Abstracts
BACKGROUND: Physical exercise induces hemodynamic stress. OBJECTIVE: To evaluate if voluntary running and forced running induced different levels of stress protein (Hsp72) in the myocardium of female Wistar rats. METHODS: Female rats were randomly assigned to the following groups: forced treadmill running group (FR; n= 6), voluntary running group (VR; n=6) and control group (C; n=6). VR group animals had free access to running wheels, and those from FR group underwent a running program on a treadmill (18 m/min, 60 min/day, 5 days/wk) for 8 weeks. Left ventricle (LV) and right ventricle (RV) fragments were collected at sacrifice, and the relative immunoblot contents of stress protein (Hsp72) were determined. RESULTS: VR animals ran on average 4.87 km/wk, and FR rats ran 4.88 km/wk. Animals from VR and FR groups had less body weight gain (p<0.05) than those from C group (81.67 ± 11.95g vs 81.17 ± 10.18g vs 111.50 ± 2.26g, respectively). Heart weight/body weight ratio was not significantly different (p>0.05) among VR, FR and C groups (4.54 ± 0.79 mg/g vs 4.94 ± 0.89 mg/g vs 4.34 ± 0.87 mg/g, respectively). FR group animals had levels of Hsp72 (p<0.05) higher than those from VR, both in LV (287.45 ± 35.86 % vs 135.59 ± 5.10 %, respectively) and RV (241.31 ± 25.83 % vs 137.91 ± 45.20 %, respectively). CONCLUSION: Voluntary running and forced running induced different levels of Hsp72 in the myocardium of female Wistar rats.
Physical activity; running; rats; stress; myocardium
FUNDAMENTO: O exercício físico promove estresse hemodinâmico. OBJETIVO: Testar se programas de treinamento com corridas voluntária e forçada induzem níveis distintos de expressão de Hsp72 no miocárdio de ratas Wistar. MÉTODOS: Ratas Wistar foram alocadas em três grupos (n = 6, cada): treinadas com corrida voluntária (TCV), treinadas com corrida forçada (TCF) e grupo controle (C). Os animais do TCV tiveram livre acesso à roda de corrida voluntária, enquanto os do TCF foram submetidos à corrida forçada em esteira (18 m/min, 0% inclinação, 60 m/min, 5 dias/sem) durante oito semanas. Fragmentos dos ventrículos esquerdo (VE) e direito (VD) foram coletados para análise dos níveis de Hsp72. RESULTADOS: As ratas do grupo TCV correram, em média, 4,87 km, e as do TCF, 4,88 km por semana. Os animais dos grupos TCV e TCF ganharam menos peso (p < 0,05) que os do grupo C (81,67 ± 11,95 g vs 81,17 ± 10,18 g vs 111,50 ± 2,26 g, respectivamente). O peso relativo do coração não foi diferente (p > 0,05) entre os grupos TCV, TCF e C (4,54 ± 0,79 mg/g vs 4,94 ± 0,89 mg/g vs 4,34 ± 0,87 mg/g, respectivamente). Ratas treinadas com corrida forçada apresentaram níveis de Hsp72 maiores (p < 0,05) que as que correram voluntariamente, no VE (287,45 ± 35,86% vs 135,59 ± 5,10%, respectivamente) e no VD (241,31 ± 25,83% vs 137,91 ± 45,20%, respectivamente). CONCLUSÃO: Os programas de treinamento com corrida voluntária e forçada induziram níveis distintos de expressão de Hsp72 no miocárdio de ratas Wistar.
Atividade física; corrida; ratos; estresse; miocárdio
FUNDAMENTO: El ejercicio físico promueve estrés hemodinámico. OBJETIVO: Probar si programas de entrenamiento con carreras voluntaria y forzada inducen niveles distintos de expresión de Hsp72 en el miocardio de ratas hembra Wistar. MÉTODOS: Ratas hembra Wistar fueron distribuidas en tres grupos (n = 6, cada uno): entrenadas con carrera voluntaria (ECV), entrenadas con carrera forzada (ECF) y grupo control (C). Los animales del ECV tuvieron libre acceso a la rueda de carrera voluntaria, mientras que los del ECF fueron sometidos a carrera forzada en cinta sin fin (18 m/min, 0% inclinación, 60 m/min, 5 días/sem) durante ocho semanas. Fragmentos de los ventrículos izquierdo (VI) y derecho (VD) se recolectaron para análisis de los niveles de Hsp72. RESULTADOS: Las ratas del grupo ECV corrieron, en promedio 4,87 km, y las del ECF, 4,88 km por semana. Los animales de los grupos ECV y ECF ganaron menos peso (p<0,05) que los del grupo C (81,67 ± 11,95 g vs. 81,17 ± 10,18 g vs. 111,50 ± 2,26 g, respectivamente). El peso relativo del corazón no fue diferente (p>0,05) entre los grupos ECV, ECF y C (4,54 ± 0,79 mg/g vs. 4,94 ± 0,89 mg/g vs. 4,34 ± 0,87 mg/g, respectivamente). Las ratas entrenadas con carrera presentaron niveles de Hsp72 mayores (p<0,05) que las que corrieron voluntariamente, en el VI (287,45 ± 35,86% vs. 135,59 ± 5,10%, respectivamente) y en el VD (241,31 ± 25,83% vs. 137,91 ± 45,20%, respectivamente). CONCLUSIÓN: Los programas de entrenamiento con carreras voluntaria y forzada inducen niveles distintos de expresión de Hsp72 en el miocardio de ratas Wistar.
Actividad física; carrera; ratones; estrés; miocardio
ORIGINAL ARTICLE
Different levels of Hsp72 in female rat myocardium in response to voluntary exercise and forced exercise
Stéphano Freitas Soares Melo; Wellington Lunz; Elizabeth Pacheco Batista Fontes; Cristina Maria Ganns Chaves Dias; Miguel Araujo Carneiro Júnior; Anselmo Gomes de Moura; Ricardo Junqueira Del Carlo; Antonio Jose Natali
Universidade Federal de Viçosa (UFV), Viçosa, MG - Brazil
Mailing address
SUMMARY
BACKGROUND: Physical exercise induces hemodynamic stress.
OBJECTIVE: To evaluate if voluntary running and forced running induced different levels of stress protein (Hsp72) in the myocardium of female Wistar rats.
METHODS: Female rats were randomly assigned to the following groups: forced treadmill running group (FR; n= 6), voluntary running group (VR; n=6) and control group (C; n=6). VR group animals had free access to running wheels, and those from FR group underwent a running program on a treadmill (18 m/min, 60 min/day, 5 days/wk) for 8 weeks. Left ventricle (LV) and right ventricle (RV) fragments were collected at sacrifice, and the relative immunoblot contents of stress protein (Hsp72) were determined.
RESULTS: VR animals ran on average 4.87 km/wk, and FR rats ran 4.88 km/wk. Animals from VR and FR groups had less body weight gain (p<0.05) than those from C group (81.67 ± 11.95g vs 81.17 ± 10.18g vs 111.50 ± 2.26g, respectively). Heart weight/body weight ratio was not significantly different (p>0.05) among VR, FR and C groups (4.54 ± 0.79 mg/g vs 4.94 ± 0.89 mg/g vs 4.34 ± 0.87 mg/g, respectively). FR group animals had levels of Hsp72 (p<0.05) higher than those from VR, both in LV (287.45 ± 35.86 % vs 135.59 ± 5.10 %, respectively) and RV (241.31 ± 25.83 % vs 137.91 ± 45.20 %, respectively).
CONCLUSION: Voluntary running and forced running induced different levels of Hsp72 in the myocardium of female Wistar rats.
Key-words: Physical activity; running; rats; stress; myocardium.
Introduction
Exposure of cells to various stressful situations induces the expression of heat shock proteins (HSP), also known as stress proteins. These proteins confers cellular tolerance to multiple stress agents, provide protection against protein denaturation and help remove damaged proteins1,2.
Both acute and chronic physical exercise are stress agents that may induce the Hsp72 expression, which is an inducible form of the HSP family with molecular weight of 70kDa (Hsp70), in various tissues, including skeletal muscle and cardiac muscle3-10. There are evidences that exercise-induced Hsp72 expression in the myocardium is associated with cardiac protection from cardiovascular stress events7,11-14, despite some opposite results15. However, exercise-induced Hsp72 expression appears to be gender specific16-18 and intensity dependent6.
Since forced exercise for animal models cause changes in the neuroendocrine and immunological responses commonly associated with stress19-21, forced exercise-induced Hsp72 expression may be affected by stress factors that are unrelated to exercise. The voluntary running model, in which the animals have free access to the exercise equipment from their cages and run voluntarily22, on the contrary, induces lower levels of stress in the animals, when compared to forced running and swimming4,23. Therefore, the objective of this study was to evaluate if voluntary and forced running training programs induce different levels of Hsp72 expression in the myocardium of female Wistar rats.
Methods
Experimental animals and animal handling
We used a total of 18 young female Wistar rats (7 weeks of age). Three groups were formed at different times, namely: voluntary running trained group (VR, n = 6) forced running trained group (FR, n = 6) and control group (C, n = 6). All animals were handled identically, but those of group C did not exercise. The animals were housed in individual stainless steel cages (25 x 20 x 18 cm) and received water and food ad libitum. The rats were kept in a controlled environment of a fixed 12:12-h light-dark cycle, with the room temperature maintained at approximately 22º C during the entire period of the experiment. The animals were provided by the Central Biotery of the Center for Biological Sciences and Health, Federal University of Viçosa, and we followed the rules established by the 'Guide for the Care and Use of Laboratory Animals' (Institute of Laboratory Animal Resources, National Academy of Sciences, Washington, DC 1996) and the Ethical Principles in Animal Experimentation of the Brazilian College of Animal Experimentation (COBEA).
Voluntary running training program
The first group to be exercised was the voluntary running group, in which the rats had free access to rotating wheels attached to their stainless steel cages (25 x 20 x 18 cm), for 8 weeks. The rotating wheel had an inner circumference of 1.53 m, and the number of revolutions was recorded by a mechanical revolution counter, and the distances traveled by the animals were calculated by the number of revolutions. It is noteworthy that rodents are nocturnal animals, and, therefore, they normally exercise during the dark phase of the light-dark cycle.
Forced running training program
The training protocol for the other group of rats (FR group) was established after the total distance and the weekly distances traveled by VR rats were recorded, so as to ensure that the distances traveled by both groups were similar. Thus, FR rats underwent a forced running program for eight weeks (adapted from Samelman)5. The animals initiated the running program on a motor treadmill (Insight Instrumentos Científicos, Ribeirão Preto, SP, Brazil) at an intensity of 15 m/min, 0% grade, for 15 minutes on the first day, five days a week. This period was increased by five minutes a day, so that in the last day of the second week, the animals ran for 60 minutes. So, by the first day of the third week, their speed had risen to 17 m/min. From the fourth to the eighth week, the exercise sessions lasted 60 minutes a day, at 18 m/min, 0% grade, five days a week. This running program was applied during the dark phase of the light-dark cycle, so as not to differ from the VR group. The animals were motivated to run by gentle tapping on their backs. It is noteworthy that these animals were housed in individual stainless steel cages (25 x 20 x 18 cm), in the same way as the VR and C groups. Thus, the voluntary movement of the animals in the cages, aside from the training period, was not considered sufficient enough to cause adaptations that could be confused with those caused by voluntary and forced running. Therefore, in none of the three groups was it necessary to take account of the movement of the animals in their cages, because they were considered similar for all animals.
Specimen collection
To avoid the acute response to exercise and preserve the chronic response, all animals were euthanized 48 hours after the last exercise session. Fragments of the left and right ventricles were collected, wrapped in aluminum foil, identified and immediately frozen in liquid nitrogen, and stored at minus 80ºC for later analysis.
Protein extraction
Specimen fragments were taken from the freezer and, after being weighed, they were macerated to obtain a tissue extract to which was added the buffer (composition: 15 mM Tris HCL, 600 mM NaCl, 1 mM PMSF, pH 7.5). 1200 µl of extraction buffer were added to 60 mg of extract (1:20 ratio). The samples were then placed in eppendorfs, which were identified and kept on ice for approximately 5 minutes. After this incubation period, the cell debris were discarded by centrifugation at 14,000 g, at 4ºC, for 20 minutes, and the supernatant was stored at minus 20ºC. Total protein extracts were quantified according to the Bradford method24, using the 3 recommended repetitions.
SDS-PAGE Electrophoresis
Electrophoreses in polyacrylamide (PAGE) gel containing the detergent sodium dodecyl sulfate (SDS) have been conducted essentially as described by Laemmli25. The protein extract was incubated for 5 minutes at 100ºC in sample buffer [10% glycerol (v/v), 2.3% SDS, 0.25% bromophenol blue, 5% 2-mercaptoethanol (v/v) and 0.0625 mol/L Tris-HCl, pH 6.8] before it was loaded onto the gel. The electrophoresis was conducted for approximately 16 hours, at 48 V, in running buffer [0.025 mol/L Tris-HCl, 0.2 mol/L glycine, 1 mmol/L EDTA, and 3.5 mmol/L SDS]. This procedure was conducted to obtain 3 gels. One of these gels was stained with a staining solution [40% methanol (v/v), 7.5% CH3COOH (v/v) and 0.01% coomassie brilliant blue R-250], for approximately 12 hours, and decolorized with a decolorizing solution [10% methanol (v/v) and 7.5% acetic acid (v/v)]. The immunoblotting technique was applied to the other 2 gels to determine if the monoclonal anti-Heat Shock Protein 70 antibody against animal Hsp72 was able to recognize the protein of interest.
Imunoblotting
The proteins were transferred to a nitrocellulose membrane, using the BioRad (USA) transfer system, according to the manufacturer's instructions. After the transfer (for approximately 1 hour, at a constant amperage of 0.70A), the nitrocellulose membrane was immersed in a blocking solution (caseinnon-fat dry milk, BioRad) for one hour. Then, four washes were performed for 15 minutes each, at room temperature with TBS-T [0.01 mol/L Tris-HCl, 1.5 mmol/L NaCl, 0.1% Tween-20 (v/v), pH 7.6]. The membrane was incubated with the monoclonal anti-Heat Shock Protein 70 (SIGMA) antibody at a 1:5,000 dilution for three hours under stirring. After the incubation period, the membrane was washed four times with TBS-T, for 15 minutes each, and then incubated with Anti-Mouse IgG antibody (alkaline phosphatase - SIGMA) at a 1:10,000 dilution, for approximately two hours. The membrane was extensively washed with TBS-T, four times again for 15 minutes each, and subsequently incubated with the enzyme buffer (0.1 mol/L Tris-HCl, pH 9.8, 0.1 mol/L NaCl, 0.5 mol/L MgCl2) for ten minutes. The alkaline phosphatase activity was detected, using the NBT (nitro-blue tetrazolium, GIBCO/BRL) and BCIP (5-bromo-4-chloro-3-indolyl phosphate, GIBCO/BRL) substrates. The immunoblot bands were quantified by computerized densitometry using a Molecular Dynamics Personal Densitometer equipped with the program Image Quant, version 5.2 (Molecular Dynamics - USA).
Statistical analysis
Mean body weights, mean weight gains and mean relative heart weights were compared using one-way ANOVA followed by post hoc Tukey's test. Hsp72 level comparisons were performed using the Mann-Whitney test because the data did not follow the normal distribution (Kolmogorov-Smirnov, p <0.05). The tests were performed using SigmaStat (version 3.0 - Richmond - Ca) up to a 5% level of significance.
This study was funded by the Foundation for the Support of Research of the State of Minas Gerais (FAPEMIG - CDS 947/2002).
Results
VR group animals voluntarily traveled different distances, according to their individual capacities, whereas in the FR group all animals were forced to travel the same distance. Thus, the distances traveled by the animals in the VR group are presented as mean values (mean ± SEM), and the distances traveled by the animals in the FR group as absolute values. VR group animals traveled 4.87 ± 0.55 km/week and a total of 38.96 ± 3.55 km, whereas FR group animals, in the same period, traveled 4.88 km/week and a total of 39.03 km, so there were no differences between the distances traveled in both models.
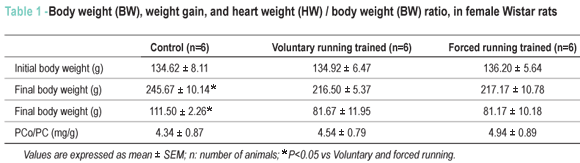
After eight weeks of training, VR group and FR group animals had significantly lower weight gain than those in the control group (Table 1). Relative heart weight, an index of cardiac hypertrophy, had no statistically significant difference among the groups (Table 1).
Figure 1 shows Hsp72 and Hsp73 accumulation in the LV of two Wistar rats of each group. The expression of Hsp73, a constitutive Hsp70 family member, was similar in the three groups (p> 0.05) and was not affected by the exercise programs used in this study. However, there was an induction of Hsp72 expression in response to forced and voluntary exercise programs, but at different levels. In LVs and RVs of control group animals, we observed an expression of small amounts of Hsp72, presented as arbitrary units (282.19 ± 20.02 vs 287 ± 32.05, respectively), probably due to some environmental stress. These values were used as a baseline for comparison of the effects of the two exercise protocols, in percentage. The rats who were forced to run on a treadmill showed higher levels of Hsp72 (p <0.05) than those who ran voluntarily, in the LV (287.45 ± 35.86% vs 135.59 ± 5.10%, respectively) and in the RV (241.31 ± 25.83% vs 137.91 ± 45.20%, respectively). However, there was no significant difference in the Hsp72 expression between the LV and the RV (Figure 1B).
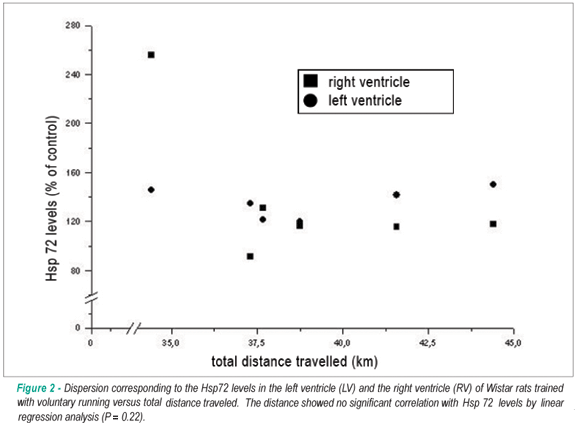
It was observed that the rats had an interindividual variation in total distance traveled of approximately 3.5 kilometers, but there was no significant correlation (p = 0.22) between the total distance traveled and the induction of Hsp72 expression (Figure 2).
Discussion
We investigated if voluntary and forced running training programs induced different levels of Hsp72 expression in the myocardium of female Wistar rats. The data showed that the exercise protocols used in this study induced different levels of Hsp72 expression in the myocardium of female rats, both in LV and RV, and this expression was higher in the forced treadmill running program than in the voluntary running program.
VR group and FR group animals traveled similar distances. However, it should be noted that the implementation of the running programs was different in each model. In the treadmill forced running program, the animals ran on a continuous basis because the speed of the treadmill remained constant during the whole training session. In the voluntary running program, the animals exercised intermittently, by running short periods (60-90s) for about 5 minutes, interspersed with periods of rest26. Therefore, only in the forced running program it was possible to calculate the work done by the rats, which is a function of animal body mass, running time and treadmill speed and incline27. Although body mass, distances traveled and treadmill incline were similar in both models, speed and running time were different. We considered this lack of precision in the equivalence between the work done by VR and FR groups as a limitation of this study. However, the exercise programs reduced weight gain in these animals when compared to that of sedentary control animals. This indicates that exercise programs affect rat bodily functions because weight reduction may reflect an increase in metabolic rates or in energy expenditure28. Physiologically it is known that similar body weight does not necessarily mean equivalent body composition and energy metabolism. However, previous studies showed that aerobic exercise training at different intensities resulted in different changes in body weight of rats29 ;it also showed that different types of exercise caused similar weight gain reductions in rats, in comparison to sedentary rats (running on a treadmill versus swimming 30; running on a treadmill versus voluntary running)4,31. Therefore, it is reasonable to speculate that our voluntary and forced running protocols resulted in similar metabolic rates, which enables us to make comparisons between the two groups.
Relative heart weight did not differ in the three experimental groups. This suggests that the exercise protocols used in this study were not sufficient enough to promote cardiac hypertrophy. However, adaptations of cardiac muscle to chronic exercise, including the induction of Hsp72 expression were observed in animals with and without cardiac hypertrophy12,31.
The main finding of this study was that the exercise protocols used induced Hsp72 expression in the myocardium of female rats, differently in LV and RV, and significantly higher in forced running than in voluntary running. These increased levels of Hsp72 reflect an increase in the level of intracellular stress generated by the forced running program, when compared to the voluntary running program. Although our data did not specify the mechanisms responsible for the observed increase in Hsp72 expression, several physiological and metabolic events occur at cellular level (e.g. increased temperature, hypoxia, oxidative stress, increased concentration of calcium and pH reduction) during exercise, and they can induce Hsp72 expression10,32.
Some studies have credited the high levels of Hsp72 expression in response to forced running to the intensity of the exercise31. Indeed, Milne and Noble6 showed that the induction of Hsp72 expression in cardiac and skeletal muscles is dependent on the intensity of the exercise, and speeds above 24 m/minute are the most efficient for inducing Hsp72 expression. However, since in this study the speed during forced treadmill running was 18 m/min, the distances traveled in both protocols were similar, and the animals could run voluntarily at speeds exceeding 40 m/min33 the increased Hsp72 expression observed in FR group animals appears to have been caused by other factors besides the exercise load. One of these factors could be the level of stress provided by the animal model of exercise. There is evidence that the level of stress suffered by animals in voluntary running is lower in comparison to that of running on a treadmill4,23. In the present study, we did not use electric shock to force the animals to run on a treadmill, which increases Hsp72 expression34, but the animals were stimulated with taps on the back.Furthermore, chronic forced exercise can cause mental stress to the animals35 and other negative changes that are indicative of chronic stress (e.g. reduction of serum corticosteroid-binding globulin, adrenal hypertrophy, thymus involution, suppression of lymphocyte proliferation, and antigen-specific IgM)20. In voluntary running, on the other hand, the animals were not forced to run. There are reports that voluntary running training reduced the levels of oxidative damage to the DNA4 and reduced the behavioral depression induced by stress in rats36.
Furthermore, we observed that the rats showed an interindividual variation in total distance traveled of approximately 3.5 km. However, the distance traveled showed no significant correlation with the induction of Hsp72 expression (Figure 2). This may be an indication that in the voluntary running model, the intensity of exercise exposes the animals to less stress. In fact, it has been reported that voluntary running may counteract the suppression of immune function induced by stress and the negative impacts of stress on behavior and immune function of rats36,37. Recently, Hill et al38 demonstrated that chronic voluntary running increased the capacity for GABA synthesis in the brain of rats, which may result in changes in sensitivity to stress during exercise.
We noticed that there was no significant regional distinction (LV vs RD) in the induction of Hsp72 expression. This observation confirms the results reported in previous studies5,31. Despite the changes occurred in the heart muscle in response to exercise are more pronounced in the left ventricle, which reflects the distinct intracellular stress imposed by the mechanical load under which the fibers work during the exercise, all myocardial fibers are recruited at each heartbeat, and the heart's workload grows linearly as a function of increased contractility and heart rate in both ventricles.
Our results provide evidence that female rats also respond to the stress caused by exercise, by increasing the induction of Hsp72 expression in the myocardium. It has been observed that the induction of Hsp72 expression in response to exercise is greater in male rats, suggesting that the presence of estrogen in female rats is sufficient to inhibit Hsp72 expression16-18. However, the myocardium of female Sprague-Dawley rats exhibited two times more Hsp72 than those of male rats39, and the heart muscle of rats showed no sexual dimorphism in the Hsp72 expression in response to electric shock35.
Finally, even though we did not evaluate the effects of exercise protocols on cardiac protection, the increased Hsp72 expression observed here may have physiologically significant effects on myocardial protection against stress events (e.g. ischemia/reperfusion)16,18. Although some studies have reported that the Hsp70 response to acute and chronic exercise (running on a treadmill), mediated by gender-specific hormones, resulted in cardiac protection, preferably in male rats, Chicco et al40 demonstrated that female rats that voluntarily ran for eight weeks showed elevated levels of Hsp72, which were associated with an improvement in the cardiac dysfunction, induced by doxorubicin.
Conclusion
We concluded that the training programs with the voluntary and forced running programs used in this study induced different levels of Hsp72 expression in the myocardium of female Wistar rats.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
This study was funded by FAPEMIG.
Study Association
This study is not associated with any post-graduation program.
References
- 1. Li GC, Li LG, Liu YK, Mak JY, Chen LL, Lee WM. Thermal response of rat fibroblasts stably transfected with the human 70-kDa heat shock protein-encoding gene. Proc Natl Acad Sci USA. 1991; 88 (5): 1681-5.
- 2. Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992; 72 (4): 1063-81.
- 3. Locke M, Noble EG, Atkinson BG. Exercising mammals synthesize stress proteins. Am J Physiol. 1990; 258 (4 Pt 1): C723-9.
- 4. Asami S, Hirano T, Yamaguchi R, Tsurudome Y, Itoh H, Kasai H. Effects of forced and spontaneous exercise on 8-hydroxydeoxyguanosine levels in rat organs. Biochem Biophys Res Commun. 1998; 243 (3): 678-82.
- 5. Samelman TR. Heat shock protein expression is increased in cardiac and skeletal muscles of Fischer 344 rats after endurance training. Exp Physiol. 2000; 85 (1): 92-102.
- 6. Milne KJ, Noble EG. Exercise-induced elevation of HSP70 is intensity dependent. J Appl Physiol. 2002; 93 (2): 561-8.
- 7. Campisi J, Leem TH, Greenwood BN, Hansen MK, Moraska A, Higgins K, et al. Habitual physical activity facilitates stress-induced HSP72 induction in brain, peripheral, and immune tissues. Am J Physiol Regul Integr Comp Physiol. 2003; 284 (2): R520-30.
- 8. Atalay M, Oksala NK, Laaksonen DE, Khanna S, Nakao C, Lappalainen J, et al. Exercise training modulates heat shock protein response in diabetic rats. J Appl Physiol. 2004; 97 (2): 605-11.
- 9. Lunz W, Oliveira EC, Neves MT, Fontes EP, Dias CM, Natali AJ. Anabolic steroid- and exercise-induced cardiac stress protein (HSP72) in the rat. Braz J Med Biol Res. 2006; 39 (7): 889-93.
- 10. Whitham M, Fortes MB. Heat shock protein 72: release and biological significance during exercise. Front Biosci. 2008; 13: 1328-39.
- 11. Locke M, Tanguay RM, Klabunde RE, Ianuzzo CD. Enhanced postischemic myocardial recovery following exercise induction of HSP 72. Am J Physiol. 1995; 269 (1 Pt 2): H320-5.
- 12. Powers SK, Demirel HA, Vincent HK, Coombes JS, Naito H, Hamilton KL, et al. Exercise training improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. Am J Physiol. 1998; 275 (5 Pt 2): R1468-77.
- 13. Demirel HA, Powers SK, Zergeroglu MA, Shanely RA, Hamilton K, Coombes J, et al. Short-term exercise improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. J Appl Physiol. 2001; 91 (5): 2205-12.
- 14. Melling CW, Thorp DB, Milne KJ, Krause MP, Noble EG. Exercise-mediated regulation of Hsp70 expression following aerobic exercise training. Am J Physiol Heart Circ Physiol. 2007; 293(6):H3692-8.
- 15. Taylor RP, Harris MB, Starnes JW. Acute exercise can improve cardioprotection without increasing heat shock protein content. Am J Physiol. 1999; 276(3 Pt 2):H1098-102.
- 16. Paroo Z, Haist JV, Karmazyn M, Noble EG. Exercise improves postischemic cardiac function in males but not females: consequences of a novel sex-specific heat shock protein 70 response. Circ Res. 2002; 90(8):911-7.
- 17. Brown DA, Johnson MS, Armstrong CJ, Lynch JM, Caruso NM, Ehlers LB, et al. Short-term treadmill running in the rat: what kind of stressor is it? J Appl Physiol. 2007; 103(6):1979-85.
- 18. Thorp DB, Haist JV, Leppard J, Milne KJ, Karmazyn M, Noble EG. Exercise training improves myocardial tolerance to ischemia in male but not in female rats. Am J Physiol Regul Integr Comp Physiol. 2007; 293(1):R363-71.
- 19 Harri M, Kuusela P. Is swimming exercise or cold exposure for rats? Acta Physiol Scand. 1986; 126(2):189-97.
- 20. Moraska A, Deak T, Spencer RL, Roth D, Fleshner M. Treadmill running produces both positive and negative physiological adaptations in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol. 2000; 279(4):R1321-9.
- 21. Linthorst AC, Penalva RG, Flachskamm C, Holsboer F, Reul JM. Forced swim stress activates rat hippocampal serotonergic neurotransmission involving a corticotropin-releasing hormone receptor-dependent mechanism. Eur J Neurosci. 2002; 16(12):2441-52.
- 22. Shyu BC, Andersson SA, Thoren P. Spontaneous running in wheels: a microprocessor assisted method for measuring physiological parameters during exercise in rodents. Acta Physiol Scand. 1984; 121(2):103-9.
- 23. Rupp H. Differential effect of physical exercise routines on ventricular myosin and peripheral catecholamine stores in normotensive and spontaneously hypertensive rats. Circ Res. 1989; 65(2):370-7.
- 24. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72:248-54.
- 25. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970; 227: 680-5.
- 26. Eikelboom R, Mills R. A microanalysis of wheel running in male and female rats. Physiol Behav. 1988; 43(5):625-30.
- 27. Brooks GA, White TP. Determination of metabolic and heart rate responses of rats to treadmill exercise. J Appl Physiol. 1978; 45(6):1009-15.
- 28. Brooks GA, Fahey TD. Exercise physiology: human bioenergetics and its applications. New York: Macmillan; 1984.
- 29 Chang FL, Huang TH, Hsieh SS, Yang RS, Lin CC. The effects of different endurance training intensity on systematic and peripheral citrate synthase activity. Med Sci Sports Exerc. 2001; S33:295.
- 30 Huang TH, Lin SC, Chang FL, Hsieh SS, Liu SH, Yang RS. Effects of different exercise modes on mineralization, structure, and biomechanical properties of growing bone. J Appl Physiol. 2003; 95(1):300-7.
- 31. Noble EG, Moraska A, Mazzeo RS, Roth DA, Olsson MC, Moore RL, et al. Differential expression of stress proteins in rat myocardium after free wheel or treadmill run training. J Appl Physiol. 1999; 86(5):1696-701.
- 32. Noble EG. Heat shock proteins and their induction with exercise. In: Locke M, Noble EG, editors. Exercise and stress response: the role of stress proteins. Barcelona: Boca Raton; 2002. p. 43-78.
- 33. Rodnick KJ, Reaven GM, Haskell WL, Sims CR, Mondon CE. Variations in running activity and enzymatic adaptations in voluntary running rats. J Appl Physiol. 1989; 66(3):1250-7.
- 34. Nickerson M, Kennedy SL, Johnson JD, Fleshner M. Sexual dimorphism of the intracellular heat shock protein 72 response. J Appl Physiol. 2006; 101(2):566-75.
- 35. Maier SF. Learned helplessness and animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 1984; 8(3):435-46.
- 36. Moraska A, Fleshner M. Voluntary physical activity prevents stress-induced behavioral depression and anti-KLH antibody suppression. Am J Physiol Regul Integr Comp Physiol. 2001; 281(2):R484-9.
- 37. Dishman RK, Warren JM, Youngstedt SD, Yoo H, Bunnell BN, Mougey EH, et al. Activity-wheel running attenuates suppression of natural killer cell activity after footshock. J Appl Physiol. 1995; 78(4):1547-54.
- 38. Hill LE, Droste SK, Nutt DJ, Linthorst AC, Reul JM. Voluntary exercise alters GABAA receptor subunit and glutamic acid decarboxylase-67 gene expression in the rat forebrain. J Psychopharmacol. 2008; 00(00):1-12
- 39. Voss MR, Stallone JN, Li M, Cornelussen RN, Knuefermann P, Knowlton AA. Gender differences in the expression of heat shock proteins: the effect of estrogen. Am J Physiol Heart Circ Physiol. 2003; 285(2):H687-92.
- 40. Chicco AJ, Schneider CM, Hayward R. Voluntary exercise protects against acute doxorubicin cardiotoxicity in the isolated perfused rat heart. Am J Physiol Regul Integr Comp Physiol. 2005; 289(2):R424-R31.
Publication Dates
-
Publication in this collection
06 Jan 2010 -
Date of issue
Nov 2009
History
-
Accepted
02 Dec 2008 -
Received
26 Sept 2008 -
Reviewed
03 Nov 2008