ABSTRACT
Forest fragmentation has been intense in the eastern Amazon region, which has negatively affected wildlife populations. The speed of deforestation in this region underscores the urgent need to understand the effects of such changes on populations of endemic species, and to implement measures for ecosystem conservation. We analyzed the extent to which fragmented forests are still connected in the Xingu Area of Endemism, in the eastern Brazilian Amazon, and assigned conservation priority to fragments most important for connectivity maintenance. We structurally classified the Xingu landscape using the Morphological Spatial Pattern Analysis and ranked each fragment according to its importance using an Index of Connectivity. Our data revealed important differences in conservation potential across the region. Although most of the study area already receives some degree of protection, future conservation actions should prioritize the connection of habitat fragments to maximize dispersal potential and minimize genetic isolation of biodiversity components. We produced a map of prioritary areas for connectivity maximization. These areas include fragments with large core areas and high-quality fragments that provide connection among habitats which, together, should maintain crucial corridors for gene flow in a biologically-rich region of the Amazon.
Keywords:
functional connectivity; spatial analysis; integral index of connectivity
RESUMO
A fragmentação florestal tem sido intensa na parte leste da Amazônia, afetando negativamente as populações silvestres. O desmatamento rápido nesta região intensifica a necessidade de entender como estas mudanças afetam as populações de espécies endêmicas e de implementar medidas de conservação de ecossistemas. Nós analisamos o nível de conexão que ainda existe entre os fragmentos florestais na Área de Endemismo Xingu, na Amazônia Oriental, e atribuímos prioridade de conservação aos fragmentos mais importantes para a manutenção da conectividade entre fragmentos. Analisamos estruturalmente a paisagem do Xingu usando a Análise Morfológica de Padrão Espacial e classificamos cada fragmento de acordo com sua importância usando um Índice de Conectividade. Nossos resultados indicam grande diferença no potencial de conservação ao longo da área de estudo. Apesar de que grande parte da área de estudo já possui algum tipo de proteção, futuras ações de conservação deveriam priorizar a conexão entre fragmentos de hábitat, para maximizar o potencial de dispersão e minimizar o isolamento genético de componentes da biodiversidade. Produzimos um mapa de áreas prioritárias para maximizar a conectividade. Essas áreas incluem fragmentos com grandes áreas-núcleo e fragmentos de alta qualidade que promovem conexão entre habitats que, conjuntamente, podem formar corredores cruciais para o fluxo gênico em uma região de alta diversidade biológica na Amazônia.
Palavras-chave:
conectividade funcional; análise espacial; índice integral de conectividade
INTRODUCTION
The conservation of biodiversity in the Amazon is considered an important environmental goal, particularly because its landscape has become increasingly modified since the 1970s (Laurance et al. 2002Laurance, W.F.; Lovejoy, T.E.; Vasconcelos, H.L.; Bruna, E.M.; Didham, R.K.; Stouffer, P.C.; et al. 2002. Ecosystem decay of Amazonian forest fragments: a 22-year investigation. Biological Conservation , 16: 605-618.; Nepstad et al. 2006Nepstad, D.C.; Stickler, C.M.; Almeida, O.T. 2006. Globalization of the Amazon soy and beef industries: opportunities for conservation. Conservation Biology, 20: 1595-1603.). Many species in the region are not widely distributed, but occur in areas of limited endemism, which, in many cases, makes them unique and irreplaceable (Silva et al. 2005Silva, J.M.; Rylands, A.; Fonseca, G. 2005. O destino das areas de endemismo da Amazonia. Megadiversidade, 1: 124-131.). The conservation of these areas is particularly relevant because habitats of endemic species define the smallest and most-basic biogeographic unit on which to construct hypotheses about processes related to biodiversity (Almeida et al. 2014Almeida, A.S.; Vieira, I.C.G.; Barros, M.N.R.; Rocha, D.P.N. 2014. Áreas de endemismo Belém e Xingu: configuração e espacialização do uso da terra e da cobertura vegetal. In: Emilio, T.; Luizão, F. (Org.). Cenários para a Amazônia: Clima, Biodiversidade e Uso da Terra. 1st ed., Editora INPA, Manaus, p.57-66.).
The Xingu Area of Endemism (XAE), in the eastern Brazilian Amazon, is among the most degraded areas in the region, as it has been under large-scale deforestation pressure. The large-scale conversion of forest into agriculture and pasture has been sponsored by the Brazilian government, and intensified in the 2000s due to growing markets for export-oriented agricultural commodities (Nepstad et al. 2006Nepstad, D.C.; Stickler, C.M.; Almeida, O.T. 2006. Globalization of the Amazon soy and beef industries: opportunities for conservation. Conservation Biology, 20: 1595-1603.; Barona et al. 2010Barona, E.; Ramankutty, N.; Hyman, G.; Coomes, O.T. 2010. The role of pasture and soybean in deforestation of the Brazilian Amazon. Environmental Research Letters, 5: 24002.; DeFries et al. 2013DeFries, R.; Herold, M.; Verchot, L.; Macedo, M.N.; Shimabukuro, Y. 2013. Export-oriented deforestation in Mato Grosso: harbinger or exception for other tropical forests? Philosophical Transactions of the Royal Society B: Biological Sciences, 368: 20120173.). Despite the decline in deforestation between 2006 and 2010 (Macedo et al. 2012Macedo, M.N.; DeFries, R.S.; Morton, D.C.; Stickler, C.M.; Galford, G.L.; Shimabukuro, Y.E. 2012. Decoupling of deforestation and soy production in the southern Amazon during the late 2000s. Proceedings of the National Academy of Sciences, 109: 1341-1346.), the XAE remained particularly vulnerable to changes associated with land use (Coe et al. 2013Coe, M.T.; Marthews, T.R.; Costa, M.H.; Galbraith, D.R.; Greenglass, N.L.; Imbuzeiro, H.M.A.; et al. 2013. Deforestation and climate feedbacks threaten the ecological integrity of south-southeastern Amazonia. Philosophical Transactions of the Royal Society B: Biological Sciences, 368: 20120155.), and large-scale forest fragmentation (Alves 2001Alves, D.S. 2001. O processo de desmatamento na Amazônia. Parcerias Estratégicas, 6: 259-275. ; Crist et al. 2005Crist, M.R.; Wilmer, B.; Aplet, G.H. 2005. Assessing the value of roadless areas in a conservation reserve strategy: biodiversity and landscape connectivity in the northern Rockies. Journal of Applied Ecology, 42: 181-191.).
The connection among the remaining forest fragments is fundamental for important ecological functions, such as animal dispersion and gene flow (Crist et al. 2005Crist, M.R.; Wilmer, B.; Aplet, G.H. 2005. Assessing the value of roadless areas in a conservation reserve strategy: biodiversity and landscape connectivity in the northern Rockies. Journal of Applied Ecology, 42: 181-191.), which places the study of connectivity among the essential topics in conservation biology (Calabrese and Fagan 2004Calabrese, J.M.; Fagan, W.F. 2004. A comparison-shopper’s guide to connectivity metrics. Frontiers Ecology and the Environment, 2: 529-536.). Connectivity studies can be divided into two types: (a) structural connectivity, which describes the physical connection among fragments, such as inter-fragment distances and the existence and characteristics of corridors; and (b) functional connectivity, which addresses the capacity for species dispersal across different elements of the landscape (Forero-Medina and Vieira 2007Forero-Medina, G.; Vieira, M.V. 2007. Conectividade funcional e a importância da interação organismo-paisagem. Oecologia Brasiliensis, 11: 493-502.). Thus, connectivity can be seen both as an independent variable affecting ecological processes and populations, and as a variable dependent on species behavior and landscape structure (Goodwin 2003Goodwin, B.J. 2003. Is landscape connectivity a dependent or independent variable? Landscape Ecology, 18: 687-699.).
It is important to incorporate landscape metrics and spatial connectivity maintenance in analyses for conservation area prioritization, as these parameters provide the theoretical and empirical basis for the promotion of species persistence and the continuity of evolutionary processes in a region (Forero-Medina and Vieira 2007Forero-Medina, G.; Vieira, M.V. 2007. Conectividade funcional e a importância da interação organismo-paisagem. Oecologia Brasiliensis, 11: 493-502.). In this context, we used two approaches for evaluating connectivity of remnant forest patches in the XAE. We classified and spatially analyzed remaining forest fragments in the XAE, then we evaluated the connectivity of patches in the landscape using an index of connectivity and identified key fragments for maintaining connectivity in the region.
MATERIAL AND METHODS
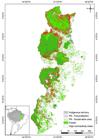
The Xingu Area of Endemism (XAE) is located in the state of Pará (Brazil), in the eastern Amazon region (1ºS−14ºS, 48ºW−54ºW) (Figure 1). Its boundaries are determined by natural barriers, mainly the rivers Tocantins, Itacaiunas, Araguaia and Xingu. Vegetation of the XAE is comprised of fragments of ombrophilous forest, which is essential habitat for endemic species in the region. Surveys conducted until 2012 indicated at least 259 mammal, 759 bird, 220 reptile and amphibian, and 467 fish species in the lower and middle Xingu region (Brasil 2012Brasil. 2012. Plano de Ação Nacional para a Conservação das Espécies Endêmicas e Ameaçadas de Extinção da Fauna da Região do Baixo e Médio Xingu. Ministério do Meio Ambiente, Brasília. ( (http://www.icmbio.gov.br/portal/images/stories/docs-plano-de-acao/pan-xingu/matriz-xingu.pdf
). Accessed on 25 Oct 2018.
http://www.icmbio.gov.br/portal/images/s...
). The National Action Plan for Conservation lists 21 endangered animal species, which makes the region an important conservation target (Brasil 2012Brasil. 2012. Plano de Ação Nacional para a Conservação das Espécies Endêmicas e Ameaçadas de Extinção da Fauna da Região do Baixo e Médio Xingu. Ministério do Meio Ambiente, Brasília. ( (http://www.icmbio.gov.br/portal/images/stories/docs-plano-de-acao/pan-xingu/matriz-xingu.pdf
). Accessed on 25 Oct 2018.
http://www.icmbio.gov.br/portal/images/s...
).
Map of the Xingu Area of Endemism, showing its location in the eastern Brazilian Amazon. The red outline marks the forest remnants with significant potential connectivity, as determined by the delta Integral Index of Connectivity (dIIC). PA = protected area. This figure is in color in the electronic version.
For the construction of our binary conceptual model (habitat and non-habitat), we used the land-cover data provided by PRODES (with 60 m of spatial resolution) for 2014 (INPE 2016INPE. 2016. Instituto Nacional de Pesquisas Espaciais. PRODES - Projeto de Monitoramento do Desmatamento na Amazônia Legal por Satélite. ( (http://www.obt.inpe.br/OBT/assuntos/programas/amazonia/prodes
). Accessed on 03/03/2016.
http://www.obt.inpe.br/OBT/assuntos/prog...
). We extracted data referring to the area covered by ombrophilous forest (habitat), constructed polygons that represent forest fragments and selected those fragments with more than 50 ha in order to simplify the analysis. Data on 19 protected areas and 20 indigenous lands of the XAE were provided by the Brazilian government (Brazil 2016).
We structurally classified the XAE landscape using the “Morphological Spatial Pattern Analysis” (MSPA) algorithm. This algorithm consists of a personalized sequence of mathematical operators derived from a geometric description of connectivity among specific landscape features and configurations (Soille and Vogt 2009Soille, P.; Vogt, P. 2009. Morphological segmentation of binary patterns. Pattern Recognition Letters, 30: 456-459.). Based on this geometric concept, we used MSPA to classify each forest fragment within one of seven spatial-configuration categories: (1) core area (an area of core habitat that excludes forest edges); (2) edge (an area located within 300 m of the perimeter boundary of a fragment, which is the transition from forest to non-forest); (3) islet (a small fragment that does not have any core habitat area); (4) loop (an area that connects a forest fragment to itself); (5) bridge (an area that connects various fragments); (6) perforation (an area located at the internal boundary of a fragment); and (7) branch (an area that connects non-core forest habitat elements). We defined edge as 300 m because this is considered to be the distance over which the greatest changes (transitions) in biota from forest to non-forest occur (Laurance et al. 2002Laurance, W.F.; Lovejoy, T.E.; Vasconcelos, H.L.; Bruna, E.M.; Didham, R.K.; Stouffer, P.C.; et al. 2002. Ecosystem decay of Amazonian forest fragments: a 22-year investigation. Biological Conservation , 16: 605-618.). We executed the MSPA algorithm using the Guidos Toolbox package (Soille and Vogt 2009).
To analyze connectivity, we used the Integral Index of functional Connectivity (IIC) (Pascual-Hortal and Saura 2006Pascual-Hortal, L.; Saura, S. 2006. Comparison and development of new graph-based landscape connectivity indices: towards the priorization of habitat patches and corridors for conservation. Landscape Ecology, 21: 959-967.; Saura and Pascual-Hortal 2007Saura, S.; Pascual-Hortal, L. 2007. A new habitat availability index to integrate connectivity in landscape conservation planning: Comparison with existing indices and application to a case study. Landscape and Urban Planning, 83: 91-103.; Saura and Torné 2009Saura, S.; Torné, J. 2009. Conefor Sensinode 2.2: A software package for quantifying the importance of habitat patches for landscape connectivity. Environmental Modelling & Software, 24: 135-139.), which uses a potential dispersal distance to rank each fragment according to its importance to landscape connectivity. This is considered to be one of the most robust indexes, as it reacts to all types of landscape change in a consistent and desirable way (Pascual-Hortal and Saura 2006). The index is based on graph theory and uses the size, proximity and topology of the fragments as parameters (Pascual-Hortal and Saura 2006). These properties make the analysis more sensitive to spatial patterns and enable the creation of models that link species dispersal capacity to the landscape’s spatial structure (Pascual-Hortal and Saura 2006). The IIC consists of three sub-indices (IIC-Intra, IIC-Flux, and IIC-Conn), which differ in the way the contribution of a fragment to overall landscape connectivity is considered. IIC-Intra indicates each fragment’s internal contribution to connectivity based on its area. IIC-flux analyzes dispersal flow by considering each fragment’s location within the network of fragments and its area. IIC-Conn indicates each fragment’s contribution as a linking element, using only its topology (Saura and Rubio 2010Saura, S.; Rubio, L. 2010. A common currency for the different ways in which patches and links can contribute to habitat availability and connectivity in the landscape. Ecography, 33: 523-537.). We used 800 and 3300 m as dispersal values in order to estimate connectivity for species with intermediate and high capacity for dispersal, respectively (Umetsu et al. 2008Umetsu, F.; Paul Metzger, J.; Pardini, R. 2008. Importance of estimating matrix quality for modeling species distribution in complex tropical landscapes: a test with Atlantic forest small mammals. Ecography, 31: 359-370.; Jesus et al. 2012Jesus, F.M.; Pivello, V.R.; Meirelles, S.T.; Franco, G.A.D.C.; Metzger, J.P. 2012. The importance of landscape structure for seed dispersal in rain forest fragments. Journal of Vegetation Science, 23: 1126-1136.). We used delta Integral Index of Connectivity (dIIC) values, which correspond to the relative importance of each fragment in providing connectivity between habitats (Pascual-Hortal and Saura 2006; Saura and Rubio 2010). The indices (dIIC, dIIC-Intra, dIIC-Flux and dIIC-Conn) provided delta values that allowed this information to be used to develop plans for biodiversity conservation and management (Pascual-Hortal and Saura 2006; Saura and Rubio 2010). The forest fragments were grouped into five classes, using Jenks Natural Breaks Optimization, also known simply as “natural breaks” (Jenks and Caspall 1971Jenks, G.F.; Caspall F.C. 1971. Error on choroplethic maps: definition, measurement, reduction. Annals of the Association of American Geographers, 61: 217-244.). The classification followed an order of importance, with class 1 being the most important and 5 the least important. We defined the fragments with higher values (classes 1, 2 or 3) as the most important for connectivity. The connectivity values were calculated with the Conefor software (Pascual-Hortal and Saura 2006; Saura and Rubio 2010), that allows quantifying the importance of habitat areas and links for the maintenance or improvement of connectivity. The distribution of the values in five classes was developed with the geographic information system software ArcGIS 10.0 (ESRI 2010ESRI. 2010. ArcGIS geographic information system, Environmental Systems Research Institute.).
For the construction of the model of prioritary forest areas for connectivity, we filtered out fragments with the highest levels of connectivity (classes 1-3) in at least one of the two dispersal potentials (800 and 3300 m) analyzed for the IIC. The outline of these fragments served as the basis to indicate the priority areas for conservation from the point of view of functional connectivity of the region’s landscape. Finally, we developed a map comparing the importance of the fragments as classified by the IIC (based on the 800−m dispersal potential) with the priority areas for conservation indicated by the Brazilian government in 2006 (Brasil 2007Brasil. 2007. Ministério do Meio Ambiente. Secretaria Nacional de Biodiversidade e Florestas. Departamento de Conservação da Biodiversidade. Áreas Prioritárias para Conservação, Uso Sustentável e Repartição de Benefícios da Biodiversidade Brasileira: Atualização - Portaria MMA n°9, de 23 de janeiro de 2007.29-38.). We used only the 800−m dispersal potential for this map because we assumed that this parameter also accounts for species with higher dispersal capacity.
We also analyzed the variation of values attributed by the dIIC index (for 800−m dispersal potential) in relation to the area of forest fragments. The relation between the variables was represented using the boxplot graph tool in the Systat 12.0 software (CSIL 2017CSIL. 2017. SYSTAT 12.0 - Powerful Statistical Analysis and Graphics Software. Cranes Software International Limited.).
We also quantified the proportion of protected areas (indigenous lands, sustainable use reserves and integral conservation units) by class (dIIC) to evaluate the efficiency of the existing network of protected areas. This analysis was developed with the help of ArcGIS 10.0, based on the calculation of the area of each fragment and the comparison between our functional connectivity models and the list of priority areas for conservation in 2006 (Brasil 2007Brasil. 2007. Ministério do Meio Ambiente. Secretaria Nacional de Biodiversidade e Florestas. Departamento de Conservação da Biodiversidade. Áreas Prioritárias para Conservação, Uso Sustentável e Repartição de Benefícios da Biodiversidade Brasileira: Atualização - Portaria MMA n°9, de 23 de janeiro de 2007.29-38.).
RESULTS
We identified 6376 fragments larger than 50 ha, which, together, covered more than 20 million ha and comprised approximately 50% of the total area (39.06 million ha) of the XAE. Most fragments were small and the four largest comprised 66.7% of the total area of fragments.
The MSPA analysis indicated 74% of forest fragments as core habitat, 10% as edge, 2% as perforations in core areas, and 14% as connecting segments (bridges, loops, branches, and islands). Under both dispersal scenarios (800 and 3300 m), the amount of core area was higher than any of the other connectivity classes (Figure 2).
Classification of landscape spatial structure in the Xingu Area of Endemism (eastern Brazilian Amazon): (A) for classes defined by a 800-m dispersal threshold using the Integral Index of Connectivity algorithm; (B) for classes defined by a 3300-m dispersal threshold using the Integral Index of Connectivity algorithm; and (C) for the entire Xingu Area of Endemism without relation to the integral index of connectivity. This figure is in color in the electronic version.
In general, larger-sized fragments were more important for connecting forest patches in the XAE landscape (Figures 3, 4, and 5). However, the relative importance of fragments (based on dIIC subindices) differed between the two potential threshold dispersal distance scenarios, as the fragments located in the southern part of the XAE were more important for species with lower dispersal capacity (800-m scenario) than for species with higher dispersal capacity (3300-m scenario)(Figures 4 and 5).
Boxplot showing the variation of connectivity values of the forest fragments in the Xingu Endemism Area (based on a 800-m dispersal threshold) for each area class, as determined (A) by the delta Integral Index of Connectivity (dIIC) and its subindices: (B) delta Integral Index of Connectivity-Intra; (C) delta Integral Index of Connectivity- Flux; (D) delta Integral Index of Connectivity-Conn. The areas were grouped into five classes, using Jenks Natural Breaks Optimization, also known simply as “natural breaks”. The columns show the mean and quartiles, the bars the range of values. Circles indicate atypical values. Asteriscs refer to very discrepant values.
Classification of forest remnants in the Xingu Endemism Area under the 800 m dispersal threshold scenario based on their connectivity importance as determined by (A) delta Integral Index of Connectivity (dIIC) and its subindices: (B) delta Integral Index of Connectivity-Intra; (C) delta Integral Index of Connectivity- Flux; (D) delta Integral Index of Connectivity-Conn. This figure is in color in the electronic version.
Classification of forest remnants in the Xingu Endemism Area under the 3300 m dispersal threshold scenario based on their connectivity importance as determined by (A) delta Integral Index of Connectivity (dIIC) and its subindices: (B) delta Integral Index of Connectivity-Intra; (C) delta Integral Index of Connectivity- Flux; (D) delta Integral Index of Connectivity-Conn. This figure is in color in the electronic version.
According to the IIC index, under either dispersal scenario, few fragments were classified as highly important for dispersal (i.e., belonging to classes 1, 2 and 3) (Table 1). Under the 800 m dispersal scenario, four fragments had high importance level for subindex dIIC-Intra, four for subindex dIIC-Flux (regarding location and potential contribution to gene flow), and 18 for subindex dIIC-Conn (important fragments as binding elements) (Table 1). Under the 3300 m dispersal scenario, only four fragments (all with large core areas) had high dIIC-Intra subindex scores, six had high dIIC-Flux subindex scores, and 16 had high IIC-Conn subindex scores.
Number of fragments in the Xingu Area of Endemism defined by delta Integral Index of Connectivity (dIIC) sub-indices, importance class, and dispersal distance.
Overall, 47.5% of the current forest area in the XAE is under protection status, most of it as indigenous land (Table 2). Our results show that, in general, these protected areas provide outstanding connectivity among fragments (Figure 1). Under both dispersal-distance scenarios, less than half of the IIC class 1 fragments are protected, but nearly 90% of the IIC class 2 fragments are under some protection (Table 2). Although our results show that a considerable part of remaining forested areas in the XAE already receive some level of protection, there is no protection for a large portion of fragments located in the northern region of the XAE, which provide the highest potential for connectivity for both dispersal distance scenarios (Figure 1).
Proportion of protected areas in the Xingu Area of Endemism calculated for the whole XAE and for each class of importance for connectivity, based on the Delta Index of Connectivity (dIIC) for two dispersal distances (800 and 3300 m). IL = indigenous land, PASU = protected area of sustainable use, FPPA = full protection protected area, XAE = Xingu Area of Endemism.
DISCUSSION
We identified forest fragments with high potential for providing connectivity to other fragments in the northern and southern portions of the Xingu Area of Endemism. Our results also showed that the eastern and western regions of the XAE differ in their conservation potential. Many small forest fragments occur in the eastern region, while the western region supports large fragments with core habitats that have a high connectivity potential. This spatial distribution pattern of fragments should make it easier to design a conservation network of fragments to maintain suitable connectivity among important habitats.
The highest-scoring fragments, according to our dIIC connectivity index, contained large core areas. This condition is relevant when designing protected areas because the amount of remaining habitat usually determines the potential for biota to persist in a given landscape (Lindenmayer et al. 2007Lindenmayer, D.; Hobbs, R.J.; Montague-Drake, R.; Alexandra, J.; Bennett, A.; Burgman, M.; et al. 2007. A checklist for ecological management of landscapes for conservation. Ecology Letters, 11: 78-91.). We also found that several fragments with low connectivity (class 5), mainly in the eastern portion of the XAE, should be taken into account when developing regional protection plans, as they provide important conduits to migration between core areas and allow the maintenance of the rescue effect, to reduce the probability of species extinctions (Odum and Barret 2008Odum, E.P.; Barrett, G.W. 2008. Fundamentos de Ecologia. 5th ed. Cengage Learning, São Paulo, 613p.).
Our results indicated four very large forest fragments in the XAE, which favors the persistence of species, as species richness of an isolated patch is directly proportional to its area and indirectly related to the distance to other patches (MacArthur and Wilson 2001MacArthur, R.H.; Wilson, E.O. 2001. The Theory of Island Biogeography. Princeton University Press, Princeton, 226p.). This relationship between size and distance has been confirmed in many tropical landscapes (Vieira et al. 2009Vieira, M.V.; Olifiers, N.; Delciellos, A.C.; Antunes, V.Z.; Bernardo, L.R.; Grelle, C.E.V.; et al. 2009. Land use vs. fragment size and isolation as determinants of small mammal composition and richness in Atlantic Forest remnants. Biological Conservation , 142: 1191-1200.; Banks-Leite et al. 2012Banks-Leite, C.; Ewers, R.M.; Metzger, J.P. 2012. Unraveling the drivers of community dissimilarity and species extinction in fragmented landscapes. Ecology, 93: 2560-2569.; Almeida-Gomes et al. 2014Almeida-Gomes, M.; Rocha, C.F.D. 2014. Diversity and distribution of lizards in fragmented Atlantic forest landscape in Southeastern Brazil. Journal of Herpetology, 48: 423-429.; Almeida-Gomes et al. 2016Almeida-Gomes, M.; Vieira, M.V.; Rocha, C.F.D.; Metzger, J.P.; De Coster, G. 2016. Patch size matters for amphibians in tropical fragmented landscapes. Biological Conservation, 195: 89-96.). In addition, as predicted by metapopulation theory, it is likely that these larger fragments function as source habitat for individuals dispersing to smaller fragments (Odum and Barret 2008Odum, E.P.; Barrett, G.W. 2008. Fundamentos de Ecologia. 5th ed. Cengage Learning, São Paulo, 613p.), which further enhances their importance for biodiversity conservation. Larger fragments minimize detrimental effects of disturbance due to their higher ratio of core area to perimeter (Odum and Barret 2008). Based on the neutral theory of biodiversity, we can assume that the decrease in size and the increase in distance from larger fragments in the XAE likely directly influence species composition of fragments, as stochastic processes affecting population abundance can be compromised by lack of connectivity in the fragment network (Hubbell 2005Hubbell, S.P. 2005. The neutral theory of biodiversity and biogeography and Stephen Jay Gould. Paleobiology, 31: 122-132.). It is also likely that changes in the spatial structure of the landscape influence species extinction processes in ways similar to those observed in other tropical regions that support species adapted to large areas (Odum and Barret 2008).
The dispersal scenario with a lower distance threshold resulted in more fragments classified with higher IIC scores, which was mainly due to dispersal scores calculated for the southernmost XAE fragments, which potentially provide important dispersal connections to other fragments, thus allowing for species with lower dispersal capacity to disperse to other areas. However, detailed information on dispersal capacity of populations relative to landscape structure is scarce (Pütz et al. 2011Pütz, S.; Groeneveld, J.; Alves, L.F.; Metzger, J.P.; Huth, A. 2011. Fragmentation drives tropical forest fragments to early successional states: A modelling study for Brazilian Atlantic forests. Ecological Modelling, 222: 1986-1997.) and future studies should take into account other important factors, such as the quality of the landscape matrix (Umetsu et al. 2008Umetsu, F.; Paul Metzger, J.; Pardini, R. 2008. Importance of estimating matrix quality for modeling species distribution in complex tropical landscapes: a test with Atlantic forest small mammals. Ecography, 31: 359-370.).
The four largest forest fragments in the XAE are likely important for species assemblages with a wide range of dispersal capacities (e.g. 800 and 3300 m), primarily because large fragments maintain a high degree of internal connectivity. Changes in the structure (size, distance and location in the network of fragments) of large fragments could detrimentally impact populations of seed dispersers (particularly birds, and mammals), which could lead to other negative changes in the ecosystem (Umetsu et al. 2008Umetsu, F.; Paul Metzger, J.; Pardini, R. 2008. Importance of estimating matrix quality for modeling species distribution in complex tropical landscapes: a test with Atlantic forest small mammals. Ecography, 31: 359-370.; Jesus et al. 2012Jesus, F.M.; Pivello, V.R.; Meirelles, S.T.; Franco, G.A.D.C.; Metzger, J.P. 2012. The importance of landscape structure for seed dispersal in rain forest fragments. Journal of Vegetation Science, 23: 1126-1136.). Despite differences in species dispersal abilities, most species respond in the same way (population decline size, extinction, among others) to alterations in landscape structure, which reinforces the need for creating and protecting larger areas of core habitat to maintain biodiversity (Crouzeilles et al. 2014Crouzeilles, R.; Prevedello, J.A.; Figueiredo, M. de S.L.; Lorini, M.L.; Grelle, C.E.V. 2014. The effects of the number, size and isolation of patches along a gradient of native vegetation cover: how can we increment habitat availability? Landscape Ecology, 29: 479-489.).
Currently, a map of priority conservation areas produced by the Brazilian Ministry of the Environment is used to provide strategic support to resource managers when creating new protected areas, prioritizing units of high biological importance and that are under intense anthropogenic pressure (Brasil 2007Brasil. 2007. Ministério do Meio Ambiente. Secretaria Nacional de Biodiversidade e Florestas. Departamento de Conservação da Biodiversidade. Áreas Prioritárias para Conservação, Uso Sustentável e Repartição de Benefícios da Biodiversidade Brasileira: Atualização - Portaria MMA n°9, de 23 de janeiro de 2007.29-38.; Brasil 2017Brasil. 2017. Unidades de conservação. Ministério do Meio Ambiente. ( (http://areasprioritarias.mma.gov.br/2-atualizacao-das-areas-prioritarias
). Accessed on 25 Oct 2018.
http://areasprioritarias.mma.gov.br/2-at...
). However, the process of prioritizing areas for protection needs to be improved so that all attributes essential for maintaining biodiversity are included as part of the selection criteria, such as information on an area’s potential for providing connectivity among remnant forest habitats.
Until 2016, considerable progress had been made in conserving forest tracts in the XAE, including the establishment of protected areas on public and private lands, and the demarcation of indigenous lands (Almeida et al. 2014Almeida, A.S.; Vieira, I.C.G.; Barros, M.N.R.; Rocha, D.P.N. 2014. Áreas de endemismo Belém e Xingu: configuração e espacialização do uso da terra e da cobertura vegetal. In: Emilio, T.; Luizão, F. (Org.). Cenários para a Amazônia: Clima, Biodiversidade e Uso da Terra. 1st ed., Editora INPA, Manaus, p.57-66.; Brasil 2017Brasil. 2017. Unidades de conservação. Ministério do Meio Ambiente. ( (http://areasprioritarias.mma.gov.br/2-atualizacao-das-areas-prioritarias
). Accessed on 25 Oct 2018.
http://areasprioritarias.mma.gov.br/2-at...
). The large high-value fragments in the western portion of the XAE, including the indigenous lands of Trincheira Bacaja, Arawet, Koatinemo, Apyterewa and Kayap, form the largest area of protected remnant forest in the region. Despite the intense fragmentation in the eastern region of the XAE, some of the fragments there still provide important connectivity to more northerly fragments, as is the case of the indigenous land of the Parakanã tribe and other surrounding protected areas. Also, more attention should focus on the connection formed by the Xicrin indigenous land on, the Caeté River, and the surrounding protected areas, to the indigenous lands of Kayape and Apyterewa.
The second largest fragment, located in the northern part of the XAE and ranked among the most important for connectivity, is currently not well protected. Although several protection units have been designated in this area (Caxiuanã National Forest, Gurupá Melgaço Extractivist Reserve, Arioca Pruanã Extractivist Reserve, and Marajó Achipelago Environmental Protection Area), they represent but a small fraction of the total forest remnants in the area that remain unprotected. Our IIC-Intra and IIC-Flux indices ranked these areas highly for both dispersal scenarios, thus it is important not only because it is large, but also because it has a high potential for providing species connectivity to the most important fragments in the XAE.
In 2006, during workshops held by the Brazilian government to identify priority areas for conservation in the Amazon, stakeholders recognized the urgent need for further protection areas in the XAE (Figure 6). The need to urgently promote better environmental protection in the area was acknowledged by assigning a strong threat status to remnant habitat patches. Workshop participants also recognized the importance of creating a corridor between the northern fragments and the most important core fragments in the XAE, so that our findings are in agreement with their proposals.
Map of the Xingu Area of Endemism showing currently existing protection areas (indigenous lands, sustainable use reserves, and integral protection reserves), areas identified as threatened, and areas proposed by the Brazilian government (as of 2006) for prioritary conservation actions. The numbers correspond to the names of protection areas on the left. PA = protected area. This figure is in color in the electronic version.
The non-protection of part of the XAE is a result of its potential for exploration of natural resources for various economic activities and the resulting conflicts of interest, which have shaped the occupation and transformation of the region over the last decades (Almeida et al. 2014Almeida, A.S.; Vieira, I.C.G.; Barros, M.N.R.; Rocha, D.P.N. 2014. Áreas de endemismo Belém e Xingu: configuração e espacialização do uso da terra e da cobertura vegetal. In: Emilio, T.; Luizão, F. (Org.). Cenários para a Amazônia: Clima, Biodiversidade e Uso da Terra. 1st ed., Editora INPA, Manaus, p.57-66.). Deforestation has progressed continuously in the XAE due to bland environmental conservation policies and a general lack of governance (Almeida et al. 2014). Thus, land use diagnostics and planning, as well as the strengthening of environmental protection and sustainable development policies, are urgent issues for the region.
We propose that conservation of remnant forest fragments could initially focus on two strategies that consider landscape connectivity. The first would be to protect species richness and population viability by prioritizing protection of those fragments with a high potential for internal connectivity (high scores of the IIC-Intra index) that are currently not well protected (Saura and Rubio 2010Saura, S.; Rubio, L. 2010. A common currency for the different ways in which patches and links can contribute to habitat availability and connectivity in the landscape. Ecography, 33: 523-537.). The fragments in the northern portion of the XAE would be a priority in this context. The second would be to protect the fragments that have the highest potential for providing connectivity (highest scores of the IIC-Conn index), such as those that allow for connection between fragments in the southern and central-western XAE. Our quantitative rankings of potential corridors provided a clearer evaluation of the importance of fragments for the preservation of biodiversity in the XAE.
Approximately half of the forest remnants of the XAE are already under some type of protection, and, according to the criteria used in this study, most of the existing protected areas have a high index of connectivity. However, it is necessary to complement the network of protected areas in order to conserve large fragments close to one another, and to keep the landscape functionally linked from the north to the south of the XAE. Populations of many species would become less vulnerable to random demographic and environmental events, minimizing local or regional extinction through connectivity maintenance (Ayres et al. 2005Ayres, J.M.; Fonseca, G.A.B. da; Rylands, A.B.; Queiroz, H.L.; Pinto, L.P.; Masterson, D.; Cavalcanti, R.B. 2005. Os Corredores Ecológicos das Florestas Tropicais do Brasil. Sociedade Civil Mamirauá, Belém, 255p.).
CONCLUSIONS
Our results showed that the environmental degradation level in the Xingu Area of Endemism, in the eastern Amazon region, is unevenly distributed. While the eastern region of the XAE is intensely fragmented, the western region possesses a large amount of core area and a representative portion of this area is under some type of protection. Future conservation actions should concentrate on maintaining and increasing important habitat protection, and on preserving connections among forest fragments to prevent species from becoming genetically isolated. Our map of fragment prioritization includes prioritary fragments for connectivity conservation with large core area, connector quality and also those important for gene flow, thus forming a north-south axis of connectivity in the XAE. We provided important information for the conservation of biodiversity in the XAE.
ACKNOWLEDGMENTS
We thank the Graduate Program in Biodiversity and Evolution of Museu Paraense Emilio Goeldi (MPEG) for supporting the development of this study. We also thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) through the Programa de Capacitação Institucional - PCI/CNPQ/MCTI (#454792/2015-8) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for providing scholarships.
REFERENCES
- Almeida-Gomes, M.; Rocha, C.F.D. 2014. Diversity and distribution of lizards in fragmented Atlantic forest landscape in Southeastern Brazil. Journal of Herpetology, 48: 423-429.
- Almeida-Gomes, M.; Vieira, M.V.; Rocha, C.F.D.; Metzger, J.P.; De Coster, G. 2016. Patch size matters for amphibians in tropical fragmented landscapes. Biological Conservation, 195: 89-96.
- Almeida, A.S.; Vieira, I.C.G.; Barros, M.N.R.; Rocha, D.P.N. 2014. Áreas de endemismo Belém e Xingu: configuração e espacialização do uso da terra e da cobertura vegetal. In: Emilio, T.; Luizão, F. (Org.). Cenários para a Amazônia: Clima, Biodiversidade e Uso da Terra 1st ed., Editora INPA, Manaus, p.57-66.
- Alves, D.S. 2001. O processo de desmatamento na Amazônia. Parcerias Estratégicas, 6: 259-275.
- Ayres, J.M.; Fonseca, G.A.B. da; Rylands, A.B.; Queiroz, H.L.; Pinto, L.P.; Masterson, D.; Cavalcanti, R.B. 2005. Os Corredores Ecológicos das Florestas Tropicais do Brasil Sociedade Civil Mamirauá, Belém, 255p.
- Banks-Leite, C.; Ewers, R.M.; Metzger, J.P. 2012. Unraveling the drivers of community dissimilarity and species extinction in fragmented landscapes. Ecology, 93: 2560-2569.
- Barona, E.; Ramankutty, N.; Hyman, G.; Coomes, O.T. 2010. The role of pasture and soybean in deforestation of the Brazilian Amazon. Environmental Research Letters, 5: 24002.
- Brasil. 2007. Ministério do Meio Ambiente. Secretaria Nacional de Biodiversidade e Florestas. Departamento de Conservação da Biodiversidade. Áreas Prioritárias para Conservação, Uso Sustentável e Repartição de Benefícios da Biodiversidade Brasileira: Atualização - Portaria MMA n°9, de 23 de janeiro de 2007.29-38.
- Brasil. 2012. Plano de Ação Nacional para a Conservação das Espécies Endêmicas e Ameaçadas de Extinção da Fauna da Região do Baixo e Médio Xingu. Ministério do Meio Ambiente, Brasília. ( (http://www.icmbio.gov.br/portal/images/stories/docs-plano-de-acao/pan-xingu/matriz-xingu.pdf ). Accessed on 25 Oct 2018.
» http://www.icmbio.gov.br/portal/images/stories/docs-plano-de-acao/pan-xingu/matriz-xingu.pdf - Brasil. 2017. Unidades de conservação. Ministério do Meio Ambiente. ( (http://areasprioritarias.mma.gov.br/2-atualizacao-das-areas-prioritarias ). Accessed on 25 Oct 2018.
» http://areasprioritarias.mma.gov.br/2-atualizacao-das-areas-prioritarias - Calabrese, J.M.; Fagan, W.F. 2004. A comparison-shopper’s guide to connectivity metrics. Frontiers Ecology and the Environment, 2: 529-536.
- Coe, M.T.; Marthews, T.R.; Costa, M.H.; Galbraith, D.R.; Greenglass, N.L.; Imbuzeiro, H.M.A.; et al 2013. Deforestation and climate feedbacks threaten the ecological integrity of south-southeastern Amazonia. Philosophical Transactions of the Royal Society B: Biological Sciences, 368: 20120155.
- Crist, M.R.; Wilmer, B.; Aplet, G.H. 2005. Assessing the value of roadless areas in a conservation reserve strategy: biodiversity and landscape connectivity in the northern Rockies. Journal of Applied Ecology, 42: 181-191.
- Crouzeilles, R.; Prevedello, J.A.; Figueiredo, M. de S.L.; Lorini, M.L.; Grelle, C.E.V. 2014. The effects of the number, size and isolation of patches along a gradient of native vegetation cover: how can we increment habitat availability? Landscape Ecology, 29: 479-489.
- CSIL. 2017. SYSTAT 12.0 - Powerful Statistical Analysis and Graphics Software. Cranes Software International Limited.
- DeFries, R.; Herold, M.; Verchot, L.; Macedo, M.N.; Shimabukuro, Y. 2013. Export-oriented deforestation in Mato Grosso: harbinger or exception for other tropical forests? Philosophical Transactions of the Royal Society B: Biological Sciences, 368: 20120173.
- ESRI. 2010. ArcGIS geographic information system, Environmental Systems Research Institute.
- Forero-Medina, G.; Vieira, M.V. 2007. Conectividade funcional e a importância da interação organismo-paisagem. Oecologia Brasiliensis, 11: 493-502.
- Goodwin, B.J. 2003. Is landscape connectivity a dependent or independent variable? Landscape Ecology, 18: 687-699.
- Hubbell, S.P. 2005. The neutral theory of biodiversity and biogeography and Stephen Jay Gould. Paleobiology, 31: 122-132.
- INPE. 2016. Instituto Nacional de Pesquisas Espaciais. PRODES - Projeto de Monitoramento do Desmatamento na Amazônia Legal por Satélite. ( (http://www.obt.inpe.br/OBT/assuntos/programas/amazonia/prodes ). Accessed on 03/03/2016.
» http://www.obt.inpe.br/OBT/assuntos/programas/amazonia/prodes - Jenks, G.F.; Caspall F.C. 1971. Error on choroplethic maps: definition, measurement, reduction. Annals of the Association of American Geographers, 61: 217-244.
- Jesus, F.M.; Pivello, V.R.; Meirelles, S.T.; Franco, G.A.D.C.; Metzger, J.P. 2012. The importance of landscape structure for seed dispersal in rain forest fragments. Journal of Vegetation Science, 23: 1126-1136.
- Laurance, W.F.; Lovejoy, T.E.; Vasconcelos, H.L.; Bruna, E.M.; Didham, R.K.; Stouffer, P.C.; et al 2002. Ecosystem decay of Amazonian forest fragments: a 22-year investigation. Biological Conservation , 16: 605-618.
- Lindenmayer, D.; Hobbs, R.J.; Montague-Drake, R.; Alexandra, J.; Bennett, A.; Burgman, M.; et al 2007. A checklist for ecological management of landscapes for conservation. Ecology Letters, 11: 78-91.
- MacArthur, R.H.; Wilson, E.O. 2001. The Theory of Island Biogeography Princeton University Press, Princeton, 226p.
- Macedo, M.N.; DeFries, R.S.; Morton, D.C.; Stickler, C.M.; Galford, G.L.; Shimabukuro, Y.E. 2012. Decoupling of deforestation and soy production in the southern Amazon during the late 2000s. Proceedings of the National Academy of Sciences, 109: 1341-1346.
- Nepstad, D.C.; Stickler, C.M.; Almeida, O.T. 2006. Globalization of the Amazon soy and beef industries: opportunities for conservation. Conservation Biology, 20: 1595-1603.
- Odum, E.P.; Barrett, G.W. 2008. Fundamentos de Ecologia 5th ed. Cengage Learning, São Paulo, 613p.
- Pascual-Hortal, L.; Saura, S. 2006. Comparison and development of new graph-based landscape connectivity indices: towards the priorization of habitat patches and corridors for conservation. Landscape Ecology, 21: 959-967.
- Pütz, S.; Groeneveld, J.; Alves, L.F.; Metzger, J.P.; Huth, A. 2011. Fragmentation drives tropical forest fragments to early successional states: A modelling study for Brazilian Atlantic forests. Ecological Modelling, 222: 1986-1997.
- Saura, S.; Pascual-Hortal, L. 2007. A new habitat availability index to integrate connectivity in landscape conservation planning: Comparison with existing indices and application to a case study. Landscape and Urban Planning, 83: 91-103.
- Saura, S.; Torné, J. 2009. Conefor Sensinode 2.2: A software package for quantifying the importance of habitat patches for landscape connectivity. Environmental Modelling & Software, 24: 135-139.
- Saura, S.; Rubio, L. 2010. A common currency for the different ways in which patches and links can contribute to habitat availability and connectivity in the landscape. Ecography, 33: 523-537.
- Silva, J.M.; Rylands, A.; Fonseca, G. 2005. O destino das areas de endemismo da Amazonia. Megadiversidade, 1: 124-131.
- Soille, P.; Vogt, P. 2009. Morphological segmentation of binary patterns. Pattern Recognition Letters, 30: 456-459.
- Umetsu, F.; Paul Metzger, J.; Pardini, R. 2008. Importance of estimating matrix quality for modeling species distribution in complex tropical landscapes: a test with Atlantic forest small mammals. Ecography, 31: 359-370.
- Vieira, M.V.; Olifiers, N.; Delciellos, A.C.; Antunes, V.Z.; Bernardo, L.R.; Grelle, C.E.V.; et al 2009. Land use vs. fragment size and isolation as determinants of small mammal composition and richness in Atlantic Forest remnants. Biological Conservation , 142: 1191-1200.
-
CITE AS:
Castro, R.B.; Pereira, J.L.G.; Saturnino, R.; Monteiro, P.S.D.; Albernaz, A.L.K.M. 2020. Identification of priority areas for landscape connectivity maintenance in the Xingu Area of Endemism in Brazilian Amazonia Acta Amazonica 50: 68-79.
Edited by
Associate Editor:
Publication Dates
-
Publication in this collection
27 Feb 2020 -
Date of issue
Jan-Mar 2020
History
-
Received
30 Aug 2019 -
Accepted
02 Oct 2019