Abstract
INTRODUCTION:
Oral infection by Trypanosoma cruzi is currently the most important route of transmission of acute Chagas disease (ACD) in the North region of Brazil, and the reported outbreaks are usually related to ingestion of contaminated food, especially unprocessed açaí pulp.
METHODS
A retrospective cohort study was performed to analyze the epidemiological profile of individuals with suspected cases of ACD in the municipality of Breves, located in the state of Pará, Brazil. Therefore, notifications of suspected cases of ACD were collected from the Municipal Health Department of Breves from January 2007 to December 2017.
RESULTS
A total of 265 individuals were registered, and the majority were male (54.7%; 145/265). Age ranged from nine months to 79 years, with a greater number of notifications for individuals aged between 1 and 39 years (71.3%; 189/265). Most of them had a low level of education (74.3%, 197/265), were living in rural and urban areas (58.9%; 156/265 and 37.7%; 100/265, respectively). Infection occurred mainly in the domestic environment (96.2%; 255/265) through oral transmission (98.1%; 260/265). There were a greater number of notifications in November, December and January.
CONCLUSIONS
These data showed that oral transmission of T. cruzi has become increasingly high in the study region, and health education programs need to be implemented as strategies to ensure good manufacturing practices of unprocessed food.
Keywords:
Trypanosoma cruzi; Açaí; Acute infection
INTRODUCTION
American trypanosomiasis or Chagas disease, an anthropozoonosis caused by Trypanosoma cruzi, is one of the 17 neglected tropical diseases that still persist in the poorest societies and affect approximately six million people worldwide11. Organização Mundial de Saúde (OMS). Chagas disease (American trypanosomiasis) 2018. Available from: http://www.who.int/chagas/epidemiology/en.
http://www.who.int/chagas/epidemiology/e...
. Transmission to human beings can occur in several ways: vectorial, blood transfusion, congenital including oral transmission, and also through transplants of organs from infected people, laboratory accidents and sexual intercourse22. Barreto-de-Albuquerque J, Silva-dos-Santos D, Stein JV, De Meis J. Oral versus intragastric inoculation: Similar Pathways of Trypanosoma cruzi Experimental infection? From target tissues, parasite evasion, and immune response. Immunol. 2018;9:1734..
Vector transmission, which happens only in the endemic countries of Latin America, was considered as the main transmission route of the parasite for several decades33. Deane MP, Lenzi HL, Jansen A. Trypanosoma cruzi: vertebrate and invertebrate cycles in the same mammal host, the opossum Didelphis marsupialis. Mem Inst Oswaldo Cruz. 1984;79(4):513-5.. Currently, as a result of larger migration flows, chagasic infection is no longer found only in endemic areas of Latin America; it is now also diagnosed in countries considered to be non-endemic. Thus, T. cruzi infection has become a major global health problem, with treatment deficiencies and absence of effective vaccines44. Thakare R, Dasgupta A, Chopra S. An update on benznidazole for the treatment of patients with Chagas disease. Drugs Today. (Barc) 2018;54(1):15-23.. However, transmission remains active in some ecologically viable regions. The main problems include poor maintenance of vector surveillance, new epidemiological situations, oral transmission, wild and secondary vectors, resistance to insecticides, urbanization and migration of infected individuals, restrictive policies such as instability, decentralization of health programs, and millions of existing cases requiring medical attention55. Dias JCP. Elimination of Chagas disease transmission: perspectives. Mem Inst Oswaldo Cruz . 2009;104(supl. 1):41-5..
Oral transmission of T. cruzi to humans was first reported in Brazil in 1967 in the municipality of Teutônia, state of Rio Grande do Sul66. Nery-Guimarães F, Silva NN, Calusell DT, Mello AL, Rapone T, Snell T, et al. Um surto epidêmico de doença de Chagas de provável transmissão digestiva, ocorrido em Teutônia (Estrela, Rio Grande do Sul). Hospital. 1968;73:1767-804.. From 1968 to 2000, 50% of acute cases of Chagas disease in the Amazon region were attributed to oral transmission77. Benchimol-Barbosa PR. The oral transmission of Chagas’ disease: an acute form of infection responsible for regional outbreaks. Int J Cardiol. 2006;112(1):132-3., and between 2000 and 2010, the rate reached 70%88. Shikanai-Yasuda MA, Carvalho NB. Oral transmission of Chagas disease. Clin Infect Dis. 2012;54(6):845-52.. This route of transmission is frequent in the Brazilian Amazon region99. Valente SAS, Valente VC, Fraiha-Neto H. Considerations on the epidemiology and transmission of Chagas disease in the Brazilian Amazon. Mem Inst Oswaldo Cruz . 1999;94(Suppl. 1):395-8.
10. Coura JR, Junqueira AC, Fernandes O, Valente SA, Miles MA. Emerging Chagas disease in Amazonian Brazil. Trends Parasitol. 2002;18(4):171-6.-1111. Pinto AYN, Valente SAS, Valente VC, Ferreira Junior AG, Coura JR. Acute phase of Chagas disease in the Brazilian Amazon region. Study of 233 cases from Pará, Amapá and Maranhão observed between 1988 and 2005. Rev Soc Bras Med Trop. 2008;41(6):602-14., and it is responsible for the two largest outbreaks of acute Chagas disease reported to date1212. Alarcón-de-Noya B, Martínez J. Transmisión oral de la enfermedad de Chagas en Venezuela: un segundo brote escolar. Salus. 2009;13:9-10.,1313. Alarcón-de-Noya B, Díaz-Bello Z, Colmenares C, Ruiz-Guevara R, Mauriello L, Zavala-Jaspe R, et al. Large urban outbreak of orally-acquired acute Chagas disease, at a school in Caracas, Venezuela. J Infect Dis. 2010;201(9):1308-315.. Outbreaks of orally-transmitted T. cruzi infection have been reported in several Latin American countries, such as Colombia, Venezuela, Bolivia and French Guiana1414. Alarcón-de-Noya B, Díaz-Bello Z, Colmenares C, Ruiz-Guevara R, Mauriello L, Muñoz-Calderón A, et al. Update on oral Chagas disease outbreaks in Venezuela: epidemiological, clinical and diagnostic approaches. Mem Inst Oswaldo Cruz . 2015;110(3):377-86.. For most of the outbreaks that have been stated, the epidemiological profile indicates non-vector transmission, involving intake of juice made of local fruits1515. Díaz ML, González CI. Enfermedad de Chagas agudo: transmisión oral de Trypanosoma cruzi como una vía de transmisión re-emergente. Rev Univ Ind Santander Salud. 2014;46(2):177-88..
One of the main suspected sources of T. cruzi infection is the ingestion of tainted food, such as Euterpe oleracea Mart. (açaí), which is widely consumed as a drink made from a mixed pulp1616. Santana RAG, Guerra MGVB, Sousa DR, Couceiro K, Ortiz JV, Oliveira M, et al. Oral Transmission of Trypanosoma cruzi, Brazilian Amazon. Emerg Infect Dis. 2019;25(1):132-5.. However, this relevant transmission pathway is underestimated and understudied1717. Secretaria de Vigilância em Saúde, Ministério da Saúde. Doença de Chagas Aguda e distribuição especial dos triatomíneos de importância epidemiológica, Brasil 2012 a 2016. Secretaria de Vigilância em Saúde, Ministério da Saúde, Boletim Epidemiológico 2019; 50:2. Available from: http://portalarquivos2.saude.gov.br/images/pdf/2019/janeiro/23/2018-025.pdf.
http://portalarquivos2.saude.gov.br/imag...
. In Brazil, 24 cases of acute human Chagas disease were detected in Santa Catarina State, and all of them were related to ingestion of T. cruzi-contaminated sugarcane juice1818. Steindel M, Kramer Pacheco L, Scholl D, Soares M, Moraes MH, Eger I, et al. Characterization of Trypanosoma cruzi isolated from humans, vectors, and animal reservoirs following an outbreak of acute human Chagas disease in Santa Catarina State, Brazil. Diagn Microbiol Infect Dis. 2008;60(1):25-32.. Recently, another outbreak of ACD resulting from infection by sugarcane juice, occurred in the city of Marcelino Vieira, state of Rio Grande do Norte (RN), with 18 confirmed cases1919. Vargas A, Malta JMA, Costa VMD, Cláudio LDG, Alves RV, Cordeiro GDS, et al. Investigation of an outbreak of acute Chagas disease outside the Amazon Region, in Rio Grande do Norte State, Brazil, 2016. Cad Saúde Públ. 2018;34(1):e00006517.. Another outbreak was reported in the municipality of Ibimirim, in the state of Pernambuco, where 27 people were positive for T. cruzi after attending the same religious event. The source of contamination is still under review, but evidence points to raw foods2020. Secretaria Estadual de Saúde de Pernambuco. Secretaria Estadual de Saúde promove curso sobre Doença de Chagas [Internet]. Recife-PE, Secretaria Estadual de Saúde do Estado de Pernambuco. Available from: http://portal.saude.pe.gov.br/noticias/secretaria-executiva-de-vigilancia-em-saude/ses-promove-curso-sobre-doenca-de-chagas-0.
http://portal.saude.pe.gov.br/noticias/s...
. In the period between 2012 and 2016, 1,190 cases of ACD were confirmed and 18 deaths were recorded, with an annual mortality rate of 1.5%. Most of the cases (82%) occurred in the North region, and 12% of the municipalities in this region registered cases of ACD in five consecutive years; nine municipalities are located in the state of Pará and one, in the state of Amapá. Approximately 52% of individuals with ACD live in urban areas1717. Secretaria de Vigilância em Saúde, Ministério da Saúde. Doença de Chagas Aguda e distribuição especial dos triatomíneos de importância epidemiológica, Brasil 2012 a 2016. Secretaria de Vigilância em Saúde, Ministério da Saúde, Boletim Epidemiológico 2019; 50:2. Available from: http://portalarquivos2.saude.gov.br/images/pdf/2019/janeiro/23/2018-025.pdf.
http://portalarquivos2.saude.gov.br/imag...
. Recent data collected in the Brazilian Amazon have described the occurrence of an outbreak of acute Chagas disease (ACD) involving ten patients whose suspected source of contamination was the ingestion of açaí pulp. Analysis of the patients’ blood samples and the juice samples contained T. cruzi, genotyped as TcIV, indicating oral transmission of the etiologic agent of the infection1616. Santana RAG, Guerra MGVB, Sousa DR, Couceiro K, Ortiz JV, Oliveira M, et al. Oral Transmission of Trypanosoma cruzi, Brazilian Amazon. Emerg Infect Dis. 2019;25(1):132-5.. It should be noted that many cases of ACD may remain unnoticed, mainly as a result of the difficulty of diagnosis in the acute phase, which is usually limited by the lack of commercial kits registered with the National Health Surveillance Agency (ANVISA), and also because of clinical symptoms that are nonspecific to Chagas’ disease2121. Secretaria de Vigilância em Saúde, Ministério da Saúde. Recomendações sobre o diagnóstico parasitológico, sorológico e molecular para confirmação da doença de Chagas aguda e crônica. Rev Patol Trop. 2013;42(4):475-8..
In this context, the present study evaluates the epidemiological profile of individuals suspected of ACD in the municipality of Breves, State of Pará, Brazil, and to understand the route of infection in order to help improve public policies to prevent the oral transmission of T. cruzi.
METHODS
Study area
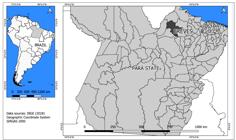
The study was carried out in the municipality of Breves, in the north of the state of Pará, in northern Brazil. The municipality is located in the southwest of Marajó Island, at latitude 01º40'56” South and longitude 50º28'49" West (Figure 1). Breves has a humid equatorial climate with high temperatures and high rainfall, with an area of approximately 9,566 km2. The population was estimated at 92,860 inhabitants in 2010. The local economy is based mainly on extractivism, especially extraction of açaí, in addition to fishing, mining, logging, production of Brazil nuts, as well as family farming and tourism1515. Díaz ML, González CI. Enfermedad de Chagas agudo: transmisión oral de Trypanosoma cruzi como una vía de transmisión re-emergente. Rev Univ Ind Santander Salud. 2014;46(2):177-88..
Study design
This is a retrospective cohort study, using secondary data provided by the Municipal Health Department of Breves, for the period from January 2007 to December 2017. All of the study informants lived in the municipality of Breves and were treated at the municipal hospital. Eight demographic variables were assessed: month of onset of clinical symptoms, age, education, sex, type of dwelling, form of transmission, place of infection and laboratory-confirmed diagnosis. Suspected cases were attributed to individuals who had clinical manifestations associated with ACD, as cases of the disease had been detected in the region, and the people infected had reported intake of fresh açaí (artisanal production).
Individuals were considered to be positive in laboratory tests when the parasite was detected by the direct parasitological method, based on the detection of trypomastigote forms in the blood. Samples from the subjects were considered to be negative in the absence of trypomastigotes during direct examination of peripheral blood. Data were analyzed using descriptive statistics, and the results were presented in tables and graphs as absolute and relative frequencies.
RESULTS
A total of 265 individuals suspected of T. cruzi infection were investigated in the municipality of Breves, in the state of Pará, Brazil, from 2007 to 2017. The socio-demographic profile of these individuals showed that the majority were male (54.7%). The subjects’ age ranged from nine months to 79 years, with a greater number of cases reported between 1-19 years (38.1%) and 20-39 years of age (33.2%). They had a low level of education, and 71.7% of them had not completed high school. Individuals lived in rural (58.9%), urban (37.7%) and peri-urban areas (2.3%) (Table 1).
Epidemiological variables of individuals notified with suspected acute Chagas disease in the municipality of Breves, Pará State, Brazil from 2007 to 2017.
There were variations in the number of cases notified between 2007 and 2014, and there was a progressive increase from 2014 to 2016 (Figure 2). Most of the individuals were infected in their household (96.2%, 255/265), and the main infection route was oral transmission of the parasite (98.1%; 260/265). The notifications were reported mainly in the months of November, December and January (Figure 3), suggesting that ACD by oral infection has a seasonal profile in this region. Laboratory diagnosis was confirmed in 64.2% (170/265) of the cases while 27.5% (73) were negative (Table 2).
Annual distribution of suspected cases of acute Chagas disease among inhabitants of the municipality of Breves, state of Pará, Brazil, from 2007 to 2017.
Distribution of suspected cases of acute Chagas disease among inhabitants of the municipality of Breves, state of Pará, Brazil, for the month of onset of symptoms in the period from 2007 to 2017.
Number of individuals notified with suspicion of acute Chagas disease, route and place of transmission, and laboratory tests in the city of Breves, Pará State, Brazil, from 2007 to 2017.
DISCUSSION
The epidemiological profile of suspected cases of acute Chagas’ disease by oral transmission showed that T. cruzi infection occurred mainly in male individuals whose age ranged from nine months to 39 years old; they had incomplete high school education, and lived in the rural area. Epidemiological data of the state of Pará, collected from the DATASUS database from 2006 to 2012, confirmed a profile for the region with a high percentage of oral transmission (68.4%) in a total of 977 patients diagnosed with ACD2222. Santos VRC, De Meis J, Savino W, Andrade JAA, Vieira JRS, Coura JR, et al. Acute Chagas disease in the state of Pará, Amazon Region: is it increasing? Mem Inst Oswaldo Cruz . 2018;113(5):e170298.. A recent study using data from the Notifiable Diseases Information System (SINAN) - Ministry of Health, showed epidemiological profile similar to the present study: 69% of respondents were aged between 18 and 60 years, and 38% were male and 31% female. These respondents represent the group most affected by oral transmission of T. cruzi in the region2222. Santos VRC, De Meis J, Savino W, Andrade JAA, Vieira JRS, Coura JR, et al. Acute Chagas disease in the state of Pará, Amazon Region: is it increasing? Mem Inst Oswaldo Cruz . 2018;113(5):e170298.. However, we cannot ignore the fact that human-vector interactions are possible even with low frequency of triatomines indoors1111. Pinto AYN, Valente SAS, Valente VC, Ferreira Junior AG, Coura JR. Acute phase of Chagas disease in the Brazilian Amazon region. Study of 233 cases from Pará, Amapá and Maranhão observed between 1988 and 2005. Rev Soc Bras Med Trop. 2008;41(6):602-14.. The municipality of Breves is located in northern Brazil, where non-domiciled triatomines are sporadically found indoors. Most cases of T. cruzi infection have been attributed to oral transmission and a few have been reported1616. Santana RAG, Guerra MGVB, Sousa DR, Couceiro K, Ortiz JV, Oliveira M, et al. Oral Transmission of Trypanosoma cruzi, Brazilian Amazon. Emerg Infect Dis. 2019;25(1):132-5..
The epidemiological scenario of Chagas disease in Breves showed that the oral route is the main form of transmission of T. cruzi, corroborating recent2222. Santos VRC, De Meis J, Savino W, Andrade JAA, Vieira JRS, Coura JR, et al. Acute Chagas disease in the state of Pará, Amazon Region: is it increasing? Mem Inst Oswaldo Cruz . 2018;113(5):e170298. and old2323. Nóbrega AA, Garcia MH, Tatto E, Obara MT, Costa E, Sobel J, et al. Oral Transmission of Chagas Disease by Consumption of Açaí Palm Fruit, Brazil. Emerg Infect Dis . 2009;15(4):653-5. data, and occurs mainly in households (96.2%). In the present study, there was a high rate of T. cruzi oral transmission, and it has become increasingly frequent in this region, when compared to previous research. In other regions, where oral transmission by T. cruzi is less frequent, for example, in the state of Pernambuco, this route of transmission has significantly decreased after 2006, a period in which Brazil received an international certificate for interrupting vector transmission by Triatoma infestans2424. Santos FL, Lorena VM, Souza WV, Gomes YM. Spatiotemporal analysis of reported cases of acute Chagas disease in the State of Pernambuco, Brazil, from 2002 to 2013. Rev Soc Bras Med Trop . 2015;48(2):181-7..
The data provided for this study by the Municipal Health Department did not allow a description of the clinical manifestations present in individuals with suspected oral transmission of the parasite. However, a recent analysis of records from the SINAN in the state of Pará showed that the most frequent clinical manifestations in orally-infected individuals were fever, asthenia, facial or lower limb edema and generalized edema2222. Santos VRC, De Meis J, Savino W, Andrade JAA, Vieira JRS, Coura JR, et al. Acute Chagas disease in the state of Pará, Amazon Region: is it increasing? Mem Inst Oswaldo Cruz . 2018;113(5):e170298..
The progressive increase in notifications of suspected ACD cases associated with oral transmission by T. cruzi, as found in this study, follows the same profile reported in Brazil: between 1968 and 2005, 437 cases of acute Chagas disease were reported; 311 of them were linked to 62 outbreaks caused by ingestion of açaí pulp. In contrast, from 2000 to 2010, there were more than 1,000 acute cases in 138 outbreaks, with 776 cases (71%) attributed to orally-transmitted epidemics1515. Díaz ML, González CI. Enfermedad de Chagas agudo: transmisión oral de Trypanosoma cruzi como una vía de transmisión re-emergente. Rev Univ Ind Santander Salud. 2014;46(2):177-88.. The number of ACD notifications was found to be period-specific, and was higher in the months of November, December and January. This finding suggests that seasonal infection by T. cruzi is associated mainly with oral transmission. The period corresponding to the peak of ACD notifications coincides with the açaí harvest months in this region. This finding reinforces the relationship between this food and oral transmission of T. cruzi. However, other factors may contribute to increased infection by this parasite; for example, longer life expectancy and the growing number of açaí consuming markets without certification for parasitological quality2525. Labello-Barbosa R. Perspectives on foodborne parasites. Adv Food Technol Nutr Sci Open J. 2016;1(4):82-3.
26. Diário Oficial do Estado do Pará (DOEPA). Decreto no 326 de 20/01/2012; Available from: http://www.normasbrasil.com.br/norma/decreto-326-2012-pa_147597.html
http://www.normasbrasil.com.br/norma/dec...
-2727. Ferreira RTB, Branquinho MR, Leite PC. Transmissão oral da doenca de Chagas pelo consumo de açaí: um desafio para a Vigilância Sanitária. Vig Sanit Debate. 2014;2:4-11.. Such factors were identified as potential sources of transmission of the parasite and, consequently, of ACD, which could become an important public health problem in the coming decades.
Historically, micro-epidemics of community-based oral infection of ACD are responsible for human cases with severe symptoms that can be followed by death2828. Pereira KS, Schmidt FL, Labello-Barbosa R, Guaraldo AMA, Franco RMB, Dias VL, et al. Transmission of Chagas disease (American trypanosomiasis) by food. Adv Food Nutr Res. 2010;59:63-85.
29. Andrade CM, Câmara ACJ, Nunes DF, Guedes PMM, Pereira WO, Chiari E, et al. Chagas disease: morbidity profile in an endemic area of Northeastern Brazil. Rev Soc Bras Med Trop . 2015;48(6):706-15.-3030. De-Góes-Costa E, Dos Santos SO, Sojo-Milano M, Amador ECC, Tatto E, Souza DSM, et al. Acute Chagas disease in the Brazilian Amazon: epidemiological and clinical features. Int J Cardiol . 2017;235;176-8.. T. cruzi oral transmission generally coincides with warm climates, which causes triatomines to remain more active, with greater mobility and hematophagy. As a consequence, there is a stronger possibility of contamination of food and the environment with feces and/or urine containing metacyclic trypomastigote forms. Depending on temperature and humidity levels, T. cruzi can stay alive for a few hours or even for days; at low temperatures, its viability can last for weeks. Thus, contaminated food kept moist and in a liquid or pasty state favors resistance and, consequently, transmission of the parasite. The açaí is an ideal source for transmission of the parasite, because its fruits are grown, harvested and manipulated with the help of artificial light for artisanal preparation in rural or peri-urban areas, where triatomines are often abundant and there is no sanitary control2525. Labello-Barbosa R. Perspectives on foodborne parasites. Adv Food Technol Nutr Sci Open J. 2016;1(4):82-3.,3131. Filigeddu MT, Górgolas M, Ramos JM. Orally-transmitted Chagas disease. Med Clin. (Barc) 2017;148:125-131.. However, the parasite can be neutralized by heating at 43ºC for 20 min3232. Barbosa R L, Pereira KS, Dias VL, Schmidt FL, Alves DP, Guaraldo AMA, et al. Virulence of Trypanosoma cruzi in açai (Euterpe oleraceae Martius) pulp following mild heat treatment. J Food Prot. 2016;79(10):1807-12..
Prevention and control measures must be adopted at all stages of the açaí production chain, both by large producers that do perform pasteurization and by artisan processors. In the state of Pará, hygienic-sanitary procedures have been established for the handling of açaí by artisan processors since 2012. These procedures are a way of reducing the initial microbial load, minimizing risks, and preventing outbreaks of foodborne diseases. The process consists of three washes of the fruits with drinking water; the second wash is performed with hypochlorite at the concentration of 150 PPM of active chlorine. Then, the bleaching step should be performed by immersing açaí in heated water at 80ºC for 10 s and then in cold water. Best practices have been introduced in the entire açaí production chain to improve the hygienic-sanitary conditions of the processing units, offering consumers a safe product, in addition to promoting public policies for social inclusion in this segment of the production chain3333. Secretaria Especial de Estado de Gestão. Governo do Pará. Decreto Nº 326, de 20 janeiro de 2012. Pará, 2012. Available from: http://www.ioepa.com.br/pages/2012/2012.01.24.DOE.pdf.
http://www.ioepa.com.br/pages/2012/2012....
.
The State of Pará is the largest producer and consumer of açaí worldwide. This fruit is traditionally consumed unprocessed and is the basis of the cuisine of Pará; it is one of the most important supplements in the Brazilian diet of North region3434. Rogez H. Açaí: preparo, composição e melhoramento da conservação. EdUFPA. 2000;313.. It is worth mentioning that irrigation of land for cultivation of açaí is a common practice throughout year in the municipality of Breves. This practice results in high availability of the fruit, which may increase the risk of infection with T. cruzi in this region. In these cases, ACD outbreaks have a high socioeconomic impact in the North of Brazil3535. Rogez H, Aguiar FS. Contaminação da bebida açaí envolvendo Trypanosoma cruzi. In: Pessoa JDC, Teixeira GHA, Tecnologias para inovação das cadeias Euterpe. Embrapa. 2012;205-228..
Of the 265 individuals suspected of ACD, 73 (27.5%) tested negative in a parasitological examination of peripheral blood for T. cruzi infection. The parasitological diagnosis of Chagas disease in the acute phase is based on the detection of the parasite, and test sensitivity depends on the number of parasites in the peripheral blood, which is usually high. For suspected cases of acute infection, different direct parasitological methods can be used for immediate and repeated reading to clarify the diagnosis3636. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Consenso Brasileiro em Doença de Chagas. Rev Soc Bras Med Trop . 2005;38(Suppl 3):1-29.
37. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Programa Nacional de Controle de Chagas. Doença de Chagas aguda: aspectos epidemiológicos, diagnóstico e tratamento: guia de consulta rápida para profissionais de saúde. Rev Patol Trop . 2007;36(3):1-32.-3838. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Guia de Vigilância em Saúde. Brasília: Ministério da Saúde; 2014.. These methods are: Strout concentration, micro-hematocrit test and buffy coat, and they are recommended as the first choice for symptomatic diagnosis of cases with more than 30 days’ evolution, because of the decline in parasitemia over time3939. Luquetti AO, Rassi A 2000. Diagnóstico laboratorial da infecção pelo Trypanosoma cruzi. In Z Brener, Z Andrade, M Barral-Netto (org.), Trypanosoma cruzi e Doença de Chagas, Editora Guanabara Koogan, Rio de Janeiro, pp. 344-348.. Finding negative results for the parasite in direct examination of peripheral blood suggests the need to use a serological test to detect anti-T.cruzi IgM antibodies. This test indicates a positive acute phase, especially when combined with epidemiological features and clinical manifestations4040. Requena-Méndez A, Aldasoro E, Lazzari E, Sicuri E, Brown M, Moore DA, et al. Prevalence of Chagas disease in Latin-American migrants living in Europe: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2015;9(2):e0003540.. Difficulties in the diagnosis of the acute phase of ACD can be explained by the lack of commercial kits registered in the National Health Surveillance Agency for obtaining positive controls for IgM3636. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Consenso Brasileiro em Doença de Chagas. Rev Soc Bras Med Trop . 2005;38(Suppl 3):1-29., as well as by the possibility of false positive results in several febrile illnesses2121. Secretaria de Vigilância em Saúde, Ministério da Saúde. Recomendações sobre o diagnóstico parasitológico, sorológico e molecular para confirmação da doença de Chagas aguda e crônica. Rev Patol Trop. 2013;42(4):475-8..
The limitation of this study refers to the lack of other data available in the database, e.g., reported clinical manifestation, individuals’ knowledge of triatomines, risk of oral infection by T. cruzi and hygiene measures that should be adopted before consumption of açaí fruits. The scope is intended for analysis of data from one municipality only, which represents a significant reduction in the sampling population. Thus, we suggest that the method used in the present study should be employed in the analysis of others municipalities in the northern region of Brazil. Underreporting and the fact that the study is retrospective are relevant limitations.
Our results demonstrated that the rate of oral transmission of T. cruzi through tainted food has become increasingly high in the study region over the years. Therefore, we recommend that high-quality basic education - mainly in the açaí harvest months, based on effective public policies - should be extended to the entire state of Pará, including islands far away from the metropolitan region. Such education could be used as a model for the entire North region. This can facilitate inspection to ensure continuous control of efficient hygiene practices in the açaí production chain by the population and by artisanal producers. Other measures, e.g., training of health professionals, prevention measures, early diagnosis in case of epidemiological risk (even if far from vector-transmission areas) are also extremely important. We also emphasize the proper care of people infected with T. cruzi as an essential strategy for a comprehensive control of Chagas infection and its eradication as a public health problem11. Organização Mundial de Saúde (OMS). Chagas disease (American trypanosomiasis) 2018. Available from: http://www.who.int/chagas/epidemiology/en.
http://www.who.int/chagas/epidemiology/e...
,3131. Filigeddu MT, Górgolas M, Ramos JM. Orally-transmitted Chagas disease. Med Clin. (Barc) 2017;148:125-131.. These findings call for better hygiene control in fresh food handling, for artisanal açaí in particular, in order to avoid infection by T. cruzi and to increase the safety and viability of consumption of this fruit, which is a great nutritional source and has high economic importance for the northern region of Brazil.
ACKNOWLEDGMENTS
The authors would like to thank the Public Health Department of the municipality of Breves in the State of Pará, Brazil, and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for granting scholarships (GHFS, NRMH, ANBS).
REFERENCES
-
1Organização Mundial de Saúde (OMS). Chagas disease (American trypanosomiasis) 2018. Available from: http://www.who.int/chagas/epidemiology/en
» http://www.who.int/chagas/epidemiology/en -
2Barreto-de-Albuquerque J, Silva-dos-Santos D, Stein JV, De Meis J. Oral versus intragastric inoculation: Similar Pathways of Trypanosoma cruzi Experimental infection? From target tissues, parasite evasion, and immune response. Immunol. 2018;9:1734.
-
3Deane MP, Lenzi HL, Jansen A. Trypanosoma cruzi: vertebrate and invertebrate cycles in the same mammal host, the opossum Didelphis marsupialis Mem Inst Oswaldo Cruz. 1984;79(4):513-5.
-
4Thakare R, Dasgupta A, Chopra S. An update on benznidazole for the treatment of patients with Chagas disease. Drugs Today. (Barc) 2018;54(1):15-23.
-
5Dias JCP. Elimination of Chagas disease transmission: perspectives. Mem Inst Oswaldo Cruz . 2009;104(supl. 1):41-5.
-
6Nery-Guimarães F, Silva NN, Calusell DT, Mello AL, Rapone T, Snell T, et al. Um surto epidêmico de doença de Chagas de provável transmissão digestiva, ocorrido em Teutônia (Estrela, Rio Grande do Sul). Hospital. 1968;73:1767-804.
-
7Benchimol-Barbosa PR. The oral transmission of Chagas’ disease: an acute form of infection responsible for regional outbreaks. Int J Cardiol. 2006;112(1):132-3.
-
8Shikanai-Yasuda MA, Carvalho NB. Oral transmission of Chagas disease. Clin Infect Dis. 2012;54(6):845-52.
-
9Valente SAS, Valente VC, Fraiha-Neto H. Considerations on the epidemiology and transmission of Chagas disease in the Brazilian Amazon. Mem Inst Oswaldo Cruz . 1999;94(Suppl. 1):395-8.
-
10Coura JR, Junqueira AC, Fernandes O, Valente SA, Miles MA. Emerging Chagas disease in Amazonian Brazil. Trends Parasitol. 2002;18(4):171-6.
-
11Pinto AYN, Valente SAS, Valente VC, Ferreira Junior AG, Coura JR. Acute phase of Chagas disease in the Brazilian Amazon region. Study of 233 cases from Pará, Amapá and Maranhão observed between 1988 and 2005. Rev Soc Bras Med Trop. 2008;41(6):602-14.
-
12Alarcón-de-Noya B, Martínez J. Transmisión oral de la enfermedad de Chagas en Venezuela: un segundo brote escolar. Salus. 2009;13:9-10.
-
13Alarcón-de-Noya B, Díaz-Bello Z, Colmenares C, Ruiz-Guevara R, Mauriello L, Zavala-Jaspe R, et al. Large urban outbreak of orally-acquired acute Chagas disease, at a school in Caracas, Venezuela. J Infect Dis. 2010;201(9):1308-315.
-
14Alarcón-de-Noya B, Díaz-Bello Z, Colmenares C, Ruiz-Guevara R, Mauriello L, Muñoz-Calderón A, et al. Update on oral Chagas disease outbreaks in Venezuela: epidemiological, clinical and diagnostic approaches. Mem Inst Oswaldo Cruz . 2015;110(3):377-86.
-
15Díaz ML, González CI. Enfermedad de Chagas agudo: transmisión oral de Trypanosoma cruzi como una vía de transmisión re-emergente. Rev Univ Ind Santander Salud. 2014;46(2):177-88.
-
16Santana RAG, Guerra MGVB, Sousa DR, Couceiro K, Ortiz JV, Oliveira M, et al. Oral Transmission of Trypanosoma cruzi, Brazilian Amazon. Emerg Infect Dis. 2019;25(1):132-5.
-
17Secretaria de Vigilância em Saúde, Ministério da Saúde. Doença de Chagas Aguda e distribuição especial dos triatomíneos de importância epidemiológica, Brasil 2012 a 2016. Secretaria de Vigilância em Saúde, Ministério da Saúde, Boletim Epidemiológico 2019; 50:2. Available from: http://portalarquivos2.saude.gov.br/images/pdf/2019/janeiro/23/2018-025.pdf
» http://portalarquivos2.saude.gov.br/images/pdf/2019/janeiro/23/2018-025.pdf -
18Steindel M, Kramer Pacheco L, Scholl D, Soares M, Moraes MH, Eger I, et al. Characterization of Trypanosoma cruzi isolated from humans, vectors, and animal reservoirs following an outbreak of acute human Chagas disease in Santa Catarina State, Brazil. Diagn Microbiol Infect Dis. 2008;60(1):25-32.
-
19Vargas A, Malta JMA, Costa VMD, Cláudio LDG, Alves RV, Cordeiro GDS, et al. Investigation of an outbreak of acute Chagas disease outside the Amazon Region, in Rio Grande do Norte State, Brazil, 2016. Cad Saúde Públ. 2018;34(1):e00006517.
-
20Secretaria Estadual de Saúde de Pernambuco. Secretaria Estadual de Saúde promove curso sobre Doença de Chagas [Internet]. Recife-PE, Secretaria Estadual de Saúde do Estado de Pernambuco. Available from: http://portal.saude.pe.gov.br/noticias/secretaria-executiva-de-vigilancia-em-saude/ses-promove-curso-sobre-doenca-de-chagas-0
» http://portal.saude.pe.gov.br/noticias/secretaria-executiva-de-vigilancia-em-saude/ses-promove-curso-sobre-doenca-de-chagas-0 -
21Secretaria de Vigilância em Saúde, Ministério da Saúde. Recomendações sobre o diagnóstico parasitológico, sorológico e molecular para confirmação da doença de Chagas aguda e crônica. Rev Patol Trop. 2013;42(4):475-8.
-
22Santos VRC, De Meis J, Savino W, Andrade JAA, Vieira JRS, Coura JR, et al. Acute Chagas disease in the state of Pará, Amazon Region: is it increasing? Mem Inst Oswaldo Cruz . 2018;113(5):e170298.
-
23Nóbrega AA, Garcia MH, Tatto E, Obara MT, Costa E, Sobel J, et al. Oral Transmission of Chagas Disease by Consumption of Açaí Palm Fruit, Brazil. Emerg Infect Dis . 2009;15(4):653-5.
-
24Santos FL, Lorena VM, Souza WV, Gomes YM. Spatiotemporal analysis of reported cases of acute Chagas disease in the State of Pernambuco, Brazil, from 2002 to 2013. Rev Soc Bras Med Trop . 2015;48(2):181-7.
-
25Labello-Barbosa R. Perspectives on foodborne parasites. Adv Food Technol Nutr Sci Open J. 2016;1(4):82-3.
-
26Diário Oficial do Estado do Pará (DOEPA). Decreto no 326 de 20/01/2012; Available from: http://www.normasbrasil.com.br/norma/decreto-326-2012-pa_147597.html
» http://www.normasbrasil.com.br/norma/decreto-326-2012-pa_147597.html -
27Ferreira RTB, Branquinho MR, Leite PC. Transmissão oral da doenca de Chagas pelo consumo de açaí: um desafio para a Vigilância Sanitária. Vig Sanit Debate. 2014;2:4-11.
-
28Pereira KS, Schmidt FL, Labello-Barbosa R, Guaraldo AMA, Franco RMB, Dias VL, et al. Transmission of Chagas disease (American trypanosomiasis) by food. Adv Food Nutr Res. 2010;59:63-85.
-
29Andrade CM, Câmara ACJ, Nunes DF, Guedes PMM, Pereira WO, Chiari E, et al. Chagas disease: morbidity profile in an endemic area of Northeastern Brazil. Rev Soc Bras Med Trop . 2015;48(6):706-15.
-
30De-Góes-Costa E, Dos Santos SO, Sojo-Milano M, Amador ECC, Tatto E, Souza DSM, et al. Acute Chagas disease in the Brazilian Amazon: epidemiological and clinical features. Int J Cardiol . 2017;235;176-8.
-
31Filigeddu MT, Górgolas M, Ramos JM. Orally-transmitted Chagas disease. Med Clin. (Barc) 2017;148:125-131.
-
32Barbosa R L, Pereira KS, Dias VL, Schmidt FL, Alves DP, Guaraldo AMA, et al. Virulence of Trypanosoma cruzi in açai (Euterpe oleraceae Martius) pulp following mild heat treatment. J Food Prot. 2016;79(10):1807-12.
-
33Secretaria Especial de Estado de Gestão. Governo do Pará. Decreto Nº 326, de 20 janeiro de 2012. Pará, 2012. Available from: http://www.ioepa.com.br/pages/2012/2012.01.24.DOE.pdf
» http://www.ioepa.com.br/pages/2012/2012.01.24.DOE.pdf -
34Rogez H. Açaí: preparo, composição e melhoramento da conservação. EdUFPA. 2000;313.
-
35Rogez H, Aguiar FS. Contaminação da bebida açaí envolvendo Trypanosoma cruzi In: Pessoa JDC, Teixeira GHA, Tecnologias para inovação das cadeias Euterpe. Embrapa. 2012;205-228.
-
36Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Consenso Brasileiro em Doença de Chagas. Rev Soc Bras Med Trop . 2005;38(Suppl 3):1-29.
-
37Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Programa Nacional de Controle de Chagas. Doença de Chagas aguda: aspectos epidemiológicos, diagnóstico e tratamento: guia de consulta rápida para profissionais de saúde. Rev Patol Trop . 2007;36(3):1-32.
-
38Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Guia de Vigilância em Saúde. Brasília: Ministério da Saúde; 2014.
-
39Luquetti AO, Rassi A 2000. Diagnóstico laboratorial da infecção pelo Trypanosoma cruzi In Z Brener, Z Andrade, M Barral-Netto (org.), Trypanosoma cruzi e Doença de Chagas, Editora Guanabara Koogan, Rio de Janeiro, pp. 344-348.
-
40Requena-Méndez A, Aldasoro E, Lazzari E, Sicuri E, Brown M, Moore DA, et al. Prevalence of Chagas disease in Latin-American migrants living in Europe: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2015;9(2):e0003540.
Publication Dates
-
Publication in this collection
11 Sept 2020 -
Date of issue
2020
History
-
Received
25 Mar 2020 -
Accepted
24 June 2020