Abstract
INTRODUCTION:
The Amazon tropical rainforest has the most dense and diverse ecosystem worldwide. A few studies have addressed rodent-borne diseases as potential hazards to humans in this region.
METHODS:
A retrospective survey was conducted using enzyme-linked immunosorbent assay for detecting mammarenavirus and orthohantavirus antibodies in 206 samples collected from rural settlers of the Brazilian Western Amazonian region.
RESULTS:
Six (2.91%) individuals in the age group of 16 to 36 years were found to possess antibodies against mammarenavirus.
CONCLUSION:
Evidence of previous exposure to mammarenavirus in the rural population points to its silent circulation in this region.
Keywords:
Zoonoses; Brazilian Amazonian region; Mammarenavirus; Hantavirus; Rodent-borne disease
The Amazon region (Amazon River Basin) is a vast territory that encompasses parts of nine South American countries, including a large portion of Brazil. This region has the largest tropical rainforest in the world with a climate that is characterized by high temperature and humidity levels, copious rainfalls, and the most dense and diverse ecosystem worldwide11. Penna G, Pinto LF, Soranz D, Glatt R. High Incidence of Diseases Endemic to the Amazon Region of Brazil, 2001-2006. Emerg Infect Dis. 2009;15(4):626-32..
Demographic density in the Brazilian Amazonian region is low (4.7 persons/km2), and many areas are nearly bereft of healthcare facilities. Paradoxically, an intense urbanization process has been taking place in this region in the last few years11. Penna G, Pinto LF, Soranz D, Glatt R. High Incidence of Diseases Endemic to the Amazon Region of Brazil, 2001-2006. Emerg Infect Dis. 2009;15(4):626-32.,22. Ilacqua RC, Chaves LSM, Bergo ES, Conn JE, Sallum MAM, Laporta GZ. A method for estimating the deforestation timeline in rural settlements in a scenario of malaria transmission in frontier expansion in the Amazon Region. Mem Inst Oswaldo Cruz. 2018;113(9):e170522. . Since the 1970s, the Brazilian government has been creating rural settlements in the Amazon, and in this region, agriculture and farming are the two main economic activities conducted for livelihood22. Ilacqua RC, Chaves LSM, Bergo ES, Conn JE, Sallum MAM, Laporta GZ. A method for estimating the deforestation timeline in rural settlements in a scenario of malaria transmission in frontier expansion in the Amazon Region. Mem Inst Oswaldo Cruz. 2018;113(9):e170522. .
The conditions in the Brazilian Amazonian region are favorable for the transmission of numerous tropical infectious agents, which pose particular risks to the health of the population as they are exposed to hazardous housing and working conditions22. Ilacqua RC, Chaves LSM, Bergo ES, Conn JE, Sallum MAM, Laporta GZ. A method for estimating the deforestation timeline in rural settlements in a scenario of malaria transmission in frontier expansion in the Amazon Region. Mem Inst Oswaldo Cruz. 2018;113(9):e170522. . Recently, a new mammarenavirus was identified in rodents that were captured in Acre state, Brazil. This virus seems to be the result of reassortment between two distinct mammarenavirus clades and is the first known mammarenavirus identified from this region33. Fernandes J, Guterres A, de Oliveira RC, Chamberlain J, Lewandowski K, Teixeira BR, et al. Xapuri virus, a novel mammarenavirus: natural reassortment and increased diversity between New World viruses. Emerg Microbes Infect. 2018;7(1):120.. Only a few studies have addressed rodent-borne diseases, such as mammarenavirus and orthohantavirus infections, as potential hazards to humans in this region, although the human and rodent infections, mostly related to orthohantavirus, have previously been reported in the Northern region of Brazil33. Fernandes J, Guterres A, de Oliveira RC, Chamberlain J, Lewandowski K, Teixeira BR, et al. Xapuri virus, a novel mammarenavirus: natural reassortment and increased diversity between New World viruses. Emerg Microbes Infect. 2018;7(1):120.
4. Gimaque JBL, Bastos MS, Braga WSM, Oliveira CMC, Castilho MC, Figueiredo RMP, et al. Serological evidence of hantavirus infection in rural and urban regions in the state of Amazonas, Brazil. Mem Inst Oswaldo Cruz . 2012;107(1):135-7.
5. de Oliveira RC, Cordeiro-Santos M, Guterres A, Fernandes J, de Melo AX, João GA, et al. Rio Mamoré virus and hantavirus pulmonar syndrome, Brazil. Emerg Infect Dis . 2014;20:1568-70.
6. Fernandes J, Silva TACD, Oliveira RC, Guterres A, Oliveira EC, Terças ACP, et al. Silent arenavirus infection in individuals living in Colniza, Mato Grosso, Brazil. Rev Soc Bras Med Trop 2018;51(6):881-2.
7. Fernandes J, de Oliveira RC, Guterres A, Barreto-Vieira DF, Terças ACP, Teixeira BR, et al. Detection of Latino virus (Arenaviridae: Mammarenavirus) naturally infecting Calomys callidus. Acta Trop. 2015;179:19-24.-88. Nunes ML, Oliveira SV, Elkhoury MR, Fonseca LX, Pereira SVC, Caldas EP, et al. Evidência de circulação de hantavirus em área silenciosa da Região Amazônica. Rev Pan-Amaz Saude. 2017;6(4):63-7.. In the present study, we aimed to retrospectively examine the seroprevalence of mammarenavirus and orthohantavirus in a rural population located in the Brazilian Western Amazonian region.
The surveyed area, known as “Ramal do Granada” (9o41’S-9o49’S, 67o05’W-67o07’W), is a sparsely populated rubber tapper settlement in the Acrelândia municipality of the Acre state that is a part of the Pedro Peixoto Agricultural Settlement Project. The Ramal do Granada has a linear extension of approximately 30 km, and includes households along an unpaved road with an economy based on agriculture, and mainly the livestock. Blood samples were collected during a cross-sectional survey in 2004 and were stored in a -20 °C freezer99. da Silva-Nunes M, Codeço CT, Malafronte RS, da Silva NS, Juncansen C, Muniz PT, et al. Malaria on the Amazonian frontier: transmission dynamics, risk factors, spatial distribution, and prospects for control. Am J Trop Med Hyg. 2008;79(4):624-35.. In total, 206 serum samples were subjected to serological analysis using the enzyme-linked immunosorbent assay (ELISA), according to a previously published protocol1010. Fernandes J, Oliveira RC, Coelho TA, Martins RMB, Caetano KAA, Horta MAP, et al. Rodent-borne viruses survey in rural settlers from Central Brazil. Mem Inst Oswaldo Cruz . 2018;114:e180448.. Antigens were derived from the Vero C76 cells (ATCC® CRL-1587™) infected with Junín mammarenavirus (Clade B New World mammarenaviruses) or Maciel orthohantavirus (Andes orthohantavirus group). The cut-off (0.2) was determined by estimating the mean optical densities (OD) of the negative controls with three standard deviations at 1:100 dilution1010. Fernandes J, Oliveira RC, Coelho TA, Martins RMB, Caetano KAA, Horta MAP, et al. Rodent-borne viruses survey in rural settlers from Central Brazil. Mem Inst Oswaldo Cruz . 2018;114:e180448.. The results were used to analyze the data together with the information that was gathered through a structured questionnaire99. da Silva-Nunes M, Codeço CT, Malafronte RS, da Silva NS, Juncansen C, Muniz PT, et al. Malaria on the Amazonian frontier: transmission dynamics, risk factors, spatial distribution, and prospects for control. Am J Trop Med Hyg. 2008;79(4):624-35.. The protocols implemented in this study were approved by the Research Ethics Committee for Experimentation in Human Beings of the Instituto de Ciências Biomédicas, Universidade de São Paulo as reported previously99. da Silva-Nunes M, Codeço CT, Malafronte RS, da Silva NS, Juncansen C, Muniz PT, et al. Malaria on the Amazonian frontier: transmission dynamics, risk factors, spatial distribution, and prospects for control. Am J Trop Med Hyg. 2008;79(4):624-35., and by the Fundação Oswaldo Cruz/Instituto Oswaldo Cruz under the approval number CAAE 61629416.2.1001.5248.
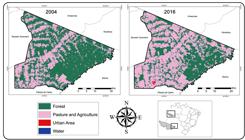
Data regarding the use of land of the Acrelândia municipality was obtained from MapBiomas v.3.1 (http://www.mapbiomas.org/) and was used to construct the comparative maps between 2004 and the more recently available data from 2016. Geoprocessing was performed using the Quantum Gis® program (QGIS Development Team, 2017)1111. QGIS Development Team, 2017. QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org.
http://qgis.osgeo.org...
. We used the resultant report of QGIS to calculate the extension (km²) covered by the forest and modified (pasture/agriculture) areas between 2004 and 2016. Aiming to test the relationship between social factors, including time of residence, house material, type of sewage coverage, presence of pets, profession, sex, and age as shown in Table 1, and the outcome variable (positivity for anti-mammarenavirus and /or anti-orthohantavirus IgG antibodies), Chi-squared tests (X2) were performed with statistical significance defined at p-value < 0.05. Data analysis was performed using the statistical package of R Studio (version 1.1.463).
The age of the 206 individuals that participated in this study was from a few months to 90 years (24.43 median years). Most of the participants were female 51.5 % (106), farmers 28.6 % (59), and students 28.2 % (58) who were living in the wood houses 82.5 % (170), and had no access to sewerage and garbage collection. Variables are presented in Table 1.
Mammarenavirus seropositivity and Chi-square test (p-value) as per the categorical variable in Ramal do Granada population, Acre state, Brazil.
None of these individuals exhibited the presence of antibodies against orthohantavirus. Low prevalence ratios of 1.1 % and 0.8 % in the Amazon basin rural population from Peru and Brazil, respectively, have been reported previously44. Gimaque JBL, Bastos MS, Braga WSM, Oliveira CMC, Castilho MC, Figueiredo RMP, et al. Serological evidence of hantavirus infection in rural and urban regions in the state of Amazonas, Brazil. Mem Inst Oswaldo Cruz . 2012;107(1):135-7.,1212. Oré RMC, Forshey BM, Huaman A, Villaran MV, Long KC, Kochel TJ, et al. Serologic Evidence for Human Hantavirus Infection in Peru. Vector Borne Zoonotic Dis. 2012;12(8):683-9.. The low seroprevalence to orthohantavirus observed in this study could be probably due to low agricultural activities in these particular region and the main activity being carried out was cattle raising (Table 1). Evidence of orthohantavirus circulation in wild rodent species, including Oligoryzomys microtis and Proechimys cuvieri, have been recorded in Acre state, although no orthohantavirus pulmonary syndrome cases among the individuals have been reported yet88. Nunes ML, Oliveira SV, Elkhoury MR, Fonseca LX, Pereira SVC, Caldas EP, et al. Evidência de circulação de hantavirus em área silenciosa da Região Amazônica. Rev Pan-Amaz Saude. 2017;6(4):63-7.. Therefore, more studies including large number of individuals are required in Acre state in order to better evaluate the impact of orthohantavirus infections in humans and rodents.
Mammarenavirus antibodies were detected in six young and adult individuals (age between 16 to 36 years), with an overall seroprevalence rate of 2.91 %. The seropositivity rate was slightly higher in females (3.8 %) than in males (2.0 %). It is noteworthy that five of the six individuals with antibodies against mammarenavirus mentioned that they performed hunting and fishing for their livelihood. No significant association was found between mammarenavirus seropositivity and work activities or other variables (Table 1), probably because of the low seroprevalence ratio, however, the prevalence observed was higher than those found in other previous studies that were conducted in Brazil and Colombia66. Fernandes J, Silva TACD, Oliveira RC, Guterres A, Oliveira EC, Terças ACP, et al. Silent arenavirus infection in individuals living in Colniza, Mato Grosso, Brazil. Rev Soc Bras Med Trop 2018;51(6):881-2.,1010. Fernandes J, Oliveira RC, Coelho TA, Martins RMB, Caetano KAA, Horta MAP, et al. Rodent-borne viruses survey in rural settlers from Central Brazil. Mem Inst Oswaldo Cruz . 2018;114:e180448.,1313. Arroyave E, Londoño AF, Quintero JC, Agudelo-Flórez P, Arboleda M, Díaz FJ, et al. [Etiology and epidemiological characterization of non-malarial febrile syndrome in three municipalities of Urabá (Antioquia), Colombia]. Biomedica. 2013;33(Suppl 1):99-107..
To date, only a few cases of Brazilian hemorrhagic fever, which is caused by the Sabiá mammarenavirus, has been described in São Paulo region, southeastern Brazil66. Fernandes J, Silva TACD, Oliveira RC, Guterres A, Oliveira EC, Terças ACP, et al. Silent arenavirus infection in individuals living in Colniza, Mato Grosso, Brazil. Rev Soc Bras Med Trop 2018;51(6):881-2.,1414. Coimbra TLM, Nassar ES, Burattini MN, de Souza LT, Ferreira I, Rocco IM, et al. New arenavirus isolated in Brazil. Lancet. 1994;343(8894):391-2.. However, five mammarenaviruses have been identified in rodents during the surveys that were conducted in the Brazilian Amazonian region and are listed as follows: (1) Amaparí virus (Neacomys guianae); (2) Cupixi virus (Hylaeamys megacephalus); (3) Flexal virus (unidentified oryzomyini); (4) Latino virus (Calomys callidus); (5) the most recently identified Xapuri virus (Neacomys musseri), demonstrating the potential for mammarenavirus emergence in this region33. Fernandes J, Guterres A, de Oliveira RC, Chamberlain J, Lewandowski K, Teixeira BR, et al. Xapuri virus, a novel mammarenavirus: natural reassortment and increased diversity between New World viruses. Emerg Microbes Infect. 2018;7(1):120.,77. Fernandes J, de Oliveira RC, Guterres A, Barreto-Vieira DF, Terças ACP, Teixeira BR, et al. Detection of Latino virus (Arenaviridae: Mammarenavirus) naturally infecting Calomys callidus. Acta Trop. 2015;179:19-24.,1414. Coimbra TLM, Nassar ES, Burattini MN, de Souza LT, Ferreira I, Rocco IM, et al. New arenavirus isolated in Brazil. Lancet. 1994;343(8894):391-2..
The area under study has a history of urbanization similar to the other regions of the Amazon basin, which started with the rubber boom in the early 20th century followed by other extractive activities, such as mining and lumber industries1616. Moutinho PR, Gil LH, Cruz RB, Ribolla PE. Population dynamics, structure and behavior of Anopheles darlingi in a rural settlement in the Amazon rainforest of Acre, Brazil. Malar J. 2011;10:174.. In 2004, the Acrelândia municipality had 983.3008 km² as the forested area (62.8 % of the total municipality area) and 580.2529 km² as the pasture and agriculture area (37.1 %). In 2016, a decrease in the forested area and an increase in the modified area were observed, leading to the existence of 657.6028 km² of forested area (42.0 %) and 901.6469 km² of pasture and agriculture area (57.6 %), as shown in Figure 1. Over the last several decades, agriculture has been the main factor that is responsible for the continued deforestation in the Pedro Peixoto settlement (Figure 1), possibly due to the poor technology applied for farming. This probably led to an increase in the contact between humans and wildlife, and a higher probability of the emergence of infectious diseases in this region22. Ilacqua RC, Chaves LSM, Bergo ES, Conn JE, Sallum MAM, Laporta GZ. A method for estimating the deforestation timeline in rural settlements in a scenario of malaria transmission in frontier expansion in the Amazon Region. Mem Inst Oswaldo Cruz. 2018;113(9):e170522. ,1515. Mota BE, Trindade GS, Diniz TC, da Silva-Nunes M, Braga EM, Urbano-Ferreira M, et al. Seroprevalence of orthopoxvirus in an Amazonian rural village, Acre, Brazil. Arch Virol. 2010;155(7):1139-44.,1616. Moutinho PR, Gil LH, Cruz RB, Ribolla PE. Population dynamics, structure and behavior of Anopheles darlingi in a rural settlement in the Amazon rainforest of Acre, Brazil. Malar J. 2011;10:174.. As reported in the previous studies, the high prevalence of zoonotic infections associated with Ramal do Granada inhabitants is suggestive of the fact that they are previously exposed to a wide variety of pathogens99. da Silva-Nunes M, Codeço CT, Malafronte RS, da Silva NS, Juncansen C, Muniz PT, et al. Malaria on the Amazonian frontier: transmission dynamics, risk factors, spatial distribution, and prospects for control. Am J Trop Med Hyg. 2008;79(4):624-35.,1515. Mota BE, Trindade GS, Diniz TC, da Silva-Nunes M, Braga EM, Urbano-Ferreira M, et al. Seroprevalence of orthopoxvirus in an Amazonian rural village, Acre, Brazil. Arch Virol. 2010;155(7):1139-44.. Many of these diseases, such as dengue, yellow fever, and malaria, are responsible for hundreds of cases, and could be easily misdiagnosed as mammarenavirus cases, especially because of the lack of healthcare services and healthcare professional training and distribution, even with the current advances in the Brazilian public health care system11. Penna G, Pinto LF, Soranz D, Glatt R. High Incidence of Diseases Endemic to the Amazon Region of Brazil, 2001-2006. Emerg Infect Dis. 2009;15(4):626-32.. Similar ecological and economic scenarios were reported during the emergence of Venezuelan hemorrhagic fever that is caused by Guanarito virus. This virus was first recognized during a dengue fever outbreak in Venezuela when the health authorities and physicians noticed “atypical” dengue hemorrhagic cases that continued to occur in the Portuguesa state, although these cases have decreased all over the country with time66. Fernandes J, Silva TACD, Oliveira RC, Guterres A, Oliveira EC, Terças ACP, et al. Silent arenavirus infection in individuals living in Colniza, Mato Grosso, Brazil. Rev Soc Bras Med Trop 2018;51(6):881-2.,1414. Coimbra TLM, Nassar ES, Burattini MN, de Souza LT, Ferreira I, Rocco IM, et al. New arenavirus isolated in Brazil. Lancet. 1994;343(8894):391-2..
Comparative maps depicting the use of land between 2004 and 2016 in the Acrelândia municipality, Acre state, Brazil.
Historically, the Northern and Northeastern regions of Brazil, which include most of the Amazon River basin, exhibits the highest social inequalities and prevalence of infectious diseases11. Penna G, Pinto LF, Soranz D, Glatt R. High Incidence of Diseases Endemic to the Amazon Region of Brazil, 2001-2006. Emerg Infect Dis. 2009;15(4):626-32.,22. Ilacqua RC, Chaves LSM, Bergo ES, Conn JE, Sallum MAM, Laporta GZ. A method for estimating the deforestation timeline in rural settlements in a scenario of malaria transmission in frontier expansion in the Amazon Region. Mem Inst Oswaldo Cruz. 2018;113(9):e170522. . Although additional investigations are required to be conducted, the identification of evidence of exposure to mammarenavirus infection in the Amazon basin indicates the occurrence of silent circulation of these emergent viruses in this region, and urges to include these viruses in the syndromic surveillance approach for febrile hemorrhagic diseases. Further studies in this region will help to better understand the mechanism by which the Amazon rural population is exposed to these zoonotic agents, and to characterize the circulating mammarenavirus species responsible for the human infections.
ACKNOWLEDGEMENTS
We gratefully acknowledge Dr. Marcelo Alves Pinto (Laboratório de Desenvolvimento Tecnológico em Virologia - Instituto Oswaldo Cruz/FIOCRUZ) for collaborating in this study.
REFERENCES
-
1Penna G, Pinto LF, Soranz D, Glatt R. High Incidence of Diseases Endemic to the Amazon Region of Brazil, 2001-2006. Emerg Infect Dis. 2009;15(4):626-32.
-
2.Ilacqua RC, Chaves LSM, Bergo ES, Conn JE, Sallum MAM, Laporta GZ. A method for estimating the deforestation timeline in rural settlements in a scenario of malaria transmission in frontier expansion in the Amazon Region. Mem Inst Oswaldo Cruz. 2018;113(9):e170522.
-
3Fernandes J, Guterres A, de Oliveira RC, Chamberlain J, Lewandowski K, Teixeira BR, et al. Xapuri virus, a novel mammarenavirus: natural reassortment and increased diversity between New World viruses. Emerg Microbes Infect. 2018;7(1):120.
-
4Gimaque JBL, Bastos MS, Braga WSM, Oliveira CMC, Castilho MC, Figueiredo RMP, et al. Serological evidence of hantavirus infection in rural and urban regions in the state of Amazonas, Brazil. Mem Inst Oswaldo Cruz . 2012;107(1):135-7.
-
5de Oliveira RC, Cordeiro-Santos M, Guterres A, Fernandes J, de Melo AX, João GA, et al. Rio Mamoré virus and hantavirus pulmonar syndrome, Brazil. Emerg Infect Dis . 2014;20:1568-70.
-
6Fernandes J, Silva TACD, Oliveira RC, Guterres A, Oliveira EC, Terças ACP, et al. Silent arenavirus infection in individuals living in Colniza, Mato Grosso, Brazil. Rev Soc Bras Med Trop 2018;51(6):881-2.
-
7Fernandes J, de Oliveira RC, Guterres A, Barreto-Vieira DF, Terças ACP, Teixeira BR, et al. Detection of Latino virus (Arenaviridae: Mammarenavirus) naturally infecting Calomys callidus. Acta Trop. 2015;179:19-24.
-
8Nunes ML, Oliveira SV, Elkhoury MR, Fonseca LX, Pereira SVC, Caldas EP, et al. Evidência de circulação de hantavirus em área silenciosa da Região Amazônica. Rev Pan-Amaz Saude. 2017;6(4):63-7.
-
9da Silva-Nunes M, Codeço CT, Malafronte RS, da Silva NS, Juncansen C, Muniz PT, et al. Malaria on the Amazonian frontier: transmission dynamics, risk factors, spatial distribution, and prospects for control. Am J Trop Med Hyg. 2008;79(4):624-35.
-
10Fernandes J, Oliveira RC, Coelho TA, Martins RMB, Caetano KAA, Horta MAP, et al. Rodent-borne viruses survey in rural settlers from Central Brazil. Mem Inst Oswaldo Cruz . 2018;114:e180448.
-
11QGIS Development Team, 2017. QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org
» http://qgis.osgeo.org -
12Oré RMC, Forshey BM, Huaman A, Villaran MV, Long KC, Kochel TJ, et al. Serologic Evidence for Human Hantavirus Infection in Peru. Vector Borne Zoonotic Dis. 2012;12(8):683-9.
-
13Arroyave E, Londoño AF, Quintero JC, Agudelo-Flórez P, Arboleda M, Díaz FJ, et al. [Etiology and epidemiological characterization of non-malarial febrile syndrome in three municipalities of Urabá (Antioquia), Colombia]. Biomedica. 2013;33(Suppl 1):99-107.
-
14Coimbra TLM, Nassar ES, Burattini MN, de Souza LT, Ferreira I, Rocco IM, et al. New arenavirus isolated in Brazil. Lancet. 1994;343(8894):391-2.
-
15Mota BE, Trindade GS, Diniz TC, da Silva-Nunes M, Braga EM, Urbano-Ferreira M, et al. Seroprevalence of orthopoxvirus in an Amazonian rural village, Acre, Brazil. Arch Virol. 2010;155(7):1139-44.
-
16Moutinho PR, Gil LH, Cruz RB, Ribolla PE. Population dynamics, structure and behavior of Anopheles darlingi in a rural settlement in the Amazon rainforest of Acre, Brazil. Malar J. 2011;10:174.
-
Financial Support: This research was supported by FIOCRUZ, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior- Brasil (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro - FAPERJ and Conselho Nacional de Desenvolvimento Científico e Tecnológico - Brasil (CNPq), grant number 404762/2016-6.
Publication Dates
-
Publication in this collection
22 June 2020 -
Date of issue
2020
History
-
Received
29 Oct 2019 -
Accepted
19 Mar 2020