Abstracts
Chronic Chagas' cardiomyopathy (CCM) causes ventricular arrhythmias and sudden death, and constitutes the most frequent cause of death in many endemic areas1,2. The circadian variation in the incidence of ventricular arrhythmias and sudden death differs according to the substrate (e.g., morning and evening peaks in ischemic heart disease and non-Chagasic dilated cardiomyopathy). Third generation implantable cardioverter defibrillators (ICDs) have the ability to store the time and date of each ventricular tachycardia (VT) episode, enabling the patterns of ventricular tachyarrhythmia occurrence to be analyzed. The aim of our study was to evaluate the circadian variation of spontaneous VT in recipients of an ICD with CCM.
Ventricular tachycardia; chagasic cardiomyopath
Cardiomiopatia chagásica crônica (CCC) causa arritmias ventriculares e morte súbita, sendo a mais freqüente causa de óbito em muitas áreas endêmicas1,2. A variação circadiana na incidência de arritmias ventriculares e morte súbita difere de acordo com o substrato (p. ex: picos matinais e noturnos na cardiopatia isquêmica e na cardiomiopatia dilatada não-chagásica). Cardioversores-desfibriladores implantáveis de terceira geração (CDI) conseguem registrar o dia e a hora de cada episódio de taquicardia ventricular (TV), permitindo uma análise dos padrões de ocorrência de taquiarritmias. O objetivo deste estudo foi avaliar a variação circadiana da TV espontânea em portadores de CCC tratados com CDI.
Taquicardia ventricular; cardiomiopatia chagásica
ORIGINAL ARTICLE
Circadian pattern of ventricular tachycardia episodes in patients with chagas heart disease
Mauricio Abello; Jorge González-Zuelgaray; Maria E. Daglio; Carlos Lopez; Sebastián Garraza; Ariel Szyszko
Section of Electrophysiology, Division of Cardiology "Argerich Hospital" - Buenos Aires, Argentina
Mailing Address Mailing Address: Mauricio Abello Arcos 1737, 13 "A" 1426 Buenos Aires, AR E-mail: maseabello@yahoo.com.ar
SUMMARY
Chronic Chagas' cardiomyopathy (CCM) causes ventricular arrhythmias and sudden death, and constitutes the most frequent cause of death in many endemic areas1,2. The circadian variation in the incidence of ventricular arrhythmias and sudden death differs according to the substrate (e.g., morning and evening peaks in ischemic heart disease and non-Chagasic dilated cardiomyopathy). Third generation implantable cardioverter defibrillators (ICDs) have the ability to store the time and date of each ventricular tachycardia (VT) episode, enabling the patterns of ventricular tachyarrhythmia occurrence to be analyzed. The aim of our study was to evaluate the circadian variation of spontaneous VT in recipients of an ICD with CCM.
Key words: Ventricular tachycardia, chagasic cardiomyopath.
Methods
This was a retrospective cohort study in the whole population of patients with CCM who have undergone ICD implantation in our institution since May 1998. All patients (n=22) had 2 out of 3 specific serologic tests positive for Chagas' disease (enzyme-linked immunosorbent assay, indirect immunofluorescence reaction and indirect hemagglutination). The presence of ischemic heart disease was ruled out by negative stress testing or coronary angiography before ICD implantation.
Seven different types of ICDs were implanted in the study patients (ICD models 1746, 1782, 1810, 1821, 1831, 1851 and 1861; Guidant Corp., St. Paul, MN, USA). A single and a dual chamber ICD were implanted in 8 and 7 patients, respectively.
Spontaneous VT was defined as a sudden change in rate with stable cycle length that does not fluctuates > 10% during tachycardia. VT discrimination was based on the configuration and stability of the intracardiac ECG, the RR interval analysis, and the first post pacing interval variability. Only VT that were successfully terminated by the device were accepted. Since ventricular fibrillation and polymorphic VT may have different substrates and precipitants, these episodes were not considered for study.
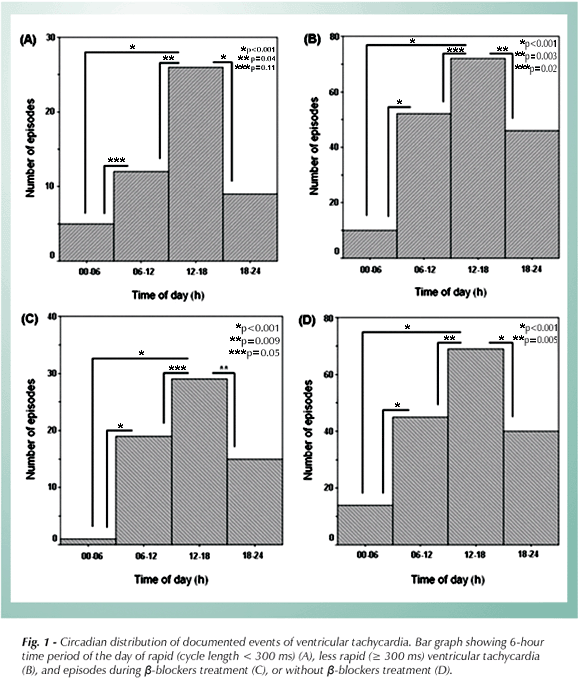
The distribution of VT detection time was examined and classified into four 6-hour time periods of the day. To avoid data bias in individuals with multiple therapies in a relatively short time frame, episodes within a 1-hour period were counted only once, and upper limits of the first 30 consecutive SMVT events per patient were used for this analysis. Tachyarrhythmias with an RR cycle length < 300 ms were classified as rapid tachyarrhythmia, those with an RR > 300 were classified as less rapid tachycardia.
Follow-up visits were scheduled for the first month following hospital discharge and then every 6 months. Clinical and ICD data were obtained by a cardiologist at each visit. All episodes were stored on floppy disks and reviewed by two independent observers. Consensus about the diagnosis of the episodes was obtained in cases of discrepancy.
Continuous variables were expressed as mean ± SD or as median and interquartile range, according to the appropriate distribution type. The level of significance was set at p = 0.05. To explore the circadian pattern, we used the histogram technique (dividing the day into 4 categories of 6-hour length). The quisquare test was used to assess the presence of circadian variation against the hypothesis of uniform distribution of episodes and to compare circadian variation in subgroups.
Results
Fifteen patients (mean age: 57±11 years, 8 females) experienced at least one episode of VT terminated by the device after a median follow-up of 18 months (interquartile range: 16-41) and constitute the subject of our study. The average left ventricular ejection fraction was 45±11%. The indications for ICD were non-syncopal VT in 8 patients (53.3%), syncope with inducible VT in 5 (33.3%), and sudden cardiac death in 2 patients (13.3%). Five patients were on beta-blockers due to compensated heart failure. Treatment schedules are listed in table 1.
A total of 695 sustained episodes was recorded and 463 were excluded due to the presence of more than one episode in the same hour or a higher number of episodes than the methodological limit of the first 30 episodes in an individual patient (376), the inappropriate nature of the therapies (65), or cycle length variation (22).
Two hundred and thirty-two VT episodes were reviewed. The median number of VT episodes was 12 (interquartile range: 7-26). The number of episodes, the mean cycle length, and the proportion of rapid or less rapid tachyarrhythmias in each patient are summarized in table 1.
Circadian pattern was demonstrated in the overall population. The episodes occurred less frequently between midnight and 6:00h with a sharp increase in the afternoon (12:00 to 18:00h) (p < 0.01). When the episodes were divided according to b-treatment or VT cycle length, all groups had a trough period between midnight and 6:00h, while a more prominent afternoon peak was observed in more rapid tachyarrhythmia episodes (Fig. 1).
Discussion
The main finding of this study is that a circadian pattern of spontaneous VT is present in patients with CCM after ICD implantation. This pattern, characterized by a prominent peak frequency between noon and 18:00h and a nadir during night (between 24:00 to 06:00h), is different from that seen in other substrates.
Previous studies of circadian rhythm in the frequency of ventricular tachyarrhythmias have described a predominant morning peak and less pronounced afternoon peak in patients with both ischemic heart disease and dilated cardiomyopathy3-5.
Few studies on circadian variation of arrhythmic events are available in patients with CCM6,7. It could be speculated that VT and ventricular premature complexes, suspected to be harbingers of VT, might have a similar circadian pattern. In this regard, Grupi et al demonstrated a large and unpredictable variability of the density of premature ventricular complexes during a 24-hour period of Holter monitoring of this group of patients7. On the other hand, Lopes et al6 reported a highly significant excess of sudden death for the period between 12:00 and 18:00h. However, none of these studies analyzed the circadian distribution of documented VT episodes treated by ICD.
Factors that could influence the circadian pattern of Chagasic VT and how they differ from other substrates remain unclear. A likely hypothesis could lie in an autonomic imbalance, a frequent finding in patients with CCM8. The demonstrated presence of-adrenergic receptor antibodies generated by parasitic activity could reduce the normal morning surge of sympathetic drive eliminating the morning peak of events seen in other substrates9. Moreover, we can speculate that patients receiving b-blockers might contribute to attenuating adrenergic influence. However, the circadian pattern was similar, regardless of the use of b-blockers in this study. The high proportion of patients receiving amiodarone, which has well-known b-blocking properties, could have also contributed to attenuating that adrenergic influence. Further investigation is warranted to support this hypothesis.
Conclusion
Patients with CCM have a specific pattern of circadian variation in the frequency of VT events. This pattern, characterized by a prominent peak between noon and 18:00h and a nocturnal nadir suggests that the circadian pattern could differ from that seen in other substrates.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
Manuscript received February 28, 2006; revised manuscript received April 10, 2006; accepted August 27, 2006.
- 1. Rosenbaum MB. Chagasic myocardiopathy. Prog Cardiovasc Dis. 1964; 7: 199-225.
- 2. Rassi A Jr., Rassi SG, Rassi A. Sudden death in Chagas disease. Arq Bras Cardiol. 2001; 76: 75-96.
- 3. Grimm W, Walter M, Menz V, Hoffmann J, Maisch B. Circadian variation and onset mechanisms of ventricular tachyarrhythmias in patients with coronary disease versus idiopathic dilated cardiomyopathy. Pacing Clin Electrophysiol. 2000; 23: 1939-43.
- 4. Tofler GH, Gebara OC, Mittleman MA, Taylor P, Siegel W, Venditti FJ Jr., et al. Morning peak in ventricular tachyarrhythmias detected by time of implantable cardioverter/defibrillator therapy: the CPI Investigators. Circulation. 1995; 92: 1203-8.
- 5. Englund A, Behrens S, Wegscheider K, Rowland E. Circadian variation of malignant ventricular arrhythmias in patients with ischemic and nonischemic heart disease after cardioverter defibrillator implantation. European 7219 Jewel Investigators. J Am Coll Cardiol. 1999; 34: 1560-8.
- 6. Lopes ER, da Cunha LF, dos Santos TA, Resende AV, Jorge BH, Salomao LA et al. Variações circadianas e semanais na morte súbita da doença de Chagas. Arq Bras Cardiol. 1993; 61: 345-8.
- 7. Grupi CJ, Sosa EA, De Carvalho JF, Antonelli RH, Bellotti G, Pileggi F. Variabilidade espontânea da extrassistolia ventricular na cardiopatia chagásica crônica. Arq Bras Cardiol. 1991; 56: 445-50.
- 8. Junqueira LF Jr. Sobre o possível papel da disfunção autonômica cardíaca na morte súbita associada à doença de Chagas. Arq Bras Cardiol. 1991;56:429-34.
- 9. Chiale PA, Ferrari I, Mahler E, Vallazza MA, Elizari MV, Rosenbaum MB et al. Differential profile and biochemical effects of antiautonomic membrane receptor antibodies in ventricular arrhythmias and sinus node dysfunction. Circulation. 2001;103:1765-71.
Mailing Address:
Publication Dates
-
Publication in this collection
04 Apr 2007 -
Date of issue
Feb 2007
History
-
Accepted
27 Aug 2006 -
Reviewed
10 Apr 2006 -
Received
28 Feb 2006