ANATOMOPATHOLOGICAL SESSION
Instituto do Coração (InCor) HC-FMUSP, São Paulo, SP - Brazil
Mailing address
Keywords:Chest pain; hypertrophy, left ventricular; pericarditis; pericardial effusion.
Man of 37 years of age sought treatment because of severe chest pain associated with nausea and profuse sweating.
The patient had been felling chest pain for five days, which worsened with breathing and chest mobilization, and sought medical attention. The patient had obesity, hypertension and was a smoker.
In this medical evaluation, besides the clinical examination, laboratory tests were made. These (February 27) revealed hemoglobin 11.4 g/dl, hematocrit 34%, mean corpuscular volume 83 fL, leukocytes 14.000/mm³ (neutrophils 75%, eosinophils 1%, lymphocytes 13%, 11% monocytes), platelets 195.000/mm³, erythrocyte sedimentation 35 mm, C-reactive protein 87.4 mg/dl, CKMB 2.58 ng/ml, troponin I < 0.2 ng/ml, creatinine 1.34 mg/dl, urea 59 mg/dl, sodium 139 mEq/l and potassium 3.8 mEq/l.
Echocardiography (February 27) revealed aorta 35 mm, left atrium 39 mm, right ventricle diastolic diameter 28 mm, left ventricle (diastole/systole) 54/34 mm, interventricular septum and upper wall thickness of 12 mm; left ventricular ejection fraction 66%. The left ventricle was hypertrophic and had normal wall motion. The valves were normal, the aorta was normal and had mild pericardial effusion.
Pericarditis was diagnosed and is yet to be elucidated (February 27 2009).
Ibuprofen 1,200 mg and prednisone 20 mg/day were prescribed.
Five days later, the patient had an episode of severe chest pain of 1 hour in duration, accompanied by nausea and profuse sweating. Again, the patient sought medical attention.
Physical examination (March 4) revealed that the patient was in good general condition, heart rate 70 bpm, blood pressure 70/40 mm Hg. Heart and lung semiology was considered normal. Abdominal examination was normal and had no lower limb edema.
Electrocardiogram (March 4) showed sinus rhythm, heart rate 75 bpm, PR 160 ms interval, QRS 80 ms duration, SÂQRS +30° backwards and abnormalities in ventricular repolarization (fig. 1).
Laboratory tests (March 4) revealed hemoglobin 11.3 g/dl, hematocrit 35%, mean corpuscular volume 85 fL, platelet 281,000/mm³, leukocytes 14,400/mm (neutrophils 81%, eosinophils 1%, lymphocytes 11% and monocytes 7%), creatinine 0.93 mg/dl, urea 42 mg/dl, sodium 145 mEq/l, potassium 3.4 mEq/l, C-reactive Protein 172 mg/l, troponin I < 0.20 ng/ml, CKMB 2,17 ng/ml, prothrombin time (INR) 1.2 and activated partial thromboplastin time ratio 0.89.
Echocardiography (March 4) revealed preserved biventricular function, left ventricular hypertrophy in appearance, moderate to severe pericardial effusion, with no signs of restriction to ventricular filling.
Coronary angiography showed no obstructive lesions and, on ventriculography, the left ventricle was hypertrophic in appearance, and showed no abnormality in wall motion. The aortic root was normal.
After volume replacement with a solution of sodium chloride and 0.9% with the use of opioids there was improvement in pain, which worsened with inspiration, and heart rate went to 92 bpm, blood pressure rose to 165/93 mmHg, blood saturation was 94%. The only abnormality in physical examination were hypophonesis sounds. There were other painful episodes, all reversed with analgesics.
In evolution (22 hours on March 4), the patient presented a new episode of chest pain accompanied by severe dyspnoea and required orotracheal intubation for ventilatory support. After intubation, the patient suffered cardiac arrest with pulseless electrical activity. Pericardial puncture was performed with drainage of 20 ml of bloody fluid. Resuscitation maneuvers went on for 45 minutes, there was no response and the patient died.
Clinical aspects
The case concerns a 37-year-old man with severe chest pain of recent onset associated with nausea and profuse sweating. He had hypertension, smoking and obesity as cardiovascular risk factors. The patient was assessed by a medical staff, which included laboratory testing and coronary angiography. The staff ruled out myocardial ischemia, the most epidemiologically prevalent disease. The possible differential diagnoses of chest pain in this patient include pericarditis and aortic dissection, which will be commented below.
Acute pericarditis is a possible hypothesis for the case in question due to the age of the patient, the clinical characteristics of the pain, the high results in inflammatory activity and leukocytosis and the echocardiographic finding of pericardial effusion. At the time, treatment was initiated for this disease (ibuprofen and prednisone) and the patient was discharged.
The pericardium is a fibroelastic enclosure composed of two layers (visceral and parietal) separated by the pericardial space, which is populated by 15 to 50 ml of plasma ultrafiltrate, which involves most of the heart. Although patients undergoing pericardiectomy present no obvious damage, the functions of this tissue are well determined. It maintains a relatively fixed position of the heart in the mediastinum irrespective of chest movement or breathing. It also offers protection against infections and its innervation participates in important consequences, as well as the transmission of pain. Its function of restricting cardiac volume is important and should be included in the assessment of major diseases that afflict it. Because of the properties of its tissue, small forces can produce large changes in stretch and, after reaching a certain threshold, the pericardium becomes tense and no longer elastic. From that point, small changes in volume in the pericardial fluid can cause large pressure rises, which is transmitted to the heart chambers, causing damage to the hemodynamics of this organ. Losses are higher in the right chambers, which have less tolerance to pressure overloads. Similarly, the withdrawal of small volumes of fluid from the pericardial cavity leads to significant improvement in cardiac function. Another important property of the pericardium is the adaptability to accommodate chronic and significant increases in cardiac volumes, as well as pericardial fluid.
The main manifestations of pericardial diseases are pericarditis and pericardial effusion, which are different phenomena. Pericarditis is clinically manifested by a variety of signs and symptoms, and it is called acute when it lasts up to two weeks. Its incidence is not well known and is estimated to occur in 0.1% of inpatients, 5% of patients in emergency units (excluding cases of acute myocardial infarction) and 1% in necropsies1-3.
The pericardium may be affected in various systemic diseases, as well as being the primary focus of involvement. The different etiologies can be divided into groups, with variations according to the form of the disease, such as the presence or absence of symptoms, stroke volume and composition of the liquid. In general, in the idiopathic form, in which there is no specific cause, it is the most prevalent form and accounts for about 80% to 90% of cases4. Other categories are: infections, cancer, cardiac origin, radiation, trauma, both inflammatory, metabolic, drug intoxication. In infections, viruses are the most prevalent ones, including HIV, which has in pericardial involvement its main form of manifestation in the cardiovascular system. Other viral agents that often involve the pericardium are the viruses Coxsachie, influenza and hepatitis viruses. Tuberculosis is a major cause of chronic pericarditis, but it is also a differential diagnosis in the acute forms. The neoplasms involved are, in general, metastasis, especially tumors of the lung, breast and lymphoma. On some occasions, in patients with cancer, the effusions or pericarditis are not due to implants of neoplastic cells at this site, but to complications of the root disease, such as bleeding and infection, or even iatrogenic injury resulting from radiation therapy. When we refer to the cardiac origin, pericarditis related to acute myocardial infarction plays an important role, either in the early form (third to seventh day after Myocardial Infarction (AMI), or especially in the late form known as Dressler's syndrome (an autoimmune mechanism)5. That group also includes free wall rupture after myocardial infarction, due to the potential to lead to catastrophic consequences. In relation to autoimmune disorders, there is systemic lupus erythematosus, which may present serositis, such as pericarditis, as an initial manifestation; rheumatoid arthritis and systemic sclerosis, as well as mixed connective tissue disease and intestinal inflammatory diseases6,7. Uremia and hypothyroidism account for most of the metabolic causes, and the latter usually presents primary pericardial effusion.
In the patient reported in this case, there is no evidence of systemic disease that can direct the diagnosis to a secondary form of pericarditis. There is no medical history or other signs leading to neoplastic or autoimmune disorder, although it is not impossible that this will be the initial manifestation of one of these diseases. The hypothesis that there is a relationship with a possible myocardial infarction was ruled out by the team which, from the data, including electrocardiogram, cardiac enzymes, echocardiogram and, later, coronary angiography, dismissed the possibility of ischemic disease. There is no report of trauma or radiation. Thus, even without analysis of pericardial fluid by CRA or other methods, the possibility exists for the case of pericarditis caused by infectious viruses, as the good general condition of the patient rules out bacterial infection, or even that it is an idiopathic form of the disease.
Typical clinical manifestations of acute pericarditis include pleuritic chest pain, pericardial friction on cardiac auscultation and diffuse ST-segment elevation on electrocardiogram. Sometimes there is recent fever or viral syndrome history, which are absent in this case. These findings may or may not be accompanied by pericardial effusion.
The electrocardiogram is the main supplementary examination for evaluation of pericarditis. Electrocardiographic abnormalities are due to epicardial inflammation, and can be dynamic. The other characteristics include diffuse ST-segment elevation, sparing the leads AVR and V1, especially in the early stages of the disease. The PR segment depression is also typical and often saves the AVR lead. There are no reports of the electrocardiogram in the patient's first visit to to the emergency room. At that time, there were other abnormalities in complementary tests such as leukocytosis and elevated inflammatory activity tests (C-reactive protein and erythrocyte sedimentation rate). The elevation of cardiac enzymes is common, usually associated with subclinical myocarditis, but was absent in this report.
The hospitalization of patients with pericarditis is not always necessary, and the presence of predictors of complications such as tamponade or major signs of stroke, high fever and leukocytosis, trauma, high cardiac enzymes or failure in therapeutic response to anti-inflammatory steroids should be evaluated8,9. Immunosuppressed patients or those using anticoagulants should also remain hospitalized. Other treatment options are high doses of aspirin or colchicine, the latter used especially in refractory cases.
Although the treatment for acute pericarditis was initially established, the patient's unusual evolution with recurrent episodes of pain and death in pulseless electrical activity, the presence of bloody pericardial fluid on emergency pericardiocentesis and the absence of a directed investigation workup for acute aorta dissection make the latter a possible diagnosis for chest pain of the patient in question.
The most prevalent symptom in patients with aortic dissection is sudden chest pain of high intensity10,11. The quality of pain is usually described by more than half of the patients as "ripping" or "stabbing"; however, many patients may report uncommon complaints, especially the elderly, complicating the diagnostic suspicion. It is important to remember that the aortic dissection may be present in some patients as pleuritic chest pain, particularly in cases of aortic rupture to the pericardial cavity. The pain secondary to pericarditis is caused by blood or in cases of pleural involvement affecting the inflammatory aortic process.
Other less common conditions are symptoms of heart failure (HF), syncope, cerebrovascular accident (CVA), peripheral arterial ischemia, paraplegia, and cardiac arrest. HF may be secondary to aortic insufficiency, cardiac tamponade or coronary dissection, especially in the right coronary artery. Syncope can occur secondarily to tamponade or stroke.
Physical examination is essential because it can help diagnose the disease. However, the absence of suggestive abnormalities does not preclude the diagnostic possibility. Asymmetry of pulses may be present by means of involvement of the left subclavian artery or the femoral arteries. Involvement of the renal artery can lead to acute renal failure and hypertension difficult to control, and involvement of the mesenteric artery with intestinal ischemia is rare, but lethal.
Aortic regurgitation can be found in up to 50% of patients with aortic dissection. Annular dilation with failure in coaptation of the pericardial layers or diastolic prolapse of a single pericardial layer may be secondary. Auscultation a soft diastolic murmur in patients with chest pain should raise the diagnostic suspicion of aortic dissection. In this report, there was no description of evaluation of pulses or heart murmurs.
Thus, the diagnosis of aortic dissection should be considered in patients with chest pain with suggestive clinical features or when combined with findings on physical examination as described above. In patients with predisposing diseases such as Marfan's disease or Ehlers-Danlos syndrome, a high diagnostic suspicion should also be raised. Those patients who did not improve after the initial measures for chest pain, especially in the absence of ECG abnormalities in duration of pain should be also be investigated.
The finding of mediastinal widening on chest radiography can also be found and should raise the possibility of dissection. The electrocardiogram is fundamental, especially for the differential diagnosis with acute coronary syndromes.
The speed in diagnostic confirmation is crucial, since mortality from acute aortic dissection is approximately 1% per hour in the first 24 hours12. In unstable patients with signs of complications such as tamponade or intractable pain, transthoracic or transesophageal echocardiography is the method of choice for diagnostic confirmation. In less unstable patients, computed tomography angiography, angioresonance or, in the persistence of diagnostic uncertainty, aortic angiography can be performed. The diagnosis of dissection is often performed in the catheterization laboratory during aortography after coronary angiography not revealing any obstructive lesions.
Aortic dissections are classified temporally into acute (less than two weeks) or chronic (more than two weeks). Taking into consideration the involvement or not of the ascending aorta, these may be classified according to Stanford into types A or B. This is the simplest and more practical classification, more commonly used in our community. Type A dissections involve the ascending aorta, regardless of the origin of the dissection; and type B do not involve the ascending aorta. The first, when acute, should be treated surgically as soon as possible, while the latter must be treated medically, reserving surgical treatment for those experiencing complications such as rupture, intractable pain or downstream impaired organ perfusion13.
Death from aortic dissection can occur due to aortic rupture, cardiac tamponade, acute severe aortic insufficiency or involvement of coronary arteries with acute myocardial infarction. The progress of patients with recurrent pain, shock with lungs clear and cardiopulmonary arrest in Pulseless Electrical Activity (PEA), pericardiocentesis with bloody fluid, leads us to believe that cardiac tamponade secondary to acute aortic dissection may be a possible diagnosis for the patient concerned.
Cardiac tamponade occurs when there is accumulation of pericardial fluid under pressure, exceeding the reserve volume, leading to reduction of cardiac chambers and circulatory collapse14. It may be secondary to any disease involving the pericardium in the primary or secondary form, but it is more often associated with diseases with faster accumulation of pericardial fluid, not allowing it to accommodate the excess volume. Tamponade is most often found in neoplastic effusions, in infectious pericarditis, such as the bacterial or viral one, or tuberculosis, or aortic dissection type A. Cardiac tamponade is uncommon in patients with pericardial involvement secondary to rheumatic diseases like lupus erythematosus, or due to metabolic diseases such as hypothyroidism, and uremia, due to the insidious accumulation of fluid in these cases.
Cardiac tamponade is a clinical diagnosis. It is performed in patients with signs of shock, associated with jugular venous distension, hypophonesis sounds and paradoxical pulse, in the absence of signs of pulmonary congestion. It can be confirmed with tests such as echocardiography, which is the most important one, as it demonstrates hemodynamic impairment in patients without clinically manifested signs of tamponade.
As soon as cardiac tamponade is diagnosed, pericardiocentesis is an urgent and solving procedure. If hemodynamic collapse is manifest or imminent, bedside must be performed; but in less critical cases, it can be performed in the operating room by cardiothoracic surgeon as soon as possible.
The patient reported here presented five days after the first passage in the medical emergency service chest pain associated with symptoms of low output and hypotension. Lung examination corroborates the tamponade hypothesis, since the hemodynamic failure in such cases is usually not accompanied by pulmonary congestion. There are no reports of jugular venous distension, muffled heart sounds, paradoxical pulse or sinus tachycardia. Chest radiograph shows silhouette change when there is at least moderate effusion, generally not found in acute cases, unless there is accumulation of more than 200 ml of fluid. Transthoracic echocardiography is considered a Class I examination of suspected pericardial disease, according to the American Society of Echocardiography and the American Heart Association and the Society of Eruopean Cardiology15. Moderate to significant effusion is a near-universal finding in cases of tamponade, except hyperacute cases. Effusions can be loculated, which may explain the regional tamponade in the absence of circumferential effusions. The following are signs of echocardiographic hemodynamic impairment associated with cardiac tamponade: diastolic collapse of right ventricle and right atrium, reduced cardiac chambers, anteroposterior motion of the heart in effusion, left ventricular septal movement during inspiration and dilatation and reduction of less than 50% of the vena cava. In the patient concerned, in his second hospitalization, there were no signs of impairment of heart function on echocardiography that could be related to the pericardial effusion. However, the patient was hypotensive, and after fluid resuscitation, the cardiac sounds became muffled. The measure of intracavitary pressures during hemodynamic study would be of great importance in clarifying the diagnosis, since these are balanced in the tamponade during diastole, as well as an increase in pressure in the right chambers and a reduction in the left chambers during inspiration.
The patient developed recurrent chest pain and shortness of breath, requiring intubation. Positive pressure ventilation can reduce cardiac filling and should be avoided in those patients, unless it is urgent. The evolution culminated in an unfavorable outcome, with patient presenting cardiac arrest in PEA, unresponsive to resuscitation or even pericardiocentesis.
Dr. Paulo Cury Rezende and Dr. Vitor Borges Viana
Hypothetical diagnosis
Thus, we conclude that the first diagnosis for this young patient with chest pain of recent onset and fatal outcome is cardiac tamponade secondary to acute aortic dissection. This disease has a high severity and mortality, even when operated in a timely manner. Its diagnosis can be extremely difficult to achieve, even in centers with large technological resources. Emergency physicians should have this disease in mind in order to perform a diagnosis and institute treatment without delay, since mortality is age-dependent. Acute pericarditis is another possible hypothesis; however, as I said earlier, the final evolution of the patient makes us believe that this possibility is unlikely. Workup attention to signs and symptoms of aortic dissection and cardiac tamponade are essential for early diagnosis and appropriate treatment.
Dr. Paulo Cury Rezende and Dr. Vitor Borges Viana
Autopsy
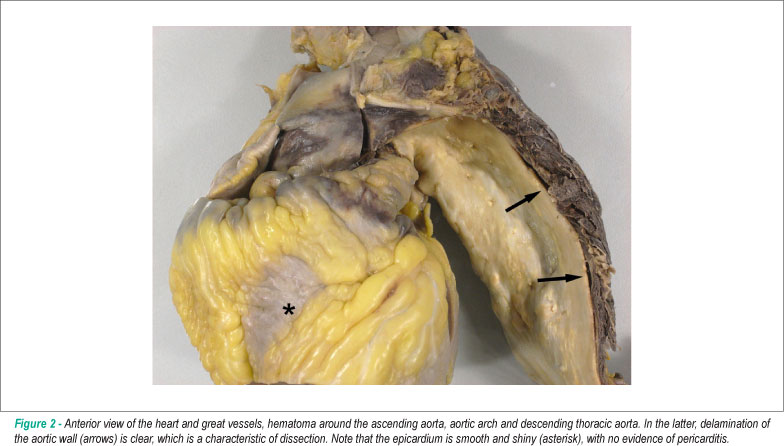
Corpse of an obese patient. After opening the cavities, visceral pallor was noted, particularly in the brain. There was 1500 ml of hemorrhagic fluid in the left pleural cavity, with lung atelectasis and massive bruising around the aortic arch of the initial segment of the descending thoracic aorta (Fig. 2 and 3) in the thoracic paravertebral region and around the esophagus. Aorta examination revealed acute dissection of the aorta wall (Fig. 1), small entry hole, longitudinal, measuring 0.6 cm, located in the aortic arch between the emergence of the left carotid and subclavian arteries. The aortic wall was in the initial route of the descending thoracic aorta, where we noticed an irregular hole, measuring 1.8 x 1.0 cm, adjacent to the hematoma in the region previously mentioned (Fig. 4). The dissection progressed in an antegrade manner across the aorta and left iliac artery. No re-entry hole was detected up to the resection margin of the autopsy. There was retrograde aortic dissection, up to 1-2 cm of the aortic valve. Histologic examination of the aorta confirmed the presence of acute dissection, with areas at the onset of organization in the abdominal segment. The pericardial sac was not tense, but at the opening, it revealed a massive hemorrhagic effusion. The parietal pericardium was thin, smooth and shiny. The heart had a smooth and shiny epicardium (Fig. 2) and sections showed marked concentric hypertrophy of the left ventricle (Fig. 4). The coronary arteries showed wall thickening with presence of atherosclerotic plaques, and maximum obstruction of about 70%-80% in the area proximal to the right coronary artery. The aorta showed moderate atherosclerosis, with areas of dilatation, calcification and partial thrombosis of the iliac arteries. The kidneys showed isolated foci of glomerular sclerosis, clear hyaline arteriolosclerosis and scar of infarction in the left kidney. The lungs showed evidence of chronic bronchitis and thin brownish pigment deposition in the alveolar macrophages, suggestive of chronic smoking. Mild steatosis, nodular hyperplasia of prostate and superficial gastritis were also found.
Dr. Luiz Alberto Benvenuti
Anatomical-pathological diagnoses
Obesity; Hypertension with concentric hypertrophy of the left ventricle; Acute aortic dissection, route; Extensive thoracic hemorrhage with hypovolemic shock (cause of death); Atherosclerosis of the aorta and coronary arteries; Nodular hyperplasia of the prostate.
Dr. Luiz Alberto Benvenuti
Comments
Case of an obese patient, 37 years old, diagnosed with acute pericarditis, who died five days after initiation of drug therapy. The autopsy showed that the clinical diagnosis was wrong, because there were no abnormalities in the pericardium. The clinical condition was due to acute aortic dissection which broke into the left hemithorax, causing severe hemorrhage and death due to hypovolemic shock. The dissection hole was small, standing in the aortic arch, which makes it difficult to view it through imaging studies. There was antegrade dissection of the thoracic and abdominal aorta and retrograde dissection of the ascending aorta, and therefore the dissection is classified as type III A according to DeBakey or type A according to Stanford. The patient had hypertension, with severe left ventricular hypertrophy, which is a known risk factor for acute dissection of the aorta16. The patient also had atherosclerosis of the aorta and coronary arteries more exuberant than usual for his age, despite the absence of ischemic heart disease.
The differential diagnosis of acute chest pain includes acute pericarditis and acute aortic dissection, among several other possibilities17. In turn, the acute aortic dissection can be confused with acute pericarditis, as in this case, with overwhelming consequences, since it is a much more serious condition, often requiring immediate surgery18. In the current case, it is also interesting to note that the patient presented important risk factors for developing cardiovascular events, which are often associated and interrelated, namely: obesity, smoking and chronic hypertension.
Dr. Luiz Alberto Benvenuti
References
- 1. Roberts WC. Pericardial disease: its morphologic features and its causes. Proc (Bayl Univ Med Cent). 2005;18(1):38-55.
- 2. Kraltein J, Frishman W. Malignant pericardial disease: diagnosis and treatment. Am Heart J. 1987;113(3):785-90.
- 3. Roberts WC. Neoplasms involving the heart, their simulators, and adverse consequences of their therapy. Proc (Bayl Univ Med Cent). 2001;14(4):358-76.
- 4. Spodick DH. Acute pericarditis: current concepts and practice. JAMA. 2003;289(22):1150-3.
- 5. Bendjelid K, Pugin J. Is Dressler syndrome dead? Chest. 2004;126(5):1680-2.
- 6. SpodicK DH. Pericarditis in systemic diseases. Cardiol Clin. 1990;8(4):709-16.
- 7. Lemos J, Santos L, Martins I, Nunes L, Santos O, Henriques P. Systemic lupus erythematosus - diagnosis in cardiology. Rev Port Cardiol. 2008;27(6):841-9.
- 8. Imazio M, Cecchi E, Demichelis B, Ierna S, Ghisio A, Pomari F, et al. Indicators of poor prognosis of acute pericarditis Circulation. 2007;115(21):2739-44.
- 9. Imazio M, Demichelis B, Parrini I, Giuggia M, Cecchi E, Gaschino G, et al. Day-hospital treatment of acute pericarditis: a management program for outpatient therapy. J Am Coll Cardiol. 2004;43(6):1042-6.
- 10. Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management. Part I: from etiology to diagnostic strategies. Circulation. 2003;108(6):628-35.
- 11. Nienaber CA, Fattori R, Mehta RH, Richartz BM, Evangelista A, Peztch M, et al. Gender-related differences in acute aortic dissection. Circulation. 2004;109(24):3014-21.
- 12. Song KJ, Kang SJ, Song JM, Kang DH, Song H, Chung CH, et al. Factors associated with in-hospital mortality in patients with acute aortic syndrome involving ascending aorta. Int J Cardiol. 2007;115(1):14-8.
- 13. Tsai TT, Bossone E, Isselbacher EM, Nienamber CA, Evangelista A, Fang J, et al (International Registry of Acute Aortic Dissection). The Clinical characteristics of hypotension in patients with acute aortic dissection. Am J Cardiol. 2005;95(1):48-52.
- 14. Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management. Part II: therapeutic management and follow-up. Circulation. 2003;108(6):772-8.
- 15. Erbel R, Alfonso F, Boileau C, Dirsch O, Eber B, Haverich A, et al; Task Force on Aortic Dissection, European Society of Cardiology. Diagnosis and management of aortic dissection. Eur Heart J. 2001;22(18):1642-81.
- 16. Larson EW, Edwards WD. Risk factors for aortic dissection: a necropsy study of 161 cases. Am J Cardiol. 1984;53(6):849-55.
- 17. Trappe HJ, Perings C. Acute chest pain. Med Klin (Munich). 2005;100(8):462-70.
- 18. Montijano Cabrera AM, Rosa Jiménez FP, Carmona Alvarez JA. [Mimesis of the aortic dissection: simulation of acute pericarditis]. Rev Clin Esp. 2006;206(5):254-5.
Case 2/2011 - Young patient, male, with pleuritic chest pain, hypotension, profuse sweating, with ECG with no acute ischemic abnormalities and negative myocardial injury markers
Publication Dates
-
Publication in this collection
02 May 2011 -
Date of issue
Apr 2011