Abstracts
BACKGROUND: The level of sympathetic nervous activity is a major determinant of prognosis in patients with heart failure. OBJECTIVE: The purpose of this investigation was to perform a proof-of-principle trial of therapeutic endoscopic left thoracic sympathetic blockade in heart failure patients to assess safety and immediate effects. METHODS: Fifteen patients with dilated cardiomyopathy and left ventricular ejection fraction (LVEF) < 40%, New York Heart Association functional class II or III, and heart rate > 65 bpm, despite either adequate betablocker use or intolerant to it, were enrolled. Ten patients underwent left infra-stellate ganglion plus T3-T4 interspinal space clipping through videothoracoscopy, while the other five patients were randomized to a control group. RESULTS: None of the treated patients had any procedure-related adverse cardiovascular events at the perioperative period. Two patients from the surgical group died due to pulmonary thromboembolism or myocardial infarction within 6 months of the initial follow-up, while three patients from the control group had heart failure progression and died or developed cardiogenic shock during the same period. Treated patients presented improvement in quality of life, level of physical activity and LVEF (from 25 ± 9% to 32 ± 8%, p=0.024) at 6 months of follow-up, whereas these parameters did not change in control patients. CONCLUSION: Endoscopic left thoracic sympathetic blockade is feasible and appears to be safe in severe heart failure patients. This initial study suggests that this procedure might be an effective alternative approach to sympathetic blockade in the treatment of dilated cardiomyopathies.
Heart failure; cardiomyopathy, dilated; stroke volume; sympathectomy; thoracoscopy
FUNDAMENTO: O nível da atividade nervosa simpática é um dos mais importantes determinantes prognósticos em pacientes com insuficiência cardíaca. OBJETIVO: O propósito dessa investigação foi realizar um estudo de viabilidade do emprego do bloqueio simpático esquerdo por toracoscopia em pacientes com insuficiência cardíaca (IC) para avaliar a segurança e os efeitos imediatos. MÉTODOS: Quinze pacientes com cardiomiopatia dilatada e fração de ejeção do ventrículo esquerdo (FEVE) < 40%, classe funcional II ou III (NYHA) e frequência cardíaca > 65 bpm, a despeito do uso adequado de beta-bloqueadores ou intolerantes a eles, forma selecionados. Dez pacientes foram submetidos à clipagem do espaço inter-espinhal em nível de T3-T4 e da porção inferior dos gânglios estrelados esquerdos através de videotoracocopia, enquanto outros cinco pacientes foram randomizados para um grupo controle. RESULTADOS: Nenhum dos pacientes operados apresentou qualquer evento cardiovascular adverso relacionado ao procedimento cirúrgico no período perioperatório. Dois pacientes do grupo cirúrgico morreram devido a tromboembolismo pulmonar ou infarto do miocárdio nos 6 meses de seguimento inicial, enquanto três pacientes do grupo controle apresentaram progressão da IC e morreram ou desenvolveram choque cardiogênico no mesmo período. Nos pacientes tratados, houve melhora na qualidade de vida, nível de atividade física e FEVE (de 25 ± 9% para 32 ± 8%, p=0,024) aos 6 meses de seguimento, enquanto esses parâmetros não se alteraram nos pacientes do grupo controle. CONCLUSÃO: O bloqueio simpático esquerdo via toracoscopia é factível e parece ser seguro em pacientes com IC grave. Esse estudo inicial sugere que esse procedimento pode ser uma abordagem alternativa eficaz para o bloqueio simpático no tratamento de cardiomiopatias dilatadas.
Insuficiência cardíaca; cardiomiopatia dilatada; volume sistólico; simpatectomia; toracoscopia
FUNDAMENTO: El nivel de la actividad nerviosa simpática es uno de los más importantes determinantes pronósticos en pacientes con insuficiencia cardíaca. OBJETIVO: El propósito de esta investigación fue realizar un estudio de viabilidad del empleo del bloqueo simpático izquierdo por toracoscopia en pacientes con insuficiencia cardíaca (IC) para evaluar la seguridad y los efectos inmediatos. MÉTODOS: Quince pacientes con cardiomiopatía dilatada y fracción de eyección del ventrículo izquierdo (FEVI) < 40%, clase funcional II o III (NYHA) y frecuencia cardíaca > 65 lpm, a despecho del uso adecuado de betabloqueantes o intolerantes a ellos, fueron seleccionados. Diez pacientes fueron sometidos a clipaje del espacio interespinal a nivel de T3-T4 y de la porción inferior de los ganglios estrellados izquierdos a través de videotoracocopia, mientras que otros cinco pacientes fueron randomizados para un grupo control. RESULTADOS: Ninguno de los pacientes operados presentó ningún evento cardiovascular adverso relacionado al procedimiento quirúrgico en el período perioperatorio. Dos pacientes del grupo quirúrgico murieron debido a tromboembolismo pulmonar o infarto de miocardio en los 6 meses de seguimiento inicial, mientras tres pacientes del grupo control presentaron progresión de la IC y murieron o desarrollaron shock cardiogénico en el mismo período. En los pacientes tratados, hubo mejora en la calidad de vida, nivel de actividad física y FEVI (de 25±9% a 32±8%, p=0,024) a los 6 meses de seguimiento, mientras que esos parámetros no se alteraron en los pacientes del grupo control. CONCLUSIÓN: El bloqueo simpático izquierdo vía toracoscopia es factible y parece ser seguro en pacientes con IC grave. Este estudio inicial sugiere que este procedimiento puede ser un abordaje alternativo eficaz para el bloqueo simpático en el tratamiento de cardiomiopatías dilatadas.
Insuficiencia cardíaca; cardiomiopatía dilatada; volumen sistólico; simpatectomía; toracoscopia
ORIGINAL ARTICLE
Instituto do Coração (Incor) do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo, SP - Brazil
Mailing address
ABSTRACT
BACKGROUND: The level of sympathetic nervous activity is a major determinant of prognosis in patients with heart failure.
OBJECTIVE: The purpose of this investigation was to perform a proof-of-principle trial of therapeutic endoscopic left thoracic sympathetic blockade in heart failure patients to assess safety and immediate effects.
METHODS: Fifteen patients with dilated cardiomyopathy and left ventricular ejection fraction (LVEF) < 40%, New York Heart Association functional class II or III, and heart rate > 65 bpm, despite either adequate betablocker use or intolerant to it, were enrolled. Ten patients underwent left infra-stellate ganglion plus T3-T4 interspinal space clipping through videothoracoscopy, while the other five patients were randomized to a control group.
RESULTS: None of the treated patients had any procedure-related adverse cardiovascular events at the perioperative period. Two patients from the surgical group died due to pulmonary thromboembolism or myocardial infarction within 6 months of the initial follow-up, while three patients from the control group had heart failure progression and died or developed cardiogenic shock during the same period. Treated patients presented improvement in quality of life, level of physical activity and LVEF (from 25 ± 9% to 32 ± 8%, p=0.024) at 6 months of follow-up, whereas these parameters did not change in control patients.
CONCLUSION: Endoscopic left thoracic sympathetic blockade is feasible and appears to be safe in severe heart failure patients. This initial study suggests that this procedure might be an effective alternative approach to sympathetic blockade in the treatment of dilated cardiomyopathies.
Key words: Heart failure; cardiomyopathy, dilated; stroke volume; sympathectomy; thoracoscopy.
Introduction
The activation of the sympathetic nervous system is an important mechanism involved in the pathophysiology of systolic heart failure. Despite being an adaptive feature to support the failing myocardium, the chronic exposure of the heart to higher norepinephrine concentrations can cause several detrimental and maladaptive effects1. Based on this fact, the use of beta-blocker therapy is currently well established in chronic heart failure treatment and it is responsible for significant clinical and survival improvement in patients with left ventricular systolic dysfunction2.
Bilateral endoscopic thoracic sympathectomy has been performed for the treatment of primary hyperhidrosis3,4 and as a palliative approach to patients with severe angina pectoris5. Furthermore, left cardiac sympathetic denervation is described for the treatment of long QT syndrome6 and catecholaminergic ventricular tachycardia7. Recent studies have demonstrated that these procedures decrease sympathetic nervous activity5,8-10; while vagal and global cardiac autonomic functions seem to be improved after them8. The most significant effects on cardiac sympathetic activity seem to be particularly related to the excision or blockade of the left stellate ganglion and subsequent sympathetic chain11. On the other hand, another experimental study suggests that unilateral right sympathectomy can potentially increase the incidence of ventricular arrhythmias, in contrast with the left sympathetic blockade12. Furthermore, clinical studies have documented that sympatholytic agent administration can be maladaptive in patients with severely compromised hemodynamics in whom a minimal adrenergic tone is important, suggesting that excessive sympathetic inhibition may be deleterious in heart failure patients13.
Based on these findings, we can speculate that the achievement of a partial sympathetic blockade including the left stellate ganglion in patients with systolic heart failure may represent an effective approach to decrease cardiac noradrenergic drive. This fact could occur while preserving some integrity of the sympathetic nervous function, a situation that can potentially provide clinical and ventricular function benefits without paradoxical deleterious effects. Therefore, the purpose of this study is to describe the technique of a reversible thoracoscopic left sympathetic blockade, which was used for the first time in the treatment of patients with severe systolic heart failure, in order to assess its feasibility and safety.
Methods
Patient selection criteria
Patients were selected by the Heart Failure Program of our Institution, among those with severe dilated cardiomyopathies and significant functional limitation with intermittent New York Heart Association functional class III or IV symptoms despite attempts to optimize medical therapy. They had had a diagnosis of dilated or ischemic cardiomyopathy for more than two years and also had reduced left ventricular function, characterized by echocardiographic ejection fraction < 0.4, and heart rate > 65 beats per minute at rest, despite either adequate beta-blocker use or intolerance to it. Patients using intravenous inotropic drugs or in persistent functional class IV were contraindicated for left sympathetic blockade, as well as those with complex or intractable arrhythmias and any life-threatening noncardiac disease. Informed consent according to our ethical and scientific review board was obtained after discussion of risks, alternatives and possible benefits of the operation.
Reversible endoscopic left sympathetic blockade
All procedures were performed under general anesthesia with single-lumen endotracheal intubation and all operations were performed by the same surgeon. The patients were placed in the supine position with the arms abducted. Surface defibrillator electrodes were routinely placed. The monitoring consisted of electrocardiogram, end-tidal CO2 analyses, pulse oximeter, direct monitoring of arterial blood pressure, a flow-direct pulmonary artery catheter and transesophageal echocardiogram.
The pleural cavity was entered through two 1.0 cm incisions at the fifth intercostal space, where we inserted the 10-mm, 30-degree thoracoscope and the second 10-mm instrument port, which was introduced under direct vision in the midaxillary line. The incision at the fifth intercostal space was performed more laterally than in the projection of the anterior axillary line, due to risk of heart injury because of the important cardiomegaly (Figure 1). The anesthesiologist provided low-volume ventilation with 100% oxygen.
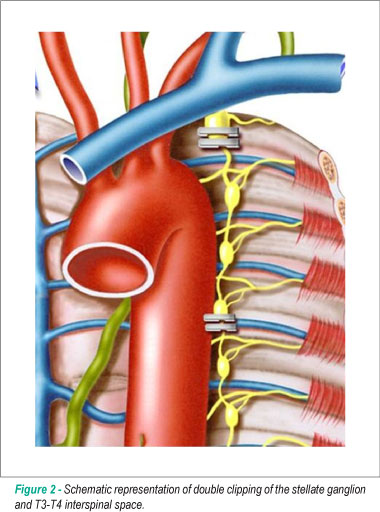
The sympathetic chain was easily identified under the transluminal parietal pleura. The parietal pleura overlying the desired level was incised. The left stellate ganglion was located in the first intercostal space and was much larger than the others, as it usually consisted of C7, C8 and T1 ganglia. The range of clipping included the lower third of the stellate ganglion and the T3-T4 interspinal space. The cephalic portion of the stellate ganglion was preserved to avoid Horner's syndrome and the electrocautery use was also avoided due to the same reason. The nerve was double-clipped at the two sites using a 10-mm Endoclip applier (Ethicon, San Angelo, Texas) (Figure 2). Hemodynamic and echocardiographic behaviors were continuously monitored during these surgical maneuvers.
After the clip application, 10 ml of 0.25% bupivacaine solution was injected along the site of the pleural dissection for postoperative analgesia. The lung was reinflated under direct vision and a small tube was inserted to remove the air through the upper incision, which was removed at the end of the operation. The two port sites were closed primarily with absorbable stitches.
Study endpoints
The primary endpoint was to assess all adverse events secondary to thoracoscopic left sympathetic blockade during the surgical procedure and for the duration of the first 6 months of follow-up. Serious adverse events were defined as fatal or life-threatening events and events requiring unexpected hospitalization. A surgical reversal (clip removal) criterion was severe worsening of heart failure symptoms in the perioperative period. The secondary endpoint was to evaluate preliminary indicators of efficacy by improvement in the quality of life using the Minnesota Living with Heart Failure questionnaire14; 6-minute walk test distance and ventricular function evaluation by Doppler-echocardiography.
Data are presented as means and standard deviation. Comparisons were made using Wilcoxon signed rank test. Randomization was performed by appropriate software.
Results
Fifteen patients were enrolled in this phase I clinical trial (12 males, mean age = 52 ± 6 years) with a diagnosis of idiopathic (8) or ischemic (7) dilated cardiomyopathy. Previous history of acute myocardial infarction was documented in seven patients and three of them had been previosuly submitted to coronary artery bypass grafting. Four patients were in NYHA functional class III and 11 in class II immediately prior to the operation. All of them were receiving maximal doses of beta-blocker therapy and angiotensin converting enzyme inhibitor. Mean left ventricular ejection fraction obtained by echocardiography (Simpson´s method) was 22 ± 5% and the mean heart rate at rest was 81 ± 3 bpm.
Ten patients were randomized in a 2:1 approach to undergo left infrastellate ganglion plus T3-T4 interspinal space clipping through videothoracoscopy, while the other five patients were randomized to a control group. There were no differences between the two groups in relation to the preoperative variables. Two patients from the surgical group were in functional class III and eight in class II, while two patients from the control group were in class III and three in class II. Mean left ventricular ejection fraction was 25 ± 9% in the patients submitted to thoracic sympathetic blockade, while it was 23 ± 8% in the control group.
All surgical procedures were performed by the same surgeon. The mean duration of the operation was 48 ± 6 minutes and the hospitalization period was two days for every patient. There were no intra or immediate postoperative complications. Normal hemodynamic and left ventricular function behaviors were observed during sympathetic chain clipping and it was not necessary to reverse the surgical procedure at the perioperative period.
During the first 6-months of follow-up, two patients from the surgical group died suddenly due to acute myocardial infarction or pulmonary thromboembolism, while they were in NYHA functional class II. Heart failure improvement was also observed in other seven patients who were in functional class I (5) or II (2) and only one patient did not improve clinically and was maintained in functional class III. Three patients described slight compensatory sweat at the plantar area.
In the control group, two patients died due to heart failure progression at 3 and 4 months of follow-up. Another patient was hospitalized due to cardiogenic shock and was submitted to intra-aortic balloon pump implantation. The remaining two patients were in functional class II at 6 months of follow-up.
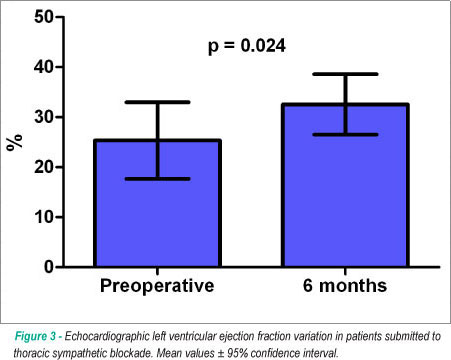
Postoperative evaluation performed in nine patients from the surgical group showed the decrease in the mean heart rate at Holter monitoring from 78 ± 8 to 72 ± 5 beats per minute (P=0.144). Minnesota Living with Heart Failure score decreased from 47 ± 16 to 39 ± 20 in the surviving patients at the 6-month evaluation (p=0.192) and the six-minute walk test distance significantly improved from 167 ± 55 to 197 ± 71 meters (p=0.029). Doppler-echocardiography documented the maintenance of left ventricular diastolic dimensions (from 70 ± 8 to 72 ± 10 mm, p=0.28) and the improvement in left ventricular ejection fraction from 25 ± 9 to 32 ± 7 % (p=0.024), as shown in Figure 3.
Discussion
Surgical procedures have been proposed as alternatives to heart transplantation to provide palliative treatment for patients with dilated cardiomyopathies. These approaches are justified by the possibility to reverse the deleterious effects of different compensatory mechanisms that are activated to preserve cardiovascular homeostasis during heart failure progression. Allied with the great success of beta-blocker therapy, this proof-of-principle trial of therapeutic endoscopic left sympathetic blockade in heart failure patients showed promising results. It demonstrated the technical feasibility and safety of applying left infra-stellate ganglion plus T3-T4 interspinal space clipping through videothoracoscopy in patients with dilated cardiomyopathy. Furthermore, despite the occurrence of two nonrelated cardiac deaths, clinical and left ventricular function improvements were documented during the first six months of follow-up in the surviving patients.
Regardless of being just a feasibility study, the modifications observed in the follow-up of patients submitted to left sympathetic blockade were similar to those documented with other well established approaches in the treatment of chronic heart failure15-17. Furthermore, as a phase I clinical trial, the current experience represents a conservative approach in the surgical achievement of a sympathetic blockade. The technique could be potentially performed with cauterization or resection of the sympathetic chain or applied bilaterally, amplifying the blockade extent.
There have been no previous studies about the effects of surgical sympathetic blockade in heart failure patients. The real extent of the sympathetic chain disruption necessary to decrease the cardiac noradrenalin drive without important deleterious effects is therefore unknown. Several studies have documented the decrease in sympathetic tone after bilateral sympathectomy for primary hyperhidrosis or angina pectoris, without significant interference with the vagal and global cardiac autonomic activities8-10. Findings on 123I-metaiodobenzylguanidine imaging studies indicate that bilateral upper-thoracic sympathectomy slightly suppresses the activation of the sympathetic nervous system, similarly to beta-blocker therapy18. The decrease in cardiac sympathetic innervation density measured by 6-[18F]Fluorodopamine positron emission tomographic scanning was also documented with bilateral upper-thoracic sympathectomy, whereas unilateral right-sided sympathectomy had no apparent effects on this finding19.
The performance of an isolated left sympathetic blockade in this study may therefore represent only a minor modification in cardiac sympathetic activity, but this surgical approach was justified in this primary clinical investigation by the great risk represented by the excessive sympathetic inhibition for heart failure patients. The clinical use of sympatholytic agents such moxonidine was terminated prematurely due to increased mortality13 and higher reductions in plasma norepinephrine concentrations after beta-blocker therapy were also related to higher mortality rates in the Beta Blocker Evaluation of Survival Trial (BEST) subgroup analysis20.
Also in favor of the isolated left sympathetic blockade is the fact that this procedure has been successfully performed for the treatment of long QT syndrome6 and catecholaminergic polymorphic ventricular tachycardia7. In this regard, left unilateral stellectomy is responsible for an increase in the ventricular refractory period similar to that obtained with the bilateral resection of stellate ganglion, whereas the isolated right stellectomy can produce a paradoxical decrease in refractoriness11. This fact can be responsible for the increase in ventricular arrhythmia incidence, a situation that need to be avoided and that is responsible for an important mechanism of death in heart failure patients.
The performance of the left sympathetic blockade using the clipping technique, instead of the sympathectomy with cauterization or resection of the sympathetic chain in this study was based on the possibility of procedure reversal in the presence of immediate deleterious effects21. Nevertheless, no procedure-related adverse events were observed in this initial trial and the intra-operative monitoring showed only a slight decrease in the peripheral vascular resistance without any period of hypotension, making it possible to perform the procedure in a more conventional and comprehensive way. The resection or cauterization of the sympathetic chain can be potentially performed in heart failure patients without any adverse complications, as it is observed in the treatment of long QT syndrome and catecholaminergic ventricular tachycardia6,7. Nevertheless, it is important to emphasize that similar results have been obtained with clipping or cauterization of the sympathetic chain in the treatment of primary hyperhidrosis22. The importance of actually interrupting the several nervous fibers that have connection with the heart is an open discussion and other studies will be necessary to adequately define the best technical approach to therapeutic sympathetic blockade in patients with heart failure.
Similar to other palliative treatments of heart failure, mortality in the follow-up can occur by different complications15-17. The occurrence of two late deaths due to myocardial infarction or pulmonary thromboembolism in this series was probably related to the underlying disease and not to the procedure itself. On the other hand, heart failure progression seems to have stabilized and the observed improvement in left ventricular function opens a real perspective for the use of this procedure as a complementary treatment for advanced heart failure due to dilated or ischemic cardiomyopathy.
In conclusion, thoracoscopic left thoracic sympathetic blockade is feasible and appears to be safe in severe heart failure patients. Exploratory data of this initial study suggest that this procedure may potentially represent an alternative approach in the treatment of dilated cardiomyopathies.
References
- 1. Merlet P, Hittinger L, Dubois-Randé JL, Castaigne A. Myocardial adrenergic dysinnervation in dilated cardiomyopathy: cornerstone or epiphenomenon? J Nucl Med. 2002; 43 (4): 536-9.
- 2. Brophy JM, Joseph L, Rouleau JL. Beta-blockers in congestive heart failure: a Bayesian meta-analysis. Ann Intern Med. 2001; 134 (7): 550-60.
- 3. de Campos JR, Kauffman P, Werebe EC, Andrade Filho LO, Kuzniec S, Jatene FB, et al. Quality of life, before and after thoracic sympathectomy: report on 378 operated patients. Ann Thorac Surg. 2003; 76 (3): 886-91.
- 4. Henteleff HJ, Kalavrouziotis D. Evidence-based review of the surgical management of hyperhidrosis. Thorac Surg Clin. 2008; 18 (2): 209-16.
- 5. Tygesen H, Wettervik C, Claes G, Drott C, Emmanuelsson H, Salem J, et al. Long-term effect of endoscopic transthoracic sympathicotomy on heart rate variability and QT dispersion in severe angina pectoris. Int J Cardiol. 1999; 70 (3): 283-92.
- 6. Schwartz PJ, Priori SG, Cerrone M, Spazzolini C, Odero A, Napolitano C, et al. Left cardiac sympathetic denervation in the management of high-risk patients affected by the long-QT syndrome. Circulation. 2004; 109 (15): 1826-33.
- 7. Collura CA, Johnson JN, Moir C, Ackerman MJ. Left cardiac sympathetic denervation for the treatment of long QT syndrome and catecholaminergic polymorphic ventricular tachycardia using video-assisted thoracic surgery. Heart Rhythm. 2009; 6 (6): 752-9.
- 8. Cruz J, Sousa J, Oliveira AG, Silva-Carvalho L. Effects of endoscopic thoracic sympathectomy for primary hyperhidrosis on cardiac autonomic nervous activity. J Thorac Cardiovasc Surg. 2009; 137 (3): 664-9.
- 9. Vigil L, Calaf N, Codina E, Fibla JJ, Gómez G, Casan P. Video-assisted sympathectomy for essential hyperhidrosis: effects on cardiopulmonary function. Chest. 2005; 128 (4): 2702-5.
- 10. Teodoriya T, Sakagami S, Ueyama T, Thompson L, Hetzer R. Influences of bilateral endoscopic transthoracic sympathicotomy on cardiac autonomic nervous activity. Eur J Cardiothorac Surg. 1999; 15 (2): 194-8.
- 11. Yanowitz F, Preston JB, Abildskov JA. Functional distribution of right and left stellate innervations to the ventricles: production of neurogenic electrocardiographic changes by unilateral alteration of sympathetic tone. Circ Res. 1966; 18 (4): 416-28.
- 12. Schwartz PJ, Verrier RL, Lown B. Effect of stellectomy and vagotomy on ventricular refractoriness in dogs. Circ Res. 1977; 40 (6): 536-40.
- 13. Coats AJ. Heart Failure 99: the MOXCON story. Int J Cardiol. 1999; 71 (2): 109-11.
- 14. Carvalho VO, Guimarães GV, Carrara D, Bacal F, Bocchi EA. Validation of the portuguese version of the Minnesota Living with Heart Failure Questionnaire. Arq Bras Cardiol. 2009; 93 (1): 39-44.
- 15. Benicio A, Moreira LF, Bacal F, Stolf NA, Oliveira SA. Reevaluation of long-term outcomes of dynamic cardiomyoplasty. Ann Thorac Surg. 2003; 76 (3): 821-7.
- 16. McAlister FA, Ezekowitz JA, Wiebe N, Rowe B, Spooner C, Crumley E, et al. Systematic review: cardiac resynchronization in patients with symptomatic heart failure. Ann Intern Med. 2004; 141 (5): 381-90.
- 17. Ribeiro GC, Lopes M, Antoniali F, Nunes A, Costa CE, Fernandes JL. Importance of the area of fibrosis at midterm evolution of patients submitted to ventricular reconstruction. Arq Bras Cardiol. 2009; 93 (6): 564-70.
- 18. Toyota S, Takimoto H, Karasawa J, Kato A, Yoshimine T. Evaluation of cardiac sympathetic nerve function by myocardial 123I-metaiodobenzylguanidine scintigraphy before and after endoscopic sympathectomy. J Neurosurg. 2004; 100 (3): 512-6.
- 19. Moak JP, Eldadah B, Holmes C, Pechnik S, Goldstein DS. Partial cardiac sympathetic denervation after bilateral thoracic sympathectomy in humans. Heart Rhythm. 2005; 2: 602-9.
- 20. Anderson JL, Krause-Steinrauf H, Goldman S, Clemson BS, Domanski MJ, Hager WD, et al. Failure of benefit and early hazard of bucindolol for Class IV heart failure. J Card Fail. 2003; 9 (4): 266-77.
- 21. Sugimura H, Spratt EH, Compeau CG, Kattail D, Shargall Y. Thoracoscopic sympathetic clipping for hyperhidrosis: long-term results and reversibility. J Thorac Cardiovasc Surg. 2009; 137 (6): 1370-6.
- 22. Coelho MS, Silva RFKC, Mezzalira G, Bergonse Neto N, Stori W de SJR, dos Santos AF, et al. T3T4 endoscopic sympathetic blockade versus T3T4 video thoracoscopic sympathectomy in the treatment of axillary hyperhidrosis. Ann Thorac Surg. 2009; 88 (6): 1780-5.
Endoscopic left sympathetic blockade in the treatment for dilated cardiomyopathy
Publication Dates
-
Publication in this collection
19 Nov 2010 -
Date of issue
Dec 2010
History
-
Received
20 Apr 2010 -
Accepted
16 Aug 2010