Abstracts
OBJECTIVE: To review the current literature on human herpesvirus 8 with particular attention to the aspects related to the etiopathogenesis of Kaposi's sarcoma. MATERIALS AND METHODS: The authors searched original research and review articles on specific aspects of human herpesvirus 8 infection, including virology, epidemiology, transmission, diagnosis, natural history, therapy, and Kaposi's sarcoma etiopathogenesis. The relevant material was evaluated and reviewed. RESULTS: Human herpesvirus 8 is a recently discovered DNA virus that is present throughout the world but with major geographic variation. In the Western world, the virus, transmitted mainly by means of sexual contact, is strongly associated with Kaposi's sarcoma and body cavity-based lymphoma and more controversially with multiple myeloma and other non-proliferative disorders. There is no specific effective treatment, but HIV protease inhibitors may play an indirect role in the clearance of human herpesvirus 8 DNA from peripheral blood mononuclear cells of HIV-infected patients. Human herpesvirus 8 DNA is present in saliva, but there are as yet no documented cases of nosocomial transmission to health care workers. The prevalence of human herpesvirus 8 among health care workers is probably similar to that in the general population. CONCLUSION: Human herpesvirus 8 appears to be, at least in Western Europe and United States, restricted to a population at risk of developing Kaposi's sarcoma. Human herpesvirus 8 certainly has the means to overcome cellular control and immune responses and thus predispose carriers to malignancy, particularly Kaposi's sarcoma. The wide diffusion of Human herpesvirus 8 in classic Kaposi's sarcoma areas appears to represent an important factor in the high incidence of the disease. However, additional co-factors are likely to play a role in the development of Kaposi's sarcoma.
Human herpesvirus 8; KSHV; Kaposi´s sarcoma; AIDS; HIV
OBJETIVO: O objetivo do presente artigo foi revisar a literatura recente em relação ao herpesvírus humano tipo 8, com ênfase especial aos aspectos relacionados à etiopatogênese do sarcoma de Kaposi. MÉTODOS: Os autores pesquisaram artigos de pesquisa original e revisões de literatura nos aspectos específicos da infecção pelo herpesvírus humano tipo 8, incluindo, virologia, epidemiologia, transmissão, diagnóstico, história natural e terapia. O material considerado relevante foi avaliado e revisado. RESULTADOS: O sarcoma de Kaposi é considerado ainda a malignidade mais comumente observada em pacientes infectados pelo HIV. Estudos epidemiológicos, assim como os baseados em técnicas de biologia molecular indicam que um agente sexualmente transmissível, independente do HIV, deve estar envolvido na etiologia do sarcoma de Kaposi, possivelmente como resultado da ação das cell signaling proteins superando os aspectos da resposta imune. O herpesvírus humano tipo 8 tem sido ainda sugerido como agente causal na patogênese de outras desordens, incluindo mieloma múltiplo, multicentric Castleman's disease, body cavity-based lymphoma, além de outras condições não-proliferativas como sarcoidose e pênfigo vulgar, embora grande parte dos estudos sorológicos apontem para uma soroprevalência em torno de 2 a 10%. O herpesvírus humano tipo 8 parece então, ser um vírus restrito a pessoas sob risco de desenvolver o sarcoma de Kaposi, associado à imunossupressão. O tratamento para o sarcoma de Kaposi é normalmente paliativo, e inclui a aplicação de vimblastina intra-lesional, crio-cirurgia, interferon-alpha e outras formas de terapia. Mais recentemente, os inibidores da protease, foram também sugeridos como possíveis agentes implicados na remissão do sarcoma de Kaposi associado ao HIV e no desaparecimento do herpesvírus humano tipo 8 das células mononucleares do sangue periférico. CONCLUSÃO: O herpesvírus humano tipo 8 está fortemente associado a todas as formas de sarcoma de Kaposi, multicentric Castleman's disease e body cavity-based lymphoma. Ainda, não existe tratamento definitivo para o sarcoma de Kaposi.
Herpesvírus humano tipo 8; KSHV; Sarcoma de Kaposi; AIDS; HIV
REVIEWS
HUMAN HERPESVIRUS 8 (HHV-8) AND THE ETIOPATHOGENESIS OF KAPOSI'S SARCOMA
Jair Carneiro Leão1 1 From the Department of Preventive Clinic and Dentistry, Science Health Center, UFPE ; Immunology Section, Adolfo Lutz Institute 2 and Department of Oral Medicine, Eastman Dental Institute, University of London 3 e 4 . , Adele Caterino-de-Araújo2 1 From the Department of Preventive Clinic and Dentistry, Science Health Center, UFPE ; Immunology Section, Adolfo Lutz Institute 2 and Department of Oral Medicine, Eastman Dental Institute, University of London 3 e 4 . , Stephen R Porter3 1 From the Department of Preventive Clinic and Dentistry, Science Health Center, UFPE ; Immunology Section, Adolfo Lutz Institute 2 and Department of Oral Medicine, Eastman Dental Institute, University of London 3 e 4 . and Crispian Scully4 1 From the Department of Preventive Clinic and Dentistry, Science Health Center, UFPE ; Immunology Section, Adolfo Lutz Institute 2 and Department of Oral Medicine, Eastman Dental Institute, University of London 3 e 4 .
RHCFAP/3090
LEÃO JC et al. - Human herpesvirus 8 (HHV-8) and the etiopathogenesis of Kaposi's sarcoma. Rev. Hosp. Clín. Fac. Med. S. Paulo 57(4):175-186, 2002.
OBJECTIVE: To review the current literature on human herpesvirus 8 with particular attention to the aspects related to the etiopathogenesis of Kaposi's sarcoma.
MATERIALS AND METHODS: The authors searched original research and review articles on specific aspects of human herpesvirus 8 infection, including virology, epidemiology, transmission, diagnosis, natural history, therapy, and Kaposi's sarcoma etiopathogenesis. The relevant material was evaluated and reviewed.
RESULTS: Human herpesvirus 8 is a recently discovered DNA virus that is present throughout the world but with major geographic variation. In the Western world, the virus, transmitted mainly by means of sexual contact, is strongly associated with Kaposi's sarcoma and body cavity-based lymphoma and more controversially with multiple myeloma and other non-proliferative disorders. There is no specific effective treatment, but HIV protease inhibitors may play an indirect role in the clearance of human herpesvirus 8 DNA from peripheral blood mononuclear cells of HIV-infected patients. Human herpesvirus 8 DNA is present in saliva, but there are as yet no documented cases of nosocomial transmission to health care workers. The prevalence of human herpesvirus 8 among health care workers is probably similar to that in the general population.
CONCLUSION: Human herpesvirus 8 appears to be, at least in Western Europe and United States, restricted to a population at risk of developing Kaposi's sarcoma. Human herpesvirus 8 certainly has the means to overcome cellular control and immune responses and thus predispose carriers to malignancy, particularly Kaposi's sarcoma. The wide diffusion of Human herpesvirus 8 in classic Kaposi's sarcoma areas appears to represent an important factor in the high incidence of the disease. However, additional co-factors are likely to play a role in the development of Kaposi's sarcoma.
DESCRIPTORS: Human herpesvirus 8. KSHV. Kaposi´s sarcoma. AIDS. HIV.
INTRODUCTION
Kaposi's sarcoma (KS) is the most common oral malignancy of HIV disease. Epidemiological studies had previously suggested that KS may have an infective etiology, and in 1994 a virus termed Kaposi's sarcoma-associated herpes virus (KSHV) or human herpes virus-8 (HHV-8) was confirmed to be the cause. Since the first description of HHV-81, more of its virology, pathogenic mechanisms, and clinical consequences have been elucidated. There is strong evidence that HHV-8 not only is the causative agent of KS2 but also of primary effusion lymphoma (PEL) (also termed body cavity-based lymphomas (BCBL)3 and multicentric Castleman disease (MCD)4. Human herpesvirus 8 has also been tenuously etiologically linked with multiple myeloma5 and non-neoplastic disorders such as sarcoidosis6.
EPIDEMIOLOGY OF HHV-8 - ASPECTS OF TRANSMISSION
Human herpesvirus 8 is principally acquired sexually. Anal intercourse may be particularly associated with transmission7. In a large study of US males, sex with other men was associated with a greater risk activity for HHV-8 acquisition than was a history of blood or blood products receipt or injecting needle usage8. Likewise, in a cohort of Danish homosexual men, HHV-8 seropositivity correlated positively with the frequency of receptive anal intercourse9. Females infected with HIV by their bisexual male partners were found to be at greater risk of developing KS than those who became HIV-infected as a consequence of vaginal intercourse with male hemophiliacs, transfusion recipients, or injecting drug users7,10.
HHV-8 is present in prostate tissue and in semen, but the frequency of carriage varies considerably between groups. Hybridization in situ revealed HHV-8 in the prostate glandular epithelium of an elderly HIV-seronegative male11. In addition, in a cohort of Italian patients of unknown HIV status, who had no apparent clinical disease other than occasional varicoceles, HHV-8 DNA was detected by polymerase chain reaction (PCR) in 12% of tissue specimens from the urogenital tract, and in 44% of prostate tissues12. In addition, 81% of ejaculates from non-HIV-infected Italian individuals were HHV-8 positive12. HHV-8 was detected in prostate tissue of men with KS by in situ hybridization; between 1% to 5% of cells expressed viral transcripts associated with HHV-8 replication, while more than 90% expressed gene products associated with viral latency, findings suggesting that the prostate may be a site for viral replication and subsequent shedding into semen13.
HHV-8 DNA was detected by PCR in semen samples of 2/15 HIV-negative and 8/15 HIV-positive men living in Central Africa14. In contrast, HHV-8 was not detected in the semen of 4 HIV-infected American males with KS15, and only 2 out of another cohort of 14 HIV-infected American homosexual men with KS were found to be HHV-8 DNA positive16. Accordingly, not all HIV-infected patients from the US had evidence of HHV-8 in semen17.
Transmission of HHV-8 in feces was suggested by the findings that oro-anal contact was associated with a higher likelihood of KS in homosexual males7. Nevertheless, supporting evidence is equivocal since HHV-8 DNA was not detectable in feces from one cohort of HIV-infected patients18 but was found in 24 out 51 duodenal and rectal biopsies from another cohort19.
Sexual transmission is unlikely to be the only route of transmission of HHV-8, particularly in geographic areas where HHV-8 infection is endemic, and where prior to the AIDS era, KS was a common tumor not subsequently found to be associated with HIV infection (e.g. in Central and East Africa)20, such as in Uganda21. A recent study found HHV-8 infection in early childhood in Uganda unassociated with antibodies to hepatitis A or C virus or with the quality of water supply, which points to a horizontal mode of transmission different to that of developed countries22.
HHV-8 has also been found in saliva of HIV-infected patients. In one study, HHV-8 DNA was found in unstimulated whole saliva from 25 of 76 HIV-infected individuals without oral KS23, and another study showed HHV-8 in the saliva of individuals having no detectable viral sequences in their peripheral blood mononuclear cells24. In addition, HHV-8 DNA was detected in the saliva of 5 of 29 (17%) symptomatic HIV-infected patients (only 1 had oral KS at the time of sampling), but in none of 15 healthy controls25 or in any of 39 HIV-negative patients26.
Parenteral transmission is another potential route of HHV-8 infection. The virus has been detected in CD19+ cells of a healthy blood donor27. But in a separate study, 13 of 14 patients were found to remain seronegative for HHV-8 despite receiving transfused blood cells from HHV-8 seropositive donors28.
Data concerning a vertical route of transmission of HHV-8 is contradictory. In one study, all 9 children born from HHV-8 IgG positive Haitian and American mothers were HHV-8 seronegative29. However, in another study, 8 children of 19 who had HHV-8 IgG seropositive mothers were also HHV-8 seropositive30. Apart from vertical transmission, intrafamilial person-to-person spread of the virus has also been suggested, since the HHV-8 seroprevalence of spouses, children, and siblings in families of KS patients is higher than in HHV-8 non-family controls31.
EPIDEMIOLOGY OF HHV-8 INFECTION - SEROLOGICAL DATA
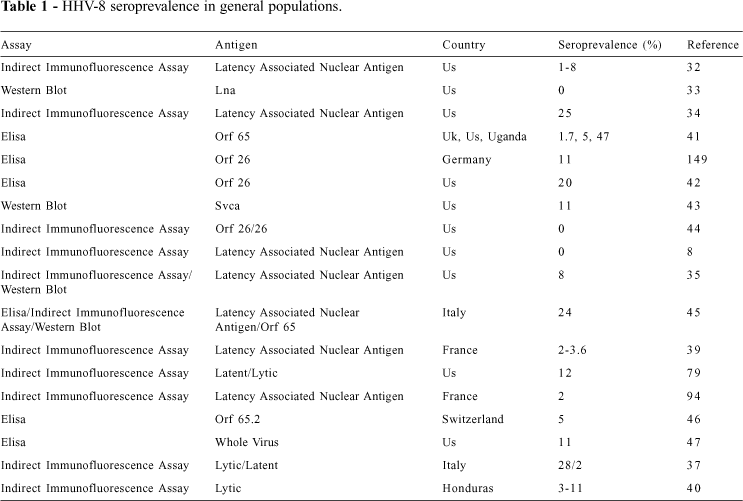
Epidemiological data based upon the detection of IgG antibodies to HHV-8 is conflicting, owing to methodological differences and, perhaps more importantly, geographical differences in HHV-8 infection. Using an indirect immunofluorescence assay (IFA) based on nuclei of BCBL-1 cells (to exclude the detection of cytoplasmic antigens)32, a seroprevalence of 1% was found in a group of HIV-negative blood donors and 8% in HIV-negative patients with syphilis. A study with indirect immunofluorescence using an uninduced cell preparation with a high cut-off to avoid non-specific background33 failed to detect HHV-8 antibodies in a large cohort of US blood donors. In contrast, an IFA based upon induced cell preparations optimized for the expression of both nuclear and cytoplasmic antigens34 found 25% of a cohort of healthy American adults and 2% to 8% of children to have IgG antibodies to HHV-8.
An IFA that detected antibodies to a latency-associated nuclear antigen (LANA) found none of 195 exclusively heterosexual men from San Francisco to have antibodies to HHV-8, while 12.5% of men who reported mostly homosexual activity, and 39.6% who were exclusively homosexual were HHV-8 positive8. More recently, using uninduced and TPA-induced BCBL-1 cells35, IFA antibody titers to HHV-8 were found to be much higher in HIV-infected Los Angeles patients with KS than in those HIV-infected without KS; furthermore, antibody positivity rate was 8% in healthy individuals, 56% in HIV-positive homosexual men, and 12% in age-matched HIV-negative controls36.
Additionally, 28% of Italian blood donors were found to be HHV-8 seropositive by IFA in a recent published study37. This is similar to another study where 23% of the control group comprised of healthy volunteers and patients with various dermatological diseases from central and southern regions of Italy had HHV-8 DNA in their PBMCs38.
Using IFA, HHV-8 antibodies were found in 16/16 French KS patients but in only 3/83 patients with non-KS dermatologic diseases and in 2/100 healthy controls39. Likewise, only 5/169 (3%) of HIV negative patients from Honduras were found to be HHV-8 positive when using a cutoff point of 1:4040.
Studies with enzyme-linked immunosorbent assay (ELISA) utilizing recombinant capsid-related proteins of HHV-8 open reading frame (ORF) 65 (which has little sequence similarity to EBV-BFRF3)41 found seroprevalence rates of 1.7% in UK, 5% in North American, 12% in Mediterranean blood donors, and up to 47% in Ugandan HIV-negative individuals. Investigations using an ELISA that utilizes 2 regions of the ORF 26 differing substantially from EBV-BDLF1, found 6 of 30 (20%) US healthy blood donors to be HHV-8 seropositive42. Another study, using small viral capsid antigen (sVCA) found 3 of 28 (11%) US blood donors to be seropositive for HHV-8, although none of 25 hemophiliac patients or 22 children (10 HIV-negative and 12 HIV-positive) were HHV-8 seropositive43. In another study, none of the 52 patients tested who were negative for HIV and hepatitis B and C virus had antibodies for HHV-844. In addition, using a latency-associated nuclear antigen (LANA) IFA and an ELISA with plates coated with capsid-related protein encoded by ORF 65, 24.1% of 779 Italian blood donors from different regions had antibodies to at least 1 antigen45.
An ELISA for detection of the ORF 65.2 antigen showed positivity in 24/26 (92.3%) of Swiss HIV-positive patients with KS, in 21/87 (24.1%) of HIV-positive patients without KS, in 11/54 (20.4%) of HIV-negative homosexual men, and in 9/178 (5.1%) blood donors46. Another ELISA assay with the whole virus lysate as the antigen was used, and a seroprevalence of 11% (10/91) was found in US blood donors. In this study, the average titer of different groups was also measured: blood donors had an average titer of 118, classic KS patients had an average titer of 14,111, and HIV-associated KS patients had 400047.
In order to standardize HHV-8 antibody assays, a blinded comparison was recently performed between 5 laboratories using 4 different IFAs and 3 ELISAs. It was found that while the HHV-8 antibody tests were adequate for epidemiological investigations, the poor specificity and sensitivity in detecting asymptomatic HHV-8 infection required further confirmatory testing using nucleic acid detection methods48.
Most serological studies (Table 1) suggest a global HHV-8 seroprevalence of 2% to 10%. Assuming a rate of 5% HHV-8 in the US and a 1970 baseline incidence of KS in men in the US (about 0.3 cases per 100,000 men), the HHV-8 rate would be only 1 case of KS for every 17,000 HHV-8 infections50.
DISEASE ASSOCIATIONS OF HUMAN HERPESVIRUS 8
Kaposi's sarcoma
Two small fragments of the HHV-8 genome, KS330Bam and KS631Bam, were originally isolated from KS tissues using representational difference analysis1. Since then, other research groups, using PCR primers derived from KS330Bam sequence, have detected HHV-8 DNA in lesional tissues of all 3 epidemiological forms of KS: HIV-related15,51-67, endemic51,52,56,58,65,67,68-70, and immunosuppressed related65,67,71-73.
Human herpesvirus 8 DNA can be amplified from KS tissue in different clinical stages of the disease74. Furthermore, semiquantitative analysis has established that the HHV-8 DNA load is higher in patients with multicentric and visceral involvement compared with those with localized disease, and that the nodular stage also has a higher viral load than do the patch and plaque stages, thereby showing a correlation between viral load and disease severity75.
The presence of HHV-8 DNA in PBMCs of HIV-infected individuals is predictive of the subsequent appearance of KS lesions76,77. In addition, antibodies to HHV-8 are associated with having KS or being at increased risk of developing KS78,79.
Body cavity-based lymphoma
Body cavity based-lymphoma (BCBL) (also termed primary effusion lymphoma (PEL)) is a rare, rapidly fatal malignancy, first described in AIDS patients.3 The disease has a distinctive presentation, with malignant peritoneal, pericardial, or pleural effusions in the absence of an identifiable tumor mass or nodal involvement. Carriage by BCBL cells of both EBV and HHV-8 infection has been observed, but association with HHV-8 alone has also been reported80-83. Notably, most cases of EBV-negative BCBL have occurred in patients who are also HIV negative, whereas BCBL in HIV positive patients are usually EBV-positive.
Multicentric Castleman's disease
Multicentric Castleman's Disease (MCD) is an atypical lymphoproliferative disorder mostly found in patients with HIV disease. HHV-8 has been found only infrequently in MCD of HIV negative patients4, making the association less strong in comparison with that of HHV-8 with KS or BCBL/primary effusion lymphoma.
Other possible disease associations of HHV-8
Lymphoproliferative disease
HHV-8 sequences have been detected in some angioimmunoblastic lymphadenopathies in non-HIV-infected patients, in particular in a distinct, benign lymphadenopathy histologically characterized by a predominantly follicular lesion with a giant hyperplasic germinal center and increased vascularity84.
HHV-8 DNA has also been detected rarely in other lymphoproliferative disorders, including non-Hodgkin's lymphoma, Hodgkin's disease, reactive lymphadenopathies85, and cutaneous lymphoma in AIDS59. The viral load is significantly higher in lymphoid tissue from HIV-infected persons as compared to HIV-seronegative individuals85, although it is still lower than in splenic tissue or PBMCs from the same patients. This suggests that the HHV-8 detected in these lesions may reflect HHV-8 carriage by non-neoplastic B cells59. Detection of HHV-8 in mature T-cell lymphoproliferative disorders is equivocal3,86-88.
Multiple Myeloma
HHV-8 has been associated with multiple myeloma (MM) in several US studies. HHV-8 DNA was found in bone marrow dendritic cells of 25% of a cohort of patients with monoclonal gammopathy, a condition that may progress to MM5. HHV-8 DNA was also found by PCR in 6 out of 7 fresh biopsy samples, and by in situ hybridization in bone marrow dendritic cells in 17 out of 20 patients with MM89. In addition, 20 of 27 (81%) patients with MM were HHV-8 seropositive using an ELISA assay, while only 22% of patients with other malignancies and 6% of blood donors were found to be HHV-8 seropositive90.
In contrast, studies in other countries have not detected HHV-8 associated with MM. Using a nested PCR and a serological assay to detect HHV-8 LANA and ORF 65 (lytic) protein, only 1 out of 20 Italian patients with MM was found to be HHV-8 seropositive91. None of 10 Swedish patients with MM were found to be HHV-8 positive by PCR or serological assay92. Furthermore, no statistically significant differences between MM patients and blood donors were observed in the frequency of HHV-8 seropositivity using an IFA assay76. Similar results have also been found by others93-97. Any significance of HHV-8 in the etiology of MM thus remains unclear.
Other conditions
HHV-8 DNA can be detected in normal skin of HIV-infected patients with KS 62,64,98, and in normal skin from patients with endemic KS12,15,65,99,100, or in iatrogenic KS101. It is also found in lesional tissue of patients with squamous cell or basal cell carcinomas66. The HHV-8 infection load appears to be lower in normal skin and in cutaneous lesions other than KS than in lesional KS tissue64,66.
Sequences of HHV-8 DNA have been detected in immunosuppressed patients with glomerulonephritis54, with pemphigus vulgaris,102 and with mycosis fungoides.103 HHV-8 sequences have also been detected in angiosarcomas104,105 and in angiolymphoid hyperplasia with eosinophilia104. However, HHV-8 DNA was not detected in immunosuppression-associated dermatofibromas, despite these sharing many histologic similarities with HIV-related KS106.
HHV-8 has been associated with sarcoidosis in Italian patients6, but not in French patients107. Furthermore, a serological assay (ELISA) detected antibodies in only 3 out 15 (20%) of Swiss patients108.
At present it is unclear if immunosuppression or granulomatous inflammation activate latent HHV-8 or whether the virus is indeed an etiological agent.
Virological aspects of HHV-8
Cell lines derived from BCBL frequently contain HHV-8 genomes. Their study has thus provided significant insights into the biological properties of HHV-8. The BC-1 line harbors HHV-8 but not EBV-DNA, while line BC-2 harbors both viruses3. Treatment of BC-1 with phorbol esters rapidly induces lytic growth of HHV-8, and progeny virus are then shed into the supporting medium109. The induced B cells can be observed to contain 110 nm intranuclear herpesvirus-like nucleocapsids and complete cytoplasmic virions81. The length of genome of the virus is estimated to be similar (e.g. 160-170 kb) to other gammaherpesviruses. The HHV-8 genome is, like that of EBV, maintained in latently infected B cells as extrachromosomal, episomal, monomeric circles, with induction from latency leading to the selective accumulation of linear genomic forms109. In contrast, only covalently closed, circular episomes of HHV-8 are identified in KS tissue, while linear forms, arising from viral replication, are additionally found in PBCs of KS patients110. While uninduced BCBL lines have not yet been shown to permit propagation of HHV-8, the virus can be cultured from skin lesions of patients with AIDS-associated KS using the human embryonal-kidney epithelioid line 293106, thus providing evidence that the virus is able of replicating vegetatively in vitro.
Sequencing of a 12.3-kb HHV-8 clone obtained from a genomic library derived from BC-1 revealed homology between HHV-8 with parts of the EBV genome. The sequences of some ORFs of HHV-8 are homologous to EBV-membrane antigen p140, herpesvirus saimiri (HVS) p160, cellular type D cyclins, and HVS and cellular G protein-coupled receptors80. Furthermore, transcription of these 4 ORFs can be demonstrated in BC-12. A novel abundant 1.2-kb RNA, polyadenylated nuclear RNA (called PAN RNA), has also been identified from the BC-1 line; it appears speckled in the nuclei by immunofluorescence and may be a early lytic cycle viral transcript111,112.
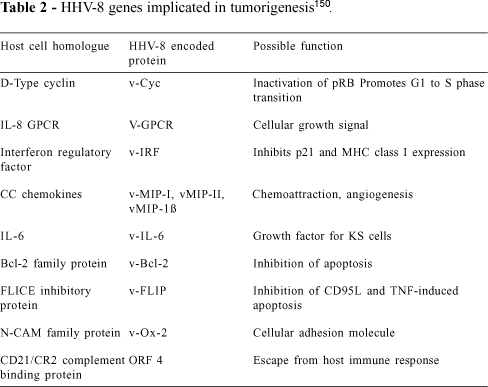
The BC-1 cell line was used to create a cosmid and phage genomic library, allowing full characterization of the HHV-8 nucleotide sequence, except for a 3 kb region at the right end of the genome113. The BC-1 HHV-8 genome is 140.5-kb long, with a unique coding region flanked by multiple 801-bp terminal repeat sequences. A genomic duplication that apparently arose in the parental tumor is present in this cell culture-derived strain. At least 81 ORFs and 5 internal repeat regions are present in the long unique region. In addition to viral structural and metabolic proteins, the virus encodes homologues as complement-binding proteins, 3 cytokines (2 macrophage inflammatory proteins (MIP), MIP-1a, MIP-1b, and interleukin-6, IL-6), dihydrofolate reductase, bcl-2, interferon regulatory factors, interleukin 8 receptor, neural cell adhesion molecule-like adhesin, and a D-type cyclin113. A subsequent study in which a 17-kb segment of HHV-8 between ORFs 11 and 17 was sequenced confirmed that the viral genome contains a single 13-kb divergent locus wherein are 9 ORFs that are homologous with, or related to, cellular proteins114. A fourth potential cytokine gene, BCK, was also identified, in addition to a viral thymidylate synthetase gene, the T1.1 abundant lytic cycle nuclear RNA gene, and 2 genes (1E1-A and 1E1-B) related to the immediate-early protein of the gamma-2 class herpesvirus bovine herpesvirus type 4114.
The herpes viral-like particles produced from BC-1 cells can further infect PBMC-derived CD19+ B cells. This suggests that HHV-8 is transmissible and B-lymphotropic115.
More recently, other BCBL-derived cell lines have been established and characterized: BC-3, BCP-1, and CRO-AP/3 do not contain EBV DNA, while CRO-AP/5 and HBL-6 contains both EBV and HHV-898,116-118. The structure of the HHV-8 gene in these cell lines has yet to be reported.
HHV-8 homology to other viral and cellular proteins
Analysis of the putative translation products of HHV-8 has revealed homology with a number of known human cell receptors, cell cycle enzymes, and chemokines important in a variety of cellular and immunological as well as homeostatic and angiogenic mechanisms. HHV-8 may thus possess a range of mechanisms capable of causing cell changes.
HHV-8 possesses several potential oncogenes. The open reading frame (ORF) K1, for instance, encodes a class I transmembrane glycoprotein with transforming properties in rodent fibroblasts119. The second HHV-8 potential oncogene is a G protein-coupled receptor (GCR), which is a putative translation product of ORF 74. These receptors are important in cellular growth and differentiation, with some GCRs being implicated in malignant transformation116. Expression of HHV-8 GCRs stimulates proliferation and causes transformation of rodent fibroblasts. Hence, the HHV-8 GCR homologue may be a mediator of KS tumorigenesis120. The closest cellular homologues to the putative HHV-8 GCR are the interleukin-8 (IL-8) receptors A and B, and the closest viral homologue is the herpesvirus samiri (HVS) ECRF3 gene, which encodes a functional IL-8 receptor121.
HHV-8 also contains a protein homologous with cell cycle controllers, such as a cyclin D-type v-cyclin that displays 53% homology to cyclin D2122. Cyclins are required for cellular division and are involved in the control of the G1S check point in the cell cycle123. HHV-8 cyclin protein is reported to possess kinase activity, which enables it to phosphorylate and thus inactivate the retinoblastoma (Rb) tumor suppressor protein. Hence HHV-8 has the potential to overcome cell-cycle control, thereby increasing the likelihood of tumor development124,125.
HHV-8 ORF 16 (vBcl-2) shares 15% to 20% amino acid identity with the Bcl-2 family113,126,127 a group of genes known to prevent apoptosis.
Other ORFs of HHV-8 have been found to encode proteins similar to macrophage inflammatory protein (MIP) chemokines (vMIP-1 from ORF K6 and vMIP-2 from ORF K4), interleukin-6 (from ORF K2), and interferon regulatory factor (vIRF) (from ORF K9)2. The HHV-8 ORF K6 protein (vMIP-1) inhibits HIV entry via the CCR5 chemokine receptor, suggesting that vMIP-1 is functional in binding CCR5 and thus contributes to interactions between HHV-8 and HIV.
Potentially, HHV-8 may exert some inhibitory action in HIV infection, which might underlie the suggestion that patients with AIDS and KS have a better prognosis than patients with AIDS but not KS128. This may not be the case, however, since a recent epidemiological study found that the presence of KS appears to accelerate the clinical course of HIV infection resulting in shorter survival times129. Furthermore, vMIP-1 is expressed only by a small subpopulation of HHV-8 infected cells (productive cells), and therefore the impact of vMIP-1 on KS angiogenesis may be limited.130 The virally-derived IL-6 (vIL-6) has been found to be expressed in some HHV-8 infected cells131.
HHV-8 may thus have the means of overcoming typical cellular strategies against viral infection including cell cycle control, apoptosis, and cell-mediated immunity (Table 2 summarizes HHV-8 genes possibly implicated in the pathogenesis of KS).
Antiviral therapy
HHV-8 replication in vitro can be inhibited by ganciclovir, foscarnet, and cidofovir, but not acyclovir133. In addition, lytic replication of HHV-8 can be inhibited by a low dose of cidofovir134,135.
Highly active antiretroviral therapy (HAART), however, is very effective in inhibiting HIV replication, increasing CD4 + cell counts and delaying, lessening, or resolving AIDS-associated opportunistic infections136,137. Preliminary data from case reports suggest that protease inhibitors may help induce remission of HIV-related KS125,138-143 and clearance of HHV-8 DNA from PBMCs138,139,141,143,145. Moreover, HAART in 2 HIV-positive patients with BCBL resulted in HHV-8 DNA being undetected in their pleural effusions146. In contrast, PrI treatment produced no effect on the clinical and virological progression of an HIV-positive patient with MCD147.
Oral health considerations of HHV-8 infection
HHV-8 is not found in saliva of healthy controls but is found in the saliva of up to 33% of patients with AIDS23 and up to 75% of HIV infected patients with KS24. Similarly, HHV-8 DNA was found only in symptomatic HIV-1-infected patients (5 [17%] of 29)25. More recently, 11 out of 24 saliva samples (45.8%) of patients with AIDS-KS were HHV-8 DNA positive, but in the control group, none of the 20 saliva specimens were positive for HHV-8 DNA38. HHV-8 has been identified in oral KS, other oral lesions (e.g. non-specific ulcers), and in normal oral mucosae in HIV disease148. Nevertheless, there are no data yet to suggest that dental health care workers are at notable risk of HHV-8 acquisition through occupational routes.
RESUMO
RHCFAP/3090
LEÃO JC e col. - Herpesvírus humano tipo 8 (HHV-8) e a etiopatogênese do sarcoma de Kaposi. Rev. Hosp. Clín. Fac. Med. S. Paulo 57(4): 175-186, 2002.
OBJETIVO: O objetivo do presente artigo foi revisar a literatura recente em relação ao herpesvírus humano tipo 8, com ênfase especial aos aspectos relacionados à etiopatogênese do sarcoma de Kaposi.
MÉTODOS: Os autores pesquisaram artigos de pesquisa original e revisões de literatura nos aspectos específicos da infecção pelo herpesvírus humano tipo 8, incluindo, virologia, epidemiologia, transmissão, diagnóstico, história natural e terapia. O material considerado relevante foi avaliado e revisado.
RESULTADOS: O sarcoma de Kaposi é considerado ainda a malignidade mais comumente observada em pacientes infectados pelo HIV. Estudos epidemiológicos, assim como os baseados em técnicas de biologia molecular indicam que um agente sexualmente transmissível, independente do HIV, deve estar envolvido na etiologia do sarcoma de Kaposi, possivelmente como resultado da ação das cell signaling proteins superando os aspectos da resposta imune. O herpesvírus humano tipo 8 tem sido ainda sugerido como agente causal na patogênese de outras desordens, incluindo mieloma múltiplo, multicentric Castleman's disease, body cavity-based lymphoma, além de outras condições não-proliferativas como sarcoidose e pênfigo vulgar, embora grande parte dos estudos sorológicos apontem para uma soroprevalência em torno de 2 a 10%. O herpesvírus humano tipo 8 parece então, ser um vírus restrito a pessoas sob risco de desenvolver o sarcoma de Kaposi, associado à imunossupressão. O tratamento para o sarcoma de Kaposi é normalmente paliativo, e inclui a aplicação de vimblastina intra-lesional, crio-cirurgia, interferon-alpha e outras formas de terapia. Mais recentemente, os inibidores da protease, foram também sugeridos como possíveis agentes implicados na remissão do sarcoma de Kaposi associado ao HIV e no desaparecimento do herpesvírus humano tipo 8 das células mononucleares do sangue periférico.
CONCLUSÃO: O herpesvírus humano tipo 8 está fortemente associado a todas as formas de sarcoma de Kaposi, multicentric Castleman's disease e body cavity-based lymphoma. Ainda, não existe tratamento definitivo para o sarcoma de Kaposi.
DESCRITORES: Herpesvírus humano tipo 8. KSHV. Sarcoma de Kaposi. AIDS. HIV.
REFERENCES
Received for publication on May 14, 2001.
- 1. CHANG Y, CESARMAN E, PESSIN MS et al. - Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 1994; 266:1865-69.
- 2. MOORE PS, BOSHOFF C, WEISS RA et al. - Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science 1995; 274:1739-44.
- 3. CESARMAN E, CHANG Y, MOORE PS et al. - Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med 1995; 332:1186-91.
- 4. SOULIER J, GROLLET L, OKSENHENDLER E et al. - Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 1995; 86:1276-80.
- 5. RETTIG MB, MA HJ, VESCIO RA et al. - Kaposi's sarcoma-associated herpesvirus infection of bone marrow dendritic cells from multiple myeloma patients. Science 1997; 276:1851-4.
- 6. DI ALBERTI L, PIATTELLI A, ARTESE L et al. - Human herpesvirus 8 variants in sarcoid tissues. Lancet 1997; 350:1655-61.
- 7. BERAL V, PETERMAN TA, BERKELMAN RL et al. - Kaposi's sarcoma among persons with AIDS: a sexually transmitted infection? Lancet 1990; 335:123-8.
- 8. MARTIN JN, GANEM DE, OSMOND DH et al. - Sexual transmission and the natural history of human herpesvirus 8 infection. N Engl J Med 1998; 338:948-54.
- 9. MELBYE M, COOK PM, HJALGRIM H et al. - Risk factors for Kaposi's-sarcoma-associated herpesvirus (KSHV/HHV-8) seropositivity in a cohort of homosexual men, 1981-1996. Int J Cancer 1998; 77:543-48.
-
10HERMANS P, LUNDGREN J, SOMMEREIJNS B et al. - Epidemiology of AIDS-related Kaposi's sarcoma in Europe over 10 years. AIDS in Europe Study Group. AIDS 1996; 10:911-7.
- 11. STASKUS KA, ZHONG W, GEBHARD K et al. - Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J Virol 1997; 71:715-9.
- 12. MONINI P, DE LELLIS L, FABRIS M et al. - Kaposi's sarcoma-associated herpesvirus DNA sequences in prostate tissue and human semen. N Engl J Med 1996; 334:1168-72.
- 13. DIAMOND C, BRODIE SJ, KRIEGER JN et al. - Human herpesvirus 8 in the prostate gland of men with Kaposi's sarcoma. J Virol 1998; 72:6223-27.
- 14. BELEC L, TEVI-BENISSAN C, MOHAMED AS et al. - Enhanced detection of human herpesvirus-8 and cytomegalovirus in semen of HIV-seropositive asymptomatic heterosexual men living in Central Africa. AIDS 1998; 12:674-6.
- 15. AMBROZIAK JA, BLACKBOURN DJ, HERNDIER BG et al. - Herpes-like sequences in HIV-infected and uninfected Kaposi's sarcoma patients. Science 1995; 268:582-3.
- 16. GUPTA P, SINGH MK, RINALDO C et al. - Detection of Kaposi's sarcoma herpesvirus DNA in semen of homosexual men with Kaposi's sarcoma. AIDS 1996; 10:1596-8.
- 17. DIAMOND C, HUANG ML, KEDES DH et al. - Absence of detectable human herpesvirus 8 in the semen of human immunodeficiency virus-infected men without Kaposi's sarcoma. J Infect Dis 1997; 176:775-7.
- 18. LIN JC, LIN SC, MAR EC et al. - Is Kaposi's-sarcoma-associated herpesvirus detectable in semen of HIV- infected homosexual men? Lancet 1995; 346:1601-2.
- 19. THOMAS JA, BROOKES LA, MCGOWAN I et al. - HHV-8 DNA in normal gastrointestinal mucosa from HIV seropositive people. Lancet 1996; 347:1137-8.
- 20. ZIEGLER JL & KATONGOLE-MBIDDE E. - Kaposi's sarcoma in childhood: an analysis of 100 cases from Uganda and relationship to HIV infection. Int J Cancer 1996; 65:200-3.
- 21. OLWENY CL, KADDUMUKASA A, ATINE I et al. - Childhood Kaposi's sarcoma: clinical features and therapy. Br J Cancer 1976; 33:555-60.
- 22. MAYAMA S, CUEVAS LE, SHELDON J et al. - Prevalence and transmission of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in Ugandan children and adolescents. Int J Cancer 1998; 77:817-20.
- 23. VIEIRA J, HUANG ML, KOELLE DM et al. - Transmissible Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in saliva of men with a history of Kaposi's sarcoma. J Virol 1997; 71:7083-87.
- 24. KOELLE DM, HUANG ML, CHANDRAN B et al. - Frequent detection of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) DNA in saliva of human immunodeficiency virus-infected men: clinical and immunologic correlates. J Infect Dis 1997; 176:94-102.
- 25. LUCHT E, BRYTTING M, BJERREGAARD L et al. - Shedding of cytomegalovirus and herpesviruses 6, 7, and 8 in saliva of human immunodeficiency virus type 1-infected patients and healthy controls. Clin Infect Dis 1998; 27:137-41.
- 26. BOLDOGH I, SZANISZLO P, BRESNAHAN WA et al. - Kaposi's sarcoma herpesvirus-like DNA sequences in the saliva of individuals infected with human immunodeficiency virus. Clin Infect Dis 1996; 23:406-7.
- 27. BLACKBOURN DJ, AMBROZIAK J, LENNETTE E et al. - Infectious human herpesvirus 8 in a healthy North American blood donor. Lancet 1997; 349:609-11.
- 28. OPERSKALSKI EA, BUSCH MP, MOSLEY JW et al. - Blood donations and viruses. Lancet 1997; 349:1327.
- 29. GOEDERT JJ, KEDES DH & GANEM D. - Antibodies to human herpesvirus 8 in women and infants born in Haiti and the USA. Lancet 1997; 349:1368.
- 30. BOURBOULIA D, WHITBY D, BOSHOFF C et al. - Serologic evidence for mother-to-child transmission of Kaposi sarcoma-associated herpesvirus infection. JAMA 1998; 280:31-32.
- 31. ANGELONI A, HESTON L, UCCINI S et al. - High prevalence of antibodies to human herpesvirus 8 in relatives of patients with classic Kaposi's sarcoma from Sardinia. J Infect Dis 1998; 177:1715-18.
- 32. KEDES DH, OPERSKALSKI E, BUSCH M et al. - The eroepidemiology of human herpesvirus 8 (Kaposi's sarcoma-associated herpevirus): Distribution of infection in KS risk groups and evidence for sexual transmission. Nature Med 1996; 2:918-24.
- 33. GAO SJ, KINGSLEY L, LI M et al. - KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi's sarcoma. Nature Med 1996; 2:925-8.
- 34. LENNETTE ET, BLACKBOURN DJ & LEVY JA. - Antibodies to human herpesvirus type 8 in the general population and in Kaposi's sarcoma patients. Lancet 1996; 348:858-61.
- 35. CHANDRAN B, SMITH MS, KOELLE DM et al. - Reactivities of human sera with human herpesvirus-8-infected BCBL-1 cells and identification of HHV-8-specific proteins and glycoproteins and the encoding cDNAs. Virology 1998; 243:208-17.
- 36. VERBEEK W, FRANKEL M, MILES S et al. - Seroprevalence of HHV-8 antibodies in HIV-positive homosexual men without Kaposi's sarcoma and their clinical follow-up. Am J Clin Pathol 1998; 109:778-83.
- 37. REZZA G, LENNETTE ET, GIULIANI M et al. - Prevalence and determinants of anti-lytic and anti-latent antibodies to human herpesvirus-8 among Italian individuals at risk of sexually and parenterally transmitted infections. Int J Cancer 1998; 77:361-5.
- 38. CATTANI P, CAPUANO M, LESNONI LP et al. - Human herpesvirus 8 in Italian HIV-seronegative patients with Kaposi sarcoma. Arch Dermatol 1998; 134:695-9.
- 39. DUPIN N, MARCELIN AG, GORIN I et al. - Prevalence of human herpesvirus 8 infection measured by antibodies to a latent nuclear antigen in patients with various dermatologic diseases. Arch Dermatol 1998; 134: 700-702.
- 40. SOSA C, KLASKALA W, CHANDRAN B et al. - Human herpesvirus 8 as a potential sexually transmitted agent in Honduras. J Infect Dis 1998; 178:547-51.
- 41. SIMPSON GR, SCHULZ TF, WHITBY D et al. - Prevalence of Kaposi's sarcoma associated herpesvirus infection measured by antibodies to recombinant capsid protein and latent immunofluorescence antigen. Lancet 1996; 348:1133-38.
- 42. DAVIS DA, HUMPHREY RW, NEWCOMB FM et al. - Detection of serum antibodies to a Kaposi's sarcoma-associated herpesvirus-specific peptide. J Infect Dis 1997; 175:1071-79.
- 43. LIN SF, SUN R, HESTON L et al. - Identification, expression, and immunogenicity of Kaposi's sarcoma-associated herpesvirus-encoded small viral capsid antigen. J Virol 1997; 71:3069-76.
- 44. SMITH MS, BLOOMER C, HORVAT R et al. - Detection of human herpesvirus 8 DNA in Kaposi's sarcoma lesions and peripheral blood of human immunodeficiency virus-positive patients and correlation with serologic measurements. J Infect Dis 1997; 176:84-93.
- 45. CALABRO ML, SHELDON J, FAVERO A et al. - Seroprevalence of Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 in several regions of Italy. J Hum Virol 1998; 1:207-13.
- 46. REGAMEY N, CATHOMAS G, SCHWAGER M et al. - High human herpesvirus 8 seroprevalence in the homosexual population in Switzerland. J Clin Microbiol 1998; 36:1784-86.
- 47. CHATLYNNE LG, LAPPS W, HANDY M et al. - Detection and titration of human herpesvirus-8-specific antibodies in sera from blood donors, acquired immunodeficiency syndrome patients, and Kaposi's sarcoma patients using a whole virus enzyme-linked immunosorbent assay. Blood 1998; 92:53-8.
- 48. RABKIN CS, SCHULZ TF, WHITBY D et al. - Interassay correlation of human herpesvirus 8 serologic tests. HHV-8 Interlaboratory Collaborative Group. J Infect Dis 1998; 178:304-9.
- 49. NEIPEL F, ALBRECHT JC & FLECKENSTEIN B. - Cell-homologous genes in the Kaposi's sarcoma-associated rhadinovirus human herpesvirus 8: determinants of its pathogenicity? J Virol 1997; 71:4187-92.
- 50. GALLO RC. - The enigmas of Kaposi's sarcoma. Science 1998; 282:1837-9.
- 51. HUANG YQ, LI JJ, KAPLAN MH et al. - Human herpesvirus-like nucleic acid in various forms of Kaposi's sarcoma. Lancet 1995; 345:759-61.
- 52. LELLIS L, FABRIS M, CASSAI E et al. - Herpesvirus-like DNA sequences in non-AIDS Kaposi's sarcoma. J Infect Dis 1995; 172:1605-7.
- 53. MOORE PS & CHANG Y. - Detection of herpesvirus-like DNA sequences in Kaposi's sarcoma in patients with and those without HIV infection. N Engl J Med 1995; 332:1181-5.
- 54. NOEL JC, HERMANS P, ANDRE J et al. - Herpesvirus-like DNA sequences and Kaposi's sarcoma: relationship with epidemiology, clinical spectrum, and histologic features. Cancer 1996; 77:2132-6.
- 55. ROIZMAN B. - New viral footprints in Kaposi's sarcoma. N Engl J Med 1995; 332:1227-8.
- 56. SCHALLING M, EKMAN M, KAAYA EE et al. - A role for a new herpes virus (KSHV) in different forms of Kaposi's sarcoma. Nature Med 1995; 1:707-8.
- 57. SU IJ, HSU YH, CHANG YC et al. - Herpesvirus-like DNA sequence in Kaposi's sarcoma from AIDS and non-AIDS patients in Taiwan. Lancet 1995; 345
- 58. CATHOMAS G, TAMM M, MCGANDY CE et al. - Detection of herpesvirus-like DNA in the bronchoalveolar lavage fluid of patients with pulmonary Kaposi's sarcoma. Eur Respir J 1996; 9:1743-6.
- 59. CORBELLINO M, POIREL L, BESTETTI G et al. - Human herpesvirus-8 in AIDS-related and unrelated lymphomas. AIDS 1996; 10:545-6.
- 60. GANEM D. - Human herpesvirus 8 and the biology of Kaposi's sarcoma. Semin Virol 1996; 7:325-32.
- 61. O'NEILL E, HENSON TH, GHORBANI AJ et al. - Herpes virus-like sequences are specifically found in Kaposi sarcoma lesions. J Clin Pathol 1996; 49:306-8.
- 62. LEBBE C, PELLET C, FLAGEUL B et al. - Sequences of human herpesvirus 8 are not detected in various non-Kaposi sarcoma vascular lesions. Arch Dermatol 1997; 133:919-20.
- 63. BOSHOFF C, WHITBY D, HATZIOANNOU T et al. - Kaposi's sarcoma associated herpesvirus in HIV-negative Kaposi's sarcoma. Lancet 1995; 345:1043-4.
- 64. DUPIN N, GRANDADAM M, CALVEZ V et al. - Herpesvirus-like DNA sequences in patients with Mediterranean Kaposi's sarcoma. Lancet 1995; 345:761-2.
- 65. LEBBE C, DE CREMOUX P, RYBOJAD M et al. - Kaposi's sarcoma and new herpesvirus. Lancet 1995; 345:1180.
- 66. RADY PL, YEN A, ROLLEFSON JL et al. - Herpesvirus-like DNA sequences in non-Kaposi's sarcoma skin lesions of transplant patients. Lancet 1995; 345:1339-40.
- 67. BUONAGURO FM, TORNESELLO ML, GIRALDO EB et al. - Herpesvirus-like DNA sequences detected in endemic, classic, iatrogenic and epidemic Kaposi's sarcoma (KS) biopsies. Int J Cancer 1996; 65:25-8.
- 68. CHANG Y, ZIEGLER J, WABINGA H et al. - Kaposi's sarcoma-associated herpesvirus and Kaposi's sarcoma in Africa. Uganda Kaposi's Sarcoma Study Group. Arch Intern Med 1996; 156:202-4.
- 69. CHUCK S, GRANT RM, MBIDDE EK et al. - Frequent presence of a novel herpesvirus genome in lesions of human immunodeficiency virus-negative Kaposi's sarcoma. J Infect Dis 1996; 173:248-51.
- 70. ETO H, KAMIDIGO NO, MURAKAMI-MORI K et al. - Short report: herpes-like DNA sequences in African-endemic and acquired immunodeficiency syndrome-associated Kaposi's sarcoma. Am J Trop Med Hyg 1996; 55:405-6.
- 71. GLUCKMAN E, PARQUET N, SCIEUX C et al. - KS-associated herpesvirus-like DNA sequences after allogeneic bone-marrow transplantation. Lancet 1995; 346
- 72. ALKAN S, KARCHER DS, ORTIZ A et al. - Human herpesvirus-8/Kaposi's sarcoma-associated herpesvirus in organ transplant patients with immunosuppression. Br J Haematol 1997; 96:412-4.
- 73. HENGHOLD WB, PURVIS SF, SCHAFFER J et al. - Kaposi sarcoma-associated herpesvirus/human herpesvirus type 8 and Epstein-Barr virus in iatrogenic Kaposi sarcoma. Arch Dermatol 1997; 133:109-11.
- 74. LUPPI M, BAROZZI P, MAIORANA A et al. - Frequency and distribution of herpesvirus-like DNA sequences (KSHV) in different stages of classic Kaposi's sarcoma and in normal tissues from an Italian population. Int J Cancer 1996; 66:427-31.
- 75. MENDEZ JC, PROCOP GW, ESPY MJ et al. - Detection and semiquantitative analysis of human herpesvirus 8 DNA in specimens from patients with Kaposi's sarcoma. J Clin Microbiol 1998; 36:2220-2.
- 76. WHITBY D, HOWARD MR, TENANT-FLOWERS M et al. - Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi's sarcoma. Lancet 1995; 346:799-802.
- 77. MOORE PS, KINGSLEY LA, HOLMBERG SD et al. - Kaposi's sarcoma-associated herpesvirus infection prior to onset of Kaposi's sarcoma. AIDS 1996; 10:175-80.
- 78. GAO SJ, KINGSLEY L, HOOVER DR et al. - Seroconversion to antibodies against Kaposi's sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi's sarcoma. N Engl J Med 1996; 335:233-41.
- 79. VERBEEK W, FRANKEL M, MILES S et al. - Seroprevalence of HHV-8 antibodies in HIV-positive homosexual men without Kaposi's sarcoma and their clinical follow-up. Am J Clin Pathol 1998; 109:778-83.
- 80. CESARMAN E, NADOR RG, AOZASA K et al. - Kaposi's sarcoma-associated herpesvirus in non-AIDS related lymphomas occurring in body cavities. Am J Pathol 1996; 149:53-7.
- 81. SAID W, CHIEN K, TAKEUCHI S et al. - Kaposi's sarcoma-associated herpesvirus (KSHV or HHV8) in primary effusion lymphoma: ultrastructural demonstration of herpesvirus in lymphoma cells. Blood 1996; 87:4937-43.
- 82. HERMINE O, MICHEL M, BUZYN-VEIL A et al. ¾ Body-cavity-based lymphoma in an HIV-seronegative patient without Kaposi's sarcoma-associated herpesvirus-like DNA sequences. N Engl J Med 1996; 334:272-3.
- 83. JONES D, BALLESTAS ME, KAYE KM et al. - Primary-effusion lymphoma and Kaposi's sarcoma in a cardiac-transplant recipient. N Engl J Med 1998; 339:444-9.
- 84. LUPPI M & TORELLI G. - Human herpesvirus 8 and interstitial pneumonitis in an HIV-negative patient. N Engl J Med 1996; 335:351-2.
- 85. BIGONI B, DOLCETTI R, DE LELLIS L et al. - Human herpesvirus 8 is present in the lymphoid system of healthy persons and can reactivate in the course of AIDS. J Infect Dis 1996; 173:542-9.
- 86. SANDER CA, SIMON M, PUCHTA U et al. - HHV-8 in lymphoproliferative lesions in skin. Lancet 1996; 348:475-6.
- 87. PASTORE C, GLOGHINI A, VOLPE G et al. - Distribution of Kaposi's sarcoma herpesvirus sequences among lymphoid malignancies in Italy and Spain. Br J Haematol 1995; 91:918-20.
- 88. PAWSON R, CATOYSKY D & SCHULZ TF. - Lack of evidence of HHV-8 in mature T-cell lymphoproliferative disorders. Lancet 1996; 348:1450-1.
- 89. SAID JW, RETTIG MR, HEPPNER K et al. - Localization of Kaposi's sarcoma-associated herpesvirus in bone marrow biopsy samples from patients with multiple myeloma. Blood 1997; 90:4278-82.
- 90. GAO SJ, ALSINA M, DENG JH et al. - Antibodies to Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in patients with multiple myeloma. J Infect Dis 1998; 178:846-9.
- 91. PARRAVICINI C, OLSEN SJ, CAPRA M et al. - Risk of Kaposi's sarcoma-associated herpes virus transmission from donor allografts among Italian posttransplant Kaposi's sarcoma patients. Blood 1997; 90:2826-9.
-
92YI Q, EKMAN M, ANTON D et al. - Blood dendritic cells from myeloma patients are not infected with Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8). Blood 1998; 92:402-4.
- 93. MACKENZIE J, SHELDON J, MORGAN G et al. - HHV-8 and multiple myeloma in the UK. Lancet 1997; 350:1144-5.
- 94. MARCELIN AG, DUPIN N, BOUSCARY D et al. - HHV-8 and multiple myeloma in France. Lancet 1997; 350:1144.
- 95. CULL GM, TIMMS JM, HAYNES AP et al. - Dendritic cells cultured from mononuclear cells and CD34 cells in myeloma do not harbour human herpesvirus 8. Br J Haematol 1998; 100:793-6.
- 96. MITTERER M, MAIR W, GATTI D et al. - Dendritic cells derived from bone marrow and CD34+ selected blood progenitor cells of myeloma patients, cultured in serum-free media, do not contain the Kaposi sarcoma herpesvirus genome. Br J Haematol 1998; 102:1338-40.
- 97. TARTE K, OLSEN SJ, YANG LZ et al. - Clinical-grade functional dendritic cells from patients with multiple myeloma are not infected with Kaposi's sarcoma-associated herpesvirus. Blood 1998; 91:1852-7.
- 98. GAIDANO G, PASTORE C, GLOGHINI A et al. - Distribution of human herpesvirus-8 sequences throughout the spectrum of AIDS-related neoplasia. AIDS 1996; 10:941-9.
- 99. UTHMAN A, BRNA C, WENINGER W et al. - No HHV8 in non-Kaposi's sarcoma mucocutaneous lesions from immunodeficient HIV-positive patients. Lancet 1996; 347:1700-1.
- 100. BOSHOFF C & WEISS RA. - Aetiology of Kaposi's sarcoma: current understanding and implications for therapy. Mol Med Today 1997; 3:488-94.
- 101. DICTOR M, RAMBECH E, WAY D et al. - Human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus) DNA in Kaposi's sarcoma lesions, AIDS Kaposi's sarcoma cell lines, endothelial Kaposi's sarcoma simulators, and the skin of immunosuppressed patients. Am J Pathol 1996; 148:2009-16.
- 102. MEMAR OM, RADY PL, GOLDBLUM RM et al. - Human herpesvirus 8 DNA sequences in blistering skin from patients with pemphigus. Arch Dermatol 1997; 133:1247-51.
- 103. UCCINI S, SIRIANNI MC, VINCENZI L et al. - Kaposi's sarcoma cells express the macrophage-associated antigen mannose receptor and develop in peripheral blood cultures of Kaposi's sarcoma patients. Am J Pathol 1997; 150:929-38.
- 104. GYULAI R, KEMENY L, KISS M et al. - Herpesvirus-like DNA sequence in angiosarcoma in a patient without HIV infection. N Engl J Med 1996; 334:540-1.
- 105. MCDONAGH DP, LIU J, GAFFEY MJ et al. - Detection of Kaposi's sarcoma-associated herpesvirus-like DNA sequence in angiosarcoma. Am J Pathol 1996; 149:1363-8.
- 106. FOREMAN K, BONISH B & NICKOLOFF B. - Absence of human herpesvirus 8 DNA sequences in patients with immunosuppression-associated dermatofibromas. Arch Dermatol 1997; 133:108-9.
- 107. BELEC L, MOHAMED AS, LECHAPT-ZALCMAN E et al. - Lack of HHV-8 DNA sequences in sarcoid tissues of French patients. Chest 1998; 114:948-9.
- 108. REGAMEY N, CATHOMAS G, SCHWAGER M, WERNLI M, HARR T & ERB P. - High human herpesvirus 8 seroprevalence in the homosexual population in Switzerland. J Clin Microbiol 1998; 36:1784-6.
- 109. RENNE R, ZHONG W, HERNDIER B et al. - Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nature Med 1996; 2:342-6.
- 110. DECKER LL, SHANKAR P, KHAN G et al. - The Kaposi sarcoma-associated herpesvirus (KSHV) is present as an intact latent genome in KS tissue but replicates in the peripheral blood mononuclear cells of KS patients. J Exp Med 1996; 184:283-8.
- 111. ZHONG W, WANG H, HERNDIER B et al. - Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc Natl Acad Sci U S A 1996; 93:6641-6.
- 112. ZHONG W & GANEM D. - Characterization of ribonucleoprotein complexes containing an abundant polyadenylated nuclear RNA encoded by Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8). J Virol 1997; 71:1207-12.
- 113. RUSSO JJ, BOHENZKY RA, CHIEN MC et al. - Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc Natl Acad Sci U S A 1996; 93:14862-7.
- 114. NICHOLAS J, RUVOLO V, ZONG J et al. - A single 13-kilobase divergent locus in the Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genome contains nine open reading frames that are homologous to or related to cellular proteins. J Virol 1997; 71:1963-74.
- 115. MESRI EA, CESARMAN E, ARVANITAKIS L et al. - Human herpesvirus-8/Kaposi's sarcoma-associated herpesvirus is a new transmissible virus that infects B cells. J Exp Med 1996; 183:2385-90.
- 116. ARVANITAKIS L, MESRI EA, NADOR RG et al. - Establishment and characterization of a primary effusion (body cavity- based) lymphoma cell line (BC-3) harboring Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood 1996; 88:2648-54.
- 117. BOSHOFF C, GAO SJ, HEALY LE et al. - Establishing a KSHV+ cell line (BCP-1) from peripheral blood and characterizing its growth in Nod/SCID mice. Blood 1998; 91:1671-9.
- 118. CARBONE A, CILIA AM, GLOGHINI A et al. - Establishment and characterization of EBV-positive and EBV-negative primary effusion lymphoma cell lines harbouring human herpesvirus type- 8. Br J Haematol 1998; 102:1081-9.
- 119. LEE H, VEAZEY R, WILLIAMS K et al. - Deregulation of cell growth by the K1 gene of Kaposi's sarcoma-associated herpesvirus. Nature Med 1998; 4:435-40.
- 120. BAIS C, SANTOMASSO B, COSO O et al. - G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 1998; 391:86-9.
- 121. AHUJA SK & MURPHY PM. - Molecular piracy of mammalian interleukin-8 receptor type B by herpesvirus saimiri. J Biol Chem 1993; 268:20691-4.
- 122. LI M, LEE H, YOON DW et al. - Kaposi's sarcoma-associated herpesvirus encodes a functional cyclin. J Virol 1997; 71:1984-91.
- 123. PETERS G. - Cell cycle. Stifled by inhibitions. Nature 1994; 371:204-5.
- 124. CHANG Y & MOORE PS. - Cyclin encoded by KS herpesvirus. Nature 1996; 382:410.
- 125. MURPHY M, ARMSTRONG D, SEPKOWITZ KA et al. - Regression of AIDS-related Kaposi's sarcoma following treatment with an HIV-1 protease inhibitor. AIDS 1997; 11:261-2.
- 126. SARID R, SATO T, BOHENZKY RA et al. - Kaposi's sarcoma-associated herpesvirus encodes a functional bcl-2 homologue. Nature Med 1997; 3:293-8.
- 127. CHENG EH, NOCHOLAS J, BELLOWS DS et al. - A Bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc Natl Acad Sci U S A 1997; 94:690-4.
- 128. MOCROFT A, YOULE M, MORCINEK J et al. - Survival after diagnosis of AIDS: a prospective observational study of 2625 patients. Royal Free/Chelsea and Westminster Hospitals Collaborative Group. BMJ 1997; 314:409-13.
- 129. BRODT HR, KAMPS BS, HELM EB et al. - Kaposi's sarcoma in HIV infection: impact on opportunistic infections and survival. AIDS 1998; 12:1475-81.
- 130. STURZL M, ASCHERL G, BLASIG C et al. - Expression of the human herpesvirus 8-encoded viral macrophage inflammatory protein-1 gene in Kaposi's sarcoma lesions. AIDS 1998; 12:1105-6.
- 131. NEIPEL F, ALBRECHT JC, ENSSER A et al. - Human herpesvirus 8 encodes a homolog of interleukin-6. J Virol 1997; 71:839-42.
- 132. KELLY GD, ENSOLI B, GUNTHEL CJ et al. - Purified Tat induces inflammatory response genes in Kaposi's sarcoma cells. AIDS 1998; 12:1753-61.
- 133. KEDES DH & GANEM D. - Sensitivity of Kaposi's sarcoma-associated herpesvirus replication to antiviral drugs. Implications for potential therapy. J Clin Invest 1997; 99:2082-6.
- 134. MEDVECZKY MM, HORVATH E, LUND T et al. - In vitro antiviral drug sensitivity of the Kaposi's sarcoma-associated herpesvirus. AIDS 1997; 11:1327-32.
- 135. NEYTS J & CLERCQ E. - Antiviral Drug susceptibility of Human Herpesvirus 8. Antimicrob Agents Chemother 1997; 41:2754-6.
- 136. LI TS, TUBIANA R, KATLAMA C et al. - Long-lasting recovery in CD4 T-cell function and viral-load reduction after highly active antiretroviral therapy in advanced HIV-1 disease. Lancet 1998; 351:1682-86.
- 137. PIALOUX G, RAFFI F, BRUN-VEZINET F et al. - A randomized trial of three maintenance regimens given after three months of induction therapy with zidovudine, lamivudine, and indinavir in previously untreated HIV-1-infected patients. N Engl J Med 1998; 339:1269-76.
- 138. BLUM L, PELLET C, AGBALIKA F et al. - Complete remission of AIDS-related Kaposi's sarcoma associated with undetectable human herpesvirus-8 sequences during anti-HIV protease therapy. AIDS 1997; 11:1653-5.
- 139. BURDICK AE, CARMIVHAEL C, RADY PL et al. - Resolution of Kaposi's sarcoma associated with undetectable level of human herpesvirus 8 DNA in a patient with AIDS after protease inhibitor therapy. J Am Acad Dermatol 1997; 37:648-9.
- 140. HAMMOUD Z, PARENTI DM & SIMON GL. - Abatement of cutaneous Kaposi's sarcoma associated with cidofovir treatment. Clin Infect Dis 1998; 26:1233.
- 141. MARTINELLI C, ZAZZI M, AMBU S et al. - Complete regression of AIDS-related Kaposi's sarcoma-associated human herpesvirus-8 during therapy with indinavir. AIDS 1998; 12:1717-9.
- 142. ABOULAFIA DM. - Regression of acquired immunodeficiency syndrome-related pulmonary Kaposi's sarcoma after highly active antiretroviral therapy. Mayo Clin Proc 1998; 73:439-43.
- 143. LEBBE C, BLUM L, PELLET C et al. - Clinical and biological impact of antiretroviral therapy with protease inhibitors on HIV-related Kaposi's sarcoma. AIDS 1998; 12:F45-F49.
- 144. RIZZIERI DA, LIU J, TRAWEEK ST & MIRALLES GD. - Clearance of HHV-8 from peripheral blood mononuclear cells with a protease inhibitor. Lancet 1997; 349:775-6.
- 145. DE MILITO A, CATUCCI M, VENTURI G et al. - Antiretroviral therapy with protease inhibitors in human immunodeficiency virus type 1- and human herpesvirus 8-coinfected patients. J Med Virol 1999; 57:140-4.
- 146. SPINA M, GAIDANO G, CARBONE A et al. - Highly active antiretroviral therapy in human herpesvirus 8-related body-cavity-based lymphoma. AIDS 1998; 12:955-6.
- 147. DUPIN N, KRIVINE A, CALVEZ V et al. - No effect of protease inhibitor on clinical and virological evolution of Castleman's disease in an HIV-1-infected patient. AIDS 1997; 11:1400-1.
- 148. DI ALBERTI L, NGUI SL, PORTER SR et al. - Presence of human herpesvirus 8 variants in the oral tissues of human immunodeficiency virus-infected persons. J Infect Dis 1997; 175:703-7.
- 149. ANDRE S, SCHATZ O, BOGNER JR et al. - Detection of antibodies against viral capsid proteins of human herpesvirus 8 in AIDS-associated Kaposi's sarcoma. J Mol Med 1997; 75:145-52.
-
150WHITBY D, SMITH NA & TALBOT SJ. - Kaposi's sarcoma and human herpesvirus 8. In: ARRAND JR AND HARPER DR, (ed.) Viruses and human cancer. Oxford, BIOS Scientific Pub Ltd 1998. p. 93-108.
Publication Dates
-
Publication in this collection
11 Sept 2002 -
Date of issue
Aug 2002
History
-
Received
14 May 2001