Abstracts
BACKGROUND: Previous studies have demonstrated that leukocytosis and hyperglycemia verified at the admission of patients with acute myocardial infarction (AMI) are associated with intrahospital mortality. However, little is known on the long-term impact of these markers. OBJECTIVE: To evaluate the short-and long-term influence of the levels of glucose and leukocytes on the prognosis of patients with AMI. METHODS: A total of 809 patients with AMI were retrospectively assessed (mean age: 63.2 ± 12.87 yrs) and prospectively and consecutively included in a specific database. RESULTS: a) At the intrahospital phase, the mean values were compared between patients that died and those who survived: Leukocytosis: 12156±5977 vs 10337±3528 (p=0.004, 95%CI = 976-2663); Glucose 176±105 mg/dl vs 140±72 mg/dl (p<0.001, 95%CI = 19.4 - 52.6), respectively. b) With the adjusted mode, the same pattern was observed [p values: 0.002 (t-ratio 3.05), 0.04 (t-ratio 2.06), respectively]. c) Long-term follow-up: the univariate analysis showed P values of 0.001 (t-ratio 3.3), <0.001 (t-ratio 4.16), respectively. The multivariate analysis showed P=0.001 (t-ratio 3.35), 0.08 (t-ratio 1.75), respectively. (d) After the exclusion of the intrahospital deaths, the leukocyte (P=0.989) and glucose levels (P=0.144) did not remain significantly correlated with mortality. The same result was observed at the multivariate analysis. CONCLUSION: The levels of glucose and leukocytes at the hospital admission of patients with AMI are excellent predictors of intrahospital mortality and poor predictors of long-term death.
Leukocytosis; glucose; myocardial infarction
FUNDAMENTO: Estudos prévios demonstraram que a leucocitose e a hiperglicemia verificadas à admissão de pacientes com IAM (infarto agudo do miocárdio), estão correlacionadas com a mortalidade intra-hospitalar. Entretanto, pouco é sabido sobre o impacto desses marcadores a longo prazo. OBJETIVO: Avaliar a curto e longo prazos, a influência dos níveis de glicose e leucócitos no prognóstico de pacientes com IAM. MÉTODOS: Foram analisados, retrospectivamente, 809 pacientes (idade média 63,2 ± 12,87 anos) com IAM, incluídos de forma prospectiva e consecutiva em banco de dados específico. RESULTADOS: a) Na fase intra-hospitalar os valores médios aferidos foram comparados entre pacientes que morreram ou sobreviveram: Leucocitose 12156±5977 vs 10337±3528 (p=0.004, 95% IC= 976-2663); Glicose 176±105 mg/dl vs 140±72 mg/dl (p<0.001, 95% IC= 19.4 - 52.6), respectivamente. b) No modo ajustado, o mesmo padrão foi verificado [valores de p: 0.002 (t-ratio 3.05), 0.04 (t-ratio 2.06), respectivamente]. c) Seguimento a longo prazo: a análise univariada revelou valores de P de 0.001 (t-ratio 3.3), <0.001 (t-ratio 4.16), respectivamente. Pela análise multivariada; P=0.001 (t-ratio 3,35), 0.08 (t-ratio 1,75), respectivamente. d) Após exclusão das mortes intra-hospitalares, os níveis leucocitários (P=0.989) e a glicemia (P=0.144) não permaneceram correlacionadas significativamente com mortalidade. O mesmo resultado foi verificado na análise multivariada. CONCLUSÃO: Níveis de leucócitos e glicemia à admissão de pacientes com IAM são excelentes preditores de mortalidade intra-hospitalar, e pobres preditores de óbitos a longo prazo.
Leucocitose; glucose; infarto do miocárdio
FUNDAMENTO: Estudios previos demostraron que tanto la leucocitosis como la hiperglucemia verificadas cuando de la admisión de pacientes con infarto agudo de miocardio (IAM), están correlacionadas con la mortalidad intrahospitalaria. Sin embargo, poco se sabe acerca del impacto de esos marcadores a largo plazo. OBJETIVO: Evaluar, a corto y largo plazos, la influencia de los niveles de glucosa y leucocitos en el pronóstico de pacientes con IAM. MÉTODOS: Se analizaron, retrospectivamente, a 809 pacientes (edad promedio 63,2 ± 12,87 años) con IAM, incluidos de forma prospectiva y consecutiva en banco de datos específico. RESULTADOS: a) En la fase intrahospitalaria se compararon los valores promedio obtenidos entre pacientes que murieron o supervivieron: leucocitosis 12.156±5.977 vs 10.337±3.528 (p=0.004, 95% IC= 976-2663); glucosa 176±105 mg/dl vs 140±72 mg/dl (p<0.001, 95% IC= 19.4 - 52.6), respectivamente. b) En el modo ajustado, se verificó el mismo estándar [valores de p: 0.002 (t-ratio 3.05), 0.04 (t-ratio 2.06), respectivamente]. c) Seguimiento a largo plazo: el análisis univariado reveló valores de P de 0.001 (t-ratio 3.3), <0.001 (t-ratio 4.16), respectivamente. Ya el análisis multivariado: P=0.001 (t-ratio 3,35), 0.08 (t-ratio 1,75), respectivamente. d) Tras la exclusión de las muertes intrahospitalarias, los niveles leucocitarios (P=0.989) y la glucemia (P=0.144) no permanecieron correlacionadas significativamente con la mortalidad. Igual resultado se verificó en el análisis multivariado. CONCLUSIÓN: Los niveles de leucocitos y glucemia al ingreso de pacientes con IAM resultan excelentes predictores de mortalidad intrahospitalaria, y pobres predictores de óbitos a largo plazo.
Leucocitosis; glucosa; infarto de miocardio
ORIGINAL ARTICLE
Instituto do Coração, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HCFMUSP), São Paulo, SP - Brazil
Mailing address
SUMMARY
BACKGROUND: Previous studies have demonstrated that leukocytosis and hyperglycemia verified at the admission of patients with acute myocardial infarction (AMI) are associated with intrahospital mortality. However, little is known on the long-term impact of these markers.
OBJECTIVE: To evaluate the short-and long-term influence of the levels of glucose and leukocytes on the prognosis of patients with AMI.
METHODS: A total of 809 patients with AMI were retrospectively assessed (mean age: 63.2 ± 12.87 yrs) and prospectively and consecutively included in a specific database.
RESULTS: a) At the intrahospital phase, the mean values were compared between patients that died and those who survived: Leukocytosis: 12156±5977 vs 10337±3528 (p=0.004, 95%CI = 976-2663); Glucose 176±105 mg/dl vs 140±72 mg/dl (p<0.001, 95%CI = 19.4 52.6), respectively. b) With the adjusted mode, the same pattern was observed [p values: 0.002 (t-ratio 3.05), 0.04 (t-ratio 2.06), respectively]. c) Long-term follow-up: the univariate analysis showed P values of 0.001 (t-ratio 3.3), <0.001 (t-ratio 4.16), respectively. The multivariate analysis showed P=0.001 (t-ratio 3.35), 0.08 (t-ratio 1.75), respectively. (d) After the exclusion of the intrahospital deaths, the leukocyte (P=0.989) and glucose levels (P=0.144) did not remain significantly correlated with mortality. The same result was observed at the multivariate analysis.
CONCLUSION: The levels of glucose and leukocytes at the hospital admission of patients with AMI are excellent predictors of intrahospital mortality and poor predictors of long-term death.
Key words: Leukocytosis; glucose; myocardial infarction.
Introduction
Patients with unstable myocardial ischemic syndromes (UMIS) must be routinely submitted to risk stratification when admitted at the hospital. The objective of this early stratification is to determine the risk and prognosis of these patients, which allows establishing a more adequate therapeutic management and clinical follow-up of the patients. In this sense, the classification by Braunwaldª and the risk scores published by the TIMI group (Thrombolysis In Myocardial Infarction)b,c, for UMIS without ST-elevation as well as for acute myocardial infarction (AMI) with ST-elevation, have been largely used for early stratification. However, other risk markers have been investigated in an attempt to make the short- and long-term prognostic assessments more accurate. Previous studies demonstrated that leukocytosis and hyperglycemia verified at the hospital admission of patients with AMI are correlated with intrahospital mortalityd-7. However, little is known about the long-term impact of these markers.
Thus, the main purpose of this study was to evaluate the short- and long-term influence of the levels of glucose and leukocytes on the prognosis of patients with AMI and compare the impact of these "new" markers with that of the "traditional" ones, such as left ventricular (LV) ejection fraction (EF) and age.
Methods
This is a unicentric study, in which all patients were selected from an Intensive Care Coronary Unit. A total of 809 patients with AMI were retrospectively assessed (mean age: 63.2 ± 12.87 yrs) and prospectively and consecutively included in a specific database, between February 1998 and July 2005. The criteria used for the AMI diagnosis were: troponin curve or fraction of creatine kinase (CK-MB) associated to at least of the following: ischemic symptoms, development of pathological Q waves at the electrocardiogram (ECG), electrocardiographic alterations indicative of ischemia (ST elevation or depression) or post-coronary intervention.
The patients were followed for up to 6.4 years (mean survival time of 5.15 years), with annual prospective assessments in relation to mortality. The follow-up of the study population was carried out annually by phone or personal interview. The patients (or their family members) were contacted by phone, visits to the office or active personal search (personal search at the workplace, residence, neighbors' houses, etc).
The values of the first leukocyte and glycemia measurements were analyzed in relation to the intrahospital and long-term prognosis. The blood collection for glycemia and leukocytosis measurement was carried out at the moment of the hospital admission.
In the adjusted models, the following variables were considered: history of angioplasty, myocardial revascularization surgery, myocardial infarction, diabetes, smoking, history of heart failure (HF), age, sex, heart rate (HR), systolic arterial pressure (SAP), ST-segment elevation, glycemia, leukocytes, EF (first echocardiogram; Simpson's method), presence of fibrinolysis and primary angioplasty.
For the intrahospital phase, the Student's t test or Kruskal-Wallis test (univariate analysis) and logistic regression (multivariate analysis) were used. For the long-term analysis, Cox proportional hazards estimation was used. The stepwise method8 (with an entry of 0.10 and removal of 0.10), was used for the logistic regression analysis.
Results
A. Study population
The data regarding the studied population are shown in Table 1.
B. Intra-hospital phase
At the intrahospital phase, 92 patients (11.37%) died. The mean time until death was 94 hours (95%CI; 60.90-127.0). The mean values measured for the variables were compared between patients that died and those who survived: Leukocytosis 12156±5977 vs 10337±3528 (p=0.004, 95% CI = 976-2663); Glucose 176±105 mg/dl vs 140±72 mg/dl (p<0.001, 95% CI = 19.40-52.60), respectively. As it can be observed, the two analyzed variables significantly correlated with the worst prognosis.
Table 2 demonstrates the results of the multivariate model. After the logistic regression analysis of 16 variables, through the stepwise model (with an entry of 0.10 and removal of 0.10), it was observed that only 5 variables significantly and independently correlated with the intrahospital mortality: glycemia, leukocytes, age, EF and SAP.
C. Late follow-up
C.1. General population
During the extra-hospital phase, there were 94 additional deaths. The probability of global survival was 77% (Kaplan-Mayer). Regarding the long-term follow-up, considering the totality of patients, significant correlations were observed between glycemia/leukocytes and mortality, as shown in Table 3. Table 4 shows that, in the multivariate model, six analyzed variables remained as prognostic factors for the long-term evolution in the global population. However, glycemia showed only a tendency to correlate with mortality.
C.2. Patients that survived the intrahospital phase
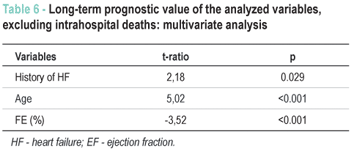
As shown in Table 5, when the intrahospital deaths were excluded, leukocytes and glycemia levels no longer correlated with the long-term evolution. In other words, the prognostic value of leukocytes and glycemia levels is restricted to the intrahospital phase, presenting no impact after hospital discharge. This behavior does not present alterations in the adjusted models, as shown in Table 6, where only the history of HF, age and EF correlate with mortality.
Discussion
In the last three decades, the treatment of AMI showed significant advancement, which resulted in the decrease of the morbimortality related to the disease9. That occurred mainly due to new pharmacological and mechanical primary reperfusion strategies, multiple anti-aggregation, in addition to the broad use of invasive stratification and the possibility of revascularization with angioplasty and stents10.
However, patients with UMIS have different characteristics, which determine their risk and prognosis variability. Some of these patients benefit from aggressive therapeutic measures, such as percutaneous invasive approach and strict glycemia control11,12.
In this sense, to identify patients at higher risk has been a constant concern in literature. In addition to the studies to validate risk scores and the variables that are traditionally associated with a poor prognosis, such as age and left ventricular dysfunction, investigators have recently worked on the identification of new prognostic variables, such as inflammatory markers, natriuretic peptide, leukocytosis and hyperglycemia, among others12-15.
Diabetic patients are recognized as being high-risk patients and having worse short- and long-term prognosis after AMI. The MONICA report showed that the mortality for diabetic and non-diabetic infarcted patients, after 28 days of evolution, was 12.6% and 7.3%, respectively16. Therefore, the inclusion of diabetes mellitus in the early risk stratification is justified.
However, the presence of hyperglycemia was also identified as a poor prognosis factor in non-diabetic infarcted patients17-19. It was recently demonstrated that hyperglycemia is still a poor prognosis factor also in patients submitted to percutaneous coronary intervention, regardless of the presence of diabetes20. It was also verified in this and in other studies21,22 that there is an association between hyperglycemia, no-reflow phenomena and ventricular remodeling, which are known factors of severity and worse prognosis in the evolution of patients with AMI23.
Similarly, another study demonstrated that hyperglycemia, but not diabetes, was a poor intrahospital prognostic factor24. Regarding the long-term follow-up, previous studies demonstrated that hyperglycemia, regardless of the presence of diabetes, was also a risk factor for mortality in patients with AMI25-27.
The data obtained in our study were similar to those found by other authors regarding the influence of glycemia on mortality. Glycemia levels at the hospital admission were significantly higher in patients that died during the hospital stay. During the follow-up of more than 6 years, hyperglycemia remained as a bad prognosis factor, although the adjusted model showed only a tendency to worse mortality. However, after the exclusion of the intrahospital deaths, the variables age, ejection fraction (EF) and history of HF remained significantly correlated with mortality, but not the levels of leukocytes or glycemia. This fact might be related to the hypotheses that hyperglycemia is not only a prognostic marker, but a direct cardiovascular system aggressor in the acute phase of AMI.
Experimental studies demonstrated that hyperglycemia is capable of causing platelet thrombosis, increasing the circulation of leukocyte adhesion molecules and decreasing the endothelium-dependent vasodilation, nitric oxide availability and the collateral coronary circulation28-31. The decrease in nitric oxide and prostacyclin levels, or even the increase in vasoconstrictors such as endothelin, are enhanced by hyperglycemia through protein kinase C activation, hexosamine increase and the activation of the pro-inflammatory nuclear factor Kappa B, with the consequent formation of superoxide radicals32. A study that induced diabetes in swine demonstrated an increase in IL-6, tumor necrosis factor (TNF), macrophage chemotactic proteins and adhesion molecules in fibroblasts of the coronary adventitia33.
Regarding the leukocytosis, some studies explored its prognostic value in AMI34. One of the most relevant ones included leukocytosis as part of a risk score for infarcted patients34. In this study, men with leukocyte levels > 9,000/microL presented a relative risk of death of 1.66 (1.35-2.05) during the 4-year follow-up. Recently, the leukocytosis in patients with AMI was directly associated with the infarction area size, presence of shock and death in six months34. In another study, patients with leukocyte levels > 10,000 microL during AMI presented a higher incidence of TIMI 0/1 flow, adverse cardiovascular events, both intrahospital and throughout five years, in comparison with patients with leukocyte levels below this range35. The increase in neutrophil levels was also associated not only to the late treatment and the presence of occluded artery, but also to the decreased reperfusion in patients with AMI36. When compared to other inflammatory markers, the increase in leukocyte levels was a predictor of adverse events similar to C-reactive protein and a better predictor than the serum amyloid A, fibrinogen and interleukin-6, throughout five years after the AMI37.
In our study, leukocytosis was associated to a worse intrahospital prognosis, even in adjusted models. However, as it occurred with hyperglycemia, during the long-term follow-up, when the intrahospital deaths are excluded, leukocytosis was no longer a long-term poor prognostic marker. The higher impact of this marker during the acute phase of AMI can be justified by the association of leukocytosis with increased acute inflammatory states, presence of more significant ischemia and difficulty in reperfusion, as mentioned before36,37.
Finally, it is worth mentioning that, despite the evidence that the adequate control of glycemia in the acute phase of AMI is beneficial11,38, the careful control of hyperglycemia is neglected in many institutions. On the other hand, attempts to control the inflammatory process have been reported with conflicting results in the literature39. Although a small study had initially pointed out a possible benefit with the use of anti-inflammatory agents in UMIS40, the excess of cardiovascular events associated to the use of non-hormonal anti-inflammatory agents in several randomized studies discouraged the use of this treatment41. The control of inflammation was also investigated considering the use of statins in UMIS. In the last years, the pleiotropic effects of these drugs have been extensively investigated, especially the anti-inflammatory action. In this sense, a recent study demonstrated the efficiency of the decrease in C-reactive protein and LDL-cholesterol in patients with UMIS, without using high doses of atorvastatin. Moreover, there was an additional cardiovascular benefit in the group of patients with higher inflammatory reduction42-44.
In conclusion, leukocyte and glycemia levels at the hospital admission of patients with AMI are excellent predictors of intrahospital mortality and poor predictors of long-term death. A previous history of HF was an independent predictor of mortality only at long-term. On the other hand, age and EF are short- and long-term independent predictors of mortality.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This study is not associated with any post-graduation program.
References
- 1. Braunwald E, Antman EM, Beasley JW, Califf RW, Cheitlin MD, Hochman JS, et al. American College of Cardiology/American Heart Association task force on practice guidelines. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction-summary article: a report of the J Am Coll Cardiol. 2002; 40: 1366-74.
- 2. Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, et al. The TIMI risk score for unstable angina/non ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000; 284: 835-42.
- 3. Morrow DA, Antman EM, Charlesworth A, Cairns R, Murphy SA, de Lemos JA, et al. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. 2000; 102: 2031-7.
- 4. Bellodi G, Manicardi V, Malavasi V, Veneri L, Bernini G, Bossini P, et al. Hyperglycemia and prognosis of acute myocardial infarction in patients without diabetes mellitus. Am J Cardiol. 1989; 64: 885-8.
- 5. O'Sullivan JJ, Conroy RM, Robinson K, Hickey N, Mulcahy R. In-hospital prognosis of patients with fasting hyperglycemia after first myocardial infarction. Diabetes Care. 1991; 14: 758-60.
- 6. Afiune Neto A, Mansur AP, Avakian SD, Gomes EP, Ramires JA. Monocytosis is an independent risk marker for coronary artery disease. Arq Bras Cardiol. 2006; 86: 240-4.
- 7. Goyal A, Mahaffey KW, Garg J, Nicolau JC, Hochman JS, Weaver WD, et al. Prognostic significance of the change in glucose level in the first 24 h after acute myocardial infarction: results from the CARDINAL study. Eur Heart J. 2006; 27: 1289-97.
- 8. Bendel RB, Afifi AA. Comparison of stopping rules in forward "stepwise" regression. J Am Stat Assoc. 1977; 72: 46-53.
- 9. Hasdai D, Behar S, Wallentin L, Danchin N, Gitt AK, Boersma E, et al. A prospective survey of the characteristics, treatments and outcomes of patients with acute coronary syndromes in Europe and the Mediterranean basin. The Euro Heart Survey of Acute Coronary Syndromes (Euro Heart Survey ACS). Eur Heart J. 2002; 15: 1190-201.
- 10. Van de Werf F, Ardissino D, Betriu A, Cokkinos DV, Falk E, Fox KA, et al. Management of acute myocardial infarction in patients presenting with ST-segment elevation. ESC task-force report. Eur Heart J. 2003; 24: 28-66.
- 11. Cannon CP, Weintraub WS, Demopoulos LA, Vicari R, Frey MJ, Lakkis N, et al. TACTICS (Treat Angina with Agrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy)-Thrombolysis in myocardial infarction 18 investigators. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001; 344: 1879-87.
- 12. Malmberg K, Ryden L, Efendic S, Herlitz J, Nicol P, Waldenstrom A, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol. 1995; 26: 57-65.
- 13. Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000; 101: 2149-53.
- 14. Lindmark E, Diderholm E, Wallentin L, Siegbahn A. Relationship between IL-6 and mortality in patients with unstable coronary artery desease: effects of an early invasive or noninvasive strategy. JAMA. 2001; 286: 2107-13.
- 15. Biasucci LM, Liuzzo G, Fantuzzi G, Caligiuri G, Rebuzzi AG, Ginnetti F, et al. Increasing levels of interleucin-1 and IL-6 during the first two days of hospitalization in unstable angina are associated with increased risk of in-hospital coronary events. Circulation. 1999; 99: 2079-84.
- 16. Richards AM, Nicholls MG, Yandle TG, Frampton C, Espiner EA, Turner JG, et al. Plasma n-terminal pro-brain natriuretic peptide and adrenomedullin: new neurohormonal predictors of left ventricular function and prognosis after myocardial infarction. Am Heart J. 1998; 135: 21-8.
- 17. Lowel H, Koenig W, Engel S, Hormann A, Keil U. The impact of diabetes mellitus on survival after myocardial infarction: can it be modified by drug treatment? Results of a population-based myocardial infarction register follow-up study. Diabetalogia. 2000; 43: 218-26.
- 18. Norhammar AM, Ryden L, Malmberg K. Admission plasma glucose: independent risk factor for long-term prognosis after myocardial infarction even in non-diabetic patients. Diabetes Care. 1999; 22: 1827-31.
- 19. Oswald GA, Smith CC, Betteridge DJ, Yudkin JS. Determinants and importance of stress hyperglycemia in non-diabetic patients with myocardial infarction. Br Med J. 1986; 293: 917-22.
- 20. Bolk J, van der Ploeg TJ, Cornel JH, Arnold AE, Sepers J, Umans VA, et al. Impaired glucose metabolism predicts mortality after a myocardial infarction. Intern J Card. 2001; 79: 207-14.
- 21. Ishihara M, Kojima S, Sakamoto T, Asada Y, Tei C, Ogawa H. Japanese acute coronary syndrome study investigators. Acute hyperglycemia is associated with adverse outcome after acute myocardial infarction in the coronary intervention era. Am Heart J. 2005; 150: 814-20.
- 22. Iwakura K, Ito H, Ikushima M, Kawano S, Okamura A, Asano K, et al. Association between hyperglycemia and the no-reflow phenomenon in patients with acute myocardial infarction. J Am Coll Cardiol. 2003; 41: 1-7.
- 23. Nicolau JC, Maia LN, Vitola JV, Mahaffey KW, Machado MN, Ramires JAF. Baseline glucose and left ventricular remodeling after acute myocardial infarction. J Diabetes Complications. 2007; 21 (5): 294-9.
- 24. Caldas MA, Tsutsui JM, Kowatsch I, Andrade JL, Nicolau JC, Ramires JF, et al. Value of myocardial contrast echocardiography for predicting left ventricular remodeling and segmental functional recovery after anterior wall acute myocardial infarction. J Am Soc Echocardiogr. 2004; 17: 923-32.
- 25. Kosuge M, Kimura K, Kojima S, Sakamoto T, Matsui K, Ogawa H. Japanese Acute Coronary Syndrome Study (JACSS) Investigators. Effects of glucose abnormalities on in-hospital outcome after coronary intervention for acute myocardial infarction. Circulation. 2005; 69: 375-9.
- 26. Macin SM, Perna ER, Augier N, Cialzeta J, Farias EF, Fontana M, et al. Clinical characteristics and long-term outcome in patients with heart failure complicating acute myocardial infarction. Rev Esp Cardiol. 2005; 58: 789-96.
- 27. Timmer JR, van der Horst IC, Ottervanger JP, Henriques JP, Hoorntje JC, de Boer MJ, et al. Myocardial Infarction Study Group. Prognostic value of admission glucose in non-diabetic patients with myocardial infarction. Am Heart J. 2004; 148: 399-404.
- 28. Bolk J, van der Ploeg T, Cornel JH, Arnold AE, Sepers J, Umans VA. Impaired glucose metabolism predicts mortality after a myocardial infarction. Int J Cardiol. 2001; 79: 207-14.
- 29. Title LM, Cummings PM, Giddens K, Nassar BA. Oral glucose loading acutely attenuates endothelium-dependent vasodilation in healthy adults without diabetes: an effect prevented by vitamins C and E. J Am Coll Cardiol. 2000; 36: 2185-91.
- 30. Scechter M, Merz NB, Paul-Labrador MJ, Kaul S. Blood glucose and platelet-dependent thrombosis in patients with coronary artery disease. J Am Coll Cardiol. 2000; 35: 300-7.
- 31. Marfella R, Esposito K, Giunta R, Coppola G, De Angelis L, Farzati B, et al. Circulating adhesion molecules in humans: role of hyperglycemia and hyperinsulinemia. Circulation. 2000; 101: 2247-51.
- 32. Kersten JR, Toller WG, Tessmer JP, Pagel PS, Warltier DC. Hyperglycemia reduces coronary blood flow through a nitric oxidemediated mechanism. Am J Physiol Heart Circ Physiol. 2001; 281: H2097-104.
- 33. Moreno PR, Fuster V. New aspects in the pathogenesis of diabetic atherothrombosis. J Am Coll Cardiol. 2004; 44: 2293-30.
- 34. Zhang L, Zalewski A, Liu Y, Mazurek T, Cowan S, Martin JL. Diabetes-induced oxidative stress and low-grade inflammation in porcine coronary arteries. Circulation. 2003; 108: 472-8.
- 35. Patel MR, Mahaffey KW, Armstrong PW, Weaver WD, Tasissa G, Nicolau JC, et al. Prognostic usefulness of white blood cell count and temperature in acute myocardial infarction (from the CARDINAL Trial). Am J Cardiol. 2005; 95: 614-8.
- 36. Marchioli R, Avanzini F, Barzi F, Chieffo C, Di Castelnuovo A, Valagussa F. GISSI-Prevenzione Investigators. Assessment of absolute risk of death after myocardial infarction by use of multiple-risk-factor assessment equations: GISSI-Prevenzione mortality risk chart. Eur Heart J. 2001; 22: 2085-103.
- 37. Comparan-Nunez A, Palacios JM, Jerjes-Sanchez CD. Leucocytosis associated with higher incidence of adverse cardiovascular events in myocardial infarcts. Arch Cardiol Mex. 2005; 75 (Suppl 3): S3-61-8.
- 38. Kirtane AJ, Bui A, Murphy SA, Barron HV, Gibson CM. Association of peripheral neutrophilia with adverse angiographic outcomes in ST-elevation myocardial infarction. Am J Cardiol. 2004; 93: 532-6.
- 39. Zairis MN, Adamopoulou EN, Manousakis SJ, Lyras AG, Bibis GP, Ampartzidou OS, et al. The impact of hs C-reactive protein and other inflammatory biomarkers on long-term cardiovascular mortality in patients with acute coronary syndromes. Atherosclerosis. 2007; 194 (2): 397-402.
- 40. Diaz R, Paolasso EA, Piegas LS, Tajer CD, Moreno MG, Corvalan R, et al. On behalf of the ECLA Collaborative Group. Metabolic modulation of acute myocardial infarction: the ECLA glucoseinsulinpotassium pilot trial. Circulation. 1998; 98: 2227-34.
- 41. Altman R, Scazziota A. Papel de los antiinflamatorios en el tratamiento de los síndromes coronarios agudos: de la ateroinflamación a la aterotrombosis. Rev Esp Cardiol. 2003; 56: 9-15.
- 42. Altman R, Luciardi HL, Muntaner J, Del Río F, Berman SG, López R, et al. Efficacy assessment of meloxicam, a preferential cyclooxygenase-2 inhibitor, in acute coronary syndromes without ST-segment elevation: the Nonsteroidal Anti-Inflammatory Drugs in Unstable Angina Treatment-2 (NUT-2) pilot study. Circulation. 2002; 106: 191-5.
- 43. Mc Gettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA. 2006; 296: 1633-44.
- 44. Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, et al. Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) Investigators. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005; 352: 20-8.
Influence of leukocytes and glycemia on the prognosis of patients with acute myocardial infarction
Publication Dates
-
Publication in this collection
06 Apr 2009 -
Date of issue
Feb 2009
History
-
Received
23 Oct 2007 -
Reviewed
14 Apr 2008 -
Accepted
09 June 2008