Abstracts
Background:
The maternal cardiovascular system undergoes progressive adaptations throughout pregnancy, causing blood pressure fluctuations. However, no consensus has been established on its normal variation in uncomplicated pregnancies.
Objective:
To describe the variation in systolic blood pressure (SBP) and diastolic blood pressure (DBP) levels during pregnancy according to early pregnancy body mass index (BMI).
Methods:
SBP and DBP were measured during the first, second and third trimesters and at 30-45 days postpartum in a prospective cohort of 189 women aged 20-40 years. BMI (kg/m2) was measured up to the 13th gestational week and classified as normal-weight (<25.0) or excessive weight (≥25.0). Longitudinal linear mixed-effects models were used for statistical analysis.
Results:
A decrease in SBP and DBP was observed from the first to the second trimester (βSBP=-0.394; 95%CI: -0.600- -0.188 and βDBP=-0.617; 95%CI: -0.780- -0.454), as was an increase in SBP and DBP up to 30-45 postpartum days (βSBP=0.010; 95%CI: 0.006-0.014 and βDBP=0.015; 95%CI: 0.012-0.018). Women with excessive weight at early pregnancy showed higher mean SBP in all gestational trimesters, and higher mean DBP in the first and third trimesters. Excessive early pregnancy BMI was positively associated with prospective changes in SBP (βSBP=7.055; 95%CI: 4.499-9.610) and in DBP (βDBP=3.201; 95%CI: 1.136-5.266).
Conclusion:
SBP and DBP decreased from the first to the second trimester and then increased up to the postpartum period. Women with excessive early pregnancy BMI had higher SBP and DBP than their normal-weight counterparts throughout pregnancy, but not in the postpartum period.
Arterial Pressure; Pregnant Women; Pregnancy; Body Mass Index; Cohort StudiesCohort Studies
Fundamento:
O sistema cardiovascular materno sofre adaptações progressivas durante a gestação, acarretando flutuações da pressão arterial. Entretanto, não há consenso sobre a variação pressórica normal na gravidez saudável.
Objetivo:
Descrever a variação da pressão arterial sistólica (PAS) e diastólica (PAD) durante a gravidez e no pós-parto imediato segundo o índice de massa corporal (IMC) no início da gravidez.
Métodos:
A PAS e a PAD foram medidas no 1º, 2º e 3º trimestres gestacionais e aos 30-45 dias pós-parto em uma coorte prospectiva de 189 mulheres com idade entre 20 e 40 anos. O IMC (kg/m2) foi aferido até a 13a semana e classificado como normal (< 25,0) ou excessivo (≥ 25,0). Modelos longitudinais de efeitos mistos foram utilizados para a análise estatística.
Resultados:
Observou-se diminuição da PAS e da PAD do primeiro para o segundo trimestre (βPAS=-0,394; IC95%:-0,600- -0,188 e βPAD=-0,617; IC95%:-0,780- -0,454) e subsequente aumento de ambas até 30-45 dias após o parto (βPAS=0,010; IC95%:0,006-0,014 e βPAD=0,015; IC95%:0,012-0,018). As mulheres com IMC excessivo apresentaram média de PAS maior em todos os trimestres, e de PAD maior no primeiro e no terceiro trimestres. O IMC excessivo no início da gestação esteve positivamente associado com mudanças na PAS (βPAS=7,055; IC95%:4,499-9,610) e na PAD (βPAD=3,201; IC95%:1,136-5,266).
Conclusão:
A PAS e a PAD diminuíram do primeiro para o segundo trimestre e aumentaram do segundo trimestre até o pósparto. Mulheres com IMC excessivo no início da gestação apresentaram valores mais elevados de PAS e PAD ao longo da gravidez, mas não no pós-parto, quando comparadas às de IMC normal.
Pressão Arterial; Gestantes; Gravidez; Índice de Massa Corporal; Estudos de Coortes
Introduction
The maternal cardiovascular system undergoes progressive adaptations throughout pregnancy, including decreased vascular resistance, increased blood volume, and other metabolic changes11 Ouzounian JG, Elkayam U. Physiologic changes during normal pregnancy and delivery. Cardiol Clin. 2012;30(3):317-29.. Although the effects of these changes on systemic blood pressure (BP) have been described in many studies, there is no consensus on its normal variation in uncomplicated pregnancies22 Metoki H, Ohkubo T, Sato Y, Kawaguchi M, Nishimura M, Watanabe Y. et al. Detection of midpregnancy fall in blood pressure by out-of-office monitoring. Hypertension. 2009;53(2):e12-3, author reply e14.,33 Tranquilli AL. Mid-trimester blood pressure in pregnancy. Blood pressure fall or fall of a myth? J Hypertens. 2011;29(4):658-9..
Hypertensive disorders of pregnancy (HDP) represent a major obstetric complication that affects 5%-10% of pregnancies, depending on characteristics of the study population, and are one of the leading causes of maternal and neonatal morbidity and mortality worldwide44 Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183(1):S1-S22.. HDP include chronic hypertension, gestational hypertension, preeclampsia and eclampsia, being considered the second most common cause of direct maternal death in developed countries55 Vest AR, Cho LS.Hypertension in pregnancy. Cardiol Clin. 2012;30(3):407-23..
The etiology of HDP is not clear; however, there are several risk factors associated with their occurrence, such as body mass index (BMI)66 Ehrenthal DB, Jurkovitz C, Hoffman M, Jiang X, Weintraub WS. Prepregnancy body mass index as an independent risk factor for pregnancy-induced hypertension. J Womens Health (Larchmt). 2011;20(1):67-72.. As the prevalence of obesity increases in women of reproductive age77 Gaillard R, Bakker R, Willemsen SP, Hofman A, Steegers EA, Jaddoe VW. Blood pressure tracking during pregnancy and the risk of gestational hypertensive disorders: the Generation R Study. Eur Heart J. 2011;32(24):3088-97., BMI [weight (kg)/stature22 Metoki H, Ohkubo T, Sato Y, Kawaguchi M, Nishimura M, Watanabe Y. et al. Detection of midpregnancy fall in blood pressure by out-of-office monitoring. Hypertension. 2009;53(2):e12-3, author reply e14. (m22 Metoki H, Ohkubo T, Sato Y, Kawaguchi M, Nishimura M, Watanabe Y. et al. Detection of midpregnancy fall in blood pressure by out-of-office monitoring. Hypertension. 2009;53(2):e12-3, author reply e14.)] and its associated complications represent a relevant public health matter. Maternal obesity is a significant risk factor for morbidity and mortality for both mother and fetus88 Scott-Pillai R, Cardwell C, Hunter A, Holmes V. The impact of body mass index on maternal and neonatal outcomes: a retrospective study in a UK obstetric population, 2004-2011. BJOG. 2013;120(8):932-9.. A systematic review has demonstrated that an increase of approximately 5-7 kg/m22 Metoki H, Ohkubo T, Sato Y, Kawaguchi M, Nishimura M, Watanabe Y. et al. Detection of midpregnancy fall in blood pressure by out-of-office monitoring. Hypertension. 2009;53(2):e12-3, author reply e14. units in BMI was associated with a two-fold increased risk of preeclampsia99 O'Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14(3):368-74..
Given these previous findings, this study aims to describe the variation in systolic blood pressure (SBP) and diastolic blood pressure (DBP) levels during uncomplicated pregnancy, according to BMI.
Methods
This prospective cohort study was conducted at a prenatal care unit in Rio de Janeiro, Brazil. The enrollment of pregnant women occurred freely and continuously between November 2009 and October 2011. The follow-up period lasted until July 2012. A total of 258 women were recruited according to the following criteria: being less than 13-week pregnant at enrollment, being 20 to 40 years old and being free from any infectious or chronic diseases (except obesity). The study comprised four follow-up waves: 4th-13th week (first trimester), 14th-27th week (second trimester), 28th-40th week (third trimester), and 30-45 postpartum days. The first follow-up wave included two visits on different days; BP and BMI data were obtained during the first visit, and all other covariates used for adjustment in the analysis were collected during the second visit. Women who underwent the first follow-up visit but quit before the second visit (n=6) were excluded from the analysis, as were those with the following characteristics: twin pregnancies (n=4); diagnosis of an infectious or non-communicable disease (n=17); miscarriage (n=25); and BP not measured in the specified interval (n=17). The final sample was composed of 189 pregnant women (Figure 1).
Flowchart illustrating the process of recruitment of women attending the prenatal care at a Public Health Center. Rio de Janeiro, 2009 – 2011
Systolic and diastolic BP were measured using an automated oscillometric BP monitoring system (HEM-742, OMRON, São Paulo, Brazil) validated according to the international protocol of the European Society of Hypertension1010 Coleman A, Steel S, Shennan A. Validation of the Omron MX3 Plus oscillometric blood pressure monitoring device according to the European Society of Hypertension international protocol. Blood Press Monit. 2005;10(3):165-8.. Blood pressure was measured after the women had rested for at least five minutes and were seated comfortably with their back supported, their legs uncrossed and their feet flat on the floor. Clothing was removed from the arm in which the cuff was placed. The arm was supported at heart level, with the palm facing up and the elbow slightly flexed. The women were advised not to speak during the procedure. Different cuff sizes, based on the upper arm circumference at the time of each measurement, were used. Blood pressure was measured twice at the first trimester (in two distinct days) to determine if the women had chronic hypertension (values of SBP ≥ 140 or DBP ≥ 90 mm Hg, before the 20th gestational week). Each measurement of BP was performed in duplicate, for all follow-up waves, with an approximate 30-minute interval between measurements. The mean values of the duplicate measurements from each time-point were used for analysis1111 Pickering TG, Hall JE, Appel L J, Falkner BE , Graves J , Hill MN. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111(5): 697-716..
The women were weighed with a digital scale (Filizola Ltd., São Paulo, Brazil), and their stature was measured in duplicate with a Seca Portable Stadiometer (Seca Ltd., Hamburg, Germany). The mean of the duplicate standing height values was used to calculate BMI. Early pregnancy BMI was obtained prior to the 13th gestational week. Anthropometric measurements were standardized and performed by trained interviewers1212 Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign: Human Kinetics Pub;1988.. The BMI was classified into two categories, using the cutoff points proposed by the World Health Organization1312 Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign: Human Kinetics Pub;1988. (normal weight, 18.5–24.9 kg/m2; excessive weight, ≥25.0 kg/m2). The two BMI categories were created by joining underweight with normal-weight women and overweight with obese women; only seven women were underweight, and the sensitivity analysis showed no difference in terms of magnitude or significance in the results whether underweight women were included or excluded.
The gestational age (in weeks) was estimated using, preferably, ultrasound performed before the 26th week of pregnancy or, alternatively, the last menstrual period reported. The following variables were also considered in the analysis: maternal age (years); self-reported skin color (white/black/brown); parity (nulliparous/multiparous); current smoking status (yes/no); marital status (lives with a partner/does not live with a partner); education (<8/≥8 years) and practice of leisure time physical activity (LTPA) before pregnancy (yes/no). The dependent variables were tested for normality using the Shapiro-Wilk test. Possible differences in the distributions of the confounders, according to the BMI categories, were assessed using the chi-square test for proportions. The pattern in BP change was assessed using longitudinal linear regression models, which used SBP and DBP as dependent variables and gestational age and quadratic gestational age as time independent variables. In order to improve model adequacy, a quadratic term for gestational age was used. Prospective changes in SBP and DBP, according to early pregnancy BMI, were assessed with longitudinal linear regression after adjustment for confounders, including parity, current smoking status, marital status, years of education and LTPA. Comparisons between eligible women lost during the follow-up and the final sample were performed with chi-square test for proportions.
The statistical analyses were performed using Stata Data Analysis and Statistical Software (STATA) version 12.0 (Stata Corp., College Station, Texas, USA). Differences were considered statistically significant when the p-value < 0.05.
The study protocol was approved by the research ethics committee of the Municipal Secretary of Health of the city of Rio de Janeiro (registration number: 0139.0.314.000-09). All participants signed a two-way term of consent, which was obtained freely and spontaneously after all necessary explanations had been provided.
Results
The sample characteristics did not differ according to early pregnancy BMI categories (p>0.05). The majority of women were younger than 30 years (73.5%), brown or black (73.5%), multiparous (58.2%), lived with a partner (78.8%), had at least eight years of education (71.4%) and did not participate in LTPA prior to pregnancy (74.3%) (Table 1). None of these variables significantly differed between eligible women lost to follow-up and the final sample, indicating a non-selective loss (data not shown).
General characteristics of the study sample, according to early pregnancy Body Mass Index (BMI) of women followed at a public health center in the city of Rio de Janeiro, Brazil, 2009 - 2012
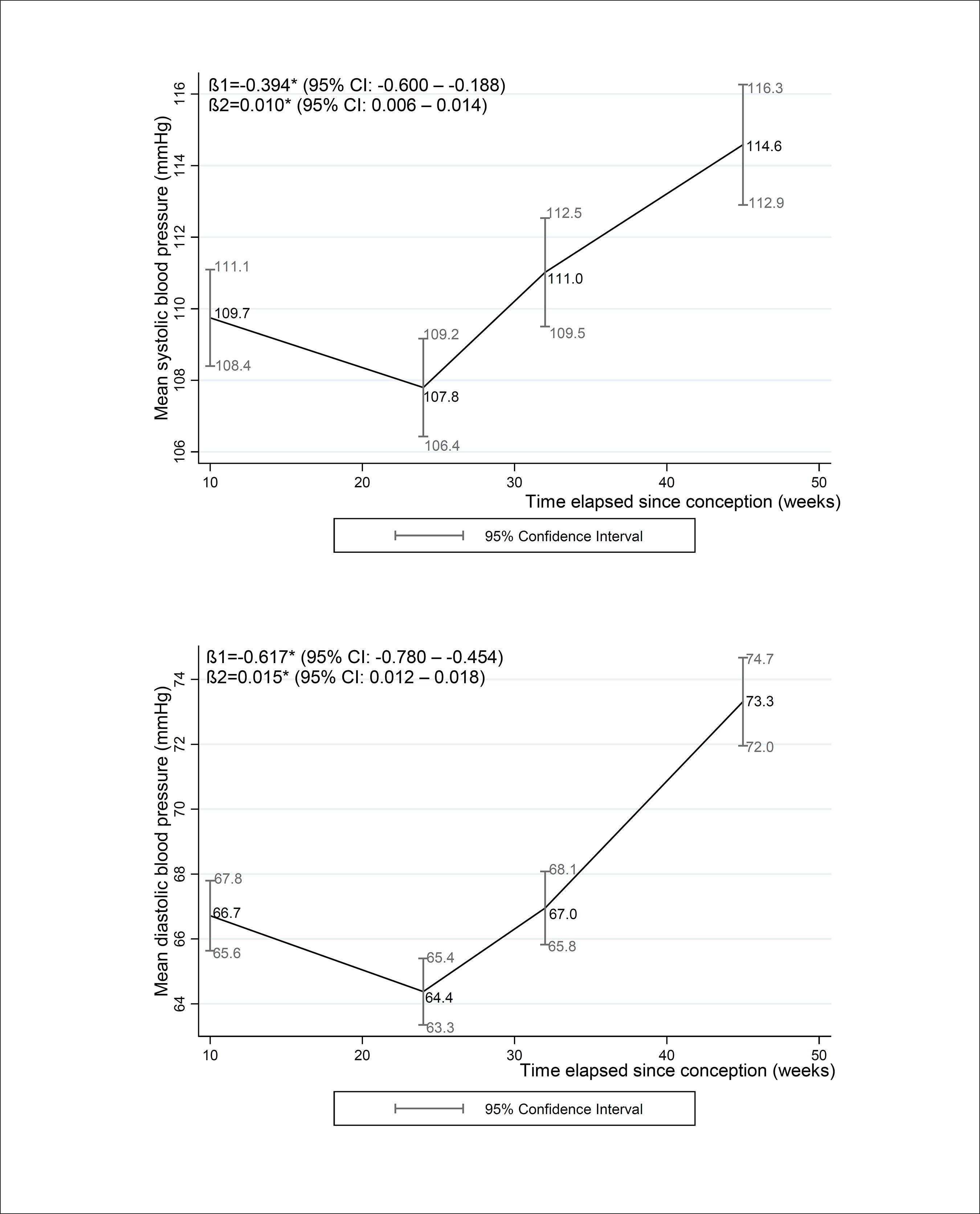
For the overall sample, the mean SBP values for the first, second and third trimesters and postpartum (95% CI) were 109.7 (108.4 – 111.1), 107.8 (106.4 – 109.2), 111.0 (109.5 – 112.5) and 114.6 (112.9 – 116.3), respectively. The mean DBP values for the first, second and third trimesters and postpartum were 66.7 (65.6 – 67.8), 64.4 (63.3 – 65.4), 67.0 (65.8 – 68.1), and 73.3 (72.0 – 74.7), respectively. The longitudinal regression coefficients for SBP and DBP decreased from the first to the second trimester [βSBP=-0.394 (-0.600 – -0.188), βDBP=-0.617 (-0.780 – -0.454)] and then increased from the second trimester to the postpartum period [βSBP=0.010 (0.006 – 0.014), βDBP=-0.015 (-0.767 – -0.442)] (Figure 2).
Mean systolic and diastolic blood pressure changes during pregnancy and 30-45 days post-partum of women followed at a public health center in the city of Rio de Janeiro, Brazil, 2009 - 2012
Note:β 1,2: the longitudinal linear regression coefficients for gestational age and quadratic gestational age, respectively; CI: Confidence interval. *p-value < 0.001 refers to maximum likelihood estimator. Mean (95% CI) gestational weeks or days postpartum and number of participants (n) in each follow-up evaluation: 1st trimester: 9.7 (9.4 – 10.0) weeks, n = 189; 2nd trimester = 23.7 (23.4 – 24.0) weeks, n=157; 3rd trimester: 32.4 (32.0 – 32.8) weeks, n = 162; postpartum: 36.4 (34.9 – 37.9) days, n = 153.
The mean SBP significantly differed among the BMI groups in all gestational trimesters. The mean DBP was significantly higher among overweight/obese women in the first and third trimesters only. Neither SBP nor DBP differed between BMI groups at postpartum. The longitudinal linear regression model showed that BMI was positively associated with prospective changes in SBP and DBP [β=7.055 (4.499 – 9.610) and β=3.201 (1.136 – 5.266), respectively] throughout pregnancy (Figure 3).
Mean systolic and diastolic blood pressure changes during pregnancy according to BMI categories of women followed at a public health center in the city of Rio de Janeiro, Brazil, 2009 - 2012
Note: β1: longitudinal linear regression coefficient for body mass index category excessive weight (reference category: normal weight) adjusted for gestational age, quadratic gestational age, parity, current smoking status, marital status, education, practice of leisure time physical activity before pregnancy. CI: confidence interval; normal-weight <25 kg/m2; excessive weight ≥ 25 kg/m2. *p-value <0.001 and **p-value: 0.002 refers to maximum likelihood estimator. Mean (95% CI) gestational weeks or days post-partum and number of participants (n) in each follow-up evaluation: 1st trimester: 9.7 (9.4 – 10.0) weeks, n = 189; 2nd trimester: 23.7 (23.4 – 24.0) weeks, n = 157; 3rd trimester: 32.4 (32.0 – 32.8) weeks, n = 162; postpartum: 36.4 (34.9 – 37.9) days, n = 153.
Discussion
The main findings of this study corroborate the known BP pattern during healthy pregnancy. The study women experienced a mid-trimester drop, followed by a progressive increase in SBP and DBP up to 30-45 postpartum days. Furthermore, our results indicated a strong association between early pregnancy BMI and SBP/DBP. Women who began pregnancy with a BMI in the overweight or obese categories presented higher values of SBP and DBP in all gestational trimesters.
Although many studies have shown this same BP pattern during pregnancy1414 Robson SC, Hunter S, Boys, RJ, Dunlop W. Serial study of factors
influencing changes in cardiac output during human pregnancy. Am J Physiol.
1989;256(4 Pt 2): H1060-5.
15 Ayala DE, Hermida RC, Mójon A, Fernández JR, Silva I, Ucieda R. Blood
pressure variability during gestation in healthy and complicated pregnancies.
Hypertension. 1997;30(3 Pt 2):611-8.
16 Thompson ML, Williams MA, Miller RS. Modelling the association of blood
pressure during pregnancy with gestational age and body mass index. Paediatr Perinat
Epidemiol. 2009;23(3):254-63.-1717 Grindheim G, Estensen M, Langesaeter E, Rosseland LA, Toska K. Changes
in blood pressure during healthy pregnancy: a longitudinal cohort study. J Hypertens.
2012;30(2):342-50., some authors have found different results, such as a mid-trimester BP
rise instead of a drop1818 Silva LM, Steegers EA, Burdorf A, Jaddoe VW, ArendsLR, Hofman A , et al.
No midpregnancy fall in diastolic blood pressure in women with a low educational
level: the Generation R Study. Hypertension. 2008;52(4):645-51.
19 Miller RS, Thompson ML, Williams MA. Trimester-specific blood pressure
levels in relation to maternal pre-pregnancy body mass index. Paediatr Perinat
Epidemiol, 2007;21(6):487-94.-2020 Nama V, Antonios TF, Onwude J, Manyonda IT. Mid-trimester blood pressure
drop in normal pregnancy: myth or reality? J Hypertens.
2011;29(4):763-8.. For this reason, the publication of new findings
is still necessary. The description of usual values and variations of SBP and DBP in
healthy pregnancies is important for prenatal practitioners to detect abnormal
variations that may be related to the onset of a disorder. Studies to elucidate this
issue should be encouraged.
Grindheim et al.1717 Grindheim G, Estensen M, Langesaeter E, Rosseland LA, Toska K. Changes in blood pressure during healthy pregnancy: a longitudinal cohort study. J Hypertens. 2012;30(2):342-50. have followed a cohort with four visits during pregnancy to evaluate BP variation. Their sample was very similar to ours in terms of age, parity, BMI, and gestational age at BP measurements. The main finding of this study was the statistically significant drop in SBP and DBP up to mid-pregnancy (22-24 weeks), followed by a progressive increase until delivery, which corroborates our results. However, their sample was smaller (n = 63) and comprised only Norwegian women, which is a very homogeneous population.1717 Grindheim G, Estensen M, Langesaeter E, Rosseland LA, Toska K. Changes in blood pressure during healthy pregnancy: a longitudinal cohort study. J Hypertens. 2012;30(2):342-50.
Nama et al.2020 Nama V, Antonios TF, Onwude J, Manyonda IT. Mid-trimester blood pressure drop in normal pregnancy: myth or reality? J Hypertens. 2011;29(4):763-8. have found a progressive increase in SBP and DBP in a sample of primiparous, healthy, white pregnant women residing in London. The authors have discussed the importance of conducting similar studies with heterogeneous populations, considering factors such as BMI. Other studies have also found progressive increases in SBP in homogeneous populations1818 Silva LM, Steegers EA, Burdorf A, Jaddoe VW, ArendsLR, Hofman A , et al. No midpregnancy fall in diastolic blood pressure in women with a low educational level: the Generation R Study. Hypertension. 2008;52(4):645-51.-1919 Miller RS, Thompson ML, Williams MA. Trimester-specific blood pressure levels in relation to maternal pre-pregnancy body mass index. Paediatr Perinat Epidemiol, 2007;21(6):487-94.. However, there are no studies that have monitored BP in healthy, adult, pregnant women from Brazil, a country composed of a very heterogeneous population.
Another point to be considered is the mean SBP and DBP values in our study. Other
similar investigations have found markedly higher values in all pregnancy
trimesters1919 Miller RS, Thompson ML, Williams MA. Trimester-specific blood pressure
levels in relation to maternal pre-pregnancy body mass index. Paediatr Perinat
Epidemiol, 2007;21(6):487-94.,2121 Bouthoorn SH, Gaillard R, Steegers EA, Hofman A, Jaddoe VW, van Lenthe
FJ. Ethnic differences in blood pressure and hypertensive complications during
pregnancy: the Generation R study. Hypertension. 2012;60(1):198-205.
22 Ohkuchi A, Iwasaki R, Suzuki H, Hirashima C, Takahashi K, Usui R, et al.
Normal and high-normal blood pressures, but not body mass index, are risk factors for
the subsequent occurrence of both preeclampsia and gestational hypertension: a
retrospective cohort study. Hypertens Res. 2006;29(3):161-7.-2323 Macdonald-Wallis C, Silverwood RJ, Fraser A, Nelson SM, Tilling K,
Lawlor DA, de Stavola BL. Gestational-age-specific reference ranges for blood
pressure in pregnancy: findings from a prospective cohort. J
Hypertens.2015;33(1):96-105., similar to
the highest BMI group in our sample. In a recent study, MacDonald-Wallis et al.2323 Macdonald-Wallis C, Silverwood RJ, Fraser A, Nelson SM, Tilling K,
Lawlor DA, de Stavola BL. Gestational-age-specific reference ranges for blood
pressure in pregnancy: findings from a prospective cohort. J
Hypertens.2015;33(1):96-105., in an attempt to establish BP reference values
during pregnancy, have found higher mean SBP and DBP values at 12 and 37 weeks of normal
pregnancies as compared to our results. Given that the possibility of changing the
cutoff points for the diagnosis of HDP has been discussed, it is important to consider
the differences in BP values for different populations and BMI categories2424 Espinoza J. The need to redefine preeclampsia. Expert Opin Med Diagn.
2012;6(4):347-57..
Although a similar pattern of variability in normal-weight and excessive-weight women was observed, those who began pregnancy as overweight or obese showed significantly higher values of SBP in all trimesters and of DBP in the first and third trimesters of pregnancy. Similar results have been observed in other populations1616 Thompson ML, Williams MA, Miller RS. Modelling the association of blood pressure during pregnancy with gestational age and body mass index. Paediatr Perinat Epidemiol. 2009;23(3):254-63.,1919 Miller RS, Thompson ML, Williams MA. Trimester-specific blood pressure levels in relation to maternal pre-pregnancy body mass index. Paediatr Perinat Epidemiol, 2007;21(6):487-94.. This indicates that the normal variation of BP is different between BMI categories. If a woman with a normal BMI has a SBP or DBP level below 140 or 90 mm Hg, respectively, but above the mean for her BMI, it may indicate an increased risk for adverse outcomes when compared with a woman who initiated pregnancy obese and has similar absolute BP values.
Some authors consider postpartum BP as the normal BP of nonpregnant women; postpartum BP is sometimes used as a pre-pregnancy measure1717 Grindheim G, Estensen M, Langesaeter E, Rosseland LA, Toska K. Changes in blood pressure during healthy pregnancy: a longitudinal cohort study. J Hypertens. 2012;30(2):342-50.. Extrapolating for our results, it can be said that there is no BP variability outside pregnancy between BMI groups. Putting together, the significant difference of BP between groups in the first trimester indicates that the BP drop in early pregnancy is probably higher among those with a lower BMI.
Some strengths and limitations of this study should be highlighted. This is the first study to assess longitudinal BP data in a group of Brazilian pregnant women. Furthermore, we used a robust statistical analysis, considering the data as repeated measures, not merely comparing means. As limitations, the evaluation of additional points during pregnancy would provide a more complete pattern of variability. Moreover, the use of ambulatory blood pressure monitoring to measure variations throughout the day would be important. Another potential limitation is the loss to follow-up of 11.3% of study participants. However, the statistical technique employed to investigate the influence of BMI on BP is efficient even when there are some values missing from the study sample2525 Pinheiro J, Bates D. Mixed effects models in S and S-Plus. New York: Springer; 2000..
Conclusion
This study provides new data about the pattern of BP variability throughout pregnancy, an issue that has been of great interest in recent years. We found that SBP and DBP decreased from early to mid-pregnancy and then increased up to 30-45 postpartum days. The findings also reinforced the role of BMI on SBP and DBP, highlighting the importance of considering this variable in studies assessing BP in pregnancy, as well as during prenatal care monitoring.
-
Sources of FundingThis study was funded by CNPq and FAPERJ.
-
Study AssociationThis study is not associated with any thesis or dissertation work.
Acknowledgments
We would like to thank our funding sources: National Council for Scientific and Technological Development (CNPq) and Carlos Chagas Filho Foundation for Research Support of Rio de Janeiro State (FAPERJ). Kac G is research fellow from CNPq. Rebelo F has received a scholarship from National School of Public Health (Oswaldo Cruz Foundation), and Farias DR and Mendes RH have received scholarships from CAPES during the development of the study.
References
-
1Ouzounian JG, Elkayam U. Physiologic changes during normal pregnancy and delivery. Cardiol Clin. 2012;30(3):317-29.
-
2Metoki H, Ohkubo T, Sato Y, Kawaguchi M, Nishimura M, Watanabe Y. et al. Detection of midpregnancy fall in blood pressure by out-of-office monitoring. Hypertension. 2009;53(2):e12-3, author reply e14.
-
3Tranquilli AL. Mid-trimester blood pressure in pregnancy. Blood pressure fall or fall of a myth? J Hypertens. 2011;29(4):658-9.
-
4Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183(1):S1-S22.
-
5Vest AR, Cho LS.Hypertension in pregnancy. Cardiol Clin. 2012;30(3):407-23.
-
6Ehrenthal DB, Jurkovitz C, Hoffman M, Jiang X, Weintraub WS. Prepregnancy body mass index as an independent risk factor for pregnancy-induced hypertension. J Womens Health (Larchmt). 2011;20(1):67-72.
-
7Gaillard R, Bakker R, Willemsen SP, Hofman A, Steegers EA, Jaddoe VW. Blood pressure tracking during pregnancy and the risk of gestational hypertensive disorders: the Generation R Study. Eur Heart J. 2011;32(24):3088-97.
-
8Scott-Pillai R, Cardwell C, Hunter A, Holmes V. The impact of body mass index on maternal and neonatal outcomes: a retrospective study in a UK obstetric population, 2004-2011. BJOG. 2013;120(8):932-9.
-
9O'Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14(3):368-74.
-
10Coleman A, Steel S, Shennan A. Validation of the Omron MX3 Plus oscillometric blood pressure monitoring device according to the European Society of Hypertension international protocol. Blood Press Monit. 2005;10(3):165-8.
-
11Pickering TG, Hall JE, Appel L J, Falkner BE , Graves J , Hill MN. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111(5): 697-716.
-
12Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign: Human Kinetics Pub;1988.
-
13Maternal anthropometry and pregnancy outcomes. A WHO Collaborative Study. Bull World Health Organ. 1995;73( Suppl): 1-98.
-
14Robson SC, Hunter S, Boys, RJ, Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol. 1989;256(4 Pt 2): H1060-5.
-
15Ayala DE, Hermida RC, Mójon A, Fernández JR, Silva I, Ucieda R. Blood pressure variability during gestation in healthy and complicated pregnancies. Hypertension. 1997;30(3 Pt 2):611-8.
-
16Thompson ML, Williams MA, Miller RS. Modelling the association of blood pressure during pregnancy with gestational age and body mass index. Paediatr Perinat Epidemiol. 2009;23(3):254-63.
-
17Grindheim G, Estensen M, Langesaeter E, Rosseland LA, Toska K. Changes in blood pressure during healthy pregnancy: a longitudinal cohort study. J Hypertens. 2012;30(2):342-50.
-
18Silva LM, Steegers EA, Burdorf A, Jaddoe VW, ArendsLR, Hofman A , et al. No midpregnancy fall in diastolic blood pressure in women with a low educational level: the Generation R Study. Hypertension. 2008;52(4):645-51.
-
19Miller RS, Thompson ML, Williams MA. Trimester-specific blood pressure levels in relation to maternal pre-pregnancy body mass index. Paediatr Perinat Epidemiol, 2007;21(6):487-94.
-
20Nama V, Antonios TF, Onwude J, Manyonda IT. Mid-trimester blood pressure drop in normal pregnancy: myth or reality? J Hypertens. 2011;29(4):763-8.
-
21Bouthoorn SH, Gaillard R, Steegers EA, Hofman A, Jaddoe VW, van Lenthe FJ. Ethnic differences in blood pressure and hypertensive complications during pregnancy: the Generation R study. Hypertension. 2012;60(1):198-205.
-
22Ohkuchi A, Iwasaki R, Suzuki H, Hirashima C, Takahashi K, Usui R, et al. Normal and high-normal blood pressures, but not body mass index, are risk factors for the subsequent occurrence of both preeclampsia and gestational hypertension: a retrospective cohort study. Hypertens Res. 2006;29(3):161-7.
-
23Macdonald-Wallis C, Silverwood RJ, Fraser A, Nelson SM, Tilling K, Lawlor DA, de Stavola BL. Gestational-age-specific reference ranges for blood pressure in pregnancy: findings from a prospective cohort. J Hypertens.2015;33(1):96-105.
-
24Espinoza J. The need to redefine preeclampsia. Expert Opin Med Diagn. 2012;6(4):347-57.
-
25Pinheiro J, Bates D. Mixed effects models in S and S-Plus. New York: Springer; 2000.
Publication Dates
-
Publication in this collection
13 Feb 2015 -
Date of issue
Apr 2015
History
-
Received
09 July 2014 -
Reviewed
07 Oct 2014 -
Accepted
02 Dec 2014