Abstract
Introduction:
Cardiac allograft vasculopathy (CAV) is a major limitation for long-term survival of patients undergoing heart transplantation (HT). Some immunosuppressants can reduce the risk of CAV.
Objectives:
The primary objective was to evaluate the variation in the volumetric growth of the intimal layer measured by intracoronary ultrasound (IVUS) after 1 year in patients who received basiliximab compared with that in a control group.
Methods:
Thirteen patients treated at a single center between 2007 and 2009 were analyzed retrospectively. Evaluations were performed with IVUS, measuring the volume of a coronary segment within the first 30 days and 1 year after HT. Vasculopathy was characterized by the volume of the intima of the vessel.
Results:
Thirteen patients included (7 in the basiliximab group and 6 in the control group). On IVUS assessment, the control group was found to have greater vessel volume (120–185.43 mm3 vs. 127.77–131.32 mm3; p = 0.051). Intimal layer growth (i.e., CAV) was also higher in the control group (27.30–49.15 mm3 [∆80%] vs. 20.23–26.69 mm3 [∆33%]; p = 0.015). Univariate regression analysis revealed that plaque volume and prior atherosclerosis of the donor were not related to intima growth (r = 0.15, p = 0.96), whereas positive remodeling was directly proportional to the volumetric growth of the intima (r = 0.85, p < 0.001).
Conclusion:
Routine induction therapy with basiliximab was associated with reduced growth of the intima of the vessel during the first year after HT.
Keywords
Vascular Diseases / physiopathology; Heart Transplantation; Antibodies, Monoclonal, Murine-Derived / admininstration & dosage; Immunosuppressive Agents
Resumo
Fundamento:
A doença vascular do enxerto (DVE) constitui uma grande limitação de sobrevida a longo prazo de pacientes submetidos a transplante cardíaco (TxC). Alguns imunossupressores diminuem o aparecimento da DVE.
Objetivos:
O principal objetivo foi avaliar, através de ultrassonografia intracoronária (USIC), a variação do crescimento volumétrico da camada íntima e comparar, após um ano, o grupo que recebeu basiliximab com um grupo de controle.
Métodos:
Treze pacientes de um único centro foram analisados retrospectivamente de 2007 a 2009. As análises foram feitas através de USIC, medindo-se o volume de um segmento coronariano nos primeiros 30 dias e um ano após o TxC. A vasculopatia foi caracterizada pelo volume da camada íntima do vaso.
Resultados:
O estudo incluiu 13 pacientes (7 no grupo com o basiliximab e 6 no grupo de controle). A análise por USIC revelou que o grupo de controle apresentou maior crescimento volumétrico do vaso (131,32 a 127,77 mm3 x 120 a 185,43 mm3 p = 0,051). O crescimento da camada íntima (CCI) também foi maior no grupo de controle [Basiliximab: 20,23 a 26,69 mm3 (∆ 33%); Controle: 27,30 a 49,15 mm3 (∆ 80% p = 0,015)]. De acordo com a regressão univariada, o volume da placa aterosclerótica prévia do doador não teve relação com o crescimento da íntima (r = 0,15, p = 0,96), enquanto que o remodelamento positivo do vaso foi diretamente proporcional ao crescimento da íntima (r = 0,85, p < 0,001).
Conclusão:
A terapia de indução de rotina com basiliximab está associada à redução do crescimento da camada íntima do vaso no primeiro ano após o transplante cardíaco.
Palavras-chave
Doenças Vasculares / fisiopatologia; Transplante Cardíaco; Anticorpos Monoclonais Murinos / administração & dosagem; Imunossupressores
Introduction
With increased survival among heart transplantation (HT) patients, mainly due to improvements in immunosuppression, the incidence of late complications, including cardiac allograft vasculopathy (CAV)11 Houser S, Muniappan A, Allan J, Sachs D, Madsen J. Cardiac allograft vasculopathy: real or a normal morphologic variant? J Heart Lung Transplant. 2007;26(2):167-73., has increased. CAV is characterized by progressive obliteration of vessels due to intimal proliferation and is considered a major cause of graft dysfunction in the first year after HT and the second most common cause of long-term death22 Stehlik J, Edwards LB, Kucheryavaya AY, Aurora P, Christie JD, Kirk R, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult heart transplant report -- 2010. J Heart Lung Transplant. 2010;29(10):1089-103..
Lymphocytes play an important role in both acute and chronic graft rejection. The immunological and non-immunological factors implicated in the pathogenesis of CAV converge by activating T lymphocytes (TL)33 Benza RL, Tallaj J. Cardiac Allograft Vasculopathy (Chronic Rejection). In: Kirklin JK, Young JB, McGiffin DC. (eds.). Heart transplantation. 3rd ed. Philadelphia: Churchill Livingstone; 2002. p. 615-65., as demonstrated by Nagano et al.44 Nagano H, Libby P, Taylor MK, Hasegawa S, Stinn JL, Becker G, et al. Coronary arteriosclerosis after T-cell-mediated injury in transplanted mouse hearts: role of interferon-gamma. Am J Pathol. 1998;152(5):1187-97.. Animal models in which these cells were blocked did not develop vasculopathy55 Uehara S, Chase CM, Colvin RB, Madsen JC, Russell PS. T-cell depletion eliminates the development of cardiac allograft vasculopathy in mice rendered tolerant by the induction of mixed chimerism. Transplant Proc. 2006;38(10):3169-71.. Thus, T lymphocyte blockade has been the objective of therapies for the prevention of CAV66 Valantine HA. Cardiac allograft vasculopathy: central role of endothelial injury leading to transplant "atheroma". Transplantation. 2003;76(6):891-9..
Basiliximab is a chimeric antibody receptor antagonist of interleukin 2 (IL-2) and is indicated in induction therapy for patients at high risk of rejection after organ transplantation77 Bacal F, Souza-Neto J, Fiorelli A, Mejia J, Marcondes-Braga FG, Mangini S, et al. II Brazilian Guidelines for cardiac transplantation. Arq Bras Cardiol. 2010;941 Suppl):e16-76.. IL-2 is a potent immunomodulator that plays an important role in the activation and maintenance of the immune response and lymphocyte proliferation88 Church AC. Clinical advances in therapies targeting the interleukin-2 receptor. QJM. 2003;96(2):91-102.; furthermore, it is a key step in the development of acute rejection99 Onrust SV, Wiseman LR. Basiliximab. Drugs. 1999;57(2):207-13.. Blockage of TL proliferation and reduced acute rejection can delay the onset of CAV1010 Young JB, Lloyd KS, Windsor NT, Cocanougher B, Weilbaecher DG, Kleiman NS, et al. Elevated soluble interleukin-2 receptor levels early after heart transplantation and long-term survival and development of coronary arteriopathy. J Heart Lung Transplant. 1991;10(2):243-50.. The aim of this study was to determine whether blockage of IL-2 with basiliximab early in the transplantation process has an effect superior to placebo in decreasing the growth of the vessel intima during the first year following HT.
Methods
We conducted a retrospective analysis of the database from a single center, including patients who underwent HT from September 2007 through March 2009. The patients were separated in two groups according to the induction therapy: those treated with basiliximab (Simulect®; Novartis, NJ, USA) and those who received no induction therapy (control group). In our institution the use of basiliximab became routine in July 2008; therefore, a comparison was made to a series of cases before and after this period. In this period, there was no difference regarding surgical technique, preservation, or other adjuvant medications. We included only patients who had clinical and ultrasound follow-up for at least 1 year. We excluded patients who did not comply with intravascular ultrasound (IVUS) follow-up or whose images in the database were inadequate to allow such analysis. The study was approved by local Ethics Committee (protocol 0005154/11).
Endpoints
The primary objective was to compare the two groups with regard to volumetric growth of the intimal layer measured by IVUS after 1 year. The secondary objective was to evaluate the remodeling of the vessel and lumen volume and donor atherosclerosis.
Immunosuppression protocol
Immunosuppression was performed in the basiliximab group at a dose of 20 mg IV, together with 500 mg methylprednisolone in three daily doses and 150 mg mycophenolate mofetil (MMF) in two doses on the day of transplantation; on the fifth day, another dose of 20 mg IV basiliximab was administered; on that day, therapy with cyclosporine was initiated. In the control group, immunosuppression was conducted with methylprednisolone and MMF at the same dosage; in addition, cyclosporine was initiated on the day of transplantation at the same dosage.
Evaluation of vasculopathy
As part of the HT protocol, patients are routinely evaluated with angiography and intracoronary ultrasound (IVUS) only at the left anterior descending (LAD) artery. This evaluation is performed 30 days after HT and then repeated annually.
Coronary angiography and IUVS were performed concurrently with an endomyocardial biopsy. To perform the procedure, a 6F introducer was introduced into the femoral artery, followed by catheterization of the left coronary artery. Unfractionated heparin (100 IU/kg) was instilled intravenously together with an intracoronary dose of isosorbide mononitrate (10 mg). The ultrasound examination was performed with an Atlantis® catheter (Boston Scientific Scimed Inc., Maple Grove, Minn.) and a 4.3 Fr catheter with a 40-MHz transducer. The IVUS catheter was positioned in the distal LAD artery; automatic pullback was performed with a velocity of 1 mm/s and an acquisition rate of 30 frames/s. The images were stored on a compact disk and analyzed using ILab® software (Boston Scientific Scimed, Inc.).
IVUS Analysis
To provide monitoring of the same segment, a 10-mm segment was selected just after the output of the first diagonal. Segment analysis was methodologically validated in a manner similar to that previously described1111 Mehran R, Mintz GS, Hong MK, Tio FO, Bramwell O, Brahimi A, et al. Validation of the in vivo intravascular ultrasound measurement of in-stent neointimal hyperplasia volumes. J Am Coll Cardiol. 1998;32(3):794-9.,1212 Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis. JAMA. 2006;295(13):1556-65.. Analysis was performed on the first computed tomography (CT) slice after the departure of the diagonal branch, marking the beginning of the segment; then each image is evaluated every 30 cuts (1-mm interval between analyses), until 10 segment images (10 mm) are completed. The analysis consists of a manual outlining of the lumen and external elastic membrane (EEM), calculating the lumen area and EEM area. Measurements were performed as standardized by the American College of Cardiology/European Society of Cardiology1313 Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37(5):1478-92.. The intimal area was calculated by subtracting the area of the lumen minus EEM. Calculation of the volume of the vessel lumen and intima was carried out using the method described by Simpson1111 Mehran R, Mintz GS, Hong MK, Tio FO, Bramwell O, Brahimi A, et al. Validation of the in vivo intravascular ultrasound measurement of in-stent neointimal hyperplasia volumes. J Am Coll Cardiol. 1998;32(3):794-9.. The volume percent was calculated according the following formula: {∑ (EEM area − lumen area)/∑ EEem area × 100.
Statistical Analysis
Continuous data were expressed as median plus 25th and 75th percentiles. Categorical data were expressed as absolute numbers. Nonparametric tests were used to evaluate differences in continuous data, and due to the small sample size, we used Mann–Whitney test for evaluation of the differences in IVUS findings. A simple linear regression model was used to assess the relationship between previous atherosclerosis and intimal growth as well as the relationship between intimal and vessel growth after 1 year, using Pearson correlation coefficients. For categorical data, the differences were evaluated using Fisher's exact test. A two sided p-value < 0.05 was required for statistical significance. Analyses were performed with SPSS 12.0 software (Chicago, IL, USA).
Results
In the period from 2007–2009, 23 HTs were performed in our institution. Two patients died during the perioperative period, and three during the first year of follow-up. Two patients were excluded from the present study due to inadequate IVUS images, and 3 patients only underwent IVUS study beyond 13 months of follow-up. We evaluated 13 patients, of whom 7 received basiliximab (basiliximab group) and 6 did not (control group). Demographic data are listed in Table 1. The patients were predominantly male (n = 10); the median age was 55 years in the basiliximab group and 47.5 years in the control group. Three patients in the control group developed acute renal failure in the postoperative period, characterized by a serum creatinine > 0.5 mg/dL, whereas no patients in the basiliximab group developed this complication. The levels of total cholesterol, triglycerides, and angiotensin receptors were similar between the groups (p = NS), and creatinine levels were somewhat higher in the control group. The use of inhibitors and statins was higher in the basiliximab group. Only a few patients received everolimus/sirolimus during follow-up: one in the basiliximab group and two in the control group. However, all patients received mycophenolate mofetil. No patient received a diagnosis of cytomegalovirus confirmed by serology. The number of rejection episodes was similar in both groups. Two patients in the basiliximab group and three in the control group underwent a biopsy with 2R; they required hospitalization and underwent pulse therapy with intravenous corticosteroids.
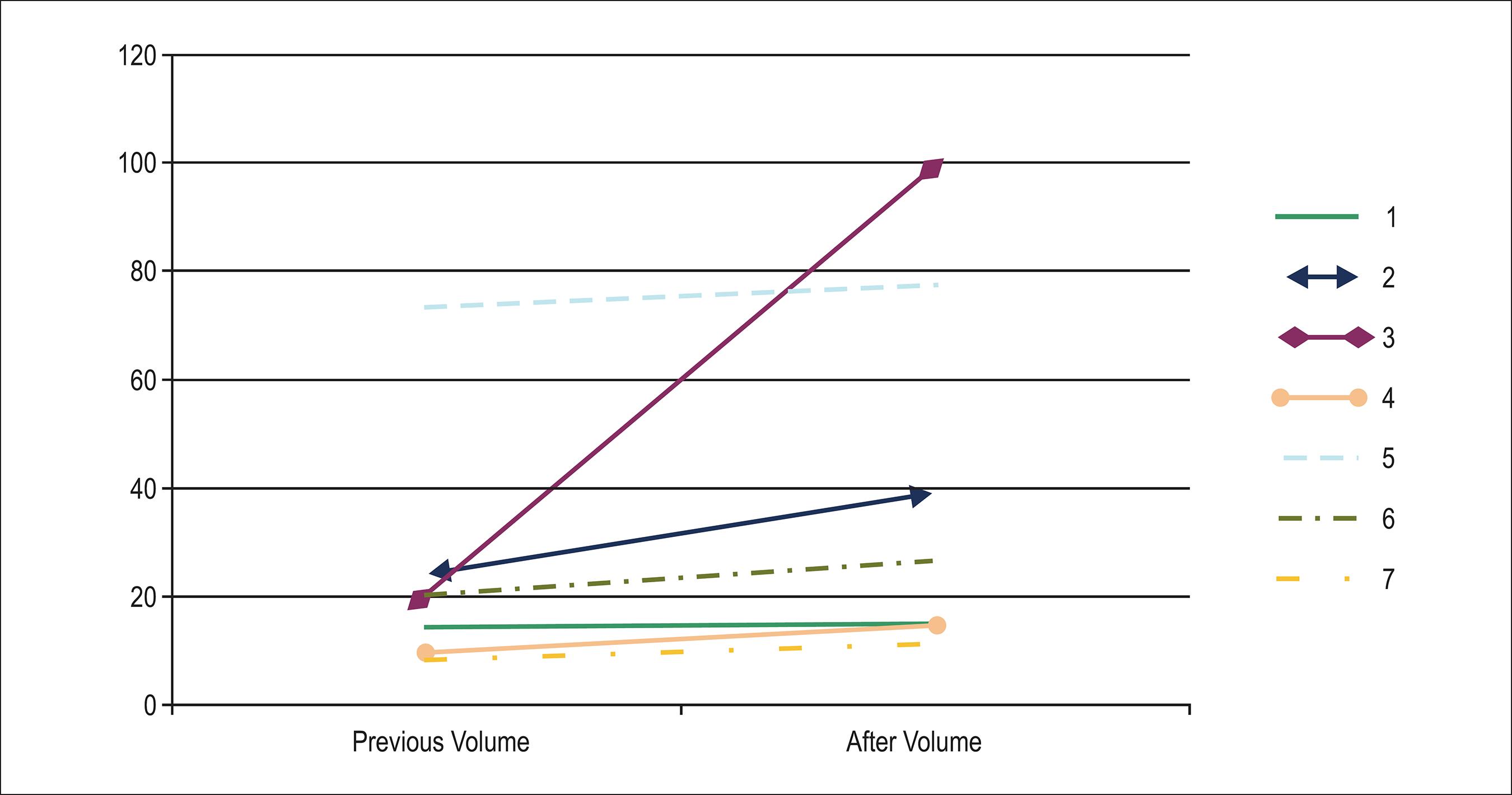
Coronary angiography performed during the first year following HT did not detect the presence of significant vascular disease (e.g., CAV), based on the new classification of the International Society of Heart and Lung Transplantation (ISHLT)1414 Mehra MR, Crespo-Leiro MG, Dipchand A, Ensminger SM, Hiemann NE, Kobashigawa JA, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant. 2010;29(7):717-27.. The data obtained by IVUS are presented in Table 2 and Graph 1. In the control group, vessel volume (delineated by the EEM) exhibited positive remodeling (increase in volume growth of 49.39 mm3), whereas in the basiliximab group, the effect was reversed (negative remodeling: –4.17 mm3), with a trend toward statistical significance (p = 0.051). The findings were similar with regard to luminal volume (-11.53 × 17.3 mm3; p = 0.051). Regarding the intimal layer (plate), a higher rate of growth (follow-up volume minus baseline volume) occurred in the control group (baseline value: 27.3 mm3; control group: 49.15 mm3; basiliximab group: 20.23–26.69 mm3; p = 0.015; Graphs 2 and 3).
Analysis of the volume change at 1 year after transplantation. A: Variation in the vessel. B: Variation in the lumen. C: Variation in the intima
In simple linear regression analysis assessment (Graph 4B), previous atherosclerosis was not associated with increased growth of the intima (r = 0.15; p = 0.96). Positive remodeling (increase in EEM) was associated with a greater increase in intimal volume (r = 0.85; p < 0.001; Graph 4A).
A. Vessel growth according to intimal growth. B. Impact of previous plaque atherosclerosis on CAV growth
Discussion
This study revealed the following findings. (1) The use of induction therapy with basiliximab was associated with less intimal tissue growth in the first year after HT. (2) In the control group, we observed greater positive remodeling, which was probably related to increased intimal growth observed in this group. (3) With simple linear regression analysis, vessel growth was proportional to the increase of the plaque regardless of induction therapy. (4) Atherosclerosis in the donor was not associated with increased growth of the intima.
Graft vascular disease begins with endothelial injury, followed by a repair process, cell proliferation, and accumulation of extracellular matrix33 Benza RL, Tallaj J. Cardiac Allograft Vasculopathy (Chronic Rejection). In: Kirklin JK, Young JB, McGiffin DC. (eds.). Heart transplantation. 3rd ed. Philadelphia: Churchill Livingstone; 2002. p. 615-65.,66 Valantine HA. Cardiac allograft vasculopathy: central role of endothelial injury leading to transplant "atheroma". Transplantation. 2003;76(6):891-9.. The degree of organ preservation, ischemia/reperfusion injury, acute rejection, and viral infection (particularly cytomegalovirus) are cited as the main non-immunological factors that affect the endothelium in the first year after HT. In response to injury, endothelial cells express cell adhesion molecules (vascular cell adhesion molecule, intercellular cell adhesion molecule, and selectins); furthermore, recruitment of inflammatory cells and release of proinflammatory cytokines occur. This results in a vicious cycle of chronic inflammation, culminating in the obliteration of the lumen1515 Yamani MH, Haji SA, Starling RC, Tuzcu EM, Ratliff NB, Cook DJ, et al. Myocardial ischemic-fibrotic injury after human heart transplantation is associated with increased progression of vasculopathy, decreased cellular rejection and poor long-term outcome. J Am Coll Cardiol. 2002;39(6):970-7.
16 Land W, Messmer K, Events E. The impact of ischemia/reperfusion injury on specific and non-specific, early and late chronic events after organ transplantation. Transplant Rev. 1996;10:108-27.-1717 Day JD, Rayburn BK, Gaudin PB, Baldwin WM 3rd, Lowenstein CJ, Kasper EK, et al. Cardiac allograft vasculopathy: the central pathogenetic role of ischemia-induced endothelial cell injury. J Heart Lung Transplant. 1995;14(6 Pt 2):S142-9..
Growth inhibition by basiliximab, which exhibits its action 4–6 weeks after infusion99 Onrust SV, Wiseman LR. Basiliximab. Drugs. 1999;57(2):207-13., reinforces the relationship between early recruitment of lymphocytes and the appearance of CAV66 Valantine HA. Cardiac allograft vasculopathy: central role of endothelial injury leading to transplant "atheroma". Transplantation. 2003;76(6):891-9.. Tori et al.1818 Tori M, Kitagawa-Sakakida S, Li Z, Izutoni H, Horiguchi K, Ito T, et al. Initial T-cell activation required for transplant vasculopathy in retransplanted rat cardiac allografts. Transplantation. 2000;70(5):737-46. and Young et al.1919 Young JB, Windsor NT, Kleiman NS, Lowry R, Cocanougher B, Lawrence EC. The relationship of soluble interleukin-2 receptor levels to allograft arteriopathy after heart transplantation. J Heart Lung Transplant. 1992;11(3 Pt 2):S79-82. observed that the infiltration and activation of lymphocytes in the first days after HT are already sufficient for the appearance of CAV. The specific activation pathway of major histocompatibility complex II and proliferation of Th1 lymphocytes are considered to be the primary route of CAV formation2020 Mitchell RN. Graft vascular disease: immune response meets the vessel wall. Annu Rev Pathol. 2009;4:19-47.. Blocking various parts of this pathway has been proven effective in reducing the appearance of CAV2121 Eisen HJ, Tuzcu EM, Dorent R, Kobashigawa J, Mancini D, Valantine-von Kaeppler HA, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003;349(9):847-58.. IL-2 also plays a major role in the activation pathway of T helper 1 (Th1) lymphocytes, and this could explain the benefit of the use of basiliximab in the first weeks after HT to interrupt the cycle of injury and repair, thus preventing the chronic inflammatory process.
The reduction of intimal growth induction therapy is not a new finding2222 Zhang R, Haverich A, Strüber M, Simon A, Bara C. Delayed onset of cardiac allograft vasculopathy by induction therapy using anti-thymocyte globulin. J Heart Lung Transplant. 2008;27(6):603-9.. Zhang et al.2222 Zhang R, Haverich A, Strüber M, Simon A, Bara C. Delayed onset of cardiac allograft vasculopathy by induction therapy using anti-thymocyte globulin. J Heart Lung Transplant. 2008;27(6):603-9. observed that induction therapy with antithymocyte antibody (antithymocyte globulin, ATG) delays the onset of CAV. However, the effect did not translate into increased long-term survival. In addition, a higher incidence of cancer is observed in patients treated with ATG, which may explain the higher late mortality rate in this group. Long-term follow-up is indicated to determine the benefit and/or clinical harm of this therapy. As basiliximab is not associated with increased infection or neoplasia99 Onrust SV, Wiseman LR. Basiliximab. Drugs. 1999;57(2):207-13.,2323 Chapman TM, Keating GM. Basiliximab: a review of its use as induction therapy in renal transplantation. Drugs. 2003;63(24):2803-35., we expect a clinical benefit.
In the global registry of the ISHLT, the use of basiliximab for induction therapy has a neutral effect on CAV (relative risk [RR]: 1.16; confidence interval [CI]: 0.99–1.37); however, CAV increased with the use of muromonab-CD3 (OKT3; RR: 1.17; p = 0.038)22 Stehlik J, Edwards LB, Kucheryavaya AY, Aurora P, Christie JD, Kirk R, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult heart transplant report -- 2010. J Heart Lung Transplant. 2010;29(10):1089-103.. This effect is probably due to selection bias. Patients with a higher risk of acute rejection in the post-transplantation period and those who have higher levels of a reactor panel of antibodies (PRA) are at greatest risk of developing CAV2424 Kobashigawa JA, Patel JK, Kittleson MM, Kawano MA, Kiyosaki KK, Davis SN, et al. The long-term outcome of treated sensitized patients who undergo heart transplantation. Clin Transplant. 2011;25(1):E61-7.,2525 Feingold B, Bowman P, Zeevi A, Girnita AL, Quivers ES, Miller SA, et al. Survival in allosensitized children after listing for cardiac transplantation. J Heart Lung Transplant. 2007;26(6):565-71.. Another example of selection bias occurs with induction therapy, correlates with IL-2 receptor antagonists, and a risk of renal dysfunction, and this medication is indicated for patients at high risk for renal failure after transplantation2626 Delgado DH, Miriuka SG, Cusimano RJ, Feindel C, Rao V, Ross HJ. Use of basiliximab and cyclosporine in heart transplant patients with pre-operative renal dysfunction. J Heart Lung Transplant. 2005;24(2):166-9..
As in atherosclerosis2727 Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316(22):1371-5., we observed positive remodeling to accommodate the increase of the intima, thus avoiding involvement of the arterial lumen. In previous studies, most intimal tissue growth and positive remodeling occurred during the first year post-HT2828 Tsutsui H, Ziada KM, Schoenhagen P, Tyisoy A, Magyar WA, Crowe TD, et al. Lumen loss in transplant coronary artery disease is a biphasic process involving early intimal thickening and late constrictive remodeling: results from a 5-year serial intravascular ultrasound study. Circulation. 2001;104(6):653-7.,2929 Yeung AC, Davis SF, Hauptman PJ, Kobashigawa JA, Miller LW, Valantine HA, et al. Incidence and progression of transplant coronary artery disease over 1 year: results of a multicenter trial with use of intravascular ultrasound. Multicenter Intravascular Ultrasound Transplant Study Group. J Heart Lung Transplant. 1995;14(6 Pt 2):S215-20.. From the second year onward, despite a lower growth of the intima, there is greater involvement of the arterial lumen due to negative vessel remodeling2828 Tsutsui H, Ziada KM, Schoenhagen P, Tyisoy A, Magyar WA, Crowe TD, et al. Lumen loss in transplant coronary artery disease is a biphasic process involving early intimal thickening and late constrictive remodeling: results from a 5-year serial intravascular ultrasound study. Circulation. 2001;104(6):653-7.. We found variation in the natural history of the process in patients treated with basiliximab. We also found a slight decrease in vessel remodeling and luminal volume reduction; however, to date, we do not know how it will progress following the second year.
In our institution, induction therapy with basiliximab is routinely performed with the goal of delaying the onset of the need for caucineurin inhibitors and minimizing the nephrotoxic effects of cyclosporin2626 Delgado DH, Miriuka SG, Cusimano RJ, Feindel C, Rao V, Ross HJ. Use of basiliximab and cyclosporine in heart transplant patients with pre-operative renal dysfunction. J Heart Lung Transplant. 2005;24(2):166-9.,3030 Anselm A, Cantarovich M, Davies R, Grenon J, Haddad H. Prolonged basiliximab use as an alternative to calcineurin inhibition to allow renal recovery late after heart transplantation. J Heart Lung Transplant. 2008;27(9):1043-5.. Candidates for HT have a high prevalence of renal dysfunction; furthermore, after HT, renal function may deteriorate, particularly because of the use of nephrotoxic drugs, low cardiac output, and impaired cardiopulmonary bypass. Moreover, acute renal failure is associated with a poor outcome22 Stehlik J, Edwards LB, Kucheryavaya AY, Aurora P, Christie JD, Kirk R, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult heart transplant report -- 2010. J Heart Lung Transplant. 2010;29(10):1089-103..
Due to low sensitivity of coronary angiography in detecting early CAV, IVUS is used in our institution for CAV research, because its high sensitivity and specificity provide an earlier diagnosis of CAV1414 Mehra MR, Crespo-Leiro MG, Dipchand A, Ensminger SM, Hiemann NE, Kobashigawa JA, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant. 2010;29(7):717-27.,3131 Cai Q, Rangasetty UC, Barbagelata A, Fujise K, Koerner MM. Cardiac allograft vasculopathy: advances in diagnosis. Cardiol Rev. 2011;19(1):30-5.. Clinically, IVUS has a good correlation with angiography; thus, it is a good prognostic tool3232 Rickenbacher PR, Pinto FJ, Lewis NP, Hunt SA, Alderman EL, Schroeder JS, et al. Prognostic importance of intimal thickness as measured by intracoronary ultrasound after cardiac transplantation. Circulation. 1995;92(12):3445-52.. Some evidence exists that early diagnosis of CAV, together with the adjustment of immunosuppressive therapy is associated with growth control. Furthermore, some studies have reported regression of CAV2121 Eisen HJ, Tuzcu EM, Dorent R, Kobashigawa J, Mancini D, Valantine-von Kaeppler HA, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003;349(9):847-58.,3333 Lamich R, Ballester M, Marti V, Brossa V, Aymat R, Carrió I, et al. Efficacy of augmented immunosuppressive therapy for early vasculopathy in heart transplantation. J Am Coll Cardiol. 1998;32(2):413-9.,3434 Dambrin C, Klupp J, Birsan T, Luma J, Suzuki T, Lam T, et al. Sirolimus (rapamycin) monotherapy prevents graft vascular disease in nonhuman primate recipients of orthotopic aortic allografts. Circulation. 2003;107(18):2369-74.. The volumetric measurement of the plate by IVUS has been previously validated by experimental1111 Mehran R, Mintz GS, Hong MK, Tio FO, Bramwell O, Brahimi A, et al. Validation of the in vivo intravascular ultrasound measurement of in-stent neointimal hyperplasia volumes. J Am Coll Cardiol. 1998;32(3):794-9. and clinical studies1212 Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis. JAMA. 2006;295(13):1556-65.. This methodology has a strong correlation with histomorphometry. Moreover, it is a robust method and requires a smaller sample to demonstrate the effectiveness of strategies that have an impact on reducing the intima1111 Mehran R, Mintz GS, Hong MK, Tio FO, Bramwell O, Brahimi A, et al. Validation of the in vivo intravascular ultrasound measurement of in-stent neointimal hyperplasia volumes. J Am Coll Cardiol. 1998;32(3):794-9..
The major limitation of this study is its small sample size, possible bias in patient selection, and retrospective nature. Thus, a prospective, multicenter, randomized study with a larger sample size, which extends clinical follow-up to assess the long-term benefit, is indicated. Furthermore, our control group had greater plaque volume, probably due to atherosclerosis of the donor; this may have affected the outcome, as suggested by a recent study by Yamasaki et al.3535 Yamasaki M, Sakurai R, Hirohata A, Honda Y, Bonneau HN, Luikart H, et al. Impact of donor-transmitted atherosclerosis on early cardiac allograft vasculopathy: new findings by three-dimensional intravascular ultrasound analysis. Transplantation. 2011;91(12):1406-11.. However, in our study, plaque volume did not correlate with higher growth of the intima (r = 024; p = 0.94), a finding that is consistent with those of previous studies3636 Botas J, Pinto FJ, Chenzbraun A, Linag D, Schroeder JS, Oesterle SN, et al. Influence of preexistent donor coronary artery disease on the progression of transplant vasculopathy: an intravascular ultrasound study. Circulation. 1995;92(5):1126-32.,3737 Kapadia SR, Nissen SE, Ziada KM, Guetta V, Crowe TD, Hobbs RE, et al. Development of transplantation vasculopathy and progression of donor-transmitted atherosclerosis: comparison by serial intravascular ultrasound imaging. Circulation. 1998;98(24):2672-8..
Conclusion
In this retrospective analysis, induction therapy with basiliximab was associated with less volumetric growth of intimal tissue (graft vasculopathy) in the first year after HT.
-
Sources of FundingThere were no external funding sources for this study.
-
Study AssociationThis article is part of the thesis of master submitted by Ricardo Wang, from Pontífica Universidade Católica do Paraná.
References
-
1Houser S, Muniappan A, Allan J, Sachs D, Madsen J. Cardiac allograft vasculopathy: real or a normal morphologic variant? J Heart Lung Transplant. 2007;26(2):167-73.
-
2Stehlik J, Edwards LB, Kucheryavaya AY, Aurora P, Christie JD, Kirk R, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult heart transplant report -- 2010. J Heart Lung Transplant. 2010;29(10):1089-103.
-
3Benza RL, Tallaj J. Cardiac Allograft Vasculopathy (Chronic Rejection). In: Kirklin JK, Young JB, McGiffin DC. (eds.). Heart transplantation. 3rd ed. Philadelphia: Churchill Livingstone; 2002. p. 615-65.
-
4Nagano H, Libby P, Taylor MK, Hasegawa S, Stinn JL, Becker G, et al. Coronary arteriosclerosis after T-cell-mediated injury in transplanted mouse hearts: role of interferon-gamma. Am J Pathol. 1998;152(5):1187-97.
-
5Uehara S, Chase CM, Colvin RB, Madsen JC, Russell PS. T-cell depletion eliminates the development of cardiac allograft vasculopathy in mice rendered tolerant by the induction of mixed chimerism. Transplant Proc. 2006;38(10):3169-71.
-
6Valantine HA. Cardiac allograft vasculopathy: central role of endothelial injury leading to transplant "atheroma". Transplantation. 2003;76(6):891-9.
-
7Bacal F, Souza-Neto J, Fiorelli A, Mejia J, Marcondes-Braga FG, Mangini S, et al. II Brazilian Guidelines for cardiac transplantation. Arq Bras Cardiol. 2010;941 Suppl):e16-76.
-
8Church AC. Clinical advances in therapies targeting the interleukin-2 receptor. QJM. 2003;96(2):91-102.
-
9Onrust SV, Wiseman LR. Basiliximab. Drugs. 1999;57(2):207-13.
-
10Young JB, Lloyd KS, Windsor NT, Cocanougher B, Weilbaecher DG, Kleiman NS, et al. Elevated soluble interleukin-2 receptor levels early after heart transplantation and long-term survival and development of coronary arteriopathy. J Heart Lung Transplant. 1991;10(2):243-50.
-
11Mehran R, Mintz GS, Hong MK, Tio FO, Bramwell O, Brahimi A, et al. Validation of the in vivo intravascular ultrasound measurement of in-stent neointimal hyperplasia volumes. J Am Coll Cardiol. 1998;32(3):794-9.
-
12Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis. JAMA. 2006;295(13):1556-65.
-
13Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37(5):1478-92.
-
14Mehra MR, Crespo-Leiro MG, Dipchand A, Ensminger SM, Hiemann NE, Kobashigawa JA, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant. 2010;29(7):717-27.
-
15Yamani MH, Haji SA, Starling RC, Tuzcu EM, Ratliff NB, Cook DJ, et al. Myocardial ischemic-fibrotic injury after human heart transplantation is associated with increased progression of vasculopathy, decreased cellular rejection and poor long-term outcome. J Am Coll Cardiol. 2002;39(6):970-7.
-
16Land W, Messmer K, Events E. The impact of ischemia/reperfusion injury on specific and non-specific, early and late chronic events after organ transplantation. Transplant Rev. 1996;10:108-27.
-
17Day JD, Rayburn BK, Gaudin PB, Baldwin WM 3rd, Lowenstein CJ, Kasper EK, et al. Cardiac allograft vasculopathy: the central pathogenetic role of ischemia-induced endothelial cell injury. J Heart Lung Transplant. 1995;14(6 Pt 2):S142-9.
-
18Tori M, Kitagawa-Sakakida S, Li Z, Izutoni H, Horiguchi K, Ito T, et al. Initial T-cell activation required for transplant vasculopathy in retransplanted rat cardiac allografts. Transplantation. 2000;70(5):737-46.
-
19Young JB, Windsor NT, Kleiman NS, Lowry R, Cocanougher B, Lawrence EC. The relationship of soluble interleukin-2 receptor levels to allograft arteriopathy after heart transplantation. J Heart Lung Transplant. 1992;11(3 Pt 2):S79-82.
-
20Mitchell RN. Graft vascular disease: immune response meets the vessel wall. Annu Rev Pathol. 2009;4:19-47.
-
21Eisen HJ, Tuzcu EM, Dorent R, Kobashigawa J, Mancini D, Valantine-von Kaeppler HA, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003;349(9):847-58.
-
22Zhang R, Haverich A, Strüber M, Simon A, Bara C. Delayed onset of cardiac allograft vasculopathy by induction therapy using anti-thymocyte globulin. J Heart Lung Transplant. 2008;27(6):603-9.
-
23Chapman TM, Keating GM. Basiliximab: a review of its use as induction therapy in renal transplantation. Drugs. 2003;63(24):2803-35.
-
24Kobashigawa JA, Patel JK, Kittleson MM, Kawano MA, Kiyosaki KK, Davis SN, et al. The long-term outcome of treated sensitized patients who undergo heart transplantation. Clin Transplant. 2011;25(1):E61-7.
-
25Feingold B, Bowman P, Zeevi A, Girnita AL, Quivers ES, Miller SA, et al. Survival in allosensitized children after listing for cardiac transplantation. J Heart Lung Transplant. 2007;26(6):565-71.
-
26Delgado DH, Miriuka SG, Cusimano RJ, Feindel C, Rao V, Ross HJ. Use of basiliximab and cyclosporine in heart transplant patients with pre-operative renal dysfunction. J Heart Lung Transplant. 2005;24(2):166-9.
-
27Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316(22):1371-5.
-
28Tsutsui H, Ziada KM, Schoenhagen P, Tyisoy A, Magyar WA, Crowe TD, et al. Lumen loss in transplant coronary artery disease is a biphasic process involving early intimal thickening and late constrictive remodeling: results from a 5-year serial intravascular ultrasound study. Circulation. 2001;104(6):653-7.
-
29Yeung AC, Davis SF, Hauptman PJ, Kobashigawa JA, Miller LW, Valantine HA, et al. Incidence and progression of transplant coronary artery disease over 1 year: results of a multicenter trial with use of intravascular ultrasound. Multicenter Intravascular Ultrasound Transplant Study Group. J Heart Lung Transplant. 1995;14(6 Pt 2):S215-20.
-
30Anselm A, Cantarovich M, Davies R, Grenon J, Haddad H. Prolonged basiliximab use as an alternative to calcineurin inhibition to allow renal recovery late after heart transplantation. J Heart Lung Transplant. 2008;27(9):1043-5.
-
31Cai Q, Rangasetty UC, Barbagelata A, Fujise K, Koerner MM. Cardiac allograft vasculopathy: advances in diagnosis. Cardiol Rev. 2011;19(1):30-5.
-
32Rickenbacher PR, Pinto FJ, Lewis NP, Hunt SA, Alderman EL, Schroeder JS, et al. Prognostic importance of intimal thickness as measured by intracoronary ultrasound after cardiac transplantation. Circulation. 1995;92(12):3445-52.
-
33Lamich R, Ballester M, Marti V, Brossa V, Aymat R, Carrió I, et al. Efficacy of augmented immunosuppressive therapy for early vasculopathy in heart transplantation. J Am Coll Cardiol. 1998;32(2):413-9.
-
34Dambrin C, Klupp J, Birsan T, Luma J, Suzuki T, Lam T, et al. Sirolimus (rapamycin) monotherapy prevents graft vascular disease in nonhuman primate recipients of orthotopic aortic allografts. Circulation. 2003;107(18):2369-74.
-
35Yamasaki M, Sakurai R, Hirohata A, Honda Y, Bonneau HN, Luikart H, et al. Impact of donor-transmitted atherosclerosis on early cardiac allograft vasculopathy: new findings by three-dimensional intravascular ultrasound analysis. Transplantation. 2011;91(12):1406-11.
-
36Botas J, Pinto FJ, Chenzbraun A, Linag D, Schroeder JS, Oesterle SN, et al. Influence of preexistent donor coronary artery disease on the progression of transplant vasculopathy: an intravascular ultrasound study. Circulation. 1995;92(5):1126-32.
-
37Kapadia SR, Nissen SE, Ziada KM, Guetta V, Crowe TD, Hobbs RE, et al. Development of transplantation vasculopathy and progression of donor-transmitted atherosclerosis: comparison by serial intravascular ultrasound imaging. Circulation. 1998;98(24):2672-8.
Publication Dates
-
Publication in this collection
23 June 2015 -
Date of issue
Aug 2015
History
-
Received
29 Sept 2014 -
Reviewed
28 Jan 2015 -
Accepted
29 Jan 2015