research grants from the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG; 23041) and Conselho Nacional de Desenvolvimento Científico e Tecnológico Chamada Universal - MCTI/CNPq N° 14/2013 (Proc. 475572/2013-0). DAD and EC received postdoctoral and researcher fellowships from the CNPq; LMCG and FCZV received visitor researcher and scholarship funds from the CNPq.
Publication Dates
-
Publication in this collection
Jul-Aug 2017
History
-
Received
10 Feb 2017 -
Accepted
10 Aug 2017