Abstract
INTRODUCTION:
Hantavirus cardiopulmonary syndrome (HCPS) is rare in Northeastern Brazil.
METHODS:
Prospective surveillance was conducted over a two-year period in Alagoas State, Northeastern Brazil. The prevalence of anti-hantavirus N-antigen IgM and IgG in human serum samples was determined by enzyme-linked immunosorbent assay testing.
RESULTS:
High avidity IgG was found in nine of 476 serum samples tested (from 102 patients with clinical manifestations compatible with HCPS, 124 patients with leptospirosis, and 250 healthy rural workers).
CONCLUSIONS:
Serologic evidence of past hantavirus infection in residents of Alagoas State indicates that hantaviruses are present in northeastern Brazil, even in areas silent for HCPS.
Keywords:
Hantavirus; Surveillance; Northeast Brazil
Hantaviruses are zoonotic viruses that were recently reclassified as belonging to the order Bunyavirales, family Hantaviridae, and genus Orthohantavirus11. International Committee on Taxonomy of Viruses (ICTV). Virus Taxonomy: 2016 Release. EC 48, Budapest, Hungary: ICTV; 2016. [Accessed in 2017 July 29]. Available from: Available from: https://talk.ictvonline.org/taxonomy/
https://talk.ictvonline.org/taxonomy/...
. These viruses cause human illnesses with a range of severity, classically referred to as hemorrhagic fever with renal syndrome, nephropathia epidemica, and hantavirus cardiopulmonary syndrome (HCPS)22. Bharadwaj M, Nofchissey R, Goade D, Koster F, Hjelle B. Humoral immune responses in the Hantavirus cardiopulmonary syndrome. J Infect Dis. 2000;182(1):43-8.. More recently, it has been suggested that these diseases could simply be referred to as hantavirus disease due to the increasing number of reports showing the overlap of symptoms during the acute course of these syndromes33. Clement J, Maes P, Lagrou K, Van Rast M, Lameire N. A unifying hypothesis and a single name for a complex globally emerging infection: hantavirus disease. Eur J Clin Microbiol Infect Dis. 2012;31(1):1-5..
In terms of virion structure, the N protein induces a strong humoral immune response and, therefore, seems to be a suitable antigen to target for serodiagnosis22. Bharadwaj M, Nofchissey R, Goade D, Koster F, Hjelle B. Humoral immune responses in the Hantavirus cardiopulmonary syndrome. J Infect Dis. 2000;182(1):43-8.. Enzyme-linked immunosorbent assay (ELISA) - both indirect immunoglobulin (IgG) and immunoglobulin (IgM) capture ELISA - is the most common serologic diagnostic method. Hantavirus antibodies are detectable early on during the onset of symptoms22. Bharadwaj M, Nofchissey R, Goade D, Koster F, Hjelle B. Humoral immune responses in the Hantavirus cardiopulmonary syndrome. J Infect Dis. 2000;182(1):43-8. and those secreted from immunologic memory plasma cells, including those antibodies with neutralizing activity, remain detectable many years after infection has occurred44. Valdivieso F, Vial P, Ferres M, Ye C, Goade D, Cuiza A, et al. Neutralizing antibodies in survivors of Sin Nombre and Andes hantavirus infection. Emerg Infect Dis. 2006;12(1):166-8..
Hantavirus cardiopulmonary syndrome has been reported in Brazil since 1993, particularly in regions in the Southern, Southeastern, and central plateau regions that have degraded ecosystems where native vegetation was originally covered by Atlantic forest and Cerrado (a type of Brazilian savanna). However, HCPS may be misdiagnosed in northeastern Brazil considering the local presence of the rodent hosts for both Juquitiba virus and Araraquara virus (Oligoryzomys nigripes and Necromys lasiurus, respectively)55. Firth C, Tokarz R, Simith DB, Nunes MRT, Bhat M, Travassos da Rosa ES, et al. Diversity and distribution of hantaviruses in South America. J Virol. 2012; 86(24):13756-66.. The combination of landscape changes resulting from sugar cane farming and the dry climate seems to favor the transmission of hantavirus and the emergence of HCPS, at least in Southeastern Brazil (especially in Ribeirão Preto in São Paulo State)66. Figueiredo LTM, Moreli ML, Souza RLM, Borges AA, Figueiredo GG, Machado AM, et al. Hantavirus pulmonary syndrome, central plateau, southeastern, and southern Brazil. Emerg Infect Dis . 2009; 15(4):561-7.. Although similar environmental conditions exist in Alagoas State (i.e. the agricultural landscape, dry climate, and the presence of at least two species of Brazilian rodent hantavirus reservoirs), this area is considered silent for hantavirus infection according to the Brazilian Ministry of Health’s official records. Thus, to investigate the circulation of hantavirus outside of areas in Northeastern Brazil where HCPS occurrence is well known, we conducted prospective surveillance for human hantavirus infection over a two-year period in both febrile patients and healthy individuals residing in Alagoas State, using ELISA testing to detect the N protein of hantavirus.
The study, conducted between July 2010 and September 2012, included 646 individuals living in Alagoas State in Northeastern Brazil. Participants were divided into four groups. Group I comprised 102 patients presenting with clinical manifestations considered compatible with HCPS who were admitted to hospitals or attended to at outpatient services in Maceió City and in Coruripe town. The criteria of the Brazilian Health Surveillance Department/Ministry of Health were used to define a suspected case of HCPS77. Ministério da Saúde (MS). Secretaria de Vigilância em Saúde. Guia de Vigilância Epidemiológica. 7ª edition. Brasília: MS; 2009. 816p.. Groups II and III comprised patients admitted to hospitals or attended to at public sector outpatient services in Alagoas state who presented with illnesses that included HCPS in the differential diagnosis. Group II comprised 124 patients with acute Leptospira spp. infection, confirmed by ELISA (Pan Bio®, Australia) IgM testing. Group III comprised 170 patients who were clinically suspected of having dengue infection; however, laboratory confirmation of dengue infection - by ELISA (Bio Rad®, France) detection of NS1 or IgM - was achieved in only 17 of these patients. Group IV comprised 250 healthy rural workers from Coruripe town who were part of a pilot serologic survey.
Informed consent was obtained from each study participant. Next, peripheral blood samples were drawn from each participant and stored at -20°C until analysis. The serum samples from groups I, II, and III were tested for anti-hantavirus IgM and IgG, whereas the samples from Group IV were tested for anti-hantavirus IgG only. Tests were performed by indirect ELISA using the N recombinant (rN) protein of Araraquara hantavirus (ARAV), produced in Escherichia coli, as the antigen. This technique has been described elsewhere88. Figueiredo LT, Moreli ML, Borges AA, de Figueiredo GG, Badra SJ, Bisordi I, et al. Evaluation of an enzyme-linked immunosorbent assay based on Araraquara virus recombinant nucleocapsid protein. Am J Trop Med Hyg. 2009;81(2):273-6.. Seropositive samples were titrated from dilutions of 1:100 to 1:6,400. All samples considered positive were tested four times to confirm the results. Serum samples obtained from patients from the Ribeirão Preto region who presented with acute HCPS - confirmed by reverse transcription polymerase chain reaction and ELISA testing99. Borges AA, Campos GM, Moreli ML, Moro Souza RL, Saggioro FP, Figueiredo GG, et al. Role of mixed Th1 and Th2 serum cytokines on pathogenesis and prognosis of hantavirus pulmonary syndrome. Microbes Infect. 2008;10(10-11):1150-7.- were preserved at -80 ºC and were used as positive controls for IgM tests. Serum samples collected after the convalescent phase of these patients were stored at -80ºC and were used as positive controls for the IgG tests. Samples considered positive for ARAV rN IgG were further tested to determine IgG avidity. This was performed by adapting the protocol previously described by Kallio-Kokko et al.1010. Kallio-Kokko H, Vapalahti O, Hedman K, Brummer-Korvenkontio M, Vaheri A. Puumala virus antibody and immunoglobulin G avidity assays based on a recombinant nucleocapsid antigen. J Clin Microbiol. 1993;31(3):677-80. and reproducing the ELISA test. For the avidity assay, after a period of incubation with ARAV rN, each serum sample was tested in quadruplicate as follows: The first duplicate was washed three times with 6M urea solution that was diluted in phosphate-buffered saline with 1% Tween 20 (PBST), while its duplicate counterpart was washed only with PBST. The IgG avidity result was calculated by comparing the absorbencies in the urea-washed wells with those in the PBST-washed wells and expressing the ratio as a percentage (IgG avidity index).
Participants in Group I ranged from >1 to 78 years of age (mean, 34.8 years; median 32.0 years); 56 (54.9%) were male and 46 (45.1%) were female. All patients in this group presented with acute symptoms and signs that met the definition for a suspected case of HCPS77. Ministério da Saúde (MS). Secretaria de Vigilância em Saúde. Guia de Vigilância Epidemiológica. 7ª edition. Brasília: MS; 2009. 816p. such as fever, myalgia, and dyspnea with or without thrombocytopenia and/or hemoconcentration; some presented with other symptoms such as chest pain, headache, arthralgia, and hemorrhagic signs. Anti-hantavirus IgM was not detected in any of these patients’ serum samples; however, one sample was reactive for anti-ARAV rN IgG with a titer 400 (Table 1) and an avidity index of 82.5% (Table 2).
Demographic and epidemiologic data of participants in the State of Alagoas whose serum samples tested positive for anti-hantavirus-N-protein immunoglobulin G.
IgG avidity results from serum samples with IgG antibodies against the N-protein of the Araraquara hantavirus.
The 124 (100 male and 24 female) patients in group II had a mean age of 30.9 years (median, 27 years); all had acute leptospirosis. Anti-hantavirus IgM was not detected in any of the 124 serum samples. However, anti-hantavirus IgG was detected in three (2.4%) serum samples taken from male patients (Table 1), with titers ranging from 400 (one sample) to 800 (two samples) and high IgG avidity indexes (>70%) (Table 2). Of note, it was possible to obtain sequential samples from two individuals, one in Group I and one in Group II; IgG prevalence was confirmed in the paired samples (Table 1).
Group III comprised 170 individuals with clinically suspected dengue infection; 71 (41.8%) were male and 99 (58.2%) were female. The mean age of Group III was 26.3 years (median, 25 years). The serologic test results of most (n=153) of the serum samples in this group were negative for dengue non-structural 1 antigen (NS1) and IgM on ELISA testing (Bio Rad®, France). All 170 samples from this group were negative for anti-hantavirus IgM and IgG.
To confirm the serologic findings obtained in groups I and II, we investigated the presence of IgG antibodies in healthy individuals from the local population who formed part of a pilot study for another serologic survey. Group IV comprised 250 healthy rural men working at a sugar mill in Coruripe town, located on the South coast of Alagoas State. Their mean age was 31.9 years. Of the 250 serum samples analyzed, five (2%) were reactive for anti-hantavirus IgG, with titers ranging from 400 to 1,600 (Table 1) and IgG avidity indexes up to 90% (Table 2). The mean age of seropositive participants was 33.8 years, and three (60%) had never worked outside of Alagoas State (Table 1). One of these patients - patient 479C - had the highest IgG titer (of 1,600) and avidity index (92.2%); he reported previously having experienced a severe disease that presented with fever, myalgia, and dyspnea, a triad of symptoms compatible with a diagnosis of HCPS.
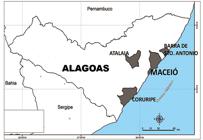
Overall, the prevalence of IgG was 1.4% (9/646). Figure 1 shows the cities where the participants who tested anti-hantavirus IgG positive, resided. The results obtained from Groups I, II, and IV demonstrate evidence of past infection with hantavirus in Alagoas State. In addition, four of the seropositive patients attested to having never worked outside of this state, suggesting that these infections occurred within the state’s geographic boundaries. The IgG prevalence in this study was lower than that of other regions in Brazil where HCPS is highly endemic, such as 13.6% in the State of Mato Grosso1111. Vieira CJSP, Silva DJF, Barreto ES, Siqueira CEH, Costa VG, Lourenço FJ, et al. Serological evidence of hantavirus infection in an urban area in Mato Grosso State, Brazil. Rev Soc Bras Med Trop. 2016;49(3):348-50.. However, an IgG prevalence of 2% was reported in Uberlandia City in Minas Gerais State1212. Limongi JE, Costa FC, Pinto RMC, Oliveira RC, Bragagnolo C, Lemos ERS, et al. Cross-sectional Survey of Hantavirus Infection, Brazil. Emerg Infect Dis . 2009;15(12):1981-3., which is another hantavirus-endemic region; this is similar to the prevalence found in the present study. The IgG prevalence found in the present study is in keeping with prevalence rates in other regions where HCPS has not been described, such as in the southern parts of Santa Catarina State (2.3%)1313. Pereira GW, Teixeira AM, Souza MS, Braga AD, Santos Jr GS, Figueiredo GG, et al. Prevalence of serum anti-bodies to hantavirus in a rural population from the Southern State of Santa Catarina, Brazil. Rev Soc Bras Med Trop . 2012;45(1):117-9., in Ceará State (2.8%)1414. Lima DM, Sabino-Santos Jr G, Oliveira ACA, Fontes RM, Colares JKB, Araújo FMC, et al. Hantavirus infection in suspected dengue cases from State of Ceará, Brazil. Rev Soc Bras Med Trop . 2011; 44(6):795-6., an in Ilhéus City in Bahia State (0.6%)1515. Moreli ML, Costa VG, Pariz FR. A seroepidemiological survey of hantavírus in Ilheus county. Am J Virol . 2012;1(1):18-23..
The Alagoas State map. Highlighted are the cities where individuals with anti-hantavirus IgG were found among febrile patients (Group I and Group II) and among healthy rural workers (Group IV). IgG: immunoglobulin G.
Since the first three cases of hantavirus infection were recognized in Brazil, cases of HCPS have rarely been reported along the coast of the Northeastern region. As most Northeastern states have never reported a case of HCPS, whether or not hantavirus is present and whether hantavirus infection is being misdiagnosed in this huge area remains unknown. This formed the basis for conducting this surveillance study in Alagoas state.
Study participants resided in several municipalities in different regions of Alagoas State as the main hospital where the surveillance was performed is a regional referral center for infectious diseases. Notably, the mean age of participants in all four study groups (30.9 years) was similar to the mean age of patients who have had HCPS in Brazil (33 years for men and 31 years for women1616. da Rosa Elkhoury M, da Silva Mendes W, Waldman EA, Dias JP, Carmo EH, Vasconcelos PFC. Hantavirus pulmonary syndrome: prognostic factors for death in reported cases in Brazil. Trans R Soc Trop Med Hyg. 2012;106(5):298-302.). Therefore, our surveillance focused on the population with the highest chance of hantavirus infection. However, the serum samples of all 396 febrile patients tested negative for anti-hantavirus IgM, and no cases of HCPS were detected during two years of surveillance. The lack of IgM positivity in the serosurvey was not associated with low ELISA sensitivity as the assay demonstrated high sensitivity (97.2%), specificity (100%), positive predictive value (100%), and negative predictive value (98.1%) for IgM detection against hantaviruses88. Figueiredo LT, Moreli ML, Borges AA, de Figueiredo GG, Badra SJ, Bisordi I, et al. Evaluation of an enzyme-linked immunosorbent assay based on Araraquara virus recombinant nucleocapsid protein. Am J Trop Med Hyg. 2009;81(2):273-6..
All positive samples were retested and titrated at least four times to confirm the results. In addition, the IgG avidity index was up to 80% in all positive samples, confirming the presence of long-term immunity to hantavirus in these subjects and reinforcing the specificity of the ELISA test. In keeping with this, Hedman et al.17 found high avidity IgG in 99% of subjects who had had symptoms of nephropathia epidemica caused by Puumala virus infection more than two years prior to blood sampling and testing17.
Although all 102 patients in Group 1 met the Brazilian Ministry of Health’s official criteria77. Ministério da Saúde (MS). Secretaria de Vigilância em Saúde. Guia de Vigilância Epidemiológica. 7ª edition. Brasília: MS; 2009. 816p.for a clinically suspected case of HCPS, this diagnosis was not considered by the treating clinicians, either at admission or during hospitalization. This could be because, to date, Alagoas and its vicinities are not officially considered at risk of hantavirus infection. On the other hand, official data from the municipal government show that, over the past decade in the City of Maceió, the Alagoas State capital, there have been 2,510 deaths from pneumonia without a defined etiological agent (MC Lima: Personal Communication, 2012). We speculate that some of these deaths may have been due to hantavirus infection.
In conclusion, this is the first study to demonstrate serologic evidence of past hantavirus infection in residents of Alagoas State. Our findings provide new data regarding hantaviruses in the Northeastern region of Brazil because Alagoas State - as well as the neighboring states on the Northeastern coast (such as the Sergipe and Paraíba States) - is currently considered a silent area for hantavirus infection. Our results, together with those of other studies1414. Lima DM, Sabino-Santos Jr G, Oliveira ACA, Fontes RM, Colares JKB, Araújo FMC, et al. Hantavirus infection in suspected dengue cases from State of Ceará, Brazil. Rev Soc Bras Med Trop . 2011; 44(6):795-6.,1515. Moreli ML, Costa VG, Pariz FR. A seroepidemiological survey of hantavírus in Ilheus county. Am J Virol . 2012;1(1):18-23., strongly suggest that at least one species or genotype of hantavirus is circulating in areas previously considered to be hantavirus free. Finally, our findings suggest that routine testing for hantavirus infection should be included in public health systems in the Northeastern states of Brazil as HCPS is probably misdiagnosed in this region.
Ethical considerations
This study was approved by the Research Ethics Committee of the Universidade Federal de Alagoas (process number 23065.009350/2010-62).
Acknowledgments
The authors are grateful to the clinical staff at the Hospital Escola Dr. Hélvio Auto (HEHA), especially to Luciana Maria de Medeiros Pacheco; Santa Casa de Misericórdia de Maceió; CENEFRON; Hospital Universitário Prof. Alberto Antunes; and to Telma Machado Lisboa Pinheiro (Laboratório Central de Saúde Pública de Alagoas - LACEN-AL) for their support with this study. We also thank Emanuelle Cavalcante Pimentel for her help with some of the serologic analyses. We are very grateful to the staff of Usina Coruripe: Israel Gomes, Lívia Jacinto, Adenilson Silva, and Maria de Fátima Fonseca for enrolling subjects during the pilot serologic survey. We also thank Ana Raquel Vasconcelos de Lima, Jaqueline Gomes de Lima, Grazielle Marques de Souza, Juliana de Melo e Silva, Rebeka Carolyne da Silva Feitosa, Danilo Machado de Melo, and Fernanda Christina Barros Farias da Silva for their assistance in collecting patients’ data and their blood samples. We are very grateful to Nicola Conran (Hemocentro/Universidade Estadual de Campinas - UNICAMP) and Juliano Bordignon (Instituto Carlos Chagas / Fundação Oswaldo Cruz - Paraná - ICC/FIOCRUZ-PR) for revising this manuscript.
REFERENCES
-
1International Committee on Taxonomy of Viruses (ICTV). Virus Taxonomy: 2016 Release. EC 48, Budapest, Hungary: ICTV; 2016. [Accessed in 2017 July 29]. Available from: Available from: https://talk.ictvonline.org/taxonomy/
» https://talk.ictvonline.org/taxonomy/ -
2Bharadwaj M, Nofchissey R, Goade D, Koster F, Hjelle B. Humoral immune responses in the Hantavirus cardiopulmonary syndrome. J Infect Dis. 2000;182(1):43-8.
-
3Clement J, Maes P, Lagrou K, Van Rast M, Lameire N. A unifying hypothesis and a single name for a complex globally emerging infection: hantavirus disease. Eur J Clin Microbiol Infect Dis. 2012;31(1):1-5.
-
4Valdivieso F, Vial P, Ferres M, Ye C, Goade D, Cuiza A, et al. Neutralizing antibodies in survivors of Sin Nombre and Andes hantavirus infection. Emerg Infect Dis. 2006;12(1):166-8.
-
5Firth C, Tokarz R, Simith DB, Nunes MRT, Bhat M, Travassos da Rosa ES, et al. Diversity and distribution of hantaviruses in South America. J Virol. 2012; 86(24):13756-66.
-
6Figueiredo LTM, Moreli ML, Souza RLM, Borges AA, Figueiredo GG, Machado AM, et al. Hantavirus pulmonary syndrome, central plateau, southeastern, and southern Brazil. Emerg Infect Dis . 2009; 15(4):561-7.
-
7Ministério da Saúde (MS). Secretaria de Vigilância em Saúde. Guia de Vigilância Epidemiológica. 7ª edition. Brasília: MS; 2009. 816p.
-
8Figueiredo LT, Moreli ML, Borges AA, de Figueiredo GG, Badra SJ, Bisordi I, et al. Evaluation of an enzyme-linked immunosorbent assay based on Araraquara virus recombinant nucleocapsid protein. Am J Trop Med Hyg. 2009;81(2):273-6.
-
9Borges AA, Campos GM, Moreli ML, Moro Souza RL, Saggioro FP, Figueiredo GG, et al. Role of mixed Th1 and Th2 serum cytokines on pathogenesis and prognosis of hantavirus pulmonary syndrome. Microbes Infect. 2008;10(10-11):1150-7.
-
10Kallio-Kokko H, Vapalahti O, Hedman K, Brummer-Korvenkontio M, Vaheri A. Puumala virus antibody and immunoglobulin G avidity assays based on a recombinant nucleocapsid antigen. J Clin Microbiol. 1993;31(3):677-80.
-
11Vieira CJSP, Silva DJF, Barreto ES, Siqueira CEH, Costa VG, Lourenço FJ, et al. Serological evidence of hantavirus infection in an urban area in Mato Grosso State, Brazil. Rev Soc Bras Med Trop. 2016;49(3):348-50.
-
12Limongi JE, Costa FC, Pinto RMC, Oliveira RC, Bragagnolo C, Lemos ERS, et al. Cross-sectional Survey of Hantavirus Infection, Brazil. Emerg Infect Dis . 2009;15(12):1981-3.
-
13Pereira GW, Teixeira AM, Souza MS, Braga AD, Santos Jr GS, Figueiredo GG, et al. Prevalence of serum anti-bodies to hantavirus in a rural population from the Southern State of Santa Catarina, Brazil. Rev Soc Bras Med Trop . 2012;45(1):117-9.
-
14Lima DM, Sabino-Santos Jr G, Oliveira ACA, Fontes RM, Colares JKB, Araújo FMC, et al. Hantavirus infection in suspected dengue cases from State of Ceará, Brazil. Rev Soc Bras Med Trop . 2011; 44(6):795-6.
-
15Moreli ML, Costa VG, Pariz FR. A seroepidemiological survey of hantavírus in Ilheus county. Am J Virol . 2012;1(1):18-23.
-
16da Rosa Elkhoury M, da Silva Mendes W, Waldman EA, Dias JP, Carmo EH, Vasconcelos PFC. Hantavirus pulmonary syndrome: prognostic factors for death in reported cases in Brazil. Trans R Soc Trop Med Hyg. 2012;106(5):298-302.
-
Financial support: The authors are very grateful to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financially supporting this study (Grant process number 478252/2010-2) and for the Programa Institucional de Bolsas de Iniciação Científica (PIBIC) research fellowships (processes: 152331/2010-7, 124383/2011-4; 100287/2012-3). We thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado de Alagoas (FAPEAL) (Grant 20110328-002-0004-0100; PIBIC-UFAL-2010-053; PIBIC-UFAL-2011-006) for Masters and PIBIC research fellowships.
Publication Dates
-
Publication in this collection
Nov-Dec 2017
History
-
Received
30 Mar 2017 -
Accepted
24 Aug 2017