Abstract
INTRODUCTION:
We evaluated clinical and epidemiological characteristics of Trypanosoma cruzi infection in Sergipe.
METHODS:
In this cross-sectional study, we collected serum samples to identify serological markers of Chagas disease. A questionnaire was used, and electrocardiogram, echocardiogram, chest radiography, and contrast radiography of esophagus and colon were performed.
RESULTS:
T. cruzi infection seroprevalence was 12.1%, mean age of subjects was 55 years, 90% had an elementary school education, 78.6% were agriculture workers, and 60.5% had electrocardiographic abnormalities.
CONCLUSIONS:
A high prevalence of T. cruzi infection was observed in mostly elderly individuals.
Keywords:
Trypanosoma cruzi; Seroprevalence; Epidemiological variables
American trypanosomiasis was first described by Carlos Chagas in 1909 and remains a serious health problem with socioeconomic repercussions. It is estimated that 6-8 million people are infected with Trypanosoma cruzi worldwide, the majority in endemic countries of Latin America with an annual incidence of 28,000 cases11. World Health Organization (WHO). Chagas disease (American trypanosomiasis). WHO; March 2017. Available at: http://www.who.int/mediacentre/factsheets/fs340/en/.
http://www.who.int/mediacentre/factsheet...
.
The Sergipe State Health Department reports that the municipality of Umbaúba is considered at high risk from Chagas disease (ChD). However, according to Martins-Melo et al.22. Martins-Melo FR, Ramos Jr AN, Alencar CH, Heukelbach J. Prevalence of Chagas disease in Brazil: a systematic review and meta-analysis. Acta Trop. 2014;130(1):167-74. in a systematic review, no data have been found regarding the disease in the State of Sergipe. Thus, this study aimed to evaluate the seroprevalence of T. cruzi infection and the clinical characteristics in infected individuals of the Umbaúba municipality.
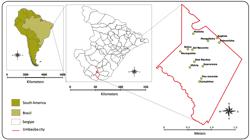
This study was conducted in 12 settlements in the rural area of the municipality of Umbaúba, located at the Southern region of the State of Sergipe, Brazil. This region covers an area of approximately 121.1 km2 with characteristic semiarid vegetation (Figure 1). This rural area is composed of 32 settlements with a resident population of 8,304 inhabitants33. Prefeitura Municipal de Umbaúba. Secretaria de Planejamento. Dados demográficos da população rural.2016..
Map of South America, showing Brazil, the State of Sergipe, the municipality of Umbaúba, and the settlements that were evaluated.
This was a cross-sectional study conducted from February 2015 to December 2016, including individuals of both sexes between the ages of 18 and 80 years. The following assumptions were adopted to calculate the sample size: a ChD prevalence of 5.9% in Sergipe according to a serological survey conducted in 197544. Camargo ME, Silva GR, Castilho EA, Silveira AC. Inquérito sorológico da prevalência da infecção chagásica no Brasil, 1975-1980. Rev Inst Med Trop São Paulo.1984; 26(4):192-204., an absolute precision of 2%, a 5% level of significance, and a potential 20% loss. The sample size was estimated as 640 individuals.
At schools and health centers from the settlements of Guararema, Palmeirinha, Matinha, Mangabeira, Eugênia, Campinhos, Vitória, Pau-Amarelo, Estiva, Macaquinho, Dois Riachos, and Sol Nascente, participants completed a questionnaire providing sociodemographic and epidemiological information. A blood sample was collected from each participant. Two serological assays were performed to detect T. cruzi infection: indirect immunofluorescence and an enzyme-linked immunosorbent assay. Both tests used Bio-Manguinho kits from Fundação Oswaldo Cruz (FIOCRUZ).
Seropositive individuals were clinically evaluated with emphasis on the cardiovascular and digestive systems. The diagnostic tests included an electrocardiogram, echocardiographic exam, chest radiography, and contrast radiography of the esophagus and colon to determine the clinical form of the disease. These exams were interpreted by the same examiner.
The sociodemographic, clinical characteristics, and results of diagnostic tests were logged in Excel spreadsheets (Microsoft Excel software, version 2010). GraphPad Prism software version 7.0 was used for statistical analysis and graphing. The χ2 test was used for categorical variables, and the significance level was set at 5%.
A total of 617 individuals participated in the study, 75 of whom were seropositive for T. cruzi with a prevalence of 12.1%, a mean [standard deviation] age of 55.0 [11] years, and a female predominance of 65.3% (n = 49). The main characteristics of the seropositive and seronegative populations are presented in Table 1.
Sociodemographic and epidemiological characteristics of positive and negative cases of Trypanosoma cruzi in Umbaúba, Sergipe, Brazil.
Clinical evaluations were performed of 71 seropositive patients; the initial sample lost four individuals: one abandoned the study, two presented clinical complications of associated diseases, and one moved out of the study area. The classification of ChD clinical forms showed 25 individuals with the cardiac form (35.2%) and 24 with the indeterminate form (33.8%) (Figure 2A). Arterial hypertension was observed in 38% (27/71) and diabetes mellitus in 11.3% (8/71) of the study participants.
In addition, electrocardiogram abnormalities were found in 60.5% (43/71) of the participants, right bundle branch block (RBBB) in 16.2% (7/43), and RBBB associated with left anterior fascicular block (LAFB) in 9.3% (4/43) (Figure 2B). The abnormality revealed by the echocardiogram test was left ventricular diastolic dysfunction in 30 individuals (42.2%). Despite the identified abnormalities, the subjects did not report any specific cardiac symptoms.
The digestive form of ChD was evident in four individuals (5.6%), two with megaesophagus and two with megacolon. The cardiodigestive form was observed in 18 individuals (25.3%), four of whom had associated megaesophagus and megacolon (Figure 2C). Megaesophagus was revealed in 21.1% (15/71) [six (40%) classified in group I, six (40%) in group II, and three (20%) in group III] and megacolon in 15.4% (11/71) of the individuals (Figure 2D and Figure 2E). Constipation was identified in 12.6% (9/71) of the individuals, although only two had megacolon.
Clinical characteristics of patients with Chagas disease. A. Percentage of patients according to clinical form. B. Percentage of patients according to the most frequent electrocardiographic changes: CDRB; RBBB; LAFB; CDLB; VES; SVES; RBBB + LAFB and AVB. C. Percentage of patients according to digestive alterations. D. Contrast radiography image of the characteristic megaesophagus. E. Contrast radiography image of the characteristic megacolon. CDRB: conduction disorder in the right branch; RBBB: right bundle branch block; LAFB: left anterior fascicular block; CDLB: conduction disorder in the left branch; VES: ventricular extrasystole; SVES: supraventricular extrasystole; RBBB + LAFB: right bundle branch block associated with left anterior fascicular block; AVB: atrioventricular block.
This rural study in the settlements of Umbaúba showed a high prevalence of T. cruzi infection. According to a national survey from 1978-1980, the prevalence of Chagas infection in Northeastern Brazil was 3.05%, and 5.9% in Sergipe44. Camargo ME, Silva GR, Castilho EA, Silveira AC. Inquérito sorológico da prevalência da infecção chagásica no Brasil, 1975-1980. Rev Inst Med Trop São Paulo.1984; 26(4):192-204.. The majority of the seropositive individuals were ≥50 years old, indicating that these patients were probably infected by vectors in the 1970s when there was a significant infestation of triatomines in the national territory.
The transmission of Chagas disease occurs in environments of precarious living conditions with poorly constructed houses, reflecting low social and economic conditions55. Silveira AC. Situação do controle da transmissão vetorial da doença de Chagas nas Américas. Cad Saude Publica. 2000;16(2):35-42.. Although most of the seropositive population currently resides in masonry houses, 16% did not have coated internal walls. Even with the construction of new houses, mud houses continue to function as storage areas favoring propitious conditions for the reproduction and maintenance of triatomines.
More than half of the seropositive individuals reported previous contact with the disease vector, which is statistically significant higher compared with the seronegative individuals. These findings have been reported by other authors66. Andrade JP, Marin Neto JA, Paola AAV, Vilas-Boas F, Oliveira GMM, Bacal F, et al. I Diretriz Latino-Americana para o diagnóstico e tratamento da cardiopatia chagásica: resumo executivo. Arq Bras Cardiol. 2011;96(6):434-42..
The majority of individuals were in the advanced age group and had developed determinate forms of the disease. These data corroborate data previously reported in the literature, showing 30% to 40% of classic forms of ChD usually developed 10-30 years after the initial infection77. Dias JCP, Ramos Jr AN, Gontijo ED, Luquetti A, Shikanai-Yasuda MA, Coura JR, et al. II Consenso Brasileiro em Doença de Chagas, 2015. Epidemiol Serv Saude. 2016;25(1):7-86..
Chronic chagasic cardiopathy was the most frequent form observed in this study, which is similar to the results of Borges-Pereira et al.88. Borges-Pereira J, Xavier SS, Pirmez C, Coura JR. Doença de Chagas em Virgem da Lapa, Minas Gerais, Brasil. IV. Aspectos clínicos e epidemiológicos do aneurisma ventricular esquerdo. Rev Soc Bras Med Trop. 1998;31(5):457-63., who retrospectively assessed 298 patients with ChD and observed 30% with heart disease. Although the study participants did not report specific cardiac complaints, arterial hypertension was observed in 38% of the participants. RBBB isolated or associated with LAFB was detected in 25% of the participants and are the most commonly reported alterations in patients with ChD99. Goldbaum M, Ajimura FY, Litvoc J, Carvalho SA, Eluf-Neto J. American trypanosomiasis and electrocardiographic alterations among industrial workers in São Paulo, Brazil. Rev Inst Med Trop São Paulo . 2004;46(6):299-302.. In the State of Sergipe, 46.3% of patients with ChD had changes electrocardiographic in the national electrocardiographic survey conducted between 1977 and 19811010. Gonçalves JG, Prata A, Dias JCP, Macedo V. O inquérito eletrocardiográfico. Rev Soc Bras Med Trop . 2011;44(2):40-6.. In this study, the frequency was higher (60.5%) and similar to that reported in the State of Goiás (63.6%), which was the highest percentage in the survey.
Digestive disorders occur in 15-20% of patients with ChD in endemic areas1111. Peñaranda-Carrillo R, Castro C, Rezende J, Prata A, Macêdo V. Estudo radiológico do esôfago de chagásicos, em 25 anos do Projeto Mambaí. Rev Soc Bras Med Trop . 2006;39(2):152-5., especially disorders of the esophagus and colon. This is mainly a result of the involvement of the enteric nervous system, particularly the Auerbach myenteric plexus with motor incoordination, sphincter achalasia, muscle hypertrophy, and dilatation. We observed a high presence of megaesophagus, similar to that reported by Castro et al. (18.6%)1212. Castro C, Penaranda-Carrilo R, Rezende J, Prata A. Estudo longitudinal do megaesôfago chagásico. Rev Soc Bras Med Trop . 2009;42(2):69-72., distributed among groups I , II and III. Because of the slow progression of dysphagia, participants in group III had already reported significant dysphagia with impaired quality of life. Two patients of this group underwent surgical treatment during the study with satisfactory results.
Although some authors1313. Macêdo V. Influência da exposição à reinfecção na evolução da doença de Chagas. Rev Pat Trop. 1976;5(1):33-116. consider a megacolon diagnosis based only on clinical criteria because of the difficulty of conducting contrast radiography and because it is an uncomfortable exam for the patient, the frequency of constipation was 12.6% in this study, and of these, only 2.8% presented megacolon on contrast radiography. However, we identified a prevalence of 15.4% of megacolon in the studied population (71 participants submitted to this test), which is similar to the prevalence reported by Andrade et al.1414. Andrade CM, Câmara ACJ, Nunes DF, Guedes PMM, Pereira WO, Chiari E, et al. Chagas disease: morbidity profile in an endemic area of Northeastern Brazil. Rev Soc Bras Med Trop . 2015;48(6):706-15. in Rio Grande do Norte (12.9%).
In conclusion, the prevalence of T. cruzi infection in the 12 settlements in the municipality of Umbaúba was 12.1%. Most seropositive individuals were in the advanced age group, showed a low level of education, and were unaware of being disease carriers. Many of these individuals had already developed one of the determinate forms of ChD, although with an absence of symptoms.
Ethical considerations
The study was approved by the Human Research Ethics Committee of the Federal University of Sergipe under protocol number CAAE-0010.0.107.000-11. All subjects were informed about the study protocol and signed the informed consent form.
Acknowledgments
The authors would like to thank the participants involved in this study and the health authorities and agents of the Umbaúba Municipal Health Secretary for their indispensable support in the field activities during the study.
REFERENCES
-
1World Health Organization (WHO). Chagas disease (American trypanosomiasis). WHO; March 2017. Available at: http://www.who.int/mediacentre/factsheets/fs340/en/
» http://www.who.int/mediacentre/factsheets/fs340/en/ -
2Martins-Melo FR, Ramos Jr AN, Alencar CH, Heukelbach J. Prevalence of Chagas disease in Brazil: a systematic review and meta-analysis. Acta Trop. 2014;130(1):167-74.
-
3Prefeitura Municipal de Umbaúba. Secretaria de Planejamento. Dados demográficos da população rural.2016.
-
4Camargo ME, Silva GR, Castilho EA, Silveira AC. Inquérito sorológico da prevalência da infecção chagásica no Brasil, 1975-1980. Rev Inst Med Trop São Paulo.1984; 26(4):192-204.
-
5Silveira AC. Situação do controle da transmissão vetorial da doença de Chagas nas Américas. Cad Saude Publica. 2000;16(2):35-42.
-
6Andrade JP, Marin Neto JA, Paola AAV, Vilas-Boas F, Oliveira GMM, Bacal F, et al. I Diretriz Latino-Americana para o diagnóstico e tratamento da cardiopatia chagásica: resumo executivo. Arq Bras Cardiol. 2011;96(6):434-42.
-
7Dias JCP, Ramos Jr AN, Gontijo ED, Luquetti A, Shikanai-Yasuda MA, Coura JR, et al. II Consenso Brasileiro em Doença de Chagas, 2015. Epidemiol Serv Saude. 2016;25(1):7-86.
-
8Borges-Pereira J, Xavier SS, Pirmez C, Coura JR. Doença de Chagas em Virgem da Lapa, Minas Gerais, Brasil. IV. Aspectos clínicos e epidemiológicos do aneurisma ventricular esquerdo. Rev Soc Bras Med Trop. 1998;31(5):457-63.
-
9Goldbaum M, Ajimura FY, Litvoc J, Carvalho SA, Eluf-Neto J. American trypanosomiasis and electrocardiographic alterations among industrial workers in São Paulo, Brazil. Rev Inst Med Trop São Paulo . 2004;46(6):299-302.
-
10Gonçalves JG, Prata A, Dias JCP, Macedo V. O inquérito eletrocardiográfico. Rev Soc Bras Med Trop . 2011;44(2):40-6.
-
11Peñaranda-Carrillo R, Castro C, Rezende J, Prata A, Macêdo V. Estudo radiológico do esôfago de chagásicos, em 25 anos do Projeto Mambaí. Rev Soc Bras Med Trop . 2006;39(2):152-5.
-
12Castro C, Penaranda-Carrilo R, Rezende J, Prata A. Estudo longitudinal do megaesôfago chagásico. Rev Soc Bras Med Trop . 2009;42(2):69-72.
-
13Macêdo V. Influência da exposição à reinfecção na evolução da doença de Chagas. Rev Pat Trop. 1976;5(1):33-116.
-
14Andrade CM, Câmara ACJ, Nunes DF, Guedes PMM, Pereira WO, Chiari E, et al. Chagas disease: morbidity profile in an endemic area of Northeastern Brazil. Rev Soc Bras Med Trop . 2015;48(6):706-15.
Publication Dates
-
Publication in this collection
Sep-Oct 2018
History
-
Received
21 Feb 2018 -
Accepted
18 July 2018