Abstract
INTRODUCTION
West Nile virus (WNV) immunoglobulin M (IgM) antibodies have been shown to persist for up to 500 days in certain patients. To evaluate the usefulness of immunoglobulin G (IgG) avidity assessment in the diagnosis of WNV infection, we analyzed 54 WNV IgM- and/or IgG-positive serum samples from 39 patients with neuroinvasive disease and 15 asymptomatic cases tested during a seroprevalence investigation.
METHODS
Serological tests (WNV IgM/IgG antibody detection, IgG avidity) were performed using commercially available enzyme-linked immunosorbent assays.
RESULTS
WNV IgM antibodies were detected in 47 (87%) samples. Acute/recent WNV infection was confirmed based on low/borderline avidity index (AI) in 44 IgM-positive samples (93.6%). In three IgM-positive samples (6.4%), high IgG AIs were detected, thus indicating persisting IgM antibodies from previous infections. All IgM-negative samples showed high AIs. Patients with WNV neuroinvasive disease tested within 30 days showed low AIs. In six patients tested 34-50 days after disease onset, AI was borderline (42%-60%), suggesting earlier WNV IgG maturation. Samples with the highest IgM values were associated with the lowest AIs (Spearman's rho coefficient -0.767, p < 0.001).
CONCLUSIONS
Our results indicate that IgG avidity differentiates current/recent WNV infection from persistent IgM seropositivity from the previous WNV transmission season both in patients with WNV neuroinvasive disease and in asymptomatic persons. A strong negative correlation between IgM antibody levels and AI indicates that in cases with very high IgM levels, determination of IgG avidity may not be necessary. As many patients showed rapid avidity maturation, low IgG avidity is indicative of WNV infection within the previous month.
Keywords:
West Nile virus; Diagnosis; IgG avidity
INTRODUCTION
West Nile virus (WNV) is a mosquito-borne virus that belongs to the genus Flavivirus of the family Flaviviridae. Most human infections (approximately 80%) caused by this virus are subclinical. Approximately 20% of infected persons develop nonspecific febrile disease (WNV fever)11. Hayes EB, Sejvar JJ, Zaki SR, Lanciotti RS, Bode AV, Campbell GL. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg Infect Dis. 2005;11(8):1174-9.. Neuroinvasive disease (meningitis, encephalitis, and acute flaccid paralysis) occurs in less than 1% of infected persons but carries a fatality rate of approximately 10%22. Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013;310(3):308-15.. Although the onset of symptoms is preceded by a viremic period, viremia persists for only a short period and the disease usually manifests after the virus is no longer detectable. Therefore, the diagnosis of WNV infection is usually based on serological methods. Usually, immunoglobulin M (IgM) is considered to be a marker of acute infection33. Hazell SL. Clinical utility of avidity assays. Expert Opin Med Diagn. 2007;1(4):511-9.. However, IgM antibodies for WNV have been shown to persist for up to 500 days or even longer in certain patients44. Papa A, Danis K, Athanasiadou A, Delianidou M, Panagiotopoulos T. Persistence of West Nile virus immunoglobulin M antibodies, Greece. J Med Virol. 2011;83(10):1857-60.. As WNV IgM antibodies may last from one transmission season to the next, an early-season reactive IgM test could result from either a recent or a past WNV infection55. Roehring JT, Nash D, Maldin B, Labowitz A, Martin DA, Lanciotti RS, et al. Persistence of virus-reactive serum immunoglobulin M antibody in confirmed West Nile virus encephalitis cases. Emerg Infect Dis 2003;9:376-9.. In addition, WNV immunoglobulin A (IgA) antibodies show a persistence pattern similar to that of IgM antibodies, and are not helpful in the diagnosis of recent WNV infections. Two studies conducted among viremic blood donors showed that IgA antibodies were remarkably persistent, particularly during the first 200 days after infection, and in 57% of cases, they persisted for more than a year66. Prince HE, Tobler LH, Yeh C, Gefter N, Custer B, Busch MP. Persistence of West Nile virus-specific antibodies in viremic blood donors. Clin Vaccine Immunol 2007;14(9):1228-30.,77. Busch MP, Kleinman SH, Tobler LH, Kamel HT, Norris PJ, Walsh I, et al. Virus and antibody dynamics in acute West Nile virus infection. J Infect Dis 2008;198(7):984-93.. Avidity testing for the differentiation of recent and past antibody response has been used for the serodiagnosis of several flaviviruses such as dengue and tick-borne encephalitis virus88. Prince HE, Yeh C, Lapé-Nixon M. Utility of IgM/IgG ratio and IgG avidity for distinguishing primary and secondary dengue virus infections using sera collected more than 30 days after disease onset. Clin Vaccine Immunol 2011;18(11):1951-6.
9. Gassmann C, Bauer G. Avidity determination of IgG directed against tick-borne encephalitis virus improves detection of current infections. J Med Virol . 1997;51(3):242-51.-1010. Vilibic-Cavlek T, Barbic L, Stevanovic V, Petrovic G, Mlinaric-Galinovic G. IgG avidity: an important serologic marker for the diagnosis of tick-borne encephalitis virus infection. Pol J Microbiol. 2016;65(1):119-21.. This test is based on the affinity maturation of immunoglobulin G (IgG) antibodies against antigens during the immune response. In the primary infection, the early IgG antibody response is of a relatively low specificity, resulting in low IgG avidity. As the immune response matures, IgG antibodies become more specific and IgG avidity continues to increase for several months after infection55. Roehring JT, Nash D, Maldin B, Labowitz A, Martin DA, Lanciotti RS, et al. Persistence of virus-reactive serum immunoglobulin M antibody in confirmed West Nile virus encephalitis cases. Emerg Infect Dis 2003;9:376-9..
The aim of this study was to evaluate the usefulness of IgG avidity determination in the diagnosis of WNV neuroinvasive disease and asymptomatic WNV infection.
METHODS
During five transmission seasons (July 2012-October 2017), a total of 54 IgM- and/or IgG-positive serum samples from Croatian patients and asymptomatic persons with confirmed WNV infection, according to the European Union criteria for WNV1111. European Centre for Disease Control and Prevention (ECDC). Meeting report. Expert consultation on West Nile virus infection. Stockholm, 21-22 April, 2009. Available at: http://ecdc.europa.eu/en/publications/Publications/0909_MER_Expert_consultation_on_WNV.pdf.
http://ecdc.europa.eu/en/publications/Pu...
, were tested for IgG avidity. Thirty-nine samples were obtained from patients with clinical symptoms of WNV neuroinvasive disease (meningitis, encephalitis, and/or acute flaccid paralysis), collected from 6 to 50 days after the onset of symptoms1212. Pem-Novosel I, Vilibic-Cavlek T, Gjenero-Margan I, Pandak N, Peric L, Barbic L, et al. First outbreak of West Nile virus neuroinvasive disease in humans, Croatia, 2012. Vector Borne Zoonotic Dis. 2014;14(1):82-4.,1313. Vilibic-Cavlek T, Kaic B, Barbic L, Pem-Novosel I, Slavic-Vrzic V, Lesnikar V, et al. First evidence of simultaneous occurrence of West Nile virus and Usutu virus neuroinvasive disease in humans in Croatia during the 2013 outbreak. Infection. 2014;42(4):689-95.. Fifteen positive samples were identified among randomly selected asymptomatic persons during a seroprevalence investigation (unpublished data of the Reference Centre for Diagnosis and Surveillance of Viral Zoonoses of the Croatian Ministry of Health). Serological tests were performed at the National Reference Laboratory for Arboviruses, Croatian Institute of Public Health. IgM and IgG antibody levels were determined using a commercially available enzyme-linked immunosorbent assay kit [anti-West Nile virus enzyme-linked immunosorbent assay (ELISA) IgM/IgG, Euroimmun, Lübeck, Germany] and interpreted according to the manufacturer’s instructions: IgM (ratio) < 0.8, negative; 0.8-1.1, borderline; >1.1, positive; and IgG (relative units; RU/mL) < 16, negative; 16-22, borderline; > 22, positive. IgG avidity was tested using urea as a denaturing agent (Avidity: West Nile virus ELISA IgG, Euroimmun, Lübeck, Germany). The avidity index (AI) was calculated and interpreted as follows: < 40%, low; 40%-60%, borderline; and > 60%, high.
In all cases, WNV infection was confirmed via a virus neutralization test (VNT) performed at the OIE Reference Centre for West Nile Disease, Istituto Zooprofilattico Sperimentale "G. Caporale", Teramo, Italy1414. Di Gennaro A, Lorusso A, Casaccia C, Conte A, Monaco F, Savini G. Serum neutralization assay can efficiently replace plaque reduction neutralization test for detection and quantitation of West Nile virus antibodies in human and animal serum samples. Clin Vaccine Immunol . 2014;21(10):1460-2., or via reverse transcription polymerase chain reaction (RT-PCR) according to the protocol described by Tang et al1515. Tang Y, Anne Hapip C, Liu B, Fang CT. Highly sensitive TaqMan RT-PCR assay for detection and quantification of both lineages of West Nile virus RNA. J Clin Virol. 2006; 36(3):177-82..
Statistical analysis
The strength and direction of a possible linear relationship between WNV IgM and AI were assessed using Spearman's correlation test. For statistical analysis, the software package Stata/IC version 11.2 (StataCorp LP, USA) was used.
Ethicals considerations
This study was approved by the Ethics Committee of the Croatian Institute of Public Health.
RESULTS
The samples were divided into two groups according to the WNV IgM/IgG results: group I (IgM-positive/equivocal and IgG-positive), 47 (87%) samples; and group II (IgM-negative and IgG-positive), 7 (13%) samples. In the IgM-positive sample group, acute/recent primary WNV infection was confirmed based on IgG avidity in 44 (93.6%) patients: low AI was detected in 36 (76.6%) patients and borderline AI in 8 (17%) patients. Three (6.4%) IgM-positive samples, as well as all 7 IgM-negative samples showed high AI, indicating a past WNV infection (Table 1).
With regard to clinical symptoms, positive/equivocal IgM antibody levels were detected in 39 (100%) patients with WNV neuroinvasive disease and in 8 (53.3%) asymptomatic subjects. Using AI, acute/recent infection was confirmed in all IgM-positive patients (low AI, 33 (84.6%) patients; borderline AI, 6 (15.4%) patients) and in 5 (62.5%) asymptomatic subjects (low AI, 3 (37.5%) patients; borderline AI, 2 (25.0%) patients). Three (37.5%) IgM-positive and all 7 IgM-negative asymptomatic subjects showed high AIs (Table 1).
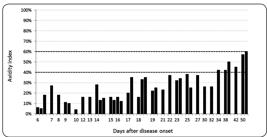
The AIs of WNV IgM-/IgG-positive patients are presented in Figure 1. Within 30 days after the disease onset, all patients showed low AIs. Six patients tested on days 34, 38, 42, and 50 showed borderline AIs (42-60%).
IgG avidity in patients with West Nile virus neuroinvasive disease. IgG: immunoglobulin G.
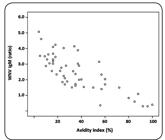
Correlation of West Nile virus IgM antibody levels and IgG avidity. IgM: immunoglobulin M; IgG: immunoglobulin G.
There was a statistically significant strong negative correlation between IgM antibody levels and AIs. Samples with the highest IgM levels were associated with the lowest AIs (Spearman's rho coefficient -0.767, p<0.001, Figure 2).
DISCUSSION
Owing to the short and low-level viremic period, serology remains an important tool for the diagnosis of human WNV infection. The detection of IgM antibodies is the most commonly used diagnostic method for confirming a current/recent infection1616. Niedrig M, Nitsche A, Donoso-Mantke O. Arthropod-borne viruses. In: Jerome KR, editor. Lennette's laboratory diagnosis of viral infections. 4th edition. New York: Informa Healthcare: 2010, p. 450-7.. A recently published study from Greece showed IgM persistence in 12% of patients 3 years after WNV infection1717. Papa A, Anastasiadou A, Delianidou M. West Nile virus IgM and IgG antibodies three years post- infection. Hippokratia. 2015;19(1):34-6.. In addition, a study from Houston found that 42%, 34%, and 23% of persons showed positive or equivocal results when tested for WNV IgM antibodies approximately 1, 6, and 8 years post infection, respectively1818. Murray KO, Garcia MN, Yan C, Gorchakov R. Persistence of detectable immunoglobulin M antibodies up to 8 years after infection with West Nile virus. Am J Trop Med Hyg. 2013;89(5):996-1000.. The presence of WNV-reactive IgM antibodies in the serum, therefore, is not necessarily diagnostic of an acute WNV infection55. Roehring JT, Nash D, Maldin B, Labowitz A, Martin DA, Lanciotti RS, et al. Persistence of virus-reactive serum immunoglobulin M antibody in confirmed West Nile virus encephalitis cases. Emerg Infect Dis 2003;9:376-9.. In this study, 87% of tested participants showed positive IgM antibody detection tests. By combining IgM levels with IgG avidity determination, a recent WNV infection was confirmed based on low/borderline AI in 93.6% of IgM-positive participants. However, 6.4% of participants showed high-avidity IgG antibodies, indicating that IgM antibodies were reflective of a previous infection.
Because the majority of WNV infections are asymptomatic, seroprevalence studies performed during/following the transmission season provide useful information on virus circulation in a particular area. In this study, WNV IgM antibodies were detected in 53.3% of asymptomatic persons. In 62.5% of them, serological response was suggestive of a WNV infection contracted during the current transmission season (low/borderline AI), whereas 37.5% demonstrated a high AI, which indicates persistent IgM antibodies from the previous transmission season. All IgM-negative asymptomatic persons showed IgG antibodies of high avidity.
During viral infections, maturation of IgG antibodies to a high avidity level usually occurs 3 to 6 months after infection33. Hazell SL. Clinical utility of avidity assays. Expert Opin Med Diagn. 2007;1(4):511-9.. Levett et al.1919. Levett PN, Sonnenberg K, Sidaway F, Shead S, Niedrig M, Steinhagen K, et al. Use of immunoglobulin G avidity for differentiation of primary from previous infections with West Nile virus. J Clin Microbiol. 2005;43(12):5873-5. found high IgG avidity in all serum samples tested six months or more after exposure to WNV, indicating that persons exposed to WNV during the preceding transmission season will mostly demonstrate high IgG avidity if tested the following spring. Low-avidity IgG antibodies were identified in 86% of the ELISA tests and 95% of indirect immunofluorescence analyses (IFA) of IgG-positive patient samples collected between 2 and 43 days after the onset of symptoms. In this study, all patients with WNV neuroinvasive disease tested within 30 days after the disease onset showed low AIs, whereas patients tested 34 to 50 days after disease onset showed borderline AIs (42-60%). Prince et al.88. Prince HE, Yeh C, Lapé-Nixon M. Utility of IgM/IgG ratio and IgG avidity for distinguishing primary and secondary dengue virus infections using sera collected more than 30 days after disease onset. Clin Vaccine Immunol 2011;18(11):1951-6. showed similar results with regard to dengue virus (DENV) infection. When considering IgG avidity, 95% of serum samples collected within 30 days after the disease onset were correctly classified as primary DENV infections. Fox et al.2020. Fox JL, Hazell SL, Tobler LH, Busch MP. Immunoglobulin G avidity in differentiation between early and late antibody responses to West Nile virus. Clin Vaccine Immunol . 2006;13(1):33-6. demonstrated even earlier maturation of IgG avidity during a WNV infection. The AI was low in WNV-positive blood donors tested within 20 days of index sampling [WNV ribonucleic acid (RNA)-positive], and was borderline in the period between approximately 30 and 60 days. Surprisingly, 33% of samples collected within 90 days exhibited high AIs. Particularly noteworthy was a cluster of samples with high AIs collected within 25 days from index sampling. In addition to rapid maturation of WNV IgG antibodies, another possibility is that these individuals were exposed to (or vaccinated against) another flavivirus in the past and produced high-avidity WNV IgG antibodies as part of an anamnestic response to common flavivirus antigens2121. Prince HE, Lapé-Nixon M, Busch MP, Tobler LH, Foster GA, Stramer SL. Utilization of follow-up specimens from viremic blood donors to assess the value of West Nile virus immunoglobulin G avidity as an indicator of recent infection. Clin Diagn Lab Immunol. 2005;12(9):1123-6..
The results of this study showed a strong negative correlation between IgM antibody levels and IgG avidity. Samples with the highest WNV IgM levels showed the lowest AI values, suggesting that in cases with very high IgM levels, the determination of IgG avidity may not be necessary.
In conclusions, our results indicate that IgG avidity differentiates current/recent WNV infection from persistent IgM seropositivity due to the previous WNV transmission season both in patients with WNV neuroinvasive disease and in asymptomatic persons. Differentiation of recent infection in patients with clinical symptoms of neuroinvasive disease is important, as WNV infection could be misdiagnosed for other viral central nervous system (CNS) infections with similar seasonal distribution (enteroviruses), as well as viruses that may present similar clinical symptoms but require specific therapy (herpes simplex virus, varicella-zoster virus). In addition, detection of a recent WNV infection in asymptomatic patients during the transmission season suggests virus circulation in the current year. High IgM antibody titer is a strong predictor for low IgG avidity. As many patients showed rapid WNV avidity maturation, a low AI is indicative of WNV infection within the previous month. However, because of the small sample size, further studies on a larger cohort are required to confirm this observation.
REFERENCES
-
1Hayes EB, Sejvar JJ, Zaki SR, Lanciotti RS, Bode AV, Campbell GL. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg Infect Dis. 2005;11(8):1174-9.
-
2Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013;310(3):308-15.
-
3Hazell SL. Clinical utility of avidity assays. Expert Opin Med Diagn. 2007;1(4):511-9.
-
4Papa A, Danis K, Athanasiadou A, Delianidou M, Panagiotopoulos T. Persistence of West Nile virus immunoglobulin M antibodies, Greece. J Med Virol. 2011;83(10):1857-60.
-
5Roehring JT, Nash D, Maldin B, Labowitz A, Martin DA, Lanciotti RS, et al. Persistence of virus-reactive serum immunoglobulin M antibody in confirmed West Nile virus encephalitis cases. Emerg Infect Dis 2003;9:376-9.
-
6Prince HE, Tobler LH, Yeh C, Gefter N, Custer B, Busch MP. Persistence of West Nile virus-specific antibodies in viremic blood donors. Clin Vaccine Immunol 2007;14(9):1228-30.
-
7Busch MP, Kleinman SH, Tobler LH, Kamel HT, Norris PJ, Walsh I, et al. Virus and antibody dynamics in acute West Nile virus infection. J Infect Dis 2008;198(7):984-93.
-
8Prince HE, Yeh C, Lapé-Nixon M. Utility of IgM/IgG ratio and IgG avidity for distinguishing primary and secondary dengue virus infections using sera collected more than 30 days after disease onset. Clin Vaccine Immunol 2011;18(11):1951-6.
-
9Gassmann C, Bauer G. Avidity determination of IgG directed against tick-borne encephalitis virus improves detection of current infections. J Med Virol . 1997;51(3):242-51.
-
10Vilibic-Cavlek T, Barbic L, Stevanovic V, Petrovic G, Mlinaric-Galinovic G. IgG avidity: an important serologic marker for the diagnosis of tick-borne encephalitis virus infection. Pol J Microbiol. 2016;65(1):119-21.
-
11European Centre for Disease Control and Prevention (ECDC). Meeting report. Expert consultation on West Nile virus infection. Stockholm, 21-22 April, 2009. Available at: http://ecdc.europa.eu/en/publications/Publications/0909_MER_Expert_consultation_on_WNV.pdf.
» http://ecdc.europa.eu/en/publications/Publications/0909_MER_Expert_consultation_on_WNV.pdf. -
12Pem-Novosel I, Vilibic-Cavlek T, Gjenero-Margan I, Pandak N, Peric L, Barbic L, et al. First outbreak of West Nile virus neuroinvasive disease in humans, Croatia, 2012. Vector Borne Zoonotic Dis. 2014;14(1):82-4.
-
13Vilibic-Cavlek T, Kaic B, Barbic L, Pem-Novosel I, Slavic-Vrzic V, Lesnikar V, et al. First evidence of simultaneous occurrence of West Nile virus and Usutu virus neuroinvasive disease in humans in Croatia during the 2013 outbreak. Infection. 2014;42(4):689-95.
-
14Di Gennaro A, Lorusso A, Casaccia C, Conte A, Monaco F, Savini G. Serum neutralization assay can efficiently replace plaque reduction neutralization test for detection and quantitation of West Nile virus antibodies in human and animal serum samples. Clin Vaccine Immunol . 2014;21(10):1460-2.
-
15Tang Y, Anne Hapip C, Liu B, Fang CT. Highly sensitive TaqMan RT-PCR assay for detection and quantification of both lineages of West Nile virus RNA. J Clin Virol. 2006; 36(3):177-82.
-
16Niedrig M, Nitsche A, Donoso-Mantke O. Arthropod-borne viruses. In: Jerome KR, editor. Lennette's laboratory diagnosis of viral infections. 4th edition. New York: Informa Healthcare: 2010, p. 450-7.
-
17Papa A, Anastasiadou A, Delianidou M. West Nile virus IgM and IgG antibodies three years post- infection. Hippokratia. 2015;19(1):34-6.
-
18Murray KO, Garcia MN, Yan C, Gorchakov R. Persistence of detectable immunoglobulin M antibodies up to 8 years after infection with West Nile virus. Am J Trop Med Hyg. 2013;89(5):996-1000.
-
19Levett PN, Sonnenberg K, Sidaway F, Shead S, Niedrig M, Steinhagen K, et al. Use of immunoglobulin G avidity for differentiation of primary from previous infections with West Nile virus. J Clin Microbiol. 2005;43(12):5873-5.
-
20Fox JL, Hazell SL, Tobler LH, Busch MP. Immunoglobulin G avidity in differentiation between early and late antibody responses to West Nile virus. Clin Vaccine Immunol . 2006;13(1):33-6.
-
21Prince HE, Lapé-Nixon M, Busch MP, Tobler LH, Foster GA, Stramer SL. Utilization of follow-up specimens from viremic blood donors to assess the value of West Nile virus immunoglobulin G avidity as an indicator of recent infection. Clin Diagn Lab Immunol. 2005;12(9):1123-6.
-
Financial support: This study was supported in part by the Croatian Science Foundation, Project No. IP-2016-06-7456: "Prevalence and Molecular Epidemiology of Emerging and Re-emerging Neuroinvasive Arboviral Infections in Croatia" (to Tatjana Vilibic-Cavlek).
Publication Dates
-
Publication in this collection
Sep-Oct 2018
History
-
Received
11 Jan 2018 -
Accepted
18 July 2018