Abstract
INTRODUCTION:
Musca domestica is resistant to many insecticides; hence, biological control is a suitable alternative.
METHODS:
We evaluated the lethality of strain Btk176 towards the larval and adult M. domestica and the histopathological effects in the larvae midgut.
RESULTS:
We observed 99% larval and 78.9% adult mortality within 48 hours of spore ingestion (dosage, 2.4×108 CFU/ml). The histopathological effects were consistent with cytotoxicity. PCR analysis showed the presence of the cry1Ba gene. Transmission electron microscopy revealed a bipyramidal parasporal body. Thurigiensin activity was not detected.
CONCLUSIONS:
The serovar, Btk176 might be a potential biocontrol agent for houseflies.
Keywords:
Biological control, housefly; Entomopathogenic bacteria; Bacillus thuringiensis var. kyushuensis
Musca domestica acts as a carrier of diverse pathogens and causes secondary myiasis in humans and animals11. Zhang J, Wang J, Chen L, Yassin AK, Kelly P, Butaye P, et al. Housefly (Musca domestica) and Blow Fly (Protophormia terraenovae) as Vectors of Bacteria Carrying Colistin Resistance Genes. Appl Environ Microbiol [Internet]. 2017; 84(1) [cited 2018 Sep 19]. Available from: Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5734023/
https://www.ncbi.nlm.nih.gov/pmc/article...
,22. Rahman A, Ishfaq A, Arshad Azmi M, Khatoon N. Cutaneous myiasis of scalp in a young girl related to Musca domestica. Dermatol Online J. 2015; 21(11).. Houseflies are notorious for their ability to develop behavioral and metabolic mechanisms to detoxify chemical insecticides33. Geden CJ. Status of biopesticides for control of house flies. J Biopest (Suppl 5) . 2012;1-11., the traditional method of pest control. To address this, alternative, non-chemical control methods have been investigated and proposed as components of integrated control programs to reduce the M. domestica population density. The bacterium B. thuringiensis var. kyushuensis demonstrates mosquitocidal activity due to the presence of the CytB toxin in the parasporal inclusions44. Held GA, Kawanishi CY, Huang YS. Characterization of the parasporal inclusion of Bacillus thuringiensis subsp. kyushuensis. J Bacteriol. 1990;172(1):481-3.. Here, we performed a preliminary evaluation of the potential of B. thuringiensis var. kyushuensis (strain Btk176) for larval and adult M. domestica control and an investigation of its histopathological effects on the larval intestinal epithelium following exposure.
Rearing of flies: Flies were collected from rubbish bins in the Amorim region, Rio de Janeiro, Brazil (latitude: -22.875707, longitude: -43.250606), transported to the laboratory and identified. Bioassays were performed with the larvae and second-generation adults, reared in ventilated shelves at 27±1ºC, 60±10% relative humidity with an artificial 12-h photoperiod. Adult M. domestica were fed with 80% sucrose. However, to stimulate oviposition and maturation, they were fed with a mix of rotten ground beef and wheat bran in equal quantities.
Bacterial strain and growth conditions: Strain Btk176 (LEMEF/IOC- Fiocruz) is maintained in the Collection of the Genus Bacillus and Correlates - IOC/Fiocruz with the code CCGB0727 (B. thuringiensis var. kyushuensis). Colonies of this strain, generated on nutrient agar, were transferred into a test tube containing nutrient yeast extract salt medium (NYSM) broth (6 ml). This pre-inoculum was incubated (incubator ES-20/60, BioSan) at 31ºC, 120 rpm for 6 h. Pre-inoculum aliquots (1 ml) were transferred to six 200 ml flasks, each containing the same culture medium (50 ml), and were incubated at 31ºC and 200 rpm for 72 h. Aliquots (10 ml) were collected at different time intervals: 24, 48, and 72 h and centrifuged at 13,000 rpm for 18 min. Supernatants were autoclaved at 121ºC for 15 min and stored in test tubes at 4ºC for subsequent assessment of the presence of thermostable exotoxin.
The culture growth was assessed daily using direct light microscopy till it exceeded 90% sporulation. Subsequently, the cultures were centrifuged (3600 rpm for 40 min at 4ºC), the supernatants were discarded, and the pellets were washed twice with 200 mM NaCl to remove any residual exotoxin. The pellets were stored and different concentrations of spores were prepared per the protocol reported by Pereira et al55. Pereira L de A, Junqueira RM, Carramaschi IN, Queiroz MMC, Zahner V. Bioactivity under laboratory conditions of Brevibacillus laterosporus towards larvae and adults of Chrysomya putoria (Diptera: Calliphoridae). J Invertebr Pathol. 2018; 158:52-4..
Bioassays: The neonatal larvae of the flies were treated with three different concentrations of the spore suspension and supernatant (Figure 1). For each test sample, five replicates were used with an additional two replicates included for microscopy. The spore suspensions or supernatant (1 ml) were mixed with the diet (2.5 g) prepared in individual plastic cups into which 10 neonatal larvae were placed. The control group was established by adding autoclaved distilled water (1 ml) to the diet66. Ferreira V dos SB, Barcellos I da S, Carramaschi IN, Santos-Mallet JR, Queiroz MMC, Zahner V. Larvicidal activity and effects on post embrionary development of laboratory reared Musca domestica (Linnaeus, 1758) (Diptera: Muscidae), treated with Brevibacillus laterosporus (Laubach) spore suspensions. J Invertebr Pathol . 2016;137:54-7.. The experimental groups were monitored daily (to register mortalities and the weight of the larvae). Additionally, we collected larvae for electron microscopy examination. Routine monitoring was conducted according to the protocol by Ferreira et al66. Ferreira V dos SB, Barcellos I da S, Carramaschi IN, Santos-Mallet JR, Queiroz MMC, Zahner V. Larvicidal activity and effects on post embrionary development of laboratory reared Musca domestica (Linnaeus, 1758) (Diptera: Muscidae), treated with Brevibacillus laterosporus (Laubach) spore suspensions. J Invertebr Pathol . 2016;137:54-7.. The results were calculated as the average of two experiments, each with five replicates.
The lethal effect exhibited by the spore suspension of strain Btk176 at concentrations of 2.4×108 CFU/ml (concentration 1), 2.4×107 CFU/ml (concentration 2), and 2.4×106 CFU/ml (concentration 3) and supernatant (SN) collected at 24, 48, and 72 h periods of growth upon M. domestica under laboratory conditions
For the bioassay with the adult flies, three cages were used for each of the test and control group, each group containing five newly emerged adults. The treatment groups were offered spore suspension (2.5 ml) mixed with either 50% sugar solution (2.5 ml) or culture supernatant (2.5 ml) mixed with 50% sugar solution (2.5 ml), provided in a Petri dish containing autoclaved cotton wool as a support. Contrastingly, the control group was offered distilled water (2.5 ml) mixed with 50% sugar solution (2.5 ml). The bioassay was monitored daily as recommended by Indrasith et al77. Indrasith LS, Suzuki N, Ogiwara K, Asano S, Hori H. Activated insecticidal crystal proteins from Bacillus thuringiensis serovars killed adult house flies. Lett Appl Microbiol. 1992;14(4):174-7.. The results were calculated based on the averages of four experiments. The bioassay data was processed and interpreted as recommended by Pereira et al55. Pereira L de A, Junqueira RM, Carramaschi IN, Queiroz MMC, Zahner V. Bioactivity under laboratory conditions of Brevibacillus laterosporus towards larvae and adults of Chrysomya putoria (Diptera: Calliphoridae). J Invertebr Pathol. 2018; 158:52-4..
Transmission electron microscopy: The larvae from each treatment and control group at 6, 12, 24, and 48 h, were collected to dissect their digestive tract for transmission electron microscopy (TEM) examination. The posterior portion of the midgut, identified by the position of the Malpighian tubules, was processed as described by Ruiu et al88. Ruiu L, Satta A, Floris I. Observations on house fly larvae midgut ultrastructure after Brevibacillus laterosporus ingestion. J Invertebr Pathol . 2012;111(3):211-6.. The dissected material was fixed with 2.5% glutaraldehyde in sodium cacodylate buffer supplemented with sucrose and stored at 4ºC for 72 h. Subsequently, the material was washed thrice in sodium cacodylate buffer (0.1 M) for 10 min each. Then, it was fixed with 1% osmium tetroxide and ferricyanide for 1 h in the dark, washed thrice in 0.1 M sodium cacodylate buffer and dehydrated successively in 30%, 50%, 70%, and 90% acetone, each step for 15 min and, finally, thrice with 100% acetone. Subsequently, the material was infiltrated with a 2:1 mixture of 100% acetone and Epoxy resin (Epon) for 4 h, posteriorly, and incubated with a 1:1 mixture overnight (approximately 12 h) followed by incubation in pure Epon for 6 h. The material (midgut) was included in the Epon and polymerized in an oven for 72 h at 60°C. The sections were prepared using the Rudolph Barth Electron Microscopy Platform (IOC/Fiocruz) using a ultramicrotome, contrasted with 5% uranyl acetate in total darkness and then with 2% lead citrate. The sections were observed using a JEOL JEM-1011 transmission electron microscope. The bacterial pellets were processed and analyzed as recommended by Macedo-Silva et al99. Macedo-Silva RM, Santos C de LP dos, Diniz VA, Carvalho JJ de, Guerra C, Côrte-Real S. Peripheral blood fibrocytes: new information to explain the dynamics of Leishmania infection. Mem Inst Oswaldo Cruz. 2014;109(1):61-9..
Polymerase chain reaction (PCR): PCR assays were performed to detect the cry1Ba target gene using the primers and cycling conditions reported by Ben-Dov et al1010. Ben-Dov E, Zaritsky A, Dahan E, Barak Z, Sinai R, Manasherob R, et al. Extended screening by PCR for seven cry-group genes from field-collected strains of Bacillus thuringiensis. Appl Environ Microbiol . 1997;63(12):4883-90..
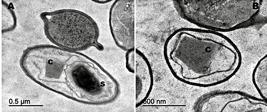
The examination of the sporulated cells of Btk176 (Figure 2) by TEM revealed the presence of bipyramidal crystals. Contrastingly, mosquitocidal strains of this serovar were observed as irregular shaped crystals44. Held GA, Kawanishi CY, Huang YS. Characterization of the parasporal inclusion of Bacillus thuringiensis subsp. kyushuensis. J Bacteriol. 1990;172(1):481-3.. Strain Btk176 demonstrated significant pathogenicity towards the larvae (Figure 1) and, to a lesser degree, towards the adults. The bioassay with the larvae resulted in maximum lethality (99%) at a concentration of 2.4×108 CFU/ml (Figure 2), 48 h after spore ingestion. The experiments examining the culture supernatants showed no significant mortality in any of the three test groups compared to the control groups (Figure 2). Regarding the recently emerged adults, the highest level of mortality was 78.9%, higher to that reported previously1111. Zimmer CR, Dias de Castro LL, Pires SM, Delgado Menezes AM, Ribeiro PB, Leivas Leite FP. Efficacy of entomopathogenic bacteria for control of Musca domestica. J Invertebr Pathol . 2013;114(3):241-4.,1212. Lonc ET, Lachowicz M, Jedryka U. Insecticidal activity of various strains of Bacillus against larvae of house ßies (Musca domestica). Wiad Parazytol. 1991; 37(3):357-65., was recorded for the suspensions containing 2.4×108 CFU/ml.
The evaluation of thuringiensin (Thu), a β-exotoxin, activity in the culture supernatants is a necessary component of any study assessing the insecticidal potential of the B. thuringiensis strains. This thermostable protein has been detected in the supernatant of some B. thuringiensis strains and is toxic towards different insect orders1313. Wiest SLF, Júnior HLP, Fiuza LM. Thuringiensin: a toxin from Bacillus thuringiensis. Bt Res [Internet]. 2015; 6(0) [cited 2019 Jan 10]. Available from: Available from: http://biopublisher.ca/index.php/bt/article/view/1920
http://biopublisher.ca/index.php/bt/arti...
. Additionally, it can show hemolytic and cytolytic activity against non-target invertebrates and even towards humans1313. Wiest SLF, Júnior HLP, Fiuza LM. Thuringiensin: a toxin from Bacillus thuringiensis. Bt Res [Internet]. 2015; 6(0) [cited 2019 Jan 10]. Available from: Available from: http://biopublisher.ca/index.php/bt/article/view/1920
http://biopublisher.ca/index.php/bt/arti...
. Thus, since the 1990s, the World Health Organization has recommended that Thu-producing strains should not be employed for the formulation of biopesticides. Here, we performed a bioassay, which is commonly used to demonstrate the absence of thuringiensin1313. Wiest SLF, Júnior HLP, Fiuza LM. Thuringiensin: a toxin from Bacillus thuringiensis. Bt Res [Internet]. 2015; 6(0) [cited 2019 Jan 10]. Available from: Available from: http://biopublisher.ca/index.php/bt/article/view/1920
http://biopublisher.ca/index.php/bt/arti...
, and provided a strong indication that strain Btk176 does not possess β-exotoxin activity. Nevertheless, specific chemical HPLC and molecular biology (PCR) tests need to be performed to provide conclusive proof that Btk176 does not produce Thu1313. Wiest SLF, Júnior HLP, Fiuza LM. Thuringiensin: a toxin from Bacillus thuringiensis. Bt Res [Internet]. 2015; 6(0) [cited 2019 Jan 10]. Available from: Available from: http://biopublisher.ca/index.php/bt/article/view/1920
http://biopublisher.ca/index.php/bt/arti...
. Only if the results of these tests turn out to be negative, we can fully discount the possibility that the toxin may be produced during in vitro culture, albeit at concentrations that do not exhibit any detectable toxicity in the bioassay. This possibility should be examined, since growth in either the insect diet or within the insect body might provide conditions that lead to unacceptable levels of Thu production.
We speculate that the entomopathogenic activity of Btk176 may be directly related to the activity of Cry1Ba as the mortality was statistically significant, the PCR amplification of the cry1Ba gene was positive, and crystalline inclusions were observed by electron microscopy (Figure 2). As our preliminary analyses have yielded promising data for this strain, we need to conduct more extensive analysis to study the mechanisms of toxicity towards houseflies. Further examination of the sequencing of the cry1Ba PCR product, confirmation of Cry1Ba expression is needed via isolation of the crystals and proteomic analysis. Additionally, bioassays using purified crystals should be performed.
The data generated from the TEM analyses demonstrated that the cytopathological effects within the insect gut included a continuous and progressive disorganization of the cell structure that was absent in the control group (Figure 3A, C, E and G). These changes are consistent with previous reports of cellular damage caused by a variety of B. thuringiensis serotypes in many different orders of insect pests1414. Song P, Wang Q, Nangong Z, Su J, Ge D. Identification of Henosepilachna vigintioctomaculata (Coleoptera: Coccinellidae) midgut putative receptor for Bacillus thuringiensis insecticidal Cry7Ab3 toxin. J Invertebr Pathol . 2012; 109(3):318-22.. A marked increase in the number of digestive vacuoles was observed at 6 h in the spore-treated groups (Figure 3B); after 12 h, the nuclear membrane demonstrated an irregular appearance and the microvilli appeared to be irregular (Figure 3D); after 24 h, the discontinuity of the microvilli increased and the formation of large vacuoles within the cytoplasm and intense cytoplasmic disorganization was observed (Figure 3F). Finally, after 48 h, we observed extrusion of the cytoplasmic content into the lumen (Figure 3H) and the microvilli were completely irregular and discontinuous (Figure 3H). Contrastingly, no histopathological changes were noted in the epithelial cells of the larvae treated with the culture supernatant (Figure 3J) compared with the control cells (Figure 3I). The histopathological damage recorded by TEM analyses of the treated larvae has been reported to be a typical consequence of the toxin binding to its specific receptor, resulting in pore formation that increases the permeability of the cell causing swelling and rupture1515. Copping LG, Menn JJ. Biopesticides: a review of their action, applications and efficacy. Pest Manag Sci. 2000;56(8):651-76..
Transmission electron microscopic image of the midgut epithelial cells of Musca domestica larvae treated for 6, 12, 24, and 48 h with spores of strain Btk176 at 2.4×107 CFU/ml and with supernatant (SN). (A) Epithelial cells obtained from the control group treated with only water for 6 h; (B) Epithelial cells from the group treated with spores of strain Btk176 at a concentration of 2.4×107 CFU/ml for 6 h; M: mitochondria, Mv: microvilli, N: nucleus, Er: endoplasmic reticulum, Dv: digestive vacuole, observed at 6200× magnification; (C) Epithelial cells from the control group treated with water for 12 h; (D) Epithelial cells from the group treated with spores of strain Btk176 at 2.4×107 CFU/ml for 12 h; Mv: microvilli, N: nucleus, C: cytoplasm, Pm: peritrophic matrix, L: lumen; observed at 7800×; (E) Epithelial cells from the control group treated with water for 24 h; (F) Epithelial cells from group treated with the spores of strain Btk176 at 2.4×107 CFU/ml for 24 h; C: cytoplasm, V: vacuoles, observed at 6200×; (G) Epithelial cells from the control group treated with water for 48 h; (H) Epithelial cells from the group treated with the spores of strain Btk176 at 2.4×107 CFU/ml treated for 48 h; M: mitochondria, Mv: microvilli, C: cytoplasm, L: lumen, Ce: cytoplasmic extrusion, observed at 9300×; (I) Epithelial cells from the control group treated with water for 48 h; (J) Epithelial cells from the supernatant treated group for 48 h - M: mitochondria, Mv: microvilli, observed at 9300×.
The potential of several B. thuringiensis strains to act as pest control agents for M. domestica has been reported since the early 1960s. Nevertheless, the evaluation of new strains that may help in overcoming problems including resistance, slow or difficult growth, limited efficiency of crystal toxin expression, and the presence of Thurigiensin is still justified. The elevated toxicity exhibited by strain Btk176, which could be achieved at a readily attainable spore concentration in the apparent absence of exotoxin production, clearly indicated the utilization of continued screening programs to uncover novel candidates for use as active ingredients in bioinsecticide products for the integrated control of the larvae and adults of M. domestica.
ACKNOWLEDGMENTS
We thank Dr. Douglas McIntosh for English revision.
REFERENCES
-
1Zhang J, Wang J, Chen L, Yassin AK, Kelly P, Butaye P, et al. Housefly (Musca domestica) and Blow Fly (Protophormia terraenovae) as Vectors of Bacteria Carrying Colistin Resistance Genes. Appl Environ Microbiol [Internet]. 2017; 84(1) [cited 2018 Sep 19]. Available from: Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5734023/
» https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5734023/ -
2Rahman A, Ishfaq A, Arshad Azmi M, Khatoon N. Cutaneous myiasis of scalp in a young girl related to Musca domestica Dermatol Online J. 2015; 21(11).
-
3Geden CJ. Status of biopesticides for control of house flies. J Biopest (Suppl 5) . 2012;1-11.
-
4Held GA, Kawanishi CY, Huang YS. Characterization of the parasporal inclusion of Bacillus thuringiensis subsp. kyushuensis J Bacteriol. 1990;172(1):481-3.
-
5Pereira L de A, Junqueira RM, Carramaschi IN, Queiroz MMC, Zahner V. Bioactivity under laboratory conditions of Brevibacillus laterosporus towards larvae and adults of Chrysomya putoria (Diptera: Calliphoridae). J Invertebr Pathol. 2018; 158:52-4.
-
6Ferreira V dos SB, Barcellos I da S, Carramaschi IN, Santos-Mallet JR, Queiroz MMC, Zahner V. Larvicidal activity and effects on post embrionary development of laboratory reared Musca domestica (Linnaeus, 1758) (Diptera: Muscidae), treated with Brevibacillus laterosporus (Laubach) spore suspensions. J Invertebr Pathol . 2016;137:54-7.
-
7Indrasith LS, Suzuki N, Ogiwara K, Asano S, Hori H. Activated insecticidal crystal proteins from Bacillus thuringiensis serovars killed adult house flies. Lett Appl Microbiol. 1992;14(4):174-7.
-
8Ruiu L, Satta A, Floris I. Observations on house fly larvae midgut ultrastructure after Brevibacillus laterosporus ingestion. J Invertebr Pathol . 2012;111(3):211-6.
-
9Macedo-Silva RM, Santos C de LP dos, Diniz VA, Carvalho JJ de, Guerra C, Côrte-Real S. Peripheral blood fibrocytes: new information to explain the dynamics of Leishmania infection. Mem Inst Oswaldo Cruz. 2014;109(1):61-9.
-
10Ben-Dov E, Zaritsky A, Dahan E, Barak Z, Sinai R, Manasherob R, et al. Extended screening by PCR for seven cry-group genes from field-collected strains of Bacillus thuringiensis Appl Environ Microbiol . 1997;63(12):4883-90.
-
11Zimmer CR, Dias de Castro LL, Pires SM, Delgado Menezes AM, Ribeiro PB, Leivas Leite FP. Efficacy of entomopathogenic bacteria for control of Musca domestica J Invertebr Pathol . 2013;114(3):241-4.
-
12Lonc ET, Lachowicz M, Jedryka U. Insecticidal activity of various strains of Bacillus against larvae of house ßies (Musca domestica). Wiad Parazytol. 1991; 37(3):357-65.
-
13Wiest SLF, Júnior HLP, Fiuza LM. Thuringiensin: a toxin from Bacillus thuringiensis Bt Res [Internet]. 2015; 6(0) [cited 2019 Jan 10]. Available from: Available from: http://biopublisher.ca/index.php/bt/article/view/1920
» http://biopublisher.ca/index.php/bt/article/view/1920 -
14Song P, Wang Q, Nangong Z, Su J, Ge D. Identification of Henosepilachna vigintioctomaculata (Coleoptera: Coccinellidae) midgut putative receptor for Bacillus thuringiensis insecticidal Cry7Ab3 toxin. J Invertebr Pathol . 2012; 109(3):318-22.
-
15Copping LG, Menn JJ. Biopesticides: a review of their action, applications and efficacy. Pest Manag Sci. 2000;56(8):651-76.
-
Financial support: This study was supported by grants from Programa de Objetivos e Metas - Instituto Oswaldo Cruz /FIOCRUZ and Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES - Finance Code 001).
Publication Dates
-
Publication in this collection
01 Aug 2019 -
Date of issue
2019
History
-
Received
20 Mar 2019 -
Accepted
03 July 2019