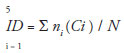Abstracts
Aniba canelilla (H.B.K.) Mez. is a tree species from Amazon that produces essential oil. The oil extraction from its leaves and stems can be an alternative way to avoid the tree cutting for production of essential oil. The aim of this study was to analyse factors that may influence the essential oil production and the biomass of resprouts after pruning the leaves and stems of A. canelilla trees. The tree crowns were pruned in the wet season and after nine months the leaves and stems of the remaining crown and the resprouts were collected, in the dry season. The results showed that the essential oil yield and chemical composition differed among the stems, leaves and resprouts. The stems' essential oil production differed between the seasons and had a higher production in the resprouting stems than the old stems of the remaining crown. The production of essential oil and leaf biomass of resprouts were differently related to the canopy openness, indicating that light increases the production of the essential oil and decreases the biomass of resprouting leaves. This study revealed that plant organs differ in their essential oil production and that the canopy openness must be taken into account when pruning the A. canelilla tree crown in order to achieve higher oil productivity.
Non timber forest products; essential oil productivity; chemical composition; precious bark
Aniba canelilla (H.B.K.) Mez. é uma espécie arbórea da Amazônia que produz óleo essencial. A extração do óleo de suas folhas e galhos pode ser uma forma alternativa de evitar a derrubada do tronco para sua produção de óleo essencial. O objetivo deste estudo foi analisar os fatores que podem influenciar a produção de óleo essencial e sua biomassa da rebrota após a poda de folhas e galhos das árvores de A. canelilla. As copas das árvores foram podadas na estação chuvosa e, após nove meses, as folhas e os galhos da copa remanescente e da rebrota foram coletadas na estação seca. Os resultados mostraram que o rendimento e a composição química de óleo essencial diferiram entre os galhos finos, as folhas e as rebrotas. A produção de óleo essencial de galhos diferiu entre as estações e teve maior produção nos galhos da rebrota do que nos galhos velhos da copa remanescente. A produção de óleo essencial e de biomassa das folhas da rebrota foram diferentemente relacionadas com a abertura de dossel, indicando que a luz aumenta a produção de óleo essencial e diminui a de biomassa nas folhas da rebrota. Este estudo revelou que as diferenças entre os órgãos da planta na produção de óleo essencial e a abertura de dossel devem ser levadas em consideração para podar a copa da árvore da A. canelilla e alcançar maior produtividade de óleo.
Produtos florestais não-madeireiros; produtividade de óleo essencial; composição química; casca preciosa
CIÊNCIAS FLORESTAIS
Biomass production and essential oil yield from leaves, fine stems and resprouts using pruning the crown of Aniba canelilla (H.B.K.) (Lauraceae) in the Central Amazon
Produção de biomassa e rendimento de óleo essencial de folhas, galhos finos e rebrotas utilizando poda da copa de Aniba canelilla (H.B.K.) (Lauraceae) na Amazônia Central
Adriana Pellegrini ManhãesI; Valdir Florêncio da Veiga-JúniorII; Larissa Silveira Moreira WiedemannII; Karenn Silveira FernandesII; Paulo de Tarso Barbosa SampaioI
I Instituto Nacional de Pesquisas da Amazônia, Avenida Efigênio Sales, 2239, Manaus, Amazonas, Brazil. E-mails: adriana-pellegrini@hotmail.com, sampaio@inpa.gov.br
IIUniversidade Federal do Amazonas, Avenida Gal. Rodrigo Octavio Jordão Ramos, 6200, Manaus, Amazonas, Brazil, CEP: 69079-000. E-mails: valdirveiga@ufam.edu.br, larissasm@yahoo.com.br, karenn.silveira@hotmail.com
ABSTRACT
Aniba canelilla (H.B.K.) Mez. is a tree species from Amazon that produces essential oil. The oil extraction from its leaves and stems can be an alternative way to avoid the tree cutting for production of essential oil. The aim of this study was to analyse factors that may influence the essential oil production and the biomass of resprouts after pruning the leaves and stems of A. canelilla trees. The tree crowns were pruned in the wet season and after nine months the leaves and stems of the remaining crown and the resprouts were collected, in the dry season. The results showed that the essential oil yield and chemical composition differed among the stems, leaves and resprouts. The stems' essential oil production differed between the seasons and had a higher production in the resprouting stems than the old stems of the remaining crown. The production of essential oil and leaf biomass of resprouts were differently related to the canopy openness, indicating that light increases the production of the essential oil and decreases the biomass of resprouting leaves. This study revealed that plant organs differ in their essential oil production and that the canopy openness must be taken into account when pruning the A. canelilla tree crown in order to achieve higher oil productivity.
Keywords: Non timber forest products, essential oil productivity, chemical composition, precious bark.
RESUMO
Aniba canelilla (H.B.K.) Mez. é uma espécie arbórea da Amazônia que produz óleo essencial. A extração do óleo de suas folhas e galhos pode ser uma forma alternativa de evitar a derrubada do tronco para sua produção de óleo essencial. O objetivo deste estudo foi analisar os fatores que podem influenciar a produção de óleo essencial e sua biomassa da rebrota após a poda de folhas e galhos das árvores de A. canelilla. As copas das árvores foram podadas na estação chuvosa e, após nove meses, as folhas e os galhos da copa remanescente e da rebrota foram coletadas na estação seca. Os resultados mostraram que o rendimento e a composição química de óleo essencial diferiram entre os galhos finos, as folhas e as rebrotas. A produção de óleo essencial de galhos diferiu entre as estações e teve maior produção nos galhos da rebrota do que nos galhos velhos da copa remanescente. A produção de óleo essencial e de biomassa das folhas da rebrota foram diferentemente relacionadas com a abertura de dossel, indicando que a luz aumenta a produção de óleo essencial e diminui a de biomassa nas folhas da rebrota. Este estudo revelou que as diferenças entre os órgãos da planta na produção de óleo essencial e a abertura de dossel devem ser levadas em consideração para podar a copa da árvore da A. canelilla e alcançar maior produtividade de óleo.
Palavras-chave: Produtos florestais não-madeireiros, produtividade de óleo essencial, composição química, casca preciosa.
INTRODUCTION
Essential oils are important non-timber forest products (NTFP) used in traditional communities and in large-scale industries. To date, more than 3000 essential oils are known but approximately 10% of these are commercially important in industries such as pharmaceutical, agronomic, food, sanitary, cosmetic and perfumery (Bakkali et al. 2008). In the Amazon region, there are approximately 350 aromatic species that show potential use in these industries (Maia and Andrade 2009). There are some Amazonian tree species that produce essential oil and its extraction in most cases involves cutting the tree's trunk to extract the oil. Essential oil extraction by tree cutting can have a negative impact on the populations of some species. Aniba rosaeodora (rosewood), for example, was included on the list of threatened species due to this extraction process (Brazilian Institute of Environment and Renewable Resources 1992).
The harvest of leaves and stems can be an alternative way for essential oil production from Amazonian tree species to avoid tree cutting. Pruning is a management technique generally used in forest plantations (Pinkard 2003; Medhurst et al. 2006), but can also be used in natural forests for non-timber purposes such as essential oil production from leaves and stems. There is a need for studies aiming to develop the management of natural forests in relation to non-timber essential oil extraction and productivity improvement.
Essential oils are a mixture of volatile and natural substances produced by aromatic plants as secondary metabolites. Several factors can influence their yield and chemical composition such as plant organs and their developmental stages (Sangwan et al. 2001). Other factors that can influence volatile emissions are the damages to leaves by mechanical, chemical or biotic injury (Figueiredo et al. 2008). Herbivory is a biotic injury that can affect chemical composition but can have any effect on essential oil yield (Edwards et al. 1993).
Environmental factors, such as light and moisture content, have strong effects on essential oil production (Sangwan et al. 2001). These factors can be the cause on variation in the seasonality (Duarte et al. 2009), although some studies did not find any differences between seasons (Rajeswara Rao et al. 1996; Lopes et al. 1997; Silva et al. 2009). One experiment with A. canelilla seedlings showed that moisture deficiency and light irradiance decreases essential oil yield in roots and leaves respectively (Atroch 2008). Thus, each species has a specific response to these diverse factors and identifying the variables that influence essential oil production of A. canelilla species in natural forests can help increase its oil productivity.
Aniba canelilla (H.B.K.) Mez. (Lauraceae), or precious bark, is a tree species that occurs in terrafirme Amazon forests and the decoction of its bark is used as a digestive in local folk medicine. The major constituent of its essential oil is the 1-nitro-2- phenylethane that is a rare molecule in natural products (Lupe 2007) and presents analgesic properties (Lima et al. 2008). Eugenol and methyleugenol are also major constituents of A. canelilla essential oil, being formerly used in the dentist, hygiene and perfume industries, and the latter in food industries (Costa 2000).
The 1-nitro-2-phenyletane compound from A. canelilla essential oil did not show any difference among seasons (Silva et al. 2009) but another study found that the concentration was higher during the wet season (Taveira et al. 2003). As in the essential oil chemical composition, the essential oil yield between plant organs also has different responses. For example, Taveira and collaborators (2003) found little variation in essential oil among barks, trunks and leaves but another study found that fine stems had the highest oil yield compared with the trunk and bark (Silva et al. 2009). The different responses in the essential oil production of A. canelilla among plant organs and seasons can be used to improve oil productivity in natural forest or cultivation conditions.
The impact and response of harvesting NTFP should be known before assessing its management practices (Ticktin 2004). Therefore, to assess the pruning response of Aniba canelilla we measured the biomass and essential oil production (yield and chemical composition) before pruning (pruned crowns) and after pruning (resprouting). To understand the essential oil production of A. canelilla the following questions were addressed: (1) Is the essential oil chemical composition different between leaves and fine stems? (2) Do plant organs (leaf, stem and resprouts) or seasons (wet and dry season) affect essential oil yield? (3) Do herbivory effects, canopy openness and plant size have any influence on essential oil yield? (4) Do canopy openness and plant size have any influence on resprouts biomass? (5) What is the productivity of essential oil of stems and leaves?
MATERIALS AND METHODS
Site description
The study site was located in the forest area of the Precious Woods Amazon (PWA) logging company at the coordinates 2º43' to 3º04'S lat and from 58º31' to 58º57' W long, Itacoatiara, Amazonas state, Brazil. It has an annual precipitation of approximately 2200 mm and the mean temperature is 26 ºC. The wet season occurs between December and May and the dry season between August and November.
Plant material
The sample area had approximately 400 ha and 46 individuals of Aniba canelilla with a diameter at breast height (DBH) superior to 130 cm were found by PWA's forest inventory time. The voucher was deposited in the INPA's herbarium with number 228004. Ten trees were randomly selected and the lower half of their crowns was pruned (fine stems were cut to a 10 cm diameter) during the wet season (February 2009) using professional climbing tree. The biomass of leaves and fine stems pruned were weighed separately after harvesting. During the dry season, nine months after the first pruning (November 2009), all resprouts and samples of leaves and fine stems of remaining crown were collected and were weighed separately.
Independent variables
The independent variables analysed were: plant organs (leaves, stems and resprouts), seasons (wet and dry), plant size (measured as DBH at a height of 130 cm), percentage of canopy openness (at ground and crown level) and herbivore damage. Two values of canopy openness were obtained for each tree using hemispherical photos taken by a Nikon Coolpix 4550 camera; the first photo was taken at the initial portion of tree crown (TC) and the other at the trunk base (TB). The pictures allowed us to measure how much light the crown and the ground receives, respectively. The pictures were converted to percentage of canopy openness by the Gap Light Analyser software (Frazer et al. 2001).
Estimates of the levels of herbivory on the leaves were determined visually by randomly selecting thirty leaves of the resprouting and the remaining crown. Each leaf was assigned to one of the following categories of damage: 0 = intact; 1 = 1-6%; 2 = 6-12%; 3 = 12-25%; 4 = 25-50%; 5 = 50-100%. The score for each leaf was used to define an index of damage (ID) per plant as follows (Benítez-Malvido et al. 2006):
Where i is the category of damage; ni is the number of leaves in the ith category of damage; Ci is the midpoint of each category.
Oil extraction and chemical composition
The leaves, stems and resprouts collected were dried in a stove at 30 ºC until constant weight was obtained, then triturated in a mill and stored in a freezer at -17 ºC until distillation began. The essential oils were extracted by hydrodistillation in a Clevenger-type apparatus over 3 h in 1.5 L of water. Water was removed from the essential oil using an anhydrous sodium sulphate and then weighed. The essential oil extraction of each sample of leaves and stems from the pruned crown was carried out in triplicate for each plant organ (170 g of dry weight) and the remaining crown and resprouts were obtained in duplicate (150 g of dry weight). The average yield (g oil/ g dried plant organ) of essential oil extraction was used for further statistical analyses.
The volatile constituents of essential oils were analysed by GC-FID using Shimadzu© GC 2010 gas chromatograph with flame ionization detector (GC-FID) on a capillary column DB-5 (30 m length, 0.25 mm i.d. and 0.25 µm film thickness). Operating conditions were: carrier gas nitrogen, flow 2.0 mL min-1; oven temperature program: 60-240 ºC at 3 ºC min-1; injection size: 0.1 μ(oil diluted in hexane 5 mg mL-1); sample injection port temperature 250 ºC; detector temperature 290 ºC; split 1:200. The essential oil chromatograms of A. canelilla from GC-FID were analysed to identify the retention index (RI) using as standard a number of hydrocarbons (C8-C18) to compare with the values in literature (Adams 2007). The analyses from GC-MS (Mass Spectra) were performed with the same conditions and column from GC-FID, using the electron impact at 70 eV, and were used to identify the major constituents of A. canelilla's essential oil using the MS Data Analyses #1 program and the Wiley 275 and Wiley 138 library.
Statistical analyses
Paired t-test was used to compare differences in the essential oil yield between plant organs and seasons. Multiple regressions with backward stepwise selection were used to identify the independent variables that may influence the resprouting biomass and the essential oil yield in leaves, stems and resprouts. The essential oil chemical composition was compared between leaves and stems using the Principal Components Analyses (PCA) by covariance. All analyses were performed using R version 2.9.1 (R Core Development Team, 2009).
RESULTS AND DISCUSSION
Essential oil chemical composition
Leaves and stems
The major constituents identified in essential oil were 1-nitro-2-phenylethane, eugenol and methyleugenol. The content of these three constituents differed among leaves and stems (Figure 1). The leaves presented higher content of eugenol (8.71 ± 4.47% [mean ± S.E.]) and methyleugenol (5.09% ± 3.70), than in fine stems (0.3 ± 0.3% and 0.42 ± 0.33% respectively). In contrast, the 1-nitro-2-phenylethane content was significantly higher in stems (92.7 ± 2.69%) and with less internal variation of this constituent than in leaves (52.2 ± 25.15%), (Figure 1).
The leaves produced a larger number of compounds than the fine stems and this higher variation may be due to its higher metabolic activity. The difference found between essential oil chemical composition of leaves and stems was an interesting result because the major constituents identified are used for different purposes. Specifically, results showed that a higher concentration of the 1-nitro-2-phenyletane constituent can be obtained from stem oil extraction and the eugenol and methyleugenol constituents from leaf oil extraction. Silva et al. (2009) identified that the methyleugenol constituent was a chemical marker of A. canelilla oil in the state of Pará, Brazil. However, our results indicate that methyleugenol also occurs in plants in the Amazonas state (Figure 1), rejecting the idea that this compound is a chemical marker for plants only in the state of Pará.
Essential oil yield
Plant organs and seasons
The difference in essential oil production between leaves and fine stems occurred only during the dry season in both the remaining crown (t = 3.461; d.f = 9; p < 0.01) (Figure 2b) and resprouting (t = 7.225; d.f = 7; p < 0.001) (Figure 2c) while in the wet season this difference did not occur (t = -0.245; d.f = 9; p = 0.8115) (Figure 2a). This can be explained by the mean increase from 0.32 ± 0.09% to 0.56 ± 0.19% (mean ± S.E.) in the essential oil yield of the stems from wet season to the dry, while the oil yield of leaves did not increase in this same period (0.26 ± 0.21% to 0.27 ± 0.23%) (Figure 2a; Figure 2b). In the dry season, the stems showed a higher essential oil yield in the resprouting (0.89 ± 0.13%) when compared to the stems of remaining crown (0.56 ± 0.19%), while the leaves did not change its essential oil production (0.29 ± 0.21% and 0.27 ± 0.23%, respectively) (Figure 2b; Figure 2c).
The results showed that the leaves and stems of A. canelilla have difference in the essential oil production and that this difference was more pronounced in the resprouting (Figure 2c). The higher essential oil yield from new stems (resprouting) than the older ones (remaining crown) indicates that the initial developmental stage of the stem can produce more essential oil. However, the leaves did not show any difference and this could be due to the presence of young leaves in the remaining crown (that represent the old leaves). It's known that peppermint species have a monoterpene accumulation related with leaf development (Gershenzon et al. 2000), but further studies should test the leaf developmental stages in A. canelilla due the difference in secondary metabolites biosynthesis in tree species.
The results showed that the essential oil production of stems was higher in the dry season when compared to the wet season of the same year, while the oil yield in leaves did not change in the same period. Other studies with A. canelilla essential oil did not find differences between seasons for plant organs such as the trunk, leaves and bark, but they did not measured essential oil yield from stems (Taveira et al. 2003; Silva et al. 2009). Our results support that leaves show no response to seasonal variation and suggest that only the stems can respond to seasonal change. Thus, the pruning can be more profitable in the dry season due to the higher essential oil yield from stems.
Plant size, canopy openness and herbivory
The essential oil yield from stems was not influenced by other analysed independent variables, which were removed by backward stepwise selection. In contrast, the resprouting leaves' essential oil yield decreased with TC canopy openness and increased with TB canopy openness (reduced model R2adj = 0.45; N= 8) (Table 1). Additionally, the oil yield from the remaining crown was influenced positively by TB canopy openness and negatively by the plant size and the herbivory (reduced model R2adj = 0.68; N= 10) (Table 1).
The results showed that the canopy openness of the tree base (TB) was the only variable that had influence in the essential oil yield both of the resprouting and of the remaining crown. This means that the light exposure in the ground can increase the essential oil yield. Different result was found by Atroch (2008), which showed a negative relationship to light exposure to the essential oil yield of A. canelilla leaves of seedlings. In this manner, the light exposure of A. canelilla individuals can have different influences in essential oil yield, changing by the age of individual (seedlings or adults) or by the area where the light can reach (crown or ground). Even though with a significant relation, the DBH was not considered a good variable because it only explains leaves of remaining crown. The herbivory and the canopy openness of the tree crown (TC) had a weak influence and also it was not considered a good predictor to essential oil yield.
Biomass
Plant size and canopy openness
The stepwise selection removed the variable DBH on the predictive model of the biomass of leaf resprouting. Thus, the result showed that the biomass of leaf resprouting has a positive relation to the canopy openness of the tree crown (TC) (t =3.238; d.f.= 1; p < 0.05) and negative to the canopy openness of tree base (TB) (t = -3.783; d.f.= 1; p < 0.01) (Figure 3).
In the predictive model of the biomass of the stem resprouting, only the canopy openness of TB variable had a significant relation (t = -2.772; d.f. = 1; p < 0.05) (Figure 4). Although, the DBH (t = 1.462; d.f. = 1; p = 0.194) and the canopy openness of TC (t = 1.383; d.f. = 1; p = 0.2160) had no influence.
Light exposure on the ground negatively influenced the resprouting biomass (leaves and stems) and this fact can be associated with the hydric stress that diminishes biomass production. However, this variable had a positive effect on essential oil yield (leaves and stems). Some plants can presents a trade-off, which means the resource allocation to one function, such as organs production, protection or reproduction can cause resource depletion to other functions (Lerdau and Gershenzon 1997). The light exposure on the ground can be an inductive variable to the trade-off in the A. canelilla because of the increase in essential oil yield (protection) and the decrease in biomass (production).
In addition, under conditions where the crown had more illumination, individuals of A. canelilla showed more investment in leaves biomass of the resprouting than its essential oil production. After pruning, some species can minimize the effects of defoliation, such as the photosynthetic enhancement capacity of the remaining foliage (Medhurst et al. 2006). The induction of the biomass production in crown light conditions in leaves and not in the stems of the resprouting, can be a response to the tree crown pruning to recover the foliage losses.
Essential oil productivity
The biomass production from all the pruned tree crowns was 102.57 ± 49.27 kg (mean ± S.E.) with 86.43% for the stems and 13.57% for the leaves, while the resprouting biomass production was 2.9 ± 1.08 kg with 45.17% for the stems and 54.83% for the leaves. The mean essential oil yield of pruned crowns was 0.27% for leaves and 0.32% for stems, whereas the mean resprouting oil yield was 0.30% for the leaves and 0.90% for the stems.
Essential oil productivity is a product of the biomass production (Kg) by its oil yield (%). As a result, the essential oil productivity was 3.7 ± 0.2 kg for all pruned tree crowns, and 0.12 ± 0.08 kg for their resprouts over a period of nine months. Thus, the oil productivity of stems was higher than leaves both in pruned tree crowns and in resprouts. This occurred due to the higher biomass production of the former and the higher oil yield of the latter. To achieve higher productivity of A. canelilla essential oil it is necessary to take biomass production and oil yield into account in the management of leaves and stems.
During the nine months, the biomass of resprouts produced only 3.24% of all essential oil extracted by the first pruning. The pruning cycle can be defined when the oil productivity from the resprout attains the same amount of oil produced in the first pruning. Studies with longer cycles are needed in order to better understand the A. canelilla response to pruning and to use this non-timber management technique to extract the essential oil from this species.
Management of Aniba canelilla from natural forests by pruning
The species A. canelilla presents a conflict in its use because it can be used for both timber and non-timber products. The PWA logging company uses this species in timber forest management, but with a low market value (PWA, personal communication), also confirmed in a study that characterized the use of this species in the Pará state (eastern Amazonia) (Herrero Jáuregui et al. 2009). However, for non-timber purposes the A. canelilla species has a medium market value (Herrero Jáuregui et al. 2009). In this case, a legal protection from logging can be put in place when non-timber economic and social value equals or exceeds the timber value (Guariguata et al. 2010).
Other alternatives are the compatible use of timber and NTFP in the same management area, however, the NTFP management may be positively or negatively affected by post-logging (Guariguata et al. 2010). One positive example is the use of logging residues (such as tree crowns or bark) to extract the A. canelilla essential oil. The increased crown illumination after logging can improve NTFP productivity (Guariguata et al. 2010), thus, the selection of A. canelilla trees for pruning should ensure minimal light exposure to the ground to improve biomass production of resprouts after defoliation, and adequate tree crown illumination to produce higher essential oil.
CONCLUSIONS
The pruning of Aniba canelilla tree crowns is a promising technique for increasing essential oil production from leaves and stems. Tree cutting is avoided for this purpose, although timber and non-timber species management are compatible in the same area. In addition, some factors can improve the essential oil productivity after pruning: more crown illumination and less light on the ground to improve the resprouting biomass. The plant organs (leaves, stems and resprouts) presented different biomass production and essential oil (yield and composition), being an important factor to be considered for non-timber management. We recommend further studies regarding longer cycles and different intensities of pruning to complement this research. This is the first study that has researched the pruning technique for essential oil extraction using leaves and stems from Amazonian trees located in its natural habitat.
ACKNOWLEDGEMENTS
We want to thank CNPq and CAPES for the financial support. Thanks to the Biomolecules laboratory of University of Amazonas State and the Amazonia Biotechnology Centre for oil extraction and analyses support. Thanks to Precious Woods Amazon, Vertical Life, AVIVE association and to the INPA for logistical and field support. Thanks to Guilherme Mazzochini for help with statistical analyses and field support and thanks to Andreé Kimber for english revison.
Recebido em: 06/07/2011
Aceito em: 15/11/2011
- Adams, R.P. 2007. Identification of essential oil components by gas chromatography/ mass spectroscopy, 4th Ed., Allured Publishing Corporation, Carol Stream, Illinois, USA. 804 pp.
- Atroch, E.M.A.C. 2008. Efeitos de fatores abióticos sobre o crescimento, características fotossintéticas e síntese de óleos voláteis em plantas jovens de espécies de lauraceae na Amazônia Central. Tese de Doutorado, Instituto Nacional de Pesquisas da Amazônia/Universidade Federal do Amazonas, Manaus, Amazonas. 109 pp.
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. 2008. Biological effects of essential oils - a review. Food and Chemical Toxicology, 46: 446-475.
- Benítez-Malvido, J.; Martínez-Ramos, M.; Camargo, J.L.C.; Ferraz, I.D.K. 2006. responses of seedling transplants to environmental variations in contrasting habitats of Central Amazonia. Journal of Tropical Ecology, 21: 397-406.
- Costa, P.R.R. 2000. Safrol e eugenol: estudo da reatividade e uso em síntese de produtos naturais biologicamente ativos e seus derivados. Química Nova, 23: 357-369.
- Duarte, A.R.; Naves, R.R.; Santos, S.C.; Seraphin, J.C.; Ferri, P.H. 2009. Seasonal influence on the essential oil variability of Eugenia dysenterica Journal of the Brazilian Chemical Society, 20: 967-974.
- Edwards, P.B.; Wanjura, W.G.; Brown, W.V. 1993. Selective herbivory by Christimas Beetles in response to intraspecific variation in Eucalyptus terpenoids. Oecologia, 95: 551-557.
- Figueiredo, A.C.; Cristina, A.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J. 2008. Factors affecting secondary metabolite production in plants: volatile components and essential oils. Flavour and Fragrance Journal, 23: 213-226.
- Frazer, G.W.; Fournier, R.A.; Trofymow, J.A.; Hall, R.J. 2001. A comparison of digital and film fisheye photography for analyses of forest canopy structure and gap light transmission. Agricultural and Forest Meteorology, 109: 249-263.
- Gershenzon, J.; Mcconkey, M. E.; Croteau, R. B. 2000. Regulation of Monoterpene Accumulation in Leaves of Peppermint. Plant Physiology, 122: 205-213.
- Guariguata, M.R.; Garcia-Fernández, C.; Sheil, D.; Nasi, R.; Herrero-Jáuregui, C.; Cronkleton, P.; Ingram, V. 2010. Compatibility of timber and non-timber Forest product management in natural tropical forests: Perspectives, challenges, and opportunities.Forest Ecology and Management, 259: 237-245.
- Herrero-Jáuregui, C.; Garcia-Fernández, C.; Sist, P.L.J.; Casado, M.A. 2009. Conflict of use for multi-purpose tree species in state of Pará, eastern Amazonia, Brazil. Biodiversity and Conservation, 18: 1019-1044.
- Lerdau, M.; Gershenzon, J. 1997. Allocation Theory and Chemical Defense, p. 265-277. In: Bazzaz, F.A., Grace, J. (Eds.), Plant Resource Alocation. Lavoisier, França.
- Lima, A.B.; Santana, M.B.; Cardoso, A.S.; Silva, J.K.R.; Maia, J.G.S.; Carvalho, J.C.T.; Souza, P.J.C. 2008. Antinociceptive activity of 1-nitro-2-phenylethane, the main component of Aniba canelilla essential oil. Phytomedicine, 16: 555-559.
- Lopes, N.P.; Kato, M.J.; Andrade, E.H.A.; Maia, J.G.S.; Yoshida, M. 1997. Circadian and seasonal variation in the essential oil from Virola surinamensis leaves. Phytochemistry, 46: 689-693.
- Lupe, F.A. 2007. Estudo da composição química de óleos essenciais de plantas aromáticas da Amazônia. Dissertação de mestrado, Universidade Estadual de Campinas, Campinas, São Paulo.
- Maia, J.G.S.; Andrade, E.H.A. 2009. Database of the Amazon aromatic plants and their essential oils. Química Nova, 32: 595-622.
- Medhurst, J.L.; Pinkard, E.A.; Beadle, C.L.; Worledge, D. 2006. Photosynthetic capacity increases in Acacia melanoxylon following form pruning in a two-species plantation. Forest Ecology and Management, 233: 250-259.
- Pinkard, E.A. 2003. Physiological and growth responses related to pattern and severity of green pruning in young Eucalyptus globules. Forest Ecology and Management, 182: 231-245.
- Rajeswara Rao, B.R.; Kaul, P.N.; Mallavarapu, G.R.; Ramesh, S. 1996. Effect of seasonal climatic changes on biomass yield and terpenoid composition on Rose-scented Geranium (Perlargonium species). Biochemical Systematics and Ecology, 24: 627-635.
- R Development Core Team, 2007. R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria.
- Sangwan, N.S.; Farooqi, A.H.A.; Shabih, F.; Sangwan, R.S. 2001. Regulation of essential oil production in plants. Plant Growth Regulation, 34: 3-21.
- Silva, J.R.; Carmo, D.F.M.; Reis, E.M.; Machado, G.M.C.; Leon, L.L.; Silva, B.O.; Ferreira, J.L.P.; Amaral, A.C.F. 2009. Chemical and biological evaluation of essential oils with economic value from Lauraceae species. Journal of the Brazilian Chemical Society, 20: 1071-1076.
- Taveira, F.S.N.; Lima, W.N.; Andrade, E.H.A.; Maia, J.G.S. 2003. Seasonal essential oil variation of Aniba canelilla Biochemical Systematics and Ecology, 31: 69-75.
- Ticktin, T. 2004. The ecological implications of harvesting non-timber forest products. Journal of Applied Ecology, 41:11-21.
Publication Dates
-
Publication in this collection
19 June 2012 -
Date of issue
Sept 2012
History
-
Received
06 July 2011 -
Accepted
15 Nov 2011