ABSTRACT
Polyembryony is the differentiation and development of multiple embryos in a single seed. This characteristic can provide advantages, as more than one embryo is produced with the same amount of resources, and the probability of establishment of at least one seedling increases. However, sibling seedlings may also increase competition, affecting development and survival. In the present study, the possible advantages and disadvantages of polyembryony were analyzed in the initial establishment of seedlings of Carapa surinamensis (Meliaceae), a tree species that produces monoembryonic or polyembryonic seeds. In this regard, the development of single seedlings was compared with a pair of seedlings emerging from polyembryonic seeds. We compared the development of seedlings attached to or detached from each other and to the seed resources. We observed two levels of competition: (a) for the seed reserves during germination and initial development, as multiple embryos of C. surinamensis share the same reserves, and (b) for external factors, mostly space for root and shoot development, and also for light. Reducing the competition for external factors by separating the siblings was not enough to reduce the effects of competition for seed reserves in the first six months of development. Nevertheless, viable seedlings were produced in all treatments. Thus, depending on sprout management in the nursery, the number of seedlings per seed can be significantly increased by detaching the seedlings, or more vigorous seedlings can be obtained when only one seedling is maintained.
Keywords:
polyembryony; andiroba; crabwood; seedling production; competition
RESUMO
Poliembrionia é a diferenciação e o desenvolvimento de múltiplos embriões em uma única semente. Esta característica pode proporcionar diversas vantagens, como aumentar o número de embriões produzidos com a mesma quantidade de recursos, e aumentar a probabilidade de estabelecimento de pelo menos uma plântula de uma única semente. Por outro lado, a competição entre plântulas pode aumentar, afetando seu desenvolvimento e sobrevivência. Neste estudo, foram analisadas as possíveis vantagens e desvantagens da poliembrionia em sementes de andiroba, Carapa surinamensis (Meliaceae), uma espécie arbórea que produz sementes monoembriônicas ou poliembriônicas. Comparamos o desenvolvimento de plântulas únicas com o de pares de plântulas provenientes de sementes poliembriônicas. As plântulas foram mantidas unidas ou separadas entre si e ligadas à ou destacadas da semente. Os resultados revelaram dois níveis de competição: (a) pelas reservas da semente durante a germinação e desenvolvimento inicial da plântula, quando embriões múltiplos de C. surinamensis compartilham as mesmas reservas, e (b) por fatores externos, principalmente espaço para o desenvolvimento da raiz e da parte aérea, e luz. A redução da competição por fatores externos, através da separação das plântulas, não foi suficiente para reduzir os efeitos da competição pelas reservas das sementes nos primeiros seis meses de desenvolvimento. Apesar disso, plântulas viáveis foram produzidas em todos os tratamentos. Assim, dependendo do manejo dos brotos em viveiro, o número de plântulas produzidas pode ser aumentado significativamente por meio da separação entre plântulas, ou o vigor das plântulas pode ser incrementado através de sua manutenção individualizada.
Palavras-chave:
poliembrionia; andiroba; crabwood; produção de plântulas; competição
INTRODUCTION
At least two species of the genus Carapa have been recorded in central Amazonia: C. guianensis (Aubl.) and C. surinamensis (Miq.). The trees occur predominantly along rivers and creeks, but also in unflooded forest (Kenfack 2011Kenfack, D. 2011. Resurrection in Carapa (Meliaceae): a reassessment of morphological variation and species boundaries using multivariate methods in a phylogenetic context. Botanical Journal of the Linnean Society, 165: 186-221.). These multiple-use trees, commonly known in Brazil as andiroba, or crabwood on the international market, are important to the local economy, their wood being employed in the construction and furniture industries (Bauch and Dünisch 2000Bauch, J.; Dünisch, O. 2000. Comparison of growth dynamics and wood characteristics of plantation-grown and primary forest Carapa guianensis in Central Amazonia. IAWA Journal, 21: 321-333.). Andiroba seed oil extraction has been known for centuries (Aublet 1775Aublet, J.B.C.F. 1775. Histoire des plantes de la Guiane françoise: rangées suivant la méthode sexuelle, avec plusieurs mémoires. 1st ed. Pierre-François Didot, London & Paris, 375p.). The oil is used for medicinal purposes (Silva et al. 2012Silva, S.G.; Nunomura, R.C.S.; Nunomura, S.M. 2012. Limonóides isolados dos frutos de Carapa guianensis Aublet (Meliaceae). Química Nova, 35: 1936-1939.) and increasingly in the cosmetics industry in recent years (Boufleuer 2004Boufleuer, N.T. 2004. Aspectos ecológicos de andiroba (Carapa guianensis Aublet, Meliaceae), visando seu manejo e conservação. Masters thesis, Universidade Federal do Acre - UFAC, Rio Branco, Brasil. 72p.; Shanley and Medina 2005Shanley, P.; Medina, G. 2005. Frutíferas e plantas úteis na vida amazônica. 2nd ed. CIFOR Imazon, Belém, 300p.; Mendonça and Ferraz 2007Mendonça, A.P.; Ferraz, I.D.K. 2007. Óleo de andiroba: processo tradicional da extração. Acta Amazonica, 37: 353-364.).
The large seeds of Carapa spp. contain a tiny embryonic axis in a homogenous mass of reserves, as the cotyledons are fused (i.e. conferruminate) and visibly indistinguishable (Harshberger 1902Harshberger, J.W. 1902. The germination of the seeds of Carapa guianensis (Aubl.). Proceedings of the Academy of Natural Sciences of Philadelphia, 54: 122-126.). Multiple embryonic axes were recorded for C. surinamensis (Fisch et al. 1995Fisch, S.T.V.; Ferraz, I.D.K.; Rodrigues, W.A. 1995. Distinguishing Carapa guianensis Aubl. from Carapa procera DC. (Meliaceae) by morphology of young seedlings. Acta Amazonica, 25: 193-200., Amoêdo and Ferraz 2017Amoêdo, S.C.; Ferraz, I.D.K. 2017. Seed quality evaluation by tetrazolium staining during a desiccation study of the recalcitrant seeds of Carapa guianensis Aubl. and Carapa surinamensis Miq. - Meliaceae. African Journal of Agricultural Research, 12: 1005-1013. ), which was previously designated as Carapa procera (see Kenfack 2011Kenfack, D. 2011. Resurrection in Carapa (Meliaceae): a reassessment of morphological variation and species boundaries using multivariate methods in a phylogenetic context. Botanical Journal of the Linnean Society, 165: 186-221.). In the region of Manaus, in the central Brazilian Amazon, two to five seedlings per seed can develop in up to 50% of the seeds (de Souza Ferreira et al. 2017de Souza Ferreira, D.N.; Camargo, J.L.C.; Ferraz, I.D.K. 2017. Multiple shoots of Carapa surinamensis seeds: Characterization and consequences in light of post-germination manipulation by rodents. South African Journal of Botany, 108: 346-351.). Despite the absence of information on the origin and development of multiple seedlings in Carapa, the phenomenon is unusual in Meliaceae (Ghosh 1972Ghosh, R.B. 1972. Studies in the embryology of the family Meliaceae. IV. Fertilisation, endosperm and embryogeny of Aphanamixis polystachya (Wall.) Parker (Syn. Amoora rohituka (W. & A.)). A medicinal plant with a discussion on its taxonomic status and horticulture. Anales de la estación experimental de Aula Dei Calcutta, 11: 396-403.; Carman 1997Carman, J.G. 1997. Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory, and polyembryony. Biological Journal of the Linnean Society, 61: 51-94.; Wilde 2007Wilde, J.J.F.E. 2007. Revision of the African genus Heckeldora (Meliaceae). Blumea, 52: 179-199.; Rai 2014Rai, Y. 2014. Seedling behaviour and growth status of endangered medicinal tree Amoora rohituka (Roxb.) in Meerut, U.P. (India). International Journal of Informative & Futuristic Research, 1: 125-132.; Hiwale 2015Hiwale, S. 2015. Neem (Azadirachta indica). In: Hiwale, S. (Ed.). Sustainable Horticulture in Semiarid Dry Lands, Springer, New Delhi, p.281-290.).
Multiple embryos can arise from diverse origins, such as adventitious polyembryony from maternal tissues or cleavage polyembryony from the fertilized embryo, thus, embryos can be genetically different (Ganeshaiah et al. 1991Ganeshaiah, K.N.; Uma Shaanker, R.; Joshi, N.V. 1991. Evolution of polyembryony: Consequences to the fitness of mother and offspring. Journal of Genetics, 70: 103-127.; Bewley et al. 2013Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M. 2013. Seeds: Physiology of Development, Germination and Dormancy. 3rd ed. Springer, New York, 392p.). Few studies have attempted to relate polyembryony to intraspecific competition of descendants from the same seed (Blanchard et al. 2010Blanchard, M.L.; Barney, J.N.; Averill, K.M.; Mohler, C.L.; DiTommaso, A. 2010. Does polyembryony confer a competitive advantage to the invasive perennial vine Vincetoxicum rossicum (Apocynaceae)? American Journal of Botany, 97: 251-260. ), but it is known that sibling seeds may differ in individual development and in relative fitness and survival (Cheplick 1992Cheplick, G.P. 1992. Sibling competition in plants. Journal of Ecology, 80: 567-575.; Martínez-Gómes and Gradziel 2003Martínez-Gómez, P.; Gradziel, T.M. 2003. Sexual polyembryony in almond. Sexual Plant Reproduction, 16: 135-139.). In addition, in seeds with fused cotyledons, there may be an optimization of resources, as the same amount of seed reserves may be distributed according to microsite conditions and fitness between seedlings. Alternatively, when seed reserves are shared, competition between seedlings may be increased (Cheplick 1992; Ladd and Cappuccino 2005Ladd, D.; Cappuccino, N. 2005. A field study of seed dispersal and seedling performance in the invasive exotic vine Vincetoxicum rossicum. Canadian Journal of Botany, 83: 1181-1188.; Mendes-Rodrigues et al. 2012Mendes-Rodrigues, C.; Sampaio, D.S.; Costa, M.G.; Caetano, A.P.S.; Ranal, M.A.; Bittencourt Júnior, N.S.; Oliveira, P.E. 2012. Polyembryony increases embryo and seedling mortality but also enhances seed individual survival in Handroanthus species (Bignoniaceae). Flora, 207: 264-274.).
As the consequences of multiple embryos of C. surinamensis for seedling development and establishment are not known, we evaluated the consequences of polyembryony for initial seedling development by comparing single and sibling seedlings from both monoembyonic and polyembryonic seeds, without focusing on their possible genetic similarities or differences. Competition was eliminated following expansion of the first leaves to compare seedling fitness after six months of development in the nursery.
MATERIAL AND METHODS
Seed collection and processing
Seeds of Carapa surinamensis were collected from three sites near Manaus, Amazonas state, Brazil: Adolpho Ducke Forest Reserve (02º55’S, 59º59’W) and the experimental forestry station Estação Experimental de Sivicultura Tropical (02º35’55”S, 60º02’14”W), both belonging to the National Institute for Amazonian Research (Instituto Nacional de Pesquisas da Amazônia - INPA), and Amorins Ranch (02°45’19”S, 59°55’58”W), a private rural property located 20 km north of Manaus.
During natural seed dispersal, seeds and mature fruits were collected beneath the mother trees and, due to the recalcitrant character of the seeds (Connor et al. 1998Connor, K.F.; Ferraz, I.D.K.; Bonner, F.T.; Vozzo, J.A. 1998. Effects of desiccation on seeds of Carapa guianensis Aubl. and Carapa procera DC. Seed Technology, 20: 71-82.), transported immediately to the laboratory in plastic bags. Seed extraction was accomplished by manual removal from the fruit valves with the help of a knife. Immediately after removal of the fruit debris, the seeds were numbered, and individual seed mass was determined for at least one thousand seeds with a precision of 0.001 g. When we were unable to set up the experiments immediately, the seeds were kept in plastic bags and stored in a cool chamber at 15 °C for a maximum period of seven days. Before planting, the seeds were submerged in water for a period of 24 h to eliminate by drowning the larvae of Hypsipyla sp., a lepidopteran known as a seed borer, which infests and damages seeds of Carapa (Ferraz et al. 2002Ferraz, I.D.K.; Camargo, J.L.C.; Sampaio, P.T.B. 2002. Sementes e plântulas de andiroba (Carapa guianensis Aubl. e Carapa procera DC.): aspectos botânicos, ecológicos e tecnológicos. Acta Amazonica, 32: 647-661.).
Experimental design
Seeds were sown in plastic trays (55 × 25 × 15 cm) at 2 cm depth in moistened vermiculite of medium granulation (Brasil Minérios®). The trays were placed in a greenhouse roofed with transparent polyethylene. During the experiment, diurnal temperature varied between 20 and 35 °C, the average relative humidity was 80%, and light incidence varied from 0.12 to 3.53 × 10 µmol m-2 s-1. Shoot emergence above the substrate was assessed daily, and each new seedling was marked with a colored tape. Tapes with different colours helped to indicate the temporal sequence of germinations of the same seed. Only seeds which produced one or two shoots were used in the experiments, and named hereafter monoembryonic or polyembryonic seeds, respectively. We employed 30 germinated seeds per treatment. As it was not possible to predict how many seedlings would germinate per seed, some treatments had a smaller final number of replicates (Table 1). Seeds which produced more than two seedlings were discarded.
Response variables of Carapa surinamensis seedlings after 180 days under different treatments. N = final sample size for each treatment. Values are the mean ± standard deviation. Data for treatments PA-2 and PD-2 are shown as averaged means of the two germinated seedlings per seed and as individual means for the first (1st) and second (2nd) emerged seedlings. Survival of seedlings were compared with a chi-square test while number of leaves and leaflets, total biomass and root/shoot ratio were compared with factorial analyses of variance using GLM. Root and shoot biomass were used for the calculation of the root/shoot ratio. For more details on treatments, see Figure 1. Table 2 summarizes the statistical analyses.
In order to evaluate whether presence or absence of a sibling seedling or seed reserves affect initial seedling development and development after the expansion of the first two eophylls, the seedlings with their respective seeds were distributed among six treatments about 15 days after germination: (1) monoembryonic seed with one seedling maintained attached to the seed (MA-1); (2) monoembryonic seed with one seedling detached from the seed (MD-1); (3) polyembryonic seed with the first seedling maintained attached to the seed and the second eliminated (PA-1); (4) polyembryonic seed with the first seedling detached from the seed and the second eliminated (PD-1); (5) polyembryonic seed with both seedlings maintained attached to the seed (PA-2); and (6) polyembryonic seed with both seedlings detached from the seed (PD-2) (Figure 1). The two seedlings in each replicate of PA-2 and PD-2 were marked as described above to identify the first and second in order of emergence.
The detachment of the seedlings and the exclusion of the second seedling were executed manually with a sharp knife, to cause as little harm as possible to the seedlings and the remaining seed. After detachment, seedlings were transplanted to pots of 1.7 L containing vermiculite, with one or two seedlings according to the treatment. Biweekly, foliar fertilization was applied to all seedlings, following the manufacturer’s recommendations (Yogen®, 2 g l-1, NPK 30:10:10).
Scheme of the six treatments applied to the seedlings of Carapa surinamensis after the development of the first pair of leaves. MA-1: monoembryonic seeds with a single seedling maintained attached to the seed; MD-1: monoembryonic seeds with a single seedling detached from the seed; PA-1: polyembryonic seeds with the first seedling attached to the seed and the second eliminated; PD-1: polyembryonic seeds with the first seedling detached from the seed and the second eliminated; PA-2: polyembryonic seeds with two seedlings, both maintained attached to the seed; PD-2: polyembryonic seeds with two seedlings, both detached from the seed.
The transplants were monitored for 180 days. At the end of this period we measured shoot height up to the shoot meristem and the number of leaves and leaflets per shoot. Root and shoot biomass were carefully rinsed in tap water, to eliminate any attached vermiculite, and then dried in an oven at 75 °C until stabilization of weight in repeated measurements every 24 h, to obtain root and shoot dry matter (precision 0.001 g). Then we calculated the root/shoot ratio by dividing the individual root dry biomass value by the shoot dry biomass value.
Statistical analyses
A chi-square test was used to compare the survival of seedlings among treatments. Response variables height, biomass, number of leaves, number of leaflets and root-shoot ratio were compared among treatments using a GLM procedure for factorial analyses of variance (p < 0.05). We used a separate model for each variable, considering the factors, with two levels each: number of seedlings developed by each seed, the presence or absence of seed reserves during development (attached or detached), and the order of germination (1st or 2nd). In treatments in which two seedlings developed (PA-2 and PD-2) we considered each seedling separately, but in the data matrices these seedlings were identified as coming from the same seed. Gamma distribution was used for data on seedling height, biomass and root-shoot ratio, and the Poisson distribution for data on leaves and leaflets. To compare the biomass of single seedlings with the sum of the biomass of seedling pairs from the same seed, we used only those where both seedlings survived until the final assessment of the experiment, and considered the number of seedlings developed by a seed and the presence or absence of seed reserves during development. A simple linear regression was used to analyze whether there was a relationship between initial seed mass and seedling dry mass.
RESULTS
Individual seed weight ranged from 6.4 to 28.4 g. Seedling survival was above 60% for all treatments (Table 1), but varied significantly among treatments (χ2 = 23.61, df = 7, p < 0.05). In the treatments in which seedlings were maintained attached to the seeds (MA-1, PA-1 and PA-2) survival was ≥ 87%, with the lowest value for the second seedling of PA-2 (PA-2-2nd). Considering the sum of the survivors in PA-2, the seed:seedling ratio in these polyembrionic seeds was 1:1.8. Seedlings detached from the seeds (MD-1, PD-1 and PD-2) had higher mortality (from 1% to 27%) in relation to the corresponding treatments with attached seedlings. The second seedlings to emerge in PD-2 had the lowest survival (60%) among all treatments. However, if we also consider the sum of survivors of PD-2, the polyembryonic seeds had a higher chance of establishing a seedling, even with a lower individual survival.
Shoot height varied significantly among treatments with significant influences of the number of seedlings developed by seed and emergence order (Figure 2, Table 2). In this way, seedling height was similar between naturally and manipulated single seedlings (MA-1 and PA-1), even when the seedlings were detached from the seeds (MD-1 and PD-1). The single seedlings (MA-1, PA-1, MD-1 and PD-1) were taller than individuals from pairs (PA-2 and PD-2) (Table 1). First seedlings had similar heights among themselves (MA-1, PA-1, MD-1, PD-1, PA-2-1st and PD-2-1st) and were taller than the second seedlings (PA-2-2nd and PD-2-2nd).
Shoot height of Carapa surinamensis seedlings after 180 days under six treatments. Data were compared with a factorial analysis of variance using GLM. Results were influenced by number of developed seedlings per seed and emergence order. Columns represent mean values, and bars the standard deviation. For more details on treatments, see Material and Methods and Figure 1. Table 2 summarizes the statistical analyses.
Summary of the results of factorial analyses of variance using GLM with response variables height, dry biomass, number of leaves, number of leaflets, root/shoot ratio and dry biomass using the sum of dry biomass of seedling pairs (1st + 2nd). The factors were number of seedling developed by seed (N seedlings), emergence order (1st or 2nd), and presence or absence of seed reserves (attached or detached). Statistically significant values are marked with * (p<0.05).
The average number of leaves also varied significantly among treatments. Seedlings in treatments with seed attachment had significantly more leaves than treatments with detached seedlings (Table 1, Table 2). For leaflets, the variation among treatments was related to the number of seedlings per seed (Table 2), with higher values for single seedlings (MA-1; MD-1; PA-1; PD-1) than for paired seedlings (PA-2, PD-2) (Table 1). By the end of the observation period, the leaves of the seedlings had not yet achieved adult morphology and were in transition between unifoliate (just one leaflet) and compound leaves (with more than one leaflet). Most of the leaves were still unifoliate and the average number of leaflets per leaf was 1.2 to 1.5.
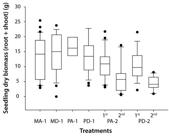
Initial seed mass was not related to seedling dry mass (F = 3.583, p = 0.061, R² = 0.027). However, seedling dry biomass varied significantly, influenced by the number of developed seedlings per seed and emergence order (Figure 3, Table 2). The second seedling to emerge in PA-2 and PD-2 had less biomass than the first seedling in all treatments (MA-1, MD-1, PA-1, PD-1, PA-2-1st and PD-2-1st). However, considering the sum of the dry biomass of first and second seedlings in PA-2 and PD-2, the values did not differ significantly from those of single-seedling treatments (MA-1, MD-1, PA-1, PD-1) (Table 2).
Dry mass of Carapa surinamensis seedlings after 180 days under six treatments. Data were compared with a factorial analysis of variance using GLM. Results were influenced by the number of developed seedlings per seed and emergence order. Bars represent 75% of the data, the central line the median, the points the minimum and maximum values, and the vertical lines the standard deviation. For more details on treatments, see Material and Methods and Figure 1. Table 2 summarizes the statistical analyses.
The root/shoot ratio was also influenced by the number of developed seedlings per seed and by emergence order (Figure 4, Table 2). Naturally single seedlings or single-seedlings after manipulation, attached or not to the seed, showed similar values for the root/shoot ratio, and these values were significantly different between paired seedlings attached or detached from the seed. The second seedling had a higher root/shoot ratio than the first (Table 1).
Root/shoot ratio of Carapa surinamensis seedlings after 180 days under six treatments. Data were compared with a factorial analysis of variance using GLM. Results were influenced by the number of developed seedlings per seed and by the presence or absence of seed reserves. Bars represent 75% of the data, the central line the median, the points the minimum and maximum values, and the vertical lines the standard deviation. For more details on treatments see Material and Methods and Figure 1. Table 2 summarizes the statistical analyses.
DISCUSSION
Polyembryony has been reported in 115 out of 348 botanical families studied so far (Carman 1997Carman, J.G. 1997. Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory, and polyembryony. Biological Journal of the Linnean Society, 61: 51-94.). This characteristic can have impacts on the life cycle of plants. Multiple embryos can result in benefits for the mother plant by increasing the probability of adding individuals to the next generation, but also can increase the energy input for seed production (Hotchkiss et al. 2008Hotchkiss, E.E.; DiTommaso, A.; Brainard, D.C.; Mohler, C.L. 2008. Survival and performance of the invasive vine Vincetoxicum rossicum (Apocynaceae) from seeds of different embryo number under two light environments. American Journal of Botany, 95: 447-453., Blanchard et al. 2010Blanchard, M.L.; Barney, J.N.; Averill, K.M.; Mohler, C.L.; DiTommaso, A. 2010. Does polyembryony confer a competitive advantage to the invasive perennial vine Vincetoxicum rossicum (Apocynaceae)? American Journal of Botany, 97: 251-260. ). Polyembryony may allow seedling establishment after partial seed damage, e.g. in white oak (Quercus alba (L.), Fagaceae), in which only the seeds with more than one embryo could develop seedlings after rodents had damaged the seeds close to the radicle (McEuen and Steele 2005McEuen, A.B.; Steele, M.A. 2005. Atypical acorns appear to allow seed escape after apical notching by squirrels. American Midland Naturalist, 154: 450-458.). However, this advantage was not observed in polyembryonic seeds of C. surinamensis (de Souza Ferreira et al. 2017de Souza Ferreira, D.N.; Camargo, J.L.C.; Ferraz, I.D.K. 2017. Multiple shoots of Carapa surinamensis seeds: Characterization and consequences in light of post-germination manipulation by rodents. South African Journal of Botany, 108: 346-351.). For individuals germinated from polyembryonic seeds, reduced biomass and survival, and increased competition may be the most important drawbacks (Ladd and Cappuccino 2005Ladd, D.; Cappuccino, N. 2005. A field study of seed dispersal and seedling performance in the invasive exotic vine Vincetoxicum rossicum. Canadian Journal of Botany, 83: 1181-1188.; Mendes-Rodrigues et al. 2011Mendes-Rodrigues, C.; Ranal, M.A.; Oliveira, P.E. 2011. Does polyembryony reduce seed germination and seedling development in Eriotheca pubescens (Malvaceae: Bombacoideae)? American Journal of Botany, 98: 1613-1622. ).
It is important to emphasise that C. surinamensis has conferruminated cotyledons, and the axes of the embryos are connected to a common seed reserve formed by fused cotyledons (Harshberger 1902Harshberger, J.W. 1902. The germination of the seeds of Carapa guianensis (Aubl.). Proceedings of the Academy of Natural Sciences of Philadelphia, 54: 122-126.; Amoêdo and Ferraz 2017Amoêdo, S.C.; Ferraz, I.D.K. 2017. Seed quality evaluation by tetrazolium staining during a desiccation study of the recalcitrant seeds of Carapa guianensis Aubl. and Carapa surinamensis Miq. - Meliaceae. African Journal of Agricultural Research, 12: 1005-1013. ). In this species, seedlings share the same seed reserves, which seems to be a rare characteristic for species with polyembryonic seeds. Therefore, comparison of our results with other species which do not share this morphological characteristic may be limited.
A higher aboveground and total biomass in monoembryonic seedlings in comparison to polyembryonic seedlings was observed in the dog-strangling vine, Vincetoxicum rossicum (Barbar) (Apocynaceae), but total polyembryonic seedling biomass per seed was greater than the biomass of monoembryonic seedlings (Blanchard et al. 2010Blanchard, M.L.; Barney, J.N.; Averill, K.M.; Mohler, C.L.; DiTommaso, A. 2010. Does polyembryony confer a competitive advantage to the invasive perennial vine Vincetoxicum rossicum (Apocynaceae)? American Journal of Botany, 97: 251-260. ). Our results indicated that total seedling biomass was equal in monoembryonic and polyembryonic seedlings, probably because the seed reserves were shared between the pairs of the polyembryonic seedlings. Competition can reduce the growth of seedlings, as was observed in the size of leaf area in dandelion, Taraxacum sp. (Asteraceae) (Koven and Jong 2001Koven, C.G.F.; Jong, G. 2001. The effect of intra-specific competition on seedlings of sexual and apomictic Taraxacum officinale. Oikos, 95: 25-30.), seedling height in golden trumpet tree, Handroanthus chrysotrichus Mart. ex DC. (Bignoniaceae) (Mendes-Rodrigues et al. 2012Mendes-Rodrigues, C.; Sampaio, D.S.; Costa, M.G.; Caetano, A.P.S.; Ranal, M.A.; Bittencourt Júnior, N.S.; Oliveira, P.E. 2012. Polyembryony increases embryo and seedling mortality but also enhances seed individual survival in Handroanthus species (Bignoniaceae). Flora, 207: 264-274.), and the number of leaves, shoot and root size and biomass in seedlings of pubescent eriotheca, Eriotheca pubescens Mart. ex. Zucc. (Malvaceae) (Mendes-Rodrigues et al. 2011). In C. surinamensis we observed reduced growth of the second seedling in polyembrionic seeds even when competition was prevented by means of seedling separation after the expansion of the first leaves. This suggests that competition started before the detachment of the seedlings. In Carapa procera (DC.) depletion of seed reserves by seedling development occurs up to two months after germination (Jansen et al. 2006Jansen, P.A.; Bongers, F.; Prins, H.H.T. 2006. Tropical rodents change rapidly germinating seeds into long-term food supplies. Oikos, 113: 449-458.), and the interval between germination of C. surinamensis siblings ranged from zero to 46 days (de Souza Ferreira et al. 2017de Souza Ferreira, D.N.; Camargo, J.L.C.; Ferraz, I.D.K. 2017. Multiple shoots of Carapa surinamensis seeds: Characterization and consequences in light of post-germination manipulation by rodents. South African Journal of Botany, 108: 346-351.).
Polyembrionic seedlings differed from monoembryonic seedlings in the root-to-shoot ratio, and reducing competition by separating siblings altered the results. Competition for seed reserves seemed to have consequences for the competitive strategy of seedlings after germination, increasing belowground biomass to prevent a decrease in survival. Likewise there was a 40 to 70% increase in belowground biomass in double or triple seedlings in comparison to singlet seedlings in Vincetoxicum rossicum, although there was no difference in root-to-shoot ratios (Blanchard et al. 2010Blanchard, M.L.; Barney, J.N.; Averill, K.M.; Mohler, C.L.; DiTommaso, A. 2010. Does polyembryony confer a competitive advantage to the invasive perennial vine Vincetoxicum rossicum (Apocynaceae)? American Journal of Botany, 97: 251-260. ).
The number of seedlings per seed had no influence on survival of V. rossicum in a three-years study (Hotchkiss et al. 2008Hotchkiss, E.E.; DiTommaso, A.; Brainard, D.C.; Mohler, C.L. 2008. Survival and performance of the invasive vine Vincetoxicum rossicum (Apocynaceae) from seeds of different embryo number under two light environments. American Journal of Botany, 95: 447-453.). In contrast, polyembrionic seeds of Handroanthus chrysotrichus had lower overall seedling survival, but polyembryonic seeds had higher survival probability of at least one seedling per seed (Mendes-Rodrigues et al. 2012Mendes-Rodrigues, C.; Sampaio, D.S.; Costa, M.G.; Caetano, A.P.S.; Ranal, M.A.; Bittencourt Júnior, N.S.; Oliveira, P.E. 2012. Polyembryony increases embryo and seedling mortality but also enhances seed individual survival in Handroanthus species (Bignoniaceae). Flora, 207: 264-274.). Second seedlings of C. surinamensis had lower survival after six months in both attached and detached treatments, which suggests that competition for seed reserves between polyembrionic seedlings until the expansion of the first leaves has drawbacks for subsequent survival. Yet, considering the production of seedlings per seed, polyembryony increased the probability of seedling establishment in comparison to monoembryonic seeds in C. surinamensis.
Even six months after eliminating competition through first seedling separation in the PD-2 treatment, development of the second seedling continued to be impaired, which may indicate that competition for resources in the early stages of embryonic development is critical for subsequent development of the seedlings. We did not include the isolation of second emerged seedlings (after eliminating the first seedling from polyembrionic seeds) in our experiment, which would have allowed a further assessment of the effect of emergence order on seedling performance. Besides, it is possible that other variables related to seedling development, which were not assessed here, such as leaf area or relative growth rate, could be influenced by the treatment factors.
The results of this study may be useful for andiroba seedling production. About 50% of the seeds of C. surinamensis are polyembryonic, and produce from two to five seedlings per seed in non-manipulated seeds, resulting in an average of 1.8 seedlings per seed (de Souza Ferreira et al. 2017de Souza Ferreira, D.N.; Camargo, J.L.C.; Ferraz, I.D.K. 2017. Multiple shoots of Carapa surinamensis seeds: Characterization and consequences in light of post-germination manipulation by rodents. South African Journal of Botany, 108: 346-351.). Instead of excluding subsequent germinations in order to achieve a more vigorous first seedling, which is the common practice in commercial seedling productions, farmers could employ the strategy used in our PD-2 treatment, waiting for multiple seedling emergence, and then detaching seedlings to be developed separately. With a survival rate of at least 60% of detached seedlings, the number of seedlings produced by the same number of seeds can be increased up to 1.5 times. As our results suggest, the isolated first seedling of polyembryonic seeds can develop with a similar vigour to that of monoembryonic seedlings. The subsequent polyembrionic seedlings, despite an initial lower height, may develop into normal plants, as all essential structures for further development were present.
CONCLUSIONS
In polyembrionic seeds of Carapa surinamensis, developing seedlings share the same seed reserves in fused cotyledons, and we detected two levels of competition between sibling seedlings. Firstly, competition for the common reserves during germination and initial development of the seedling, and secondly, competition based on external factors, mostly for space for root and shoot development and competition for light. After six months, the effects of competition for common reserves were still perceptible, and separating the siblings was not enough to overcome this reduction in initial seedling development. Nevertheless, polyembryony of C. surinamensis seeds does provide advantages, when considering the number of seedlings produced per seed under a given amount of seed reserves, improving the chances of establishment of at least one seedling. Our results suggest that seedling management by separation of siblings in the plant nursery (15 days after germination) might increase the production of seedlings and can provide economic benefits to producers, who may propagate 1.5 times the number of sprouts with the same number of seeds. On the other hand, maintaining only one seedling can allow the production of more vigorous seedlings with faster development.
ACKNOWLEDGMENTS
This work was funded by Fundação de Amparo à Pesquisa do Estado do Amazonas - FAPEAM (edital 004/2009 REDEBIO), and is part of the MSc thesis of DNSF in the Graduate Program in Ecology at Instituto Nacional de Pesquisas da Amazônia (INPA), supported by a scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). IDKF and JLCC are research fellows of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). We would like to thank the anomymous reviewers and the editor of Acta Amazonica for valuable contributions that helped us to improve the manuscript.
- Amoêdo, S.C.; Ferraz, I.D.K. 2017. Seed quality evaluation by tetrazolium staining during a desiccation study of the recalcitrant seeds of Carapa guianensis Aubl. and Carapa surinamensis Miq. - Meliaceae. African Journal of Agricultural Research, 12: 1005-1013.
- Aublet, J.B.C.F. 1775. Histoire des plantes de la Guiane françoise: rangées suivant la méthode sexuelle, avec plusieurs mémoires 1st ed. Pierre-François Didot, London & Paris, 375p.
- Bauch, J.; Dünisch, O. 2000. Comparison of growth dynamics and wood characteristics of plantation-grown and primary forest Carapa guianensis in Central Amazonia. IAWA Journal, 21: 321-333.
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M. 2013. Seeds: Physiology of Development, Germination and Dormancy 3rd ed. Springer, New York, 392p.
- Blanchard, M.L.; Barney, J.N.; Averill, K.M.; Mohler, C.L.; DiTommaso, A. 2010. Does polyembryony confer a competitive advantage to the invasive perennial vine Vincetoxicum rossicum (Apocynaceae)? American Journal of Botany, 97: 251-260.
- Boufleuer, N.T. 2004. Aspectos ecológicos de andiroba (Carapa guianensis Aublet, Meliaceae), visando seu manejo e conservação Masters thesis, Universidade Federal do Acre - UFAC, Rio Branco, Brasil. 72p.
- Carman, J.G. 1997. Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory, and polyembryony. Biological Journal of the Linnean Society, 61: 51-94.
- Cheplick, G.P. 1992. Sibling competition in plants. Journal of Ecology, 80: 567-575.
- Connor, K.F.; Ferraz, I.D.K.; Bonner, F.T.; Vozzo, J.A. 1998. Effects of desiccation on seeds of Carapa guianensis Aubl. and Carapa procera DC. Seed Technology, 20: 71-82.
- de Souza Ferreira, D.N.; Camargo, J.L.C.; Ferraz, I.D.K. 2017. Multiple shoots of Carapa surinamensis seeds: Characterization and consequences in light of post-germination manipulation by rodents. South African Journal of Botany, 108: 346-351.
- Ferraz, I.D.K.; Camargo, J.L.C.; Sampaio, P.T.B. 2002. Sementes e plântulas de andiroba (Carapa guianensis Aubl. e Carapa procera DC.): aspectos botânicos, ecológicos e tecnológicos. Acta Amazonica, 32: 647-661.
- Fisch, S.T.V.; Ferraz, I.D.K.; Rodrigues, W.A. 1995. Distinguishing Carapa guianensis Aubl. from Carapa procera DC. (Meliaceae) by morphology of young seedlings. Acta Amazonica, 25: 193-200.
- Ganeshaiah, K.N.; Uma Shaanker, R.; Joshi, N.V. 1991. Evolution of polyembryony: Consequences to the fitness of mother and offspring. Journal of Genetics, 70: 103-127.
- Ghosh, R.B. 1972. Studies in the embryology of the family Meliaceae. IV. Fertilisation, endosperm and embryogeny of Aphanamixis polystachya (Wall.) Parker (Syn. Amoora rohituka (W. & A.)). A medicinal plant with a discussion on its taxonomic status and horticulture. Anales de la estación experimental de Aula Dei Calcutta, 11: 396-403.
- Harshberger, J.W. 1902. The germination of the seeds of Carapa guianensis (Aubl.). Proceedings of the Academy of Natural Sciences of Philadelphia, 54: 122-126.
- Hiwale, S. 2015. Neem (Azadirachta indica). In: Hiwale, S. (Ed.). Sustainable Horticulture in Semiarid Dry Lands, Springer, New Delhi, p.281-290.
- Hotchkiss, E.E.; DiTommaso, A.; Brainard, D.C.; Mohler, C.L. 2008. Survival and performance of the invasive vine Vincetoxicum rossicum (Apocynaceae) from seeds of different embryo number under two light environments. American Journal of Botany, 95: 447-453.
- Jansen, P.A.; Bongers, F.; Prins, H.H.T. 2006. Tropical rodents change rapidly germinating seeds into long-term food supplies. Oikos, 113: 449-458.
- Kenfack, D. 2011. Resurrection in Carapa (Meliaceae): a reassessment of morphological variation and species boundaries using multivariate methods in a phylogenetic context. Botanical Journal of the Linnean Society, 165: 186-221.
- Koven, C.G.F.; Jong, G. 2001. The effect of intra-specific competition on seedlings of sexual and apomictic Taraxacum officinale Oikos, 95: 25-30.
- Ladd, D.; Cappuccino, N. 2005. A field study of seed dispersal and seedling performance in the invasive exotic vine Vincetoxicum rossicum Canadian Journal of Botany, 83: 1181-1188.
- Martínez-Gómez, P.; Gradziel, T.M. 2003. Sexual polyembryony in almond. Sexual Plant Reproduction, 16: 135-139.
- McEuen, A.B.; Steele, M.A. 2005. Atypical acorns appear to allow seed escape after apical notching by squirrels. American Midland Naturalist, 154: 450-458.
- Mendes-Rodrigues, C.; Ranal, M.A.; Oliveira, P.E. 2011. Does polyembryony reduce seed germination and seedling development in Eriotheca pubescens (Malvaceae: Bombacoideae)? American Journal of Botany, 98: 1613-1622.
- Mendes-Rodrigues, C.; Sampaio, D.S.; Costa, M.G.; Caetano, A.P.S.; Ranal, M.A.; Bittencourt Júnior, N.S.; Oliveira, P.E. 2012. Polyembryony increases embryo and seedling mortality but also enhances seed individual survival in Handroanthus species (Bignoniaceae). Flora, 207: 264-274.
- Mendonça, A.P.; Ferraz, I.D.K. 2007. Óleo de andiroba: processo tradicional da extração. Acta Amazonica, 37: 353-364.
- Rai, Y. 2014. Seedling behaviour and growth status of endangered medicinal tree Amoora rohituka (Roxb.) in Meerut, U.P. (India). International Journal of Informative & Futuristic Research, 1: 125-132.
- Silva, S.G.; Nunomura, R.C.S.; Nunomura, S.M. 2012. Limonóides isolados dos frutos de Carapa guianensis Aublet (Meliaceae). Química Nova, 35: 1936-1939.
- Shanley, P.; Medina, G. 2005. Frutíferas e plantas úteis na vida amazônica 2nd ed. CIFOR Imazon, Belém, 300p.
- Wilde, J.J.F.E. 2007. Revision of the African genus Heckeldora (Meliaceae). Blumea, 52: 179-199.
-
CITE AS:
Souza Ferreira, D.N. de; Camargo, J.L.C.; Ferraz, I.D.K. 2019. Do polyembryonic seeds of Carapa surinamensis (Meliaceae) have advantages for seedling development? Acta Amazonica 49: 97-104.
Edited by
Associate Editor:
Publication Dates
-
Publication in this collection
06 May 2019 -
Date of issue
Apr-Jun 2019
History
-
Received
02 May 2018 -
Accepted
03 Dec 2018