1. Introduction
Cardiovascular disease (CVD) and cancer are currently the leading causes of mortality worldwide and in Brazil.11. Abdel-Qadir H, Thavendiranathan P, Austin PC, Lee DS, Amir E, Tu JV, et al. Development and validation of a multivariable prediction model for major adverse cardiovascular events after early stage breast cancer: a population-based cohort study. Eur Heart J. 2019;40(48):3913-20.–33. Collaborators GBDB. Burden of disease in Brazil, 1990-2016: a systematic subnational analysis for the Global Burden of Disease Study. 2016. Lancet 2018;392(10149):760-75. The recent demographic and epidemiological transitions in Brazil have determined an increase in the population's life expectancy, today around 76 years, and a change in the health profile, in which chronic diseases and their complications prevail.44. Ala CK, Klein AL, Moslehi JJ. Cancer Treatment-Associated Pericardial Disease: Epidemiology, Clinical Presentation, Diagnosis, and Management. Curr Cardiol Rep. 2019;21(12):156.
These factors pose important challenges and require the development of a health policy agenda for the management of the ongoing transitions. The technological advances, the shortage of cost-effectiveness analyses and, in higher education settings, the little value attributed to health access and promotion, as well as to disease prevention, require the implementation of guidelines and consensus statements. These guidelines and consensus statements are aimed at helping the use of systematized protocols to adapt the clinical practice regardless of the geographic location of the health facilities and the heterogeneity of their resources.
Recent advances in cancer detection and treatment have resulted in an exponential increase in the number of cancer survivors around the world. According to a recent estimate, by 2026, the United States will have 20 million cancer survivors, 50% of whom will be older than 70 years.55. Alfano CM, Cheville AL, Mustian K. Developing High-Quality Cancer Rehabilitation Programs: A Timely Need. Am Soc Clin Oncol Educ Book. 2016;35:241-9.,66. Global Burden of Disease Cancer C, Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019; 5(12):1749-68 The care of an older population with history of cancer and CVD, compounded by the potential cardiovascular toxicity of the oncological treatment, requires specialists in the ‘cancer-CVD' interaction.77. Al-Kindi SG, Oliveira GH. Prevalence of Preexisting Cardiovascular Disease in Patients With Different Types of Cancer: The Unmet Need for Onco-Cardiology. Mayo Clin Proc. 2016;91(1):81-3.
In 1967, anthracycline-induced cardiotoxicity was first described.88. Tan C, Tasaka H, Yu KP, Murphy ML, Karnofsky DA. Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. Clinical evaluation with special reference to childhood leukemia. Cancer. 1967;20(3):333-53. In 1971, a study reported that anthracycline-induced cardiotoxicity was dose-dependent and that the cardiac damage might be irreversible.99. Ainger LE, Bushore J, Johnson WW, Ito J. Daunomycin: a cardiotoxic agent. J Natl Med Assoc. 1971;63(4):261-7. Some years later, risk factors for chemotherapy-related ventricular dysfunction were identified, and biomarkers, such as troponin and B-type natriuretic peptide (BNP), were related to the prediction of cardiovascular events.1010. Lipshultz SE, Lipsitz SR, Mone SM, Goorin AM, Sallan SE, Sanders SP, et al. Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332(26):1738-43.,1111. Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, Lamantia G, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109(22):2749-54. Those findings were the cornerstone of cardio-oncology.
Cardio-oncology is the field of science devoted to the early diagnosis and proper management of CVD in patients with the current or previous diagnosis of cancer. Furthermore, cardio-oncology comprises the analysis of not only the cardiovascular risks related to the oncological diagnosis, but also the patient's needs before, during and after the treatment. Cardio-oncology specialists should follow patients up since their diagnosis, through all treatment phases, and even after their cure, when the patients are called cancer survivors. The need for expansion of cardio-oncology relates directly to the epidemiology of cancer and CVD, the risk factors they share, and the multiplicity of treatments with distinct toxicities to the cardiovascular system (Figure 1).1212. Allen S, Brown V, Prabhu P, Scott M, Rockall T, Preston S, et al. A randomised controlled trial to assess whether prehabilitation improves fitness in patients undergoing neoadjuvant treatment prior to oesophagogastric cancer surgery: study protocol. BMJ Open. 2018 Dec 22;8(12):e023190.,1313. Amigoni M, Giannattasio C, Fraschini D, Galbiati M, Capra AC, Madotto F, et al. Low anthracyclines doses-induced cardiotoxicity in acute lymphoblastic leukemia long-term female survivors. Pediatr Blood Cancer. 2010;55(7):1343-7.
In 2011, the Brazilian Society of Cardiology (SBC) and the Brazilian Society of Clinical Oncology (SBOC) pioneered in joining forces to publish the I Guideline on Cardio-Oncology.1414. Anker MS, Hadzibegovic S, Lena A, Belenkov Y, Bergler-Klein J, de Boer RA, et al. Recent advances in cardio-oncology: a report from the ‘Heart Failure Association 2019 and World Congress on Acute Heart Failure 2019'. ESC Heart Fail. 2019;6(6):1140-8. In 9 years, cardio-oncology has significantly grown as a discipline because of the following factors: a) remarkable advances in cancer treatment; b) understanding of multidisciplinarity and integration of cardiology, oncology and hematology as essentials for the care of cancer patients; c) implementation of fellowship programs across the world and insertion of ‘cardio-oncology' in the curriculum of some cardiology residency training programs; d) growth of research in basic and clinical areas; and e) creation of important journals dedicated to the subject, such as JACC CardioOncology and Cardio-Oncology.1515. Anquetil C, Salem JE, Lebrun-Vignes B, Johnson DB, Mammen AL, Stenzel W, et al. Immune Checkpoint Inhibitor-Associated Myositis. Circulation. 2018;138(7):743-5.,1616. Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35(8):893-911.
It is worth noting that, in 2019, Brazil hosted the V Global Cardio-Oncology Summit, to which specialists from several countries and approximately 600 professionals (cardiologists, oncologists, hematologists, nurses, physical therapists, pharmacists, physical educators) attended. The journal Frontiers in Cardiovascular Medicine published 89 abstracts, and the JACC CardioOncology published “Proceedings From the Global Cardio-Oncology Summit - The Top 10 Priorities to Actualize for CardioOncology”.1717. Hajjar LA. 5th Global Cardio-Oncology Summit. Frontiers Media SA; 2019.,1818. Lenihan DJ, Fradley MG, Dent S, Brezden-Masley C, Carver J, Kalil Filho K, et al. Proceedings From the Global Cardio-Oncology Summit: The Top 10 Priorities to Actualize for CardioOncology. JACC: CardioOncology. 2019;1(2):256-72.
The SBC and the SBOC, aiming at knowledge updating and promotion of a rational and systematic approach to cardiovascular complications in oncology patients, have gathered a team of experts to create new strategies, issue evidence-based recommendations, and develop multiprofessional healthcare, which will provide the proper management of that increasing category of patients.
The goals of the Brazilian Cardio-Oncology Guideline - 2020 are as follows: 1) to demystify the belief of CVD as a barrier to the effective treatment of cancer patients; 2) to prevent and reduce the risks of treatment-related cardiotoxicity; 3) to promote the interaction among medical specialties (cardiology, hematology and oncology) to agree the best strategy for patient's care, weighing the risks and benefits of the treatment; 4) to propose the unification of terminologies and definitions of the cardiovascular complications of cancer patients, aiming at homogenizing care and research; 5) to disclose the evidence available on the management of cardiovascular complications in oncology patients, aiming at their early diagnosis by use of cardiovascular function monitoring before, during and after the treatment; 6) to promote proper treatment, with the participation of oncologists and hematologists, based on scientific evidence, risk analysis and care personalization, considering the patient's preferences; and 7) to boost research and knowledge spread in cardio-oncology (Figure 1).
The Brazilian Cardio-Oncology Guideline - 2020 gathers evidence on the cardiovascular complications of cancer patients available up to 2020.
2. Methods
The Brazilian Cardio-Oncology Guideline - 2020 abided by the ongoing recommendations. A team of experts in cardiology, hematology and oncology formed a committee to elaborate this manuscript. Participants were chosen based on their prominence in their fields, their participation in the International Cardio-Oncology Society (ICOS), SBC and SBOC, in addition to their scientific production.
A bibliographic search was conducted in PubMed in the period from 1975 to July 2020 with the following keywords: cardiotoxicity, cancer, immunotherapy, cardiooncology, cardiovascular complications, targeted therapy, radiotherapy, vascular toxicity, heart failure, ventricular dysfunction, pericardial disease, coronary disease, thromboembolism, arrhythmias, hypertension, individual drug names. The manuscript was sent electronically to all participants, and, after they all agreed on its content, it was formatted and sent to publication.
The classes of recommendation and levels of evidence used in this guideline were as follows:
Classes of recommendations:
Grade I – there is conclusive evidence, or, failing that, a consensus that the procedure is safe and useful/effective.
Grade II – there is conflicting evidence and/or divergent opinions on the safety and utility/effectiveness of the procedure:
-
Grade IIA: weight of the evidence/opinion is in favor of the procedure. Most experts approve;
-
Grade IIB: safety and utility/effectiveness are less well established, with no predominance of opinions in favor.
Grade III – there is evidence and/or expert consensus that the procedure is not useful/effective and, in some cases, can even be harmful.
Levels of Evidence:
Level A – data obtained from multiple, large, concordant randomized studies and/or robust meta-analyses of randomized clinical studies.
Level B – data obtained from a less robust metaanalysis, based on a single randomized trial or on non-randomized (observational) studies.
Level C – data obtained from consensus expert opinions.
3. Diagnosis and Management of Cardiovascular Complications in Cancer Patients
3.1. Initial Cardiological Assessment
The different types of cancer treatment, such as chemotherapy, immunotherapy, and radiotherapy, can result in damage to the cardiovascular system. Patients with previous CVD or cardiovascular risk factors have the highest likelihood of complications from cancer treatment. Thus, the treatment and control of cardiovascular risk factors in cancer patients are recommended.1919. Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23 Suppl 7:vii155-66.–2121. Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31(2):171-90.
The consultation of cancer patients with a cardiologist should comprise the control of cardiovascular risk factors, cardioprotective measures, adhesion to treatment, and a strategy to enable the early diagnosis of cardiac damage (I, B).
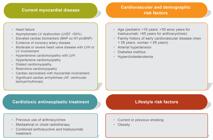
Patients with cardiovascular risk factors or already established CVD and who will undergo a potentially cardiotoxic treatment [anthracyclines, anti-HER2 (human epidermal growth factor receptor 2) agents, alkylating agents, inhibitors of vascular endothelial growth factor (VEGF) signaling, proteasome inhibitors and immune checkpoint inhibitors (ICIs)] should be assessed by a cardiologist at the beginning of therapy and followed up according to specific protocols (I, B). Table 1 shows the antineoplastic treatments most associated with cardiovascular toxicity. Figure 2 shows the factors associated with a higher risk for cardiotoxicity.
Predisposing factors for the development of cardiotoxicity in cancer patients. Adapted from Zamorano et al.2222. Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(36):2768-801.
The multiprofessional team assessing the cancer patient should weigh the risks and supposed benefits of the therapy and implement strategies to prevent cardiovascular damage (IIa, C).
Measuring and approaching the cardiovascular risk factors according to consensus and guidelines are recommended (I, A).
In the initial cardiological assessment, the following are recommended: anamnesis, physical examination, electrocardiogram (ECG), chest X-ray, complete blood count, measurement of electrolytes and biomarkers [N-terminal pro-BNP (NT-proBNP) and troponin I or high-sensitivity troponin T], folic acid, vitamins D and B12, glycemia, lipid profile, as well as kidney, liver and thyroid function (I, A) (Figure 3).
Initial cardiologist assessment. *Ideally combined with LVEF three-dimensional assessment and myocardial strain quantification by speckle tracking. CMRI: cardiac magnetic resonance imaging; CVD: cardiovascular disease; ECG: electrocardiogram; echo: echocardiography; HF: heart failure; Ica: serum ionic calcium; K: serum potassium; LV: left ventricular; LVEF: left ventricular ejection fraction; Mg: serum magnesium; Na: serum sodium; NT-proBNP: N-terminal pro-B-type natriuretic peptide; X-ray: radiography
In addition, in baseline and serial assessment according to the treatment regimen, transthoracic echocardiography with color Doppler, ideally three-dimensional, is recommended, with analysis of left ventricular ejection fraction (LVEF), diastolic function, and myocardial deformation with strain quantification by use of the speckle tracking technique (I, A).
Collaboration between cardiologists, oncologists and hematologists is recommended to ensure the proper and beneficial treatment to cancer patients (IIa, A).
3.2. Diagnosis of Cardiotoxicity in Cancer Patients
Cardiotoxicity can be diagnosed by confirming the presence of a new cardiovascular alteration (clinical and/or in biomarkers and/or in imaging) during or after treatment, once other etiologies have been excluded (I, B).
Echocardiography is the method of choice to detect myocardial dysfunction related to the oncological treatment. Three-dimensional echocardiography is the best echocardiographic method to measure LVEF in cancer patients. When not available or in the presence of limitations, biplane Simpson method is recommended (I, A).
Ventricular dysfunction related to cancer therapy is defined as a reduction ≥ 10% in LVEF to a value below the lower limit of the normal range (LVEF < 50%). A new cardiovascular imaging test should be performed in 2 to 3 weeks (I, B).
That LVEF reduction occurs in the course of treatment, and can be classified as symptomatic or asymptomatic and reversible or irreversible (I, B).
Global longitudinal strain (GLS) is a highly sensitive tool to predict later LVEF reduction. A GLS reduction ≥ 15% in regard to baseline is considered abnormal and an early marker of ventricular dysfunction (I, B).
Diastolic function analysis is recommended in oncological patients, both before therapy starts and during follow-up (IIa, C). However, there is no evidence that the treatment should be interrupted based on diastolic function.
Radionuclide ventriculography is not recommended for ventricular function assessment in cancer patients (III, B).
Cardiac magnetic resonance imaging (CMRI) is the gold-standard method to assess cardiac function. It enables structural assessment and tissue characterization, being recommended when echocardiography cannot be performed, in the presence of infiltrative diseases, for pericardial and myocardial evaluation, and for the detection of masses and tumors (IIa, B). In addition, CMRI can assess prognosis by analyzing myocardial fibrosis.
The routine use of biomarkers during a potentially cardiotoxic treatment has not been well established. Monitoring cardiotoxicity by measuring biomarkers can be considered for the early detection of myocardial damage in patients at high risk due to previous factors or those exposed to drugs, such as anthracyclines and trastuzumab (IIa, B). Neither the best time for measuring biomarkers regarding chemotherapy (during chemotherapy, 24 hours after, 48 hours after or later) nor the best management when high levels of biomarkers are detected are known. In addition, in the course of treatment, the same analysis kits of biomarkers, such as high-sensitivity troponin and NT-proBNP assays, should be used (IIa, C).
High levels of biomarkers (NT-proBNP and troponin) indicate increased risk for cardiotoxicity (I, A).
On initial assessment and throughout treatment, ECG should be performed. The QTc should be calculated by using Bazett's [QT / (RR)1/2] or Fridericia's [QT / (RR)1/3] formula, and the same method should be used for the patient's serial assessment. In cancer patients, the Fridericia's formula is preferred, because it undergoes less change in the presence of tachycardia or bradycardia (IIa, C).
Table 2 describes the cardiovascular diagnostic methods and their major advantages, uses, and limitations.
4. Ventricular Dysfunction
Ventricular dysfunction is one of the most severe complications from cancer treatment, characterized by high morbidity and mortality rates. It may appear during therapy or years after completion of therapy and even so be consequent to drug toxicity.2323. Lenihan DJ, Hartlage G, DeCara J, Blaes A, Finet JE, Lyon AR, et al. Cardio-Oncology Training: A Proposal From the International Cardioncology Society and Canadian Cardiac Oncology Network for a New Multidisciplinary Specialty. J Card Fail 2016;22(6):465-71. The classic model of ventricular dysfunction as a form of cardiotoxicity is secondary to the use of anthracyclines, which are widely used to treat sarcoma, lymphoma, leukemia, and breast cancer.2424. McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc Drugs Ther. 2017;31(1):63-75.,2525. Lyon AR, Dent S, Stanway S, Earl H, Brezden-Masley C, Cohen-Solal A, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur J Heart Fail. 2020.;28 maio. Doi;10.1002/ejhf.1920 on line
https://doi.org/10.1002/ejhf.1920...
The different chemotherapy and immunotherapy drugs associated with ventricular dysfunction result in different phenotypes in patients, ranging from asymptomatic mild and reversible dysfunction to severe, clinically manifest and irreversible heart failure (HF). Pediatric cancer survivors are up to 15 times more likely to develop HF than controls matched for other risk factors.2626. Shankar SM, Marina N, Hudson MM, Hodgson DC, Adams MJ, Landier W, et al. Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children's Oncology Group. Pediatrics. 2008;121(2):e387-96. Epub 2008 Jan 10.
Predicting cardiotoxicity is a challenge, because of the multiplicity of drugs to which patients are exposed throughout life, in addition to the often-present cardiovascular risk factors. It is worth noting the multiple drug interactions of the different therapeutic regimens, such as those of anthracyclines with cyclophosphamide and anthracyclines with trastuzumab.
In recent years, with the introduction of new chemotherapy drugs and the advent of immunotherapy, in addition to the introduction of protocols for early detection of cardiotoxicity, ventricular dysfunction has been increasingly diagnosed. Table 3 shows the antineoplastic drugs more often associated with ventricular dysfunction.
4.1. Anthracyclines
Anthracyclines consist in a group of antineoplastic drugs known to be effective in treating sarcoma, lymphoma, leukemia, and breast cancer. Their clinical use is limited by cardiotoxicity characterized by ventricular dysfunction and HF, which are the main causes of mortality in cancer survivors.
The toxicity of anthracyclines is highly variable and can occur in up to 50% of the patients, depending on the patient's risk factors and the pharmacological properties of the chemotherapy drugs, such as cumulative dose. For example, doxorubicin is associated with a 5% incidence of HF at the cumulative dose of up to 400 mg/m2, and that incidence can reach 50% if the dose exceeds 700 mg/m2.2727. Henriksen PA. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart. 2018;104(12):971-7. A recent study with 2625 patients in a 5-year follow-up has shown a 9% overall incidence of anthracycline-induced cardiotoxicity, and 98% of the cases were asymptomatic and occurred in the first year.2424. McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc Drugs Ther. 2017;31(1):63-75.
Cardiotoxicity can be acute, early or late, reversible or irreversible. Acute toxicity is characterized by the presence of supraventricular arrhythmia, left ventricular dysfunction and electrocardiographic changes, which appear right after anthracycline infusion in up to 1% of the patients, being usually reversible. Acute ventricular dysfunction can be a predictor of HF, which can be subacute or chronic. Early cardiotoxicity appears in the first year of treatment, while late cardiotoxicity appears years after treatment, on average, 7 years after completion of treatment.2828. Narezkina A, Nasim K. Anthracycline Cardiotoxicity. Circ Heart Fail. 2019;12(3):e005910.
There is no predictor of the reversibility/irreversibility of the anthracycline-induced toxicity. However, the elevation in the levels of biomarkers and its persistence can identify patients at high risk for irreversibility.2929. Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18(11):1639-42.
The tendency towards cardiotoxicity varies with the different treatment regimens, and doxorubicin is the anthracycline most associated with ventricular dysfunction. Cardiotoxicity is dose-dependent, and reducing the cumulative dose is a way to minimize it. Changes in infusion, such as prolonging its duration, splitting the dose and using liposomal formulations, can prevent cardiotoxicity.2424. McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc Drugs Ther. 2017;31(1):63-75. A recent experimental study has suggested that ischemic preconditioning might prevent doxorubicin-induced cardiotoxicity.3030. He Q, Wang F, Ryan TD, Chalasani M, Redington AN. Repeated Remote Ischemic Conditioning Reduces Doxorubicin-Induced Cardiotoxicity. JACC: CardioOncology. 2020;2(1):41-52.
Mechanistic studies have shown that anthracycline-induced ventricular dysfunction is associated with: 1) damage to the sarcoplasmic reticulum and mitochondria; 2) changes in myofibrillar structure and function; 3) total or partial loss of matrix interspersed with collagen plaques in the interstitium; 4) change in the excitation-contraction coupling and calcium flow; 5) apoptosis; 6) changes in iron metabolism; and 7) loss of the regeneration capacity of the cardiac muscle and coronary endothelial cells. The consequence is dysfunction and hypertrophy of the remaining myocytes.3131. Coelho-Filho OR, Shah RV, Mitchell R, Neilan TG, Moreno H, Jr., Simonson B, et al. Quantification of cardiomyocyte hypertrophy by cardiac magnetic resonance: implications for early cardiac remodeling. Circulation. 2013;128(11):1225-33. The common trigger for those events seems to be related to the oxidative stress caused by the production of reactive oxygen species, in addition to the inhibition of topoisomerase 2β, resulting in damage to membranes, proteins and DNA. The following observations support the importance of oxidative stress in anthracycline-induced cardiotoxicity: a) over-expression of metallothionein, a free radical scavenger, in the heart of transgenic mice minimizes the doxorubicin-induced injury; b) inhibition of the formation of peroxynitrite, a reactive oxidant produced from nitric oxide and superoxide, improves the cardiac function of mice exposed to doxorubicin; c) probucol, a strong antioxidant, prevents glutathione peroxidase reduction and reduces doxorubicin-related myocardial lipid peroxidation in a murine model; d) dexrazoxane is a chelating agent like EDTA that can prevent anthracycline damage via iron binding, which acts as a cofactor for free radicals.3232. Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments. Nat Rev Cardiol 2015;12(11):620. Diastolic dysfunction due to cumulative dose-dependent toxicity can be observed with a cumulative dose of 200 mg/m2, while systolic dysfunction is usually observed with doses over 400 mg/m2, with variability according to an individual threshold. However, impaired diastolic function has been observed with the cumulative dose of only 120 mg/m2.3333. Boyd A, Stoodley P, Richards D, Hui R, Harnett P, Vo K, et al. Anthracyclines induce early changes in left ventricular systolic and diastolic function: A single centre study. PLoS One. 2017 Apr 13;12(4):e0175544.
Table 4 shows the risk factors associated with a higher likelihood of anthracycline-induced toxicity, of which previous heart disease, cumulative dose and fast drug infusion stand out. However, in the presence of the same risk factors, there is an important variability in the occurrence of cardiotoxicity among patients, which might be related to genetic factors and interactions with unknown factors.
Polymorphisms in ATP-binding cassette (ABC) transporter genes are associated with anthracycline cardiomyopathy. Those transporters play an important role in drug resistance via cellular efflux of drugs, including anthracyclines. Reduced activity can lead to intracellular accumulation of anthracycline and cellular toxicity. Variants in that family of genes replicated in cohorts of childhood cancer patients include ABCC5 (A-1629T, rs7627754), associated with a significant LVEF reduction in T-allele homozygous survivors.3434. Chang VY, Wang JJ. Pharmacogenetics of Chemotherapy-Induced Cardiotoxicity. Curr Oncol Rep. 2018;20(7):52. In addition, a variant in histamine methyltransferase HNMT (rs17583889) confers risk in young patients exposed to anthracyclines.3535. Sachidanandam K, Gayle AA, Robins HI, Kolesar JM. Unexpected doxorubicin-mediated cardiotoxicity in sisters: possible role of polymorphisms in histamine n-methyl transferase. J Oncol Pharm Pract. 2013;19:269-72. Table 5 shows the pharmacogenetic variants that predispose to anthracycline-related cardiotoxicity.
During treatment with anthracyclines, clinical and echocardiographic monitoring is recommended at a pre-established frequency or out of protocol in the presence of HF signs and symptoms.2121. Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31(2):171-90. Ideally, the echocardiographic assessment should comprise biventricular systolic and diastolic function analysis (I, A) (Figure 4).
Echocardiographic monitoring and analysis of biomarkers in patients using anthracyclines. Echo: echocardiogram; NT-proBNP: N-terminal pro-B-type natriuretic peptide; QT: chemotherapy
4.2. HER2-targeted Therapies
Trastuzumab is a monoclonal antibody targeted at the human epidermal growth factor receptor 2 (HER2 or ErbB2). For 15-20% of the patients with breast cancer whose tumors over express HER2, therapy with trastuzumab significantly reduces mortality.3636. Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273-83.,3737. Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CE, Jr., et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32(33):3744-52. Its use is associated with a considerable risk of cardiotoxicity, clinically manifested by an asymptomatic decline in LVEF and, less commonly, by symptomatic HF.3838. Wang SY, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP, et al. Cardiovascular events, early discontinuation of trastuzumab, and their impact on survival. Breast Cancer Res Treat. 2014;146(2):411-9. After the introduction of trastuzumab, three other anti-HER2 agents were developed: lapatinib, a tyrosine kinase inhibitor of the epidermal growth factor (EGFR), ERBB1 and HER2; ado-trastuzumab emtansine (T-DM1), a conjugated antibody composed by trastuzumab, a thioester linker and an antimitotic maytansine derivative; and pertuzumab, a monoclonal antibody that binds to the subdomain II of the HER2 extracellular domain and prevents HER2 homo- and heterodimerization with other HER receptors. Although data on those new drugs are scarce, there is evidence that T-DM1 and pertuzumab are less cardiotoxic than trastuzumab.3939. Jerusalem G, Lancellotti P, Kim SB. HER2+ breast cancer treatment and cardiotoxicity: monitoring and management. Breast Cancer Res Treat. 2019;177(2):237-50.
The LVEF decline rate consequent to trastuzumab use varies in the literature. Recent studies have reported, in 15% to 40% of the patients on trastuzumab, a LVEF reduction of at least 10%, and, in 18% of the patients, a LVEF drop to less than 53%.4040. Seferina SC, de Boer M, Derksen MW, van den Berkmortel F, van Kampen RJ, van de Wouw AJ, et al. Cardiotoxicity and Cardiac Monitoring During Adjuvant Trastuzumab in Daily Dutch Practice: A Study of the Southeast Netherlands Breast Cancer Consortium. Oncologist. 2016;21(2):555-62.,4141. Tarantini L, Cioffi G, Gori S, Tuccia F, Boccardi L, Bovelli D, et al. Trastuzumab adjuvant chemotherapy and cardiotoxicity in real-world women with breast cancer. J Card Fail. 2012;18(2):113-9. Symptomatic HF has been reported in 0.6% to 8.7% of patients.4040. Seferina SC, de Boer M, Derksen MW, van den Berkmortel F, van Kampen RJ, van de Wouw AJ, et al. Cardiotoxicity and Cardiac Monitoring During Adjuvant Trastuzumab in Daily Dutch Practice: A Study of the Southeast Netherlands Breast Cancer Consortium. Oncologist. 2016;21(2):555-62.
One difference between the toxicity of anti-HER2 agents and that of anthracyclines is the reversibility of the former in most cases. The determinants of reversibility are previous cardiovascular function and the extent of LVEF decline related to treatment. A recent study has shown that all LVEF declines smaller than 10% were reversible. However, for LVEF declines greater than 10%, reversibility was observed in 91% of the patients with normal baseline cardiovascular function as compared to only 71.4% of those with reduced LVEF prior to exposure.4242. Leong DP, Cosman T, Alhussein MM, Tyagi NK, Karampatos S, Barron CC, et al. Safety of Continuing Trastuzumab Despite Mild Cardiotoxicity: A Phase I Trial. JACC: CardioOncology. 2019;1(1):1-10. Some studies have reported that, even in the presence of cardiotoxicity, 70% to 80% of patients continue receiving trastuzumab and that the highest likelihood of cardiovascular toxicity and mortality related to treatment is observed in patients with previously reduced LVEF.4343. Procter M, Suter TM, de Azambuja E, Dafni U, van Dooren V, Muehlbauer S, et al. Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol. 2010;28(21):3422-8.
Trastuzumab-induced ventricular dysfunction and clinically manifest HF are usually reversible after chemotherapy interruption and/or after beginning HF treatment. The mechanisms of the anti-HER2 therapy-induced cardiotoxicity include structural and functional changes in contractile proteins and mitochondria, but rarely lead to cellular death, explaining the potential reversibility. The interruption of trastuzumab treatment is associated with an increase in cancer recurrence, and cardiotoxicity is the major responsible for drug suspension.4444. Lidbrink E, Chmielowska E, Otremba B, Bouhlel A, Lauer S, Liste Hermoso M, et al. A real-world study of cardiac events in > 3700 patients with HER2-positive early breast cancer treated with trastuzumab: final analysis of the OHERA study. Breast Cancer Res Treat. 2019;174(1):187-96.
Table 6 shows the risk factors for cardiotoxicity induced by anti-HER2 therapy.
During treatment with trastuzumab, clinical and echocardiographic monitoring is recommended according to protocol or in the presence of HF signs and symptoms (I, A) (Figure 5).
Echocardiographic monitoring and analysis of biomarkers in patients using anti-HER2 drugs. Echo, echocardiogram; RF, risk factors; HF, heart failure; NT-proBNP, N-terminal pro-B-type natriuretic peptide
4.3. VEGF Inhibitors
The inhibition of VEGF signaling pathways benefits thousands of cancer patients, but some chemotherapy drugs of that class are associated with the risk of cardiotoxicity, which can be reversible or irreversible, particularly in the presence of concomitant or previous use of other chemotherapy drugs.4545. Choueiri TK, Mayer EL, Je Y, Rosenberg JE, Nguyen PL, Azzi GR, et al. Congestive heart failure risk in patients with breast cancer treated with bevacizumab. J Clin Oncol. 2011;29(6):632-8.–4747. Qi WX, Shen Z, Tang LN, Yao Y. Congestive heart failure risk in cancer patients treated with vascular endothelial growth factor tyrosine kinase inhibitors: a systematic review and meta-analysis of 36 clinical trials. Br J Clin Pharmacol. 2014;78(4):748-62.
The relative risk of congestive HF in bevacizumab-treated patients was 4.74 (95% CI: 1.6-11.18; p = 0.001) compared to that of the placebo group.4545. Choueiri TK, Mayer EL, Je Y, Rosenberg JE, Nguyen PL, Azzi GR, et al. Congestive heart failure risk in patients with breast cancer treated with bevacizumab. J Clin Oncol. 2011;29(6):632-8. In addition, other drugs, such as sunitinib, pazopanib and axitinib, have been associated with the development of ventricular dysfunction. A meta-analysis including 10 553 patients has reported congestive HF incidence of 3.2% (95% CI: 1.8% - 5.8%) with the use of VEGF tyrosine kinase inhibitors.4747. Qi WX, Shen Z, Tang LN, Yao Y. Congestive heart failure risk in cancer patients treated with vascular endothelial growth factor tyrosine kinase inhibitors: a systematic review and meta-analysis of 36 clinical trials. Br J Clin Pharmacol. 2014;78(4):748-62.
Systemic arterial hypertension (SAH) is a common complication of that class of chemotherapy drugs, and some studies have suggested that the proper treatment of SAH might reduce the risk of HF.4848. Waliany S, Sainani KL, Park LS, Zhang CA, Srinivas S, Witteles RM. Increase in Blood Pressure Associated With Tyrosine Kinase Inhibitors Targeting Vascular Endothelial Growth Factor. JACC: CardioOncology. 2019;1(1):24-36. The prognosis of patients who develop cardiotoxicity associated with VEGF inhibitors is hard to assess, because candidates for treatment with such drugs usually have metastatic disease and reduced life expectancy. Most cases reverse with the treatment of ventricular dysfunction. Table 7 shows the risk factors for cardiotoxicity.
4.3.1. BCR-ABL Tyrosine Kinase Inhibitors
The BCR-ABL tyrosine kinase inhibitors have changed the prognosis of patients with chronic myeloid leukemia and gastrointestinal stromal tumors. The cardiotoxicity of imatinib has not been confirmed; however, nilotinib and ponatinib may be associated with cardiotoxicity involving HF, SAH, arrhythmias and thromboembolism.4949. Herrmann J, Yang EH, Iliescu CA, Cilingiroglu M, Charitakis K, Hakeem A, et al. Vascular Toxicities of Cancer Therapies: The Old and the New--An Evolving Avenue. Circulation. 2016;133(13):1272-89.
4.4. Therapies for Multiple Myeloma
Proteasome inhibitors are relatively new drugs to treat multiple myeloma. Bortezomib and carfilzomib belong to this class of drugs and can cause cardiovascular dysfunction. Proteasomes are protein complexes responsible for degrading dysfunctional proteins, being essential for the cardiomyocyte survival. The incidence of bortezomib-related HF is 4% and it can be compounded by the use of steroids.5050. Willis MS, Patterson C. Proteotoxicity and cardiac dysfunction--Alzheimer's disease of the heart? N Engl J Med. 2013;368(5):455-64. In addition to being irreversible, carfilzomib is the most potent proteasome inhibitor and can cause HF in up to 25% of the patients.5151. Russell SD, Lyon A, Lenihan DJ, Moreau P, Joshua D, Chng W-J. Serial echocardiographic assessment of patients (pts) with relapsed multiple myeloma (RMM) receiving carfilzomib and dexamethasone (Kd) vs bortezomib and dexamethasone (Vd): a substudy of the phase 3 Endeavor Trial (NCT01568866).[abstract]. Blood; 2015;126:4250.,5252. Lendvai N, Devlin S, Patel M, Knapp KM, Ekman D, Grundberg I, et al. Biomarkers of cardiotoxicity among multiple myeloma patients subsequently treated with proteasome inhibitor therapy.[abstract]. Blood. 2015;126:4257.
4.5. BRAF and MEK Inhibitors
The combined BRAF-MEK inhibitor therapy is currently the first choice for metastatic BRAF-mutant melanoma, because it significantly improves patients' survival. So far, three BRAF inhibitors (dabrafenib, vemurafenib and encorafenib) and three MEK inhibitors (trametinib, cobimetinib and binimetinib) have been approved for the treatment of melanoma.5353. Maverakis E, Cornelius LA, Bowen GM, Phan T, Patel FB, Fitzmaurice S, et al. Metastatic melanoma - a review of current and future treatment options. Acta Derm Venereol. 2015;95(5):516-24.–5555. Johnson DB, Sosman JA. Therapeutic Advances and Treatment Options in Metastatic Melanoma. JAMA Oncol. 2015;1(3):380-6.
Several studies have reported cardiovascular adverse effects associated with those inhibitors, mainly LVEF reduction (5-11%), SAH (10-15%), and QT interval prolongation.5656. Dummer R, Ascierto PA, Gogas HJ, Arance A, Mandala M, Liszkay G, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19(5):603-15.,5757. Ascierto PA, McArthur GA, Dreno B, Atkinson V, Liszkay G, Di Giacomo AM, et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17(9):1248-60. The inhibition of BRAF and MEK interferes with cardiovascular MAPK signaling, resulting in oxidative stress, cardiac myocyte apoptosis, and angiogenesis inhibition.5656. Dummer R, Ascierto PA, Gogas HJ, Arance A, Mandala M, Liszkay G, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19(5):603-15.,5757. Ascierto PA, McArthur GA, Dreno B, Atkinson V, Liszkay G, Di Giacomo AM, et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17(9):1248-60.
A recent meta-analysis, including five randomized clinical trials and 2317 patients with melanoma and receiving BRAF and MEK inhibitors, has shown that the concomitant treatment with those inhibitors is associated with an increased risk for pulmonary embolism (4.4x), LVEF reduction (3.72x), and SAH (1.5x). There was no increase in the occurrence of arrhythmias, myocardial infarction, and QT prolongation. A higher risk for HF was detected in patients under the age of 55 years.5858. Mincu RI, Mahabadi AA, Michel L, Mrotzek SM, Schadendorf D, Rassaf T, et al. Cardiovascular Adverse Events Associated With BRAF and MEK Inhibitors: A Systematic Review and Meta-analysis. JAMA Netw Open. 2019 Aug 2;2(8):e198890.
4.6. Taxanes
Paclitaxel and docetaxel are used to treat several solid neoplasms. Cardiotoxicity is not frequent with this group of drugs, occurring in 12 out of 100 (RR: 0.9 [0.53 -1.54]).5959. Ferguson T, Wilcken N, Vagg R, Ghersi D, Nowak AK. Taxanesfor adjuvanttreatment of early breast cancer. Cochrane Database of Systematic Reviews. 2007;4:1-50. Docetaxel, in particular, seems to be associated with an increase in the occurrence of ventricular dysfunction. Some reports have suggested that taxanes should be avoided in patients with previous ventricular dysfunction, and the same non-use criteria of anthracyclines apply. Taxanes have been reported to cause sinus bradycardia, atrioventricular blocks, ventricular tachycardia, and ventricular extrasystoles. However, because taxanes are used in combination with anthracyclines, determining their cardiotoxicity potential is challenging.3636. Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273-83.,6060. Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724-34.
4.7. Immune Checkpoint Inhibitors
Immune checkpoint inhibitors have revolutionized cancer treatment. The ICIs modulate the immune system, inhibiting the apoptosis of T lymphocytes, restoring the antitumor cell response. Their anti-apoptotic action comprises the inhibition of CTLA-4 (ipilimumab), PD-1 (nivolumab, pembrolizumab), and PDL-1 (atezolizumab, durvalumab, avelumab) (Figure 6).6161. Haanen JB, Robert C. Immune Checkpoint Inhibitors. Prog Tumor Res 2015;42:55-66.
The cardiotoxicity of ICIs can be divided into two categories: inflammatory adverse effects (myocarditis, pericarditis, and vasculitis) and non-inflammatory cardiovascular toxicity (Takotsubo-like syndrome, asymptomatic non-inflammatory ventricular dysfunction, and arrhythmias). Most cases reported are severe, with mortality rates of 50% in myocarditis, 21% in pericardial disease, and 6% in vasculitis.6262. Bonaca MP, Olenchock BA, Salem JE, Wiviott SD, Ederhy S, Cohen A, et al. Myocarditis in the Setting of Cancer Therapeutics: Proposed Case Definitions for Emerging Clinical Syndromes in Cardio-Oncology. Circulation. 2019;140(2):80-91. The major causes of mortality from myocarditis are arrhythmias and cardiogenic shock.6262. Bonaca MP, Olenchock BA, Salem JE, Wiviott SD, Ederhy S, Cohen A, et al. Myocarditis in the Setting of Cancer Therapeutics: Proposed Case Definitions for Emerging Clinical Syndromes in Cardio-Oncology. Circulation. 2019;140(2):80-91.–6464. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4(12):1721-8.
The adverse events usually occur after the first or second dose of ICIs, but sporadic cardiovascular events have been reported up to 32 weeks after treatment. The prevalence of cardiovascular involvement is higher in patients on combined therapy, of the female sex, and older than 75 years. The prevalence of myocarditis varies from 0.06% to 0.3%.6262. Bonaca MP, Olenchock BA, Salem JE, Wiviott SD, Ederhy S, Cohen A, et al. Myocarditis in the Setting of Cancer Therapeutics: Proposed Case Definitions for Emerging Clinical Syndromes in Cardio-Oncology. Circulation. 2019;140(2):80-91.,6363. Varricchi G, Galdiero MR, Marone G, Criscuolo G, Triassi M, Bonaduce D, et al. Cardiotoxicity of immune checkpoint inhibitors. ESMO Open. 2017 Oct 26;2(4):e000247.
For patients who develop new cardiovascular symptoms during or right after treatment with ICIs or who have arrhythmia, conduction system abnormality or ventricular dysfunction on the echocardiogram, initiating cardiovascular investigation with measurement of biomarkers (troponin, NT-proBNP and C-reactive protein), ECG, viral panel test, strain echocardiography and CMRI is recommended to confirm the diagnosis and exclude viral myocarditis (IIa, C).
Endomyocardial biopsy should be considered in case of diagnostic suspicion even when the initial investigation is negative (IIa, C).
5. Radiotherapy
The current incidence of radiation-induced cardiotoxicity is hard to estimate, among other reasons, because of the long interval between exposure and the clinical manifestation of cardiotoxicity, the concomitant use of cardiotoxic chemotherapy, and the progressive improvement in radiation techniques in recent years with the consequent reduction in the incidence of cardiac structural damage. Some studies have reported relative risk of fatal cardiovascular events varying from 2.2% to 12.7% in lymphoma survivors and from 1% to 2.2% in breast cancer patients.6565. Jaworski C, Mariani JA, Wheeler G, Kaye DM. Cardiac complications of thoracic irradiation. J Am Coll Cardiol. 2013;61(23):2319-28.,6666. Aleman BM, van den Belt-Dusebout AW, De Bruin ML, van ‘t Veer MB, Baaijens MH, de Boer JP, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109(5):1878-86. Among survivors exposed to radiotherapy, the risk of ventricular dysfunction increases 4.9 times.6666. Aleman BM, van den Belt-Dusebout AW, De Bruin ML, van ‘t Veer MB, Baaijens MH, de Boer JP, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109(5):1878-86. Radiation-related cardiotoxicity is more frequent in patients with left-sided breast cancer6767. Correa CR, Litt HI, Hwang WT, Ferrari VA, Solin LJ, Harris EE. Coronary artery findings after left-sided compared with right-sided radiation treatment for early-stage breast cancer. J Clin Oncol. 2007;25(21):3031-7. and in those on the concomitant use of anthracyclines. The radiation-induced injury can affect the cardiac muscle, valves, pericardium, coronary arteries and conduction system,6868. Desai MY, Jellis CL, Kotecha R, Johnston DR, Griffin BP. Radiation-Associated Cardiac Disease: A Practical Approach to Diagnosis and Management. JACC Cardiovasc Imaging. 2018;11(8):1132-49. and can be diagnosed 10 to 15 years after radiotherapy.
6. Cardiotoxicity Prevention and Treatment
-
Cardiotoxicity should be prevented in all cancer patients and the cardiovascular risk factors should be recognized since the initial consultation. The following measures are recommended: smoking and alcoholism cessation, implementation of a regular diet aimed at maintaining a proper weight (body mass index between 18 and 24 kg/m2), physical exercise practice (moderate aerobic activity for 30 minutes per day at least 5 times per week), SAH control, treatment of diabetes and dyslipidemia (I, B).
-
Angiotensin-converting-enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB) are the drugs of choice to treat SAH. Statins are recommended to treat dyslipidemia, aiming at maintaining LDL levels below 100 mg/dL. Metformin is the drug of choice to treat diabetes and, when HF is associated, SGLT2 inhibitors (empaglifozin, dapaglifozin, canaglifozin) should be used. In the presence of coronary artery disease (CAD), GLP-1 agonists (liraglutide, dulaglutide and semaglutide) should be preferred (IIa, C).
-
When assessing the therapeutic proposal, the risk factors for cardiotoxicity should be identified and specific measures implemented according to the regimen (IIa, C).
-
For patients with subclinical cardiotoxicity [troponin elevation or SLG reduction (absolute ≥ 5% or relative ≥ 15%)]:
-
the use of ACEI or ARB or beta-blocker can be considered, aiming at preventing ventricular dysfunction and cardiovascular events (IIa, B);
-
repeat strain echocardiography every 3 months and measurement of biomarkers every cycle, if asymptomatic, or at any time, if symptoms appear (IIa, C);
-
chemotherapy should not be suspended based on alterations in strain and biomarkers (IIa, C);
consider referring the patient to the cardio-oncologist (IIa, C);
-
consider ruling ischemic heart disease out (IIa, C);
-
consider initiating dexrazoxane in patients who will undergo high doses of anthracyclines and at high risk for cardiotoxicity (IIa, B).
-
-
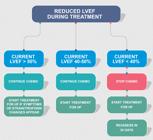
For patients with LVEF ≤ 50% and ≥ 40%, therapy with ACEI/ARB and beta-blocker is recommended before initiating a cardiotoxic treatment (I, A).
-
Patients with LVEF ≤ 40% should not receive therapy with anthracycline unless there is no effective treatment option (IIa, A).
-
Patients on chemotherapy or immunotherapy, who develop HF and LVEF < 40% during treatment, should have their antineoplastic treatment suspended temporarily based on the discussion with the cardiologist and the oncologist, and therapy for HF should begin based on guidelines and consensus statements (I, A).
-
Patients on potentially cardiotoxic drugs, who develop HF signs or symptoms, should be referred to the cardio-oncologist for clinical assessment, echocardiography, and measurement of biomarkers (IIa, C).
-
Figures 7 and 8 show the algorithms that should be considered for the management of ventricular dysfunction induced by anthracyclines and anti-HER2 (IIa, B).
-
For patients with trastuzumab-induced cardiotoxicity, after symptom stabilization and LVEF recovery to > 40%, trastuzumab reintroduction should be considered, provided that the patient is followed up by a cardio-oncologist, with serial assessment by use of echocardiography and biomarkers (IIa, B).
-
For patients with trastuzumab-induced cardiotoxicity, if symptoms do not improve and LVEF persists below 40%, trastuzumab should only be reintroduced if there is no therapeutic alternative and after thorough discussion with the oncologist (IIa, C).
-
For patients on sunitinib or other anti-VEGF drug, SAH assessment and proper control are recommended (IIa, C).
-
For patients on monoclonal antibodies or anti-VEGF tyrosine kinase inhibitors (bevacizumab, sunitinib, sorafenib, axitinib and pazopanib), the highest risk for HF occurs at the beginning of therapy. In the presence of signs and symptoms, the patient should be assessed with echocardiography and measurement of biomarkers (IIa, B). Cardio-oncologist consultation, HF treatment initiation, and discussion of drug suspension with the oncologist are recommended (IIa, C). After clinical and LVEF recovery, consider resuming chemotherapy (IIa, C).
-
For patients with HF or ventricular dysfunction, drug treatment should be instituted according to guidelines (I, A).
-
The indication for circulatory assistance device or heart transplantation follows the Brazilian Guideline on Acute and Chronic Heart Failure recommendations. Before the indication, the patient's status and oncological prognosis should be discussed with the oncologist, always considering the patient's preferences.
-
The indication for heart transplantation of cancer patients follows the Brazilian Guideline on Acute and Chronic Heart Failure recommendations (Table 8). However, patients with acute or chronic HF should only be considered for transplantation when meeting criteria of cancer remission or cure for more than 3 years (IIa, C).
-
If myocarditis due to ICIs is suspected or confirmed, the therapy with ICIs should be interrupted and corticosteroid initiated immediately (intravenous methylprednisolone, 1g per day, for 3 to 5 days, followed by prednisone, 1-2 mg/kg/day). Corticosteroid should be kept until resolution of the symptoms and normalization of troponin, systolic function and conduction abnormalities (IIa, C). In cases of pericarditis, oral corticosteroid is recommended (IIa, C). For Takotsubo syndrome, pulse therapy can be considered (IIa, C), and, for dilated cardiomyopathy, support treatment is recommended (Table 9).
-
For patients with refractory myocarditis or in severe situations with cardiogenic shock, other immunosuppressant therapies, such as antithymocyte globulin, infliximab (except for patients with HF), mycophenolate mofetil, cyclophosphamide or abatacept, should be considered (IIa, C).
-
For patients with tachyarrhythmia or bradyarrhythmia induced by ICIs, proper drug therapy and pacemaker should be considered according to the clinical characteristics (IIa, C).
-
Therapy with ICIs should be discontinued in cases of myocarditis. The decision on resuming therapy should be individualized according to cancer status, response to treatment and cardiotoxicity severity, and analyzing the risks and benefits. If ICIs are resumed, monotherapy with one anti-PD1 drug and cardiovascular surveillance are recommended (IIa, C).
-
Consider the use of dexrazoxane for patients with metastatic breast cancer and a planned high dose of anthracycline (doxorubicin > 250 mg/m2) (I, A), for patients with sarcoma and for pediatric patients with lymphoma/leukemia (IIa, A).
Algorithm for the management of heart failure and ventricular dysfunction induced by anthracyclines. chemo: chemotherapy; HF: heart failure; LVEF: left ventricular ejection fraction
Algorithm for the management of heart failure and ventricular dysfunction induced by anti-HER2 therapy. chemo: chemotherapy; HF: heart failure; LVEF: left ventricular ejection fraction
Recommendations for heart transplantation. Coordinating Committee of the Brazilian Guideline on Acute and Chronic Heart Failure6969. Comite Coordenador da Diretriz de Insuficiencia C, Rohde LEP, Montera MW, Bocchi EA, Clausell NO, Albuquerque DC, et al. Arq Bras Cardiol. 2018;111(3):436-539.
7. Arterial and Venous Thromboembolism
Thromboembolic disease is common in cancer patients, being considered the second cause of mortality in that population.
7.1. Venous Thromboembolism
Venous thromboembolism (VTE) comprises deep venous thrombosis (DVT) and pulmonary thromboembolism (PTE). It is a severe complication in cancer patients, being their second cause of death. Neoplasms are associated not only with an increase in the risk for VTE and in its severity, but also with thrombosis recurrence, which result in higher rates of treatment-related complications. Moreover, cancer patients have a 2- to 9-times higher chance of recurrence of thromboembolic events.7070. Lyman GH, Khorana AA, Falanga A, Clarke-Pearson D, Flowers C, Jahanzeb M, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. 2007;25(34):5490-505.–7272. Chee CE, Ashrani AA, Marks RS, Petterson TM, Bailey KR, Melton LJ, 3rd, et al. Predictors of venous thromboembolism recurrence and bleeding among active cancer patients: a population-based cohort study. Blood. 2014;123(25):3972-8.
Cancer induces a pro-thrombotic state because of the following: production of thrombogenic microparticles; platelet activation; its antifibrinolytic properties; and thrombin production. In addition, thrombogenesis is intensified by factors related to cancer type, disease status, concomitant use of drugs,7373. Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: Burden, mechanisms, and management. Thromb Haemost. 2017;117(2):219-30. such as erythropoiesis-stimulating agents, presence of anemia and leukocytosis, obesity and thrombogenic laboratory phenotype, such as high levels of D-dimer and prothrombin fragment 1+2.7474. Thaler J, Ay C, Pabinger I. Clinical significance of circulating microparticles for venous thromboembolism in cancer patients. Hamostaseologie. 2012;32(2):127-31.
In the past 5 years, some clinical trials on the cancer population were published, allowing the expansion of the therapeutic arsenal (Table 10).7575. Li A, Garcia DA, Lyman GH, Carrier M. Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): A systematic review and meta-analysis. Thromb Res. 2019;173:158-63.,7676. Pritchard ER, Murillo JR, Jr., Putney D, Hobaugh EC. Single-center, retrospective evaluation of safety and efficacy of direct oral anticoagulants versus low-molecular-weight heparin and vitamin K antagonist in patients with cancer. J Oncol Pharm Pract. 2019;25(1):52-9.
The recommendations for VTE management in cancer patients are:
-
The multiprofessional team caring for oncological patients should instruct them on the risk for VTE, particularly in high-risk situations, such as large surgeries and during chemotherapy (IIa, C).
-
In-patients should receive pharmacological prophylaxis, in the absence of contraindications (IIa, B).
-
Pharmacological prophylaxis should not be routinely performed for patients admitted for small procedures or chemotherapy infusion or transplantation (IIa, C).
-
For low-risk outpatients, routine anticoagulation to prevent VTE is not recommended (III, B).
-
Outpatient pharmacological prophylaxis with apixaban, rivaroxaban or enoxaparin should be provided to those at high risk for VTE, assessed with the Khorana score (≥ 2) or the CAT score (D-dimer level and cancer type) (IIa, A).
-
For outpatient pharmacological prophylaxis assessment, consider the patients' risk for bleeding (higher in gastrointestinal tumors) and their preferences (IIa, C).
-
Patients with multiple myeloma on thalidomide or lenalidomide or dexamethasone should be assessed for the use of aspirin or enoxaparin (IIa, C).
-
Patients undergoing large oncological surgeries should receive pharmacological prophylaxis against VTE (enoxaparin or low-molecular-weight heparin), starting in the preoperative period, except for those with active bleeding or at high risk for bleeding (I, A). Mechanical methods can be added to pharmacological prophylaxis; however, they should only be used as monotherapy for patients with contraindication for heparin (IIa, B).
-
The combined regimen of pharmacological and mechanical prophylaxis can improve efficacy, especially in patients at higher risk (IIa, B).
-
The pharmacological prophylaxis against thrombus for patients undergoing large oncological surgery should be extended for 7 to 10 days, and be prolonged for 4 weeks in the postoperative period in cases of open abdominal or laparoscopic surgery and of pelvic surgery in the presence of other risk factors, such as obesity, immobility, and history of VTE (IIa, B).
-
For smaller surgeries, the duration of prophylaxis should be decided on a personalized basis (IIa, C).
-
In the oncology patient, VTE can be initially treated with low-molecular-weight heparin (enoxaparin), unfractionated heparin, fondaparinux, apixaban or rivaroxaban. For patients starting treatment with parenteral anticoagulation, low-molecular-weight heparin, rather than unfractionated heparin, is preferred in the first days of treatment, provided the patient has no kidney dysfunction (creatinine clearance should be > 40 mL/min/m2) (I, A).
-
Long-term anticoagulation can be performed preferably with low-molecular-weight heparin, edoxaban, apixaban or rivaroxaban for at least 6 months (I, A).
-
Warfarin can be used in cancer patients when other drugs are not available or when other anticoagulants are contraindicated, such as for chronic kidney failure requiring dialysis (IIa, B).
-
Direct oral anticoagulants (DOACs), such as rivaroxaban and apixaban, are associated with higher bleeding rates, especially in gastrointestinal and genitourinary neoplasms (IIa, B).
-
In cancer patients, drug interactions with DOACs should be analyzed on a case-by-case basis (I, A).
-
Anticoagulation for more than 6 months should be offered to patients with active cancer, such as metastatic ones, or on chemotherapy, provided the risks and benefits are analyzed (IIa, C).
-
Based on expert opinions, in the absence of randomized studies, vena cava filters should not be inserted in patients with chronic or established (for more than 4 weeks) thrombosis or temporary contraindications for anticoagulant therapy (IIa, C).
-
Warfarin is the first option for anticoagulation in patients with chronic kidney failure requiring dialysis (IIa, B).
-
Vena cava filters can be considered for patients with high-risk acute VTE (in the past 4 weeks) and absolute contraindication for anticoagulation (IIa, C).
-
Incidental PTE and DVT should be treated the same way symptomatic VTE is, because they have similar outcomes (IIa, C).
-
The treatment of subsegmental PTE or of visceral or splanchnic venous thrombosis should be considered on a case-by-case basis, analyzing the potential benefits and risks of anticoagulation (IIa, C).
-
Cancer patients should have their risk for VTE assessed on an outpatient basis with the Khorana or the CAT score, and the benefits and risks of that strategy should be analyzed on a case-by-case basis, because they are associated with a reduction in thromboembolic events but not in mortality (IIa, B).
-
For clinically significant bleeding associated with warfarin, the treatment of choice is intravenous vitamin K (10 mg) and intravenous prothrombin complex (500 U/kg) (IIa, B).
-
For bleeding associated with rivaroxaban, edoxaban and apixaban, no specific antidote is available. Thus, the use of antifibrinolytics (intravenous tranexamic acid, 1g to 2g) and prothrombin complex (500 U/kg, intravenous) is recommended. For refractory cases, plasma (15 mL/kg), cryoprecipitate (1 U/kg) and platelet (1-2 units), by use of apheresis, are recommended (IIa, C).
There is a substantial variation in the risk for VTE in cancer patients and different clinical situations. Cancer patients should have their risk for VTE analyzed in the baseline assessment and then periodically, particularly at the beginning of antineoplastic therapy and on hospital admission. Individual risk factors, including biomarkers or cancer site, do not accurately identify cancer patients at risk for VTE. On an outpatient basis, the assessment should include the Khorana and the CAT scores (IIa, C) (Tables 11 and 12, respectively).
Khorana score7777. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902-7.
Nomogram (CAT score) to predict risk for venous thromboembolism in 6 months7878. Ay C, Dunkler D, Marosi C, Chiriac AL, Vormittag R, Simanek R, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116(24):5377-82.
7.2. Arterial Thrombosis
In an epidemiological study with 279 719 participants and comparing patients with neoplasm and controls without neoplasm, the incidence of arterial events was 4.7% in the former and 2.2% in the latter in 6 months.7979. Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, et al. Risk of Arterial Thromboembolism in Patients With Cancer. J Am Coll Cardiol. 2017;70(8):926-38. Usually, these events occur in individuals with metastatic pancreas, breast, colorectal and lung neoplasms, who are on anthracyclines, taxanes and platins. The pro-thrombotic state may favor the occurrence of embolic events secondary to atrial fibrillation. Some antineoplastic drugs, especially VEGF inhibitors, may induce thromboembolic complications. In patients on hormone therapy, higher rates of arterial thrombotic events are more often observed with aromatase inhibitors than with tamoxifen. In several cases, kinases and their pathways play a critical role in vascular and metabolic cell homeostasis. The inhibition of these kinases can cause cardiovascular sequelae, depending on the kinase type. The most worrisome vascular toxicities that might occur with the new agents include arterial ischemic events, such as acute myocardial infarction, stroke and ischemia of a limb, as well as venous thromboembolic events.7979. Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, et al. Risk of Arterial Thromboembolism in Patients With Cancer. J Am Coll Cardiol. 2017;70(8):926-38.
Recent reports have shown that the VEGF-inhibitor therapy results in adverse vascular events, such as aortic dissection, stroke, and arterial and venous thrombosis. Of the VEGF inhibitors, bevacizumab is associated with the highest VTE rate, around 12%, as compared to 2% with the other drugs.4949. Herrmann J, Yang EH, Iliescu CA, Cilingiroglu M, Charitakis K, Hakeem A, et al. Vascular Toxicities of Cancer Therapies: The Old and the New--An Evolving Avenue. Circulation. 2016;133(13):1272-89.,8080. Meyer T, Robles-Carrillo L, Robson T, Langer F, Desai H, Davila M, et al. Bevacizumab immune complexes activate platelets and induce thrombosis in FCGR2A transgenic mice. J Thromb Haemost. 2009;7(1):171-81.
8. Metabolic Syndrome Associated with Androgen Deprivation Therapy
The treatment of locally advanced prostate neoplasms is based on the hormonal control of testosterone. This blockade can be obtained surgically (orchiectomy) or through androgen deprivation therapy. Gonadotropin releasing hormone (GnRH) agonists (leuprolide, goserelin and triptorelin) and antagonists (degarelix) cause central blockade with a reduction in the levels of luteinizing and follicle stimulating hormones and testosterone. In addition, adrenal androgen receptor inhibitors (abiraterone) and direct androgen inhibitors (enzalutamide) reduce testosterone. These drugs are used with curative intention in high-risk patients with non-metastatic disease and as standard therapy for metastatic disease. Understanding the impact of these drugs on cardiovascular risk is important because many risk factors that lead to prostate cancer can result in cardiovascular disease, such as advanced age, smoking, diet, and obesity. Some studies have reported a higher prevalence of those risk factors among prostate cancer patients.
The recognized antiandrogen therapy leads to metabolic changes characterized by hyperinsulinemia, hypercholesterolemia, and body composition changes, with an increase in predominantly visceral fat and a reduction in lean mass. The metabolic syndrome resulting from the antiandrogen therapy is associated with an increase in cardiovascular complications. Modification of risk factors is recommended with lipid-lowering therapy, anti-hypertensive treatment, strict control of glycemia, and use of antiplatelet drugs (IIa, B).
9. Cardiac Arrhythmia
Several factors present in cancer patients, such as infection, electrolyte imbalance, dehydration, surgical procedures, and oncological and adjuvant therapies, predispose to the occurrence of cardiac arrhythmias.8181. Guglin M, Aljayeh M, Saiyad S, Ali R, Curtis AB. Introducing a new entity: chemotherapy-induced arrhythmia. Europace. 2009;11(12):1579-86. These arrhythmias are relatively frequent complications in cancer patients, estimated to occur in 16-36% of those patients.8282. Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53(24):2231-47.,8383. Tamargo J, Caballero R, Delpon E. Cancer chemotherapy and cardiac arrhythmias: a review. Drug Saf. 2015;38(2):129-52.
The types of cardiac arrhythmias in oncology patients comprise a wide range: sinus tachycardia, bradyarrhythmias, tachyarrhythmias, and conduction disorders. Of the supraventricular arrhythmias, the most common is atrial fibrillation. Ventricular tachycardia and ventricular fibrillation are rare, but can occur especially in the presence of QT prolongation and in patients with hypokalemia or hypomagnesemia.8484. Kishi S, Yoshida A, Yamauchi T, Tsutani H, Lee JD, Nakamura T, et al. Torsade de pointes associated with hypokalemia after anthracycline treatment in a patient with acute lymphocytic leukemia. Int J Hematol. 2000;71(2):172-9.,8585. Farmakis D, Parissis J, Filippatos G. Insights into onco-cardiology: atrial fibrillation in cancer. J Am Coll Cardiol. 2014;63(10):945-53. Table 13 lists the major drugs related to cardiac arrhythmias and their incidences.
9.1. QT Prolongation
The diagnosis of QT prolongation is electrocardiographic, and QTc should be calculated by use of the Bazett's formula [QT / (RR)1/2] or Fridericia's formula [QT / (RR)1/3]. Normal QTc values are as follows: ≤ 440 ms in men; between 450 and 460 in women. Both congenital and acquired factors can be responsible for QT prolongation, and the most cited conditions are as follows: female sex, bradycardia, electrolyte abnormalities, drug effects, myocardial ischemia, HF, myocarditis, hypothermia, and channelopathies.8686. Vorchheimer DA. What is QT interval prolongation? J Fam Pract. 2005;Suppl(6):S4-7.
The QT prolongation is a concern in cancer patients, because the oncological treatment, electrolyte disorders and concomitant medications can contribute to that prolongation and predispose to complex arrhythmias.8383. Tamargo J, Caballero R, Delpon E. Cancer chemotherapy and cardiac arrhythmias: a review. Drug Saf. 2015;38(2):129-52. Monitoring the QT interval and correcting the factors that contribute to QT prolongation should be considered in patients on medications that prolong the QT interval. Cardiotoxicity is defined in the presence of QTc prolongation > 500 ms and/or QT variation > 60ms from baseline (Table 14).8787. Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36(41):2793-867.,8888. Porta-Sanchez A, Gilbert C, Spears D, Amir E, Chan J, Nanthakumar K, et al. Incidence, Diagnosis, and Management of QT Prolongation Induced by Cancer Therapies: A Systematic Review. J Am Heart Assoc. 2017 Dec 7;6(12):e007724.
The QT interval and the risk factors associated with QT prolongation should be assessed before and after treatment with drugs known to be related to cardiac arrhythmias, such as tyrosine kinase inhibitors (crizotinib, dasatinib, sunitinib) and arsenic trioxide. Electrolyte and ECG assessments should be performed during treatment at baseline, 7-15 days after the beginning of therapy, after changes in doses in the first 3 months, and depending on the therapy frequency. Before postponing chemotherapy, the suspension of other medications related to QT prolongation, such as antiemetics, antidepressants, antiarrhythmics, antifungal drugs, antipsychotics, should be considered. In addition, correction of electrolyte disorders should be performed. Patients on arsenic trioxide should be monitored with ECG every week.2222. Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(36):2768-801.
The QT prolongation increases the incidence of ventricular arrhythmias and torsades de points.8888. Porta-Sanchez A, Gilbert C, Spears D, Amir E, Chan J, Nanthakumar K, et al. Incidence, Diagnosis, and Management of QT Prolongation Induced by Cancer Therapies: A Systematic Review. J Am Heart Assoc. 2017 Dec 7;6(12):e007724. Ventricular tachycardias are usually associated with structural cardiomyopathies [CAD, dilated cardiomyopathy, right ventricular heart diseases, congenital abnormalities, hypertrophic cardiomyopathy, and channelopathies].8989. Delacretaz E. Clinical practice. Supraventricular tachycardia. N Engl J Med. 2006;354(10):1039-51. The objective of the treatment of ventricular arrhythmias is to reduce morbidity and sudden death events, and the assessment of triggering factors is paramount. Pharmacological therapy is indicated for refractory and/or symptomatic cases.9090. Sociedade Brasileira de Cardiologia. [Guidelines for the evaluation and treatment of patients with heart arrhythmia]. Arq Bras Cardiol. 2002;79(Suppl 5):7-50.
9.2. Atrial Fibrillation
Atrial fibrillation is the most common cardiac arrhythmia of the oncology patient. Its occurrence is related to the pro-inflammatory state of these patients, the inflammatory response to oncological surgery, and the cardiotoxic effects of antineoplastic therapy.9191. Cheng WL, Kao YH, Chen SA, Chen YJ. Pathophysiology of cancer therapy-provoked atrial fibrillation. Int J Cardiol. 2016;219:186-94. Understanding the mechanisms that trigger and sustain atrial fibrillation is important for prevention.
Atrial fibrillation can be induced by several mechanisms, such as myocardial change due to electrolyte disorder, liposomal and mitochondrial injury, inflammation, pericardial disease, and increased oxidative stress inducing cell apoptosis.9292. Lopez-Fernandez T, Martin-Garcia A, Roldan Rabadan I, Mitroi C, Mazon Ramos P, Diez-Villanueva P, et al. Atrial Fibrillation in Active Cancer Patients: Expert Position Paper and Recommendations. Rev Esp Cardiol (Engl Ed). 2019;72(9):749-59.
There is great difficulty in establishing the causal relationship between arrhythmic events and each chemotherapy drug. The small number of studies published and the simultaneous administration of many drugs make it difficult to relate drug and effect. The chemotherapy drugs most commonly associated with arrhythmias are anthracyclines (doxorubicin, epirubicin), antimicrotubule agents (paclitaxel and docetaxel), antimetabolites (5-fluorouracil, capecitabine and gemcitabine), alkylating agents (cisplatin and cyclophosphamide), tyrosine kinase inhibitors (ibrutinib, ponatinib, sorafenib and sunitinib), and monoclonal antibodies (trastuzumab and cetuximab), in addition to immunotherapy drugs.
Cancer is associated with a pro-thrombotic state and can increase the risk for embolic events in patients with atrial fibrillation, who also have more bleeding complications due to treatment, and, therefore, higher morbidity and mortality. There is no consensus/guideline-based recommendation on the use of antithrombotic drugs for patients with atrial fibrillation.8585. Farmakis D, Parissis J, Filippatos G. Insights into onco-cardiology: atrial fibrillation in cancer. J Am Coll Cardiol. 2014;63(10):945-53.,9393. Melloni C, Shrader P, Carver J, Piccini JP, Thomas L, Fonarow GC, et al. Management and outcomes of patients with atrial fibrillation and a history of cancer: the ORBIT-AF registry. Eur Heart J Qual Care Clin Outcomes. 2017;3(3):192-7.
The choice of antithrombotic therapy for cancer patients should be individualized, analyzing pharmacokinetic and pharmacodynamic factors, drug interactions, and risks for thrombosis and bleeding (IIa, B).
Warfarin should be avoided in the oncology patient with atrial fibrillation, because that drug is associated with lower efficacy and higher risk of bleeding due to drug interactions, higher occurrence of liver dysfunction, dietary changes, cachexia, and malnutrition (IIa, B).
The DOACs (dabigatran, rivaroxaban, apixaban and edoxaban) are superior to warfarin in terms of efficacy and bleeding in the general population with atrial fibrillation; however, the evidence for their use in cancer patients derives from analyses of substudies and observational data (IIa, B).
Although there is no validation of the classic scores for the cancer population, anticoagulation should be initiated in those patients according to the same criteria adopted for the population without cancer: CHADS2 and CHA2DS2-VASc scores above 2 indicate anticoagulation (IIa, C) (Table 15).
Antithrombotic therapy in cancer patients should be personalized, analyzing the patient's profile, cancer type, and risk for thrombosis and bleeding, the last, for example, by use of the HAS-BLED score (IIa, C) (Table 16).
There is no prospective randomized study on DOACs in cancer patients with atrial fibrillation. Analysis of substudies from randomized clinical trials have shown those drugs to be safe and effective in cancer patients. This evidence and the results from studies on VTE and cancer, which confirm the superiority of DOACs as compared to low-molecular-weight heparin, suggest that DOACs are a feasible option of antithrombotic therapy in cancer patients with atrial fibrillation (IIa, C).
The routine use of DOACs can be considered for patients with atrial fibrillation and gastrointestinal or genitourinary tract tumors, and the potential risk for bleeding should be analyzed (IIb, B).
The management of cancer patients with atrial fibrillation should include a cardiologist since the beginning, because of the higher rates of anticoagulation use and lower incidence of ischemic and hemorrhagic complications (IIa, C).
10. Coronary Artery Disease
Cancer and CAD have several risk factors in common and often coexist in the same patient (Figure 9). The presence of risk factors, such as advanced age, smoking, diabetes, SAH, sedentary lifestyle and dyslipidemia, is elevated in cancer patients.9494. Costa I, Bittar CS, Fonseca SMR, CMPD ES, Dos Santos Rehder MHH, Rizk SI, et al. Brazilian cardio-oncology: the 10-year experience of the Instituto do Cancer do Estado de Sao Paulo. BMC Cardiovasc Disord. 2020;20(1):206. Other common factors in those patients that contribute to the development of CAD are endothelial dysfunction, oxidative stress, genetic predisposition and chronic inflammation.9595. Handy CE, Quispe R, Pinto X, Blaha MJ, Blumenthal RS, Michos ED, et al. Synergistic Opportunities in the Interplay Between Cancer Screening and Cardiovascular Disease Risk Assessment: Together We Are Stronger. Circulation. 2018;138(7):727-34.
Coronary artery disease in cardio-oncology. CAD: coronary artery disease; DAPT: dual antiplatelet therapy; VEGF: vascular endothelial growth factor
In addition, the oncological treatment contributes to the high prevalence of CAD in cancer patients.4949. Herrmann J, Yang EH, Iliescu CA, Cilingiroglu M, Charitakis K, Hakeem A, et al. Vascular Toxicities of Cancer Therapies: The Old and the New--An Evolving Avenue. Circulation. 2016;133(13):1272-89. Patients with lung cancer undergoing chemotherapy have a 5.3-time (95% CI: 2.002-14.152) increase in the risk of important coronary injury, which suggests that the treatment may be associated with anatomic complexity.9696. Yang Q, Chen Y, Gao H, Zhang J, Zhang J, Zhang M, et al. Chemotherapy-Related Anatomical Coronary-Artery Disease in Lung Cancer Patients Evaluated by Coronary-Angiography SYNTAX Score. Arq Bras Cardiol. 2020;114(6):1004-12. The major mechanisms of CAD related to oncological therapy are: vasospasm, thrombosis, and accelerated atherosclerosis.4949. Herrmann J, Yang EH, Iliescu CA, Cilingiroglu M, Charitakis K, Hakeem A, et al. Vascular Toxicities of Cancer Therapies: The Old and the New--An Evolving Avenue. Circulation. 2016;133(13):1272-89.
In patients on cisplatin isolated or associated with vincristine or bleomycin, coronary thrombosis has been observed in coronary angiography without previous atherosclerosis. These drugs can induce endothelial dysfunction/lesion, which seems to be the basic mechanism of the vasoactive alteration caused by these drugs.9797. Ito D, Shiraishi J, Nakamura T, Maruyama N, Iwamura Y, Hashimoto S, et al. Primary percutaneous coronary intervention and intravascular ultrasound imaging for coronary thrombosis after cisplatin-based chemotherapy. Heart Vessels. 2012;27(6):634-8.–9999. Karabay KO, Yildiz O, Aytekin V. Multiple coronary thrombi with cisplatin. J Invasive Cardiol. 2014;26(2):E18-20. Cisplatin leads to the death of endothelial cells via the production of procoagulant microparticles.100100. Periard D, Boulanger CM, Eyer S, Amabile N, Pugin P, Gerschheimer C, et al. Are circulating endothelial-derived and platelet-derived microparticles a pathogenic factor in the cisplatin-induced stroke? Stroke. 2007;38(5):1636-8.
Another class of chemotherapy drugs typically related to CAD in cancer patients are the antimetabolites, especially 5-fluorouracil and capecitabine. The incidence of angina or acute findings ranges from 3.9% to 12.5%.101101. Yuan C, Parekh H, Allegra C, George TJ, Starr JS. 5-FU induced cardiotoxicity: case series and review of the literature. Cardiooncology. 2019 Sep 06;5:13. The mechanism through which these drugs cause toxicity has not been completely established and several hypotheses have been raised to explain those findings, such as acute vasospasm, direct toxicity to myocytes, endothelial dysfunction, and hypercoagulable state causing thrombosis.101101. Yuan C, Parekh H, Allegra C, George TJ, Starr JS. 5-FU induced cardiotoxicity: case series and review of the literature. Cardiooncology. 2019 Sep 06;5:13.,102102. Chong JH, Ghosh AK. Coronary Artery Vasospasm Induced by 5-fluorouracil: Proposed Mechanisms, Existing Management Options and Future Directions. Interv Cardiol. 2019;14(2):89-94. Acute vasospasm is often observed and experimental studies have suggested that vasoconstriction caused by 5-fluorouracil is related to protein kinase C and endothelin-1.103103. Mosseri M, Fingert HJ, Varticovski L, Chokshi S, Isner JM. In vitro evidence that myocardial ischemia resulting from 5-fluorouracil chemotherapy is due to protein kinase C-mediated vasoconstriction of vascular smooth muscle. Cancer Res. 1993;53(13):3028-33.,104104. Thyss A, Gaspard MH, Marsault R, Milano G, Frelin C, Schneider M. Very high endothelin plasma levels in patients with 5-FU cardiotoxicity. Ann Oncol. 1992;3(1):88. Similarly to 5-fluorouracil, taxanes are another class of drugs that induce angina secondary to coronary spasms. The incidence of chest pain reported by patients on paclitaxel is approximately 0.2-4%.105105. Schrader C, Keussen C, Bewig B, von Freier A, Lins M. Symptoms and signs of an acute myocardial ischemia caused by chemotherapy with Paclitaxel (Taxol) in a patient with metastatic ovarian carcinoma. Eur J Med Res. 2005;10(11):498-501.,106106. Shah K, Gupta S, Ghosh J, Bajpai J, Maheshwari A. Acute non-ST elevation myocardial infarction following paclitaxel administration for ovarian carcinoma: a case report and review of literature. J Cancer Res Ther. 2012;8(3):442-4.
Accelerated atherosclerosis has been observed in patients being treated with second- and third-generation tyrosine kinase inhibitors. These drugs have an increased risk of coronary occlusion as compared to imatinib (OR = 3.45; 95% CI: 2.30-5.18).107107. Douxfils J, Haguet H, Mullier F, Chatelain C, Graux C, Dogne JM. Association Between BCR-ABL Tyrosine Kinase Inhibitors for Chronic Myeloid Leukemia and Cardiovascular Events, Major Molecular Response, and Overall Survival: A Systematic Review and Meta-analysis. JAMA Oncol. 2016;2(5):625-32. Of the tyrosine kinase inhibitors, ponatinib seems to be the one most often related to vascular toxicity. Arterial and venous thromboses have been reported in 27% of the patients on ponatinib, regardless of the presence of cardiovascular risk factors.107107. Douxfils J, Haguet H, Mullier F, Chatelain C, Graux C, Dogne JM. Association Between BCR-ABL Tyrosine Kinase Inhibitors for Chronic Myeloid Leukemia and Cardiovascular Events, Major Molecular Response, and Overall Survival: A Systematic Review and Meta-analysis. JAMA Oncol. 2016;2(5):625-32. Patients treated with bevacizumab have high risk for coronary ischemia as compared to a control group (RR: 2.47; 95% CI: 1.4-4.36).108108. Totzeck M, Mincu RI, Rassaf T. Cardiovascular Adverse Events in Patients With Cancer Treated With Bevacizumab: A Meta-Analysis of More Than 20 000 Patients. J Am Heart Assoc. 2017 Aug 10;6(8):e006278. Patients on ICIs have an altered inflammatory cell composition of the atherosclerotic plaque (increased ratio of CD3+ T cells to CD68+ macrophages), which may predispose to plaque progression and/or clinical coronary events.109109. Newman JL, Stone JR. Immune checkpoint inhibition alters the inflammatory cell composition of human coronary artery atherosclerosis. Cardiovasc Pathol. 2019 Nov-Dec;43:107148.
Radiotherapy is classically related to the development of CAD, usually reported later after exposure to radiation. The incidence varies in the literature, with a decreasing tendency in recent decades because of the most modern techniques that reduce direct radiation emission to the heart. The pathogenesis of CAD is multifactorial, resulting in direct myocardial injury, vascular tonus alteration, inflammatory activation, and oxidative stress.110110. Ping Z, Peng Y, Lang H, Xinyong C, Zhiyi Z, Xiaocheng W, et al. Oxidative Stress in Radiation-Induced Cardiotoxicity. Oxid Med Cell Longev. 2020;2020:3579143.,111111. Wang H, Wei J, Zheng Q, Meng L, Xin Y, Yin X, et al. Radiation-induced heart disease: a review of classification, mechanism and prevention. Int J Biol Sci. 2019;15(10):2128-38.
Because of the complexity of cancer patients with CAD, their mortality is higher than that of patients without cancer in the long run.112112. Landes U, Kornowski R, Bental T, Assali A, Vaknin-Assa H, Lev E, et al. Long-term outcomes after percutaneous coronary interventions in cancer survivors. Coron Artery Dis. 2017;28(1):5-10.,113113. Nakatsuma K, Shiomi H, Morimoto T, Watanabe H, Nakagawa Y, Furukawa Y, et al. Influence of a history of cancer on long-term cardiovascular outcomes after coronary stent implantation (an Observation from Coronary Revascularization Demonstrating Outcome Study-Kyoto Registry Cohort-2). Eur Heart J Qual Care Clin Outcomes. 2018;4(3):200-7. When approaching those patients, it is important to know the oncological prognosis, the therapeutic perspectives and the program of oncological surgeries. The control of risk factors should be reinforced in patients with evidenced CAD. The use of drug-eluted stent should be preferred in those patients when the therapeutic intervention is indicated.114114. Ganatra S, Sharma A, Levy MS. Re-Evaluating the Safety of Drug-Eluting Stents in Cancer Patients. JACC Cardiovasc Interv. 2017;10(22):2334-7. Therapy with antiplatelet agents should be maintained according to the guidelines on CAD and acute coronary syndrome management, unless contraindicated, such as in the presence of tumor bleeding.115115. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39(3):213-60.–117117. Iliescu C, Grines CL, Herrmann J, Yang EH, Cilingiroglu M, Charitakis K, et al. SCAI expert consensus statement: Evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (Endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista). Catheter Cardiovasc Interv. 2016;87(5):895-9.
Recommendations:
-
The control of risk factors (SAH, diabetes, dyslipidemias) and of body weight loss, smoking cessation and dietary guidance should be provided to all patients who will receive drugs that predispose to CAD (I, A).
-
Drug-eluted stent should be preferred in these patients (IIa, B).
-
Dual antiplatelet therapy can be maintained in patients with platelet count > 30 000, in the absence of contraindication (IIa, B).
-
The investigation of CAD is indicated in patients 5 years after mediastinal exposure to a dose of at least 30 gy (IIa, B).
-
For patients who developed acute coronary syndrome with 5-fluorouracil, assessment of the coronary anatomy and by the cardio-oncology team should be considered (IIa, B).
-
Re-exposure can be considered for patients with mild events, who are asymptomatic and whose benefit with 5-fluorouracil impacts the prognosis. The use of nitrates and vasodilators can be considered in this scenario (IIb, B).
11. Arterial Hypertension
The prevalence of SAH is higher in cancer patients and survivors as compared to that of the general population.118118. Cameron AC, Touyz RM, Lang NN. Vascular Complications of Cancer Chemotherapy. Can J Cardiol. 2016;32(7):852-62. In these patients, SAH is the major modifiable risk factor for cardiovascular events.119119. Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL. Prognostic importance of Comorbidity in a hospital-based cancer registry. Jama-J Am Med Assoc. 2004;291(20):2441-7. Chronic kidney disease, SAH, cardiovascular disease, and cancer have risk factors in common, such as smoking, obesity, and diabetes. Many types of cancer and their treatments cause or aggravate preexisting SAH, because of vascular, endothelial and renal effects.2222. Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(36):2768-801.
Periodical blood pressure measurement is recommended in cancer patients (IIa, C).
The selection of anti-hypertensive agents should consider individual risk factors, effects of the antineoplastic treatment and drug interactions. It is estimated that 35% of cancer patients will develop SAH during the treatment. Patients with history of cancer have a higher prevalence of SAH than that of the general population.9494. Costa I, Bittar CS, Fonseca SMR, CMPD ES, Dos Santos Rehder MHH, Rizk SI, et al. Brazilian cardio-oncology: the 10-year experience of the Instituto do Cancer do Estado de Sao Paulo. BMC Cardiovasc Disord. 2020;20(1):206. Patients with renal, gastric and ovarian cancer have higher blood pressure levels than those with other tumor sites. Exposure to chemotherapy is an independent risk factor for SAH.120120. Boursiquot BC, Zabor EC, Glezerman IG, Jaimes EA. Hypertension and VEGF (Vascular Endothelial Growth Factor) Receptor Tyrosine Kinase Inhibition: Effects on Renal Function. Hypertension 2017.Jul 24.HYPERTENSIONAHA.117.092275 online ahrad of print.
11.1. SAH and Chemotherapy
Therapy with anti-VEGF tyrosine kinase inhibitors and multi-targeted tyrosine kinase inhibitors aggravates and induces SAH.121121. Budolfsen C, Faber J, Grimm D, Kruger M, Bauer J, Wehland M, et al. Tyrosine Kinase Inhibitor-Induced Hypertension: Role of Hypertension as a Biomarker in Cancer Treatment. Curr Vasc Pharmacol. 2019;17(6):618-34. The mechanisms are a reduction in the production of nitric oxide and in angiogenesis, leading to an increase in systemic vascular resistance. Anti-VEGF therapy leads to fluid retention because of natriuresis impairment, in addition to inducing endothelin-1-mediated vasoconstriction and thrombotic microangiopathy, similarly to the pathophysiology of eclampsia.121121. Budolfsen C, Faber J, Grimm D, Kruger M, Bauer J, Wehland M, et al. Tyrosine Kinase Inhibitor-Induced Hypertension: Role of Hypertension as a Biomarker in Cancer Treatment. Curr Vasc Pharmacol. 2019;17(6):618-34. In a recent meta-analysis, the use of anti-VEGF tyrosine kinase inhibitors increased the risk for cardiotoxicity, such as SAH, bleeding and ventricular dysfunction. The most common vascular cardiotoxicity was SAH.122122. Li J, Gu J. Cardiovascular Toxicities with Vascular Endothelial Growth Factor Receptor Tyrosine Kinase Inhibitors in Cancer Patients: A Meta-Analysis of 77 Randomized Controlled Trials. Clin Drug Investig. 2018;38(12):1109-23.
The alkylating agents seem to induce SAH through nephrotoxicity, but there is not much evidence of their real effect on blood pressure. Cyclophosphamide has been associated with multiple vascular complications, such as pulmonary and hepatic veno-occlusive disease, thromboembolic disease, and myocardial ischemia. Pre-clinical evidence has shown endothelial injury and renin-angiotensin-aldosterone system abnormalities in animals treated with cyclophosphamide.2121. Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31(2):171-90.,2222. Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(36):2768-801. Both ifosfamide and cisplatin apparently induce SAH by causing nephrotoxicity.123123. Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of Cisplatin nephrotoxicity. Toxins (Basel). 2010;2(11):2490-518. The antimicrotubule agents affect mitosis, acting on tubulin to prevent microtubule polymerization. Experimental studies have shown that vinblastine acts on the endothelium and apoptosis, but its effect in SAH is unkonwn.124124. Stoter G, Koopman A, Vendrik CP, Struyvenberg A, Sleyfer DT, Willemse PH, et al. Ten-year survival and late sequelae in testicular cancer patients treated with cisplatin, vinblastine, and bleomycin. J Clin Oncol. 1989;7(8):1099-104.,125125. Soultati A, Mountzios G, Avgerinou C, Papaxoinis G, Pectasides D, Dimopoulos MA, et al. Endothelial vascular toxicity from chemotherapeutic agents: preclinical evidence and clinical implications. Cancer Treat Rev. 2012;38(15):473-83. Gemcitabine and proteasome inhibitors can trigger SAH associated with thrombotic microangiopathy. Adjuvant medications associated with SAH used for cancer patients are: corticosteroids, erythropoietin, calcineurin inhibitors, and anti-inflammatory drugs.
11.2 Cancer-Induced SAH
Systemic arterial hypertension can be a paraneoplastic manifestation of hepatocellular carcinoma, renal cancer, and carcinoid disease. It results from the production of renin, angiotensinogen, angiotensin I or catecholamines. Among individuals with renal cell carcinoma, the SAH prevalence exceeds 75%, and SAH is due to the nephrectomy-related loss of nephrons and particularly to the treatment with VEGF inhibitors. In addition, renal cell carcinoma can secrete vasoactive peptides, mainly endothelin-1. The presence of SAH in renal cell carcinoma may indicate more aggressive disease, with a negative impact on prognosis.126126. Bendtsen MAF, Grimm D, Bauer J, Wehland M, Wise P, Magnusson NE, et al. Hypertension Caused by Lenvatinib and Everolimus in the Treatment of Metastatic Renal Cell Carcinoma. Int J Mol Sci. 2017;18(8):1736.,127127. Liu Y, Zhou L, Chen Y, Liao B, Ye D, Wang K, et al. Hypertension as a prognostic factor in metastatic renal cell carcinoma treated with tyrosine kinase inhibitors: a systematic review and meta-analysis. BMC Urol. 2019;19(1):49. Pheochromocytoma and paraganglioma are neuroendocrine tumors of the chromaffin cells, with an annual incidence of 0.8 per 100 000 individuals. The SAH related to those tumors is caused by secretion of catecholamines (norepinephrine, epinephrine, and dopamine) and can be associated with symptoms, such as headache, palpitations, and sweating.128128. Farrugia FA, Martikos G, Tzanetis P, Charalampopoulos A, Misiakos E, Zavras N, et al. Pheochromocytoma, diagnosis and treatment: Review of the literature. Endocr Regal. 2017;51(3):168-81.
Patients with cancer and SAH have a higher incidence of heart and kidney failures, and SAH is an independent risk factor for CAD, HF, and arrhythmia, being the major modifiable risk factor to prevent HF.4747. Qi WX, Shen Z, Tang LN, Yao Y. Congestive heart failure risk in cancer patients treated with vascular endothelial growth factor tyrosine kinase inhibitors: a systematic review and meta-analysis of 36 clinical trials. Br J Clin Pharmacol. 2014;78(4):748-62.,129129. Tini G, Sarocchi M, Tocci G, Arboscello E, Ghigliotti G, Novo G, et al. Arterial hypertension in cancer: The elephant in the room. Int J Cardiol 2019;281:133-9.
Blood pressure should be properly and regularly measured in cancer patients (I, A).
Patients on anti-VEGF tyrosine kinase inhibitors, multi-targeted tyrosine kinase inhibitors, alkylating agents or high doses of steroids should undergo more frequent blood pressure monitoring (IIa, C).
The blood pressure goal in cancer patients follows the recommendations for patients without cancer: < 130 x 80 mm Hg (IIa, B).
The drug treatment should be personalized, but the presence of proteinuria or ventricular dysfunction determines the indication of ACEI or ARB (IIa, B).
If there is neither proteinuria nor ventricular dysfunction, a dihydropyridine calcium-channel blocker (amlodipine) can be initiated (IIa, C).
Diuretics should be used following a well-defined criterion and considering the risk for hypovolemia as well as for fluid and electrolyte imbalance (IIa, C).
Non-dihydropyridine calcium-channel blockers (verapamil and diltiazem) in cancer patients (III, B). Because these drugs are metabolized via CYP3A4, they can alter the serum levels of antineoplastic drugs.
Secondary causes of SAH, such as hypovolemia and pain, should be investigated (IIa, C).
-
Development: Brazilian Society of Cardiology (Sociedade Brasileira de Cardiologia – SBC)
-
Norms and Guidelines Council: Brivaldo Markman Filho, Antonio Carlos Sobral Sousa, Aurora Felice Castro Issa, Bruno Ramos Nascimento, Harry Correa Filho, Marcelo Luiz Campos Vieira
-
Norms and Guidelines Coordinator: Brivaldo Markman Filho
-
Note: These statements are for information purposes and should not replace the clinical judgment of a physician, who must ultimately determine the appropriate treatment for each patient.
Referências
-
1Abdel-Qadir H, Thavendiranathan P, Austin PC, Lee DS, Amir E, Tu JV, et al. Development and validation of a multivariable prediction model for major adverse cardiovascular events after early stage breast cancer: a population-based cohort study. Eur Heart J. 2019;40(48):3913-20.
-
2Brant LCC, Nascimento BR, Passos VMA, Duncan BB, Bensenor IJM, Malta DC, et al. Variations and particularities in cardiovascular disease mortality in Brazil and Brazilian states in 1990 and 2015: estimates from the Global Burden of Disease. Rev Bras Epidemiol. 2017;20Suppl 01(Suppl 01):116-28.
-
3Collaborators GBDB. Burden of disease in Brazil, 1990-2016: a systematic subnational analysis for the Global Burden of Disease Study. 2016. Lancet 2018;392(10149):760-75.
-
4Ala CK, Klein AL, Moslehi JJ. Cancer Treatment-Associated Pericardial Disease: Epidemiology, Clinical Presentation, Diagnosis, and Management. Curr Cardiol Rep. 2019;21(12):156.
-
5Alfano CM, Cheville AL, Mustian K. Developing High-Quality Cancer Rehabilitation Programs: A Timely Need. Am Soc Clin Oncol Educ Book. 2016;35:241-9.
-
6Global Burden of Disease Cancer C, Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019; 5(12):1749-68
-
7Al-Kindi SG, Oliveira GH. Prevalence of Preexisting Cardiovascular Disease in Patients With Different Types of Cancer: The Unmet Need for Onco-Cardiology. Mayo Clin Proc. 2016;91(1):81-3.
-
8Tan C, Tasaka H, Yu KP, Murphy ML, Karnofsky DA. Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. Clinical evaluation with special reference to childhood leukemia. Cancer. 1967;20(3):333-53.
-
9Ainger LE, Bushore J, Johnson WW, Ito J. Daunomycin: a cardiotoxic agent. J Natl Med Assoc. 1971;63(4):261-7.
-
10Lipshultz SE, Lipsitz SR, Mone SM, Goorin AM, Sallan SE, Sanders SP, et al. Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332(26):1738-43.
-
11Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, Lamantia G, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109(22):2749-54.
-
12Allen S, Brown V, Prabhu P, Scott M, Rockall T, Preston S, et al. A randomised controlled trial to assess whether prehabilitation improves fitness in patients undergoing neoadjuvant treatment prior to oesophagogastric cancer surgery: study protocol. BMJ Open. 2018 Dec 22;8(12):e023190.
-
13Amigoni M, Giannattasio C, Fraschini D, Galbiati M, Capra AC, Madotto F, et al. Low anthracyclines doses-induced cardiotoxicity in acute lymphoblastic leukemia long-term female survivors. Pediatr Blood Cancer. 2010;55(7):1343-7.
-
14Anker MS, Hadzibegovic S, Lena A, Belenkov Y, Bergler-Klein J, de Boer RA, et al. Recent advances in cardio-oncology: a report from the ‘Heart Failure Association 2019 and World Congress on Acute Heart Failure 2019'. ESC Heart Fail. 2019;6(6):1140-8.
-
15Anquetil C, Salem JE, Lebrun-Vignes B, Johnson DB, Mammen AL, Stenzel W, et al. Immune Checkpoint Inhibitor-Associated Myositis. Circulation. 2018;138(7):743-5.
-
16Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35(8):893-911.
-
17Hajjar LA. 5th Global Cardio-Oncology Summit. Frontiers Media SA; 2019.
-
18Lenihan DJ, Fradley MG, Dent S, Brezden-Masley C, Carver J, Kalil Filho K, et al. Proceedings From the Global Cardio-Oncology Summit: The Top 10 Priorities to Actualize for CardioOncology. JACC: CardioOncology. 2019;1(2):256-72.
-
19Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23 Suppl 7:vii155-66.
-
20Chow EJ, Baker KS, Lee SJ, Flowers ME, Cushing-Haugen KL, Inamoto Y, et al. Influence of conventional cardiovascular risk factors and lifestyle characteristics on cardiovascular disease after hematopoietic cell transplantation. J Clin Oncol. 2014;32(3):191-8.
-
21Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31(2):171-90.
-
22Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(36):2768-801.
-
23Lenihan DJ, Hartlage G, DeCara J, Blaes A, Finet JE, Lyon AR, et al. Cardio-Oncology Training: A Proposal From the International Cardioncology Society and Canadian Cardiac Oncology Network for a New Multidisciplinary Specialty. J Card Fail 2016;22(6):465-71.
-
24McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc Drugs Ther. 2017;31(1):63-75.
-
25Lyon AR, Dent S, Stanway S, Earl H, Brezden-Masley C, Cohen-Solal A, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur J Heart Fail. 2020.;28 maio. Doi;10.1002/ejhf.1920 on line
» https://doi.org/10.1002/ejhf.1920 -
26Shankar SM, Marina N, Hudson MM, Hodgson DC, Adams MJ, Landier W, et al. Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children's Oncology Group. Pediatrics. 2008;121(2):e387-96. Epub 2008 Jan 10.
-
27Henriksen PA. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart. 2018;104(12):971-7.
-
28Narezkina A, Nasim K. Anthracycline Cardiotoxicity. Circ Heart Fail. 2019;12(3):e005910.
-
29Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18(11):1639-42.
-
30He Q, Wang F, Ryan TD, Chalasani M, Redington AN. Repeated Remote Ischemic Conditioning Reduces Doxorubicin-Induced Cardiotoxicity. JACC: CardioOncology. 2020;2(1):41-52.
-
31Coelho-Filho OR, Shah RV, Mitchell R, Neilan TG, Moreno H, Jr., Simonson B, et al. Quantification of cardiomyocyte hypertrophy by cardiac magnetic resonance: implications for early cardiac remodeling. Circulation. 2013;128(11):1225-33.
-
32Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments. Nat Rev Cardiol 2015;12(11):620.
-
33Boyd A, Stoodley P, Richards D, Hui R, Harnett P, Vo K, et al. Anthracyclines induce early changes in left ventricular systolic and diastolic function: A single centre study. PLoS One. 2017 Apr 13;12(4):e0175544.
-
34Chang VY, Wang JJ. Pharmacogenetics of Chemotherapy-Induced Cardiotoxicity. Curr Oncol Rep. 2018;20(7):52.
-
35Sachidanandam K, Gayle AA, Robins HI, Kolesar JM. Unexpected doxorubicin-mediated cardiotoxicity in sisters: possible role of polymorphisms in histamine n-methyl transferase. J Oncol Pharm Pract. 2013;19:269-72.
-
36Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273-83.
-
37Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CE, Jr., et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32(33):3744-52.
-
38Wang SY, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP, et al. Cardiovascular events, early discontinuation of trastuzumab, and their impact on survival. Breast Cancer Res Treat. 2014;146(2):411-9.
-
39Jerusalem G, Lancellotti P, Kim SB. HER2+ breast cancer treatment and cardiotoxicity: monitoring and management. Breast Cancer Res Treat. 2019;177(2):237-50.
-
40Seferina SC, de Boer M, Derksen MW, van den Berkmortel F, van Kampen RJ, van de Wouw AJ, et al. Cardiotoxicity and Cardiac Monitoring During Adjuvant Trastuzumab in Daily Dutch Practice: A Study of the Southeast Netherlands Breast Cancer Consortium. Oncologist. 2016;21(2):555-62.
-
41Tarantini L, Cioffi G, Gori S, Tuccia F, Boccardi L, Bovelli D, et al. Trastuzumab adjuvant chemotherapy and cardiotoxicity in real-world women with breast cancer. J Card Fail. 2012;18(2):113-9.
-
42Leong DP, Cosman T, Alhussein MM, Tyagi NK, Karampatos S, Barron CC, et al. Safety of Continuing Trastuzumab Despite Mild Cardiotoxicity: A Phase I Trial. JACC: CardioOncology. 2019;1(1):1-10.
-
43Procter M, Suter TM, de Azambuja E, Dafni U, van Dooren V, Muehlbauer S, et al. Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol. 2010;28(21):3422-8.
-
44Lidbrink E, Chmielowska E, Otremba B, Bouhlel A, Lauer S, Liste Hermoso M, et al. A real-world study of cardiac events in > 3700 patients with HER2-positive early breast cancer treated with trastuzumab: final analysis of the OHERA study. Breast Cancer Res Treat. 2019;174(1):187-96.
-
45Choueiri TK, Mayer EL, Je Y, Rosenberg JE, Nguyen PL, Azzi GR, et al. Congestive heart failure risk in patients with breast cancer treated with bevacizumab. J Clin Oncol. 2011;29(6):632-8.
-
46Richards CJ, Je Y, Schutz FA, Heng DY, Dallabrida SM, Moslehi JJ, et al. Incidence and risk of congestive heart failure in patients with renal and nonrenal cell carcinoma treated with sunitinib. J Clin Oncol. 2011;29(25):3450-6.
-
47Qi WX, Shen Z, Tang LN, Yao Y. Congestive heart failure risk in cancer patients treated with vascular endothelial growth factor tyrosine kinase inhibitors: a systematic review and meta-analysis of 36 clinical trials. Br J Clin Pharmacol. 2014;78(4):748-62.
-
48Waliany S, Sainani KL, Park LS, Zhang CA, Srinivas S, Witteles RM. Increase in Blood Pressure Associated With Tyrosine Kinase Inhibitors Targeting Vascular Endothelial Growth Factor. JACC: CardioOncology. 2019;1(1):24-36.
-
49Herrmann J, Yang EH, Iliescu CA, Cilingiroglu M, Charitakis K, Hakeem A, et al. Vascular Toxicities of Cancer Therapies: The Old and the New--An Evolving Avenue. Circulation. 2016;133(13):1272-89.
-
50Willis MS, Patterson C. Proteotoxicity and cardiac dysfunction--Alzheimer's disease of the heart? N Engl J Med. 2013;368(5):455-64.
-
51Russell SD, Lyon A, Lenihan DJ, Moreau P, Joshua D, Chng W-J. Serial echocardiographic assessment of patients (pts) with relapsed multiple myeloma (RMM) receiving carfilzomib and dexamethasone (Kd) vs bortezomib and dexamethasone (Vd): a substudy of the phase 3 Endeavor Trial (NCT01568866).[abstract]. Blood; 2015;126:4250.
-
52Lendvai N, Devlin S, Patel M, Knapp KM, Ekman D, Grundberg I, et al. Biomarkers of cardiotoxicity among multiple myeloma patients subsequently treated with proteasome inhibitor therapy.[abstract]. Blood. 2015;126:4257.
-
53Maverakis E, Cornelius LA, Bowen GM, Phan T, Patel FB, Fitzmaurice S, et al. Metastatic melanoma - a review of current and future treatment options. Acta Derm Venereol. 2015;95(5):516-24.
-
54Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol. 2017;14(8):463-82.
-
55Johnson DB, Sosman JA. Therapeutic Advances and Treatment Options in Metastatic Melanoma. JAMA Oncol. 2015;1(3):380-6.
-
56Dummer R, Ascierto PA, Gogas HJ, Arance A, Mandala M, Liszkay G, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19(5):603-15.
-
57Ascierto PA, McArthur GA, Dreno B, Atkinson V, Liszkay G, Di Giacomo AM, et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17(9):1248-60.
-
58Mincu RI, Mahabadi AA, Michel L, Mrotzek SM, Schadendorf D, Rassaf T, et al. Cardiovascular Adverse Events Associated With BRAF and MEK Inhibitors: A Systematic Review and Meta-analysis. JAMA Netw Open. 2019 Aug 2;2(8):e198890.
-
59Ferguson T, Wilcken N, Vagg R, Ghersi D, Nowak AK. Taxanesfor adjuvanttreatment of early breast cancer. Cochrane Database of Systematic Reviews. 2007;4:1-50.
-
60Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724-34.
-
61Haanen JB, Robert C. Immune Checkpoint Inhibitors. Prog Tumor Res 2015;42:55-66.
-
62Bonaca MP, Olenchock BA, Salem JE, Wiviott SD, Ederhy S, Cohen A, et al. Myocarditis in the Setting of Cancer Therapeutics: Proposed Case Definitions for Emerging Clinical Syndromes in Cardio-Oncology. Circulation. 2019;140(2):80-91.
-
63Varricchi G, Galdiero MR, Marone G, Criscuolo G, Triassi M, Bonaduce D, et al. Cardiotoxicity of immune checkpoint inhibitors. ESMO Open. 2017 Oct 26;2(4):e000247.
-
64Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4(12):1721-8.
-
65Jaworski C, Mariani JA, Wheeler G, Kaye DM. Cardiac complications of thoracic irradiation. J Am Coll Cardiol. 2013;61(23):2319-28.
-
66Aleman BM, van den Belt-Dusebout AW, De Bruin ML, van ‘t Veer MB, Baaijens MH, de Boer JP, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109(5):1878-86.
-
67Correa CR, Litt HI, Hwang WT, Ferrari VA, Solin LJ, Harris EE. Coronary artery findings after left-sided compared with right-sided radiation treatment for early-stage breast cancer. J Clin Oncol. 2007;25(21):3031-7.
-
68Desai MY, Jellis CL, Kotecha R, Johnston DR, Griffin BP. Radiation-Associated Cardiac Disease: A Practical Approach to Diagnosis and Management. JACC Cardiovasc Imaging. 2018;11(8):1132-49.
-
69Comite Coordenador da Diretriz de Insuficiencia C, Rohde LEP, Montera MW, Bocchi EA, Clausell NO, Albuquerque DC, et al. Arq Bras Cardiol. 2018;111(3):436-539.
-
70Lyman GH, Khorana AA, Falanga A, Clarke-Pearson D, Flowers C, Jahanzeb M, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. 2007;25(34):5490-505.
-
71Walker AJ, Card TR, West J, Crooks C, Grainge MJ. Incidence of venous thromboembolism in patients with cancer - a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49(6):1404-13.
-
72Chee CE, Ashrani AA, Marks RS, Petterson TM, Bailey KR, Melton LJ, 3rd, et al. Predictors of venous thromboembolism recurrence and bleeding among active cancer patients: a population-based cohort study. Blood. 2014;123(25):3972-8.
-
73Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: Burden, mechanisms, and management. Thromb Haemost. 2017;117(2):219-30.
-
74Thaler J, Ay C, Pabinger I. Clinical significance of circulating microparticles for venous thromboembolism in cancer patients. Hamostaseologie. 2012;32(2):127-31.
-
75Li A, Garcia DA, Lyman GH, Carrier M. Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): A systematic review and meta-analysis. Thromb Res. 2019;173:158-63.
-
76Pritchard ER, Murillo JR, Jr., Putney D, Hobaugh EC. Single-center, retrospective evaluation of safety and efficacy of direct oral anticoagulants versus low-molecular-weight heparin and vitamin K antagonist in patients with cancer. J Oncol Pharm Pract. 2019;25(1):52-9.
-
77Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902-7.
-
78Ay C, Dunkler D, Marosi C, Chiriac AL, Vormittag R, Simanek R, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116(24):5377-82.
-
79Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, et al. Risk of Arterial Thromboembolism in Patients With Cancer. J Am Coll Cardiol. 2017;70(8):926-38.
-
80Meyer T, Robles-Carrillo L, Robson T, Langer F, Desai H, Davila M, et al. Bevacizumab immune complexes activate platelets and induce thrombosis in FCGR2A transgenic mice. J Thromb Haemost. 2009;7(1):171-81.
-
81Guglin M, Aljayeh M, Saiyad S, Ali R, Curtis AB. Introducing a new entity: chemotherapy-induced arrhythmia. Europace. 2009;11(12):1579-86.
-
82Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53(24):2231-47.
-
83Tamargo J, Caballero R, Delpon E. Cancer chemotherapy and cardiac arrhythmias: a review. Drug Saf. 2015;38(2):129-52.
-
84Kishi S, Yoshida A, Yamauchi T, Tsutani H, Lee JD, Nakamura T, et al. Torsade de pointes associated with hypokalemia after anthracycline treatment in a patient with acute lymphocytic leukemia. Int J Hematol. 2000;71(2):172-9.
-
85Farmakis D, Parissis J, Filippatos G. Insights into onco-cardiology: atrial fibrillation in cancer. J Am Coll Cardiol. 2014;63(10):945-53.
-
86Vorchheimer DA. What is QT interval prolongation? J Fam Pract. 2005;Suppl(6):S4-7.
-
87Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36(41):2793-867.
-
88Porta-Sanchez A, Gilbert C, Spears D, Amir E, Chan J, Nanthakumar K, et al. Incidence, Diagnosis, and Management of QT Prolongation Induced by Cancer Therapies: A Systematic Review. J Am Heart Assoc. 2017 Dec 7;6(12):e007724.
-
89Delacretaz E. Clinical practice. Supraventricular tachycardia. N Engl J Med. 2006;354(10):1039-51.
-
90Sociedade Brasileira de Cardiologia. [Guidelines for the evaluation and treatment of patients with heart arrhythmia]. Arq Bras Cardiol. 2002;79(Suppl 5):7-50.
-
91Cheng WL, Kao YH, Chen SA, Chen YJ. Pathophysiology of cancer therapy-provoked atrial fibrillation. Int J Cardiol. 2016;219:186-94.
-
92Lopez-Fernandez T, Martin-Garcia A, Roldan Rabadan I, Mitroi C, Mazon Ramos P, Diez-Villanueva P, et al. Atrial Fibrillation in Active Cancer Patients: Expert Position Paper and Recommendations. Rev Esp Cardiol (Engl Ed). 2019;72(9):749-59.
-
93Melloni C, Shrader P, Carver J, Piccini JP, Thomas L, Fonarow GC, et al. Management and outcomes of patients with atrial fibrillation and a history of cancer: the ORBIT-AF registry. Eur Heart J Qual Care Clin Outcomes. 2017;3(3):192-7.
-
94Costa I, Bittar CS, Fonseca SMR, CMPD ES, Dos Santos Rehder MHH, Rizk SI, et al. Brazilian cardio-oncology: the 10-year experience of the Instituto do Cancer do Estado de Sao Paulo. BMC Cardiovasc Disord. 2020;20(1):206.
-
95Handy CE, Quispe R, Pinto X, Blaha MJ, Blumenthal RS, Michos ED, et al. Synergistic Opportunities in the Interplay Between Cancer Screening and Cardiovascular Disease Risk Assessment: Together We Are Stronger. Circulation. 2018;138(7):727-34.
-
96Yang Q, Chen Y, Gao H, Zhang J, Zhang J, Zhang M, et al. Chemotherapy-Related Anatomical Coronary-Artery Disease in Lung Cancer Patients Evaluated by Coronary-Angiography SYNTAX Score. Arq Bras Cardiol. 2020;114(6):1004-12.
-
97Ito D, Shiraishi J, Nakamura T, Maruyama N, Iwamura Y, Hashimoto S, et al. Primary percutaneous coronary intervention and intravascular ultrasound imaging for coronary thrombosis after cisplatin-based chemotherapy. Heart Vessels. 2012;27(6):634-8.
-
98Jafri M, Protheroe A. Cisplatin-associated thrombosis. Anticancer Drugs. 2008;19(9):927-9.
-
99Karabay KO, Yildiz O, Aytekin V. Multiple coronary thrombi with cisplatin. J Invasive Cardiol. 2014;26(2):E18-20.
-
100Periard D, Boulanger CM, Eyer S, Amabile N, Pugin P, Gerschheimer C, et al. Are circulating endothelial-derived and platelet-derived microparticles a pathogenic factor in the cisplatin-induced stroke? Stroke. 2007;38(5):1636-8.
-
101Yuan C, Parekh H, Allegra C, George TJ, Starr JS. 5-FU induced cardiotoxicity: case series and review of the literature. Cardiooncology. 2019 Sep 06;5:13.
-
102Chong JH, Ghosh AK. Coronary Artery Vasospasm Induced by 5-fluorouracil: Proposed Mechanisms, Existing Management Options and Future Directions. Interv Cardiol. 2019;14(2):89-94.
-
103Mosseri M, Fingert HJ, Varticovski L, Chokshi S, Isner JM. In vitro evidence that myocardial ischemia resulting from 5-fluorouracil chemotherapy is due to protein kinase C-mediated vasoconstriction of vascular smooth muscle. Cancer Res. 1993;53(13):3028-33.
-
104Thyss A, Gaspard MH, Marsault R, Milano G, Frelin C, Schneider M. Very high endothelin plasma levels in patients with 5-FU cardiotoxicity. Ann Oncol. 1992;3(1):88.
-
105Schrader C, Keussen C, Bewig B, von Freier A, Lins M. Symptoms and signs of an acute myocardial ischemia caused by chemotherapy with Paclitaxel (Taxol) in a patient with metastatic ovarian carcinoma. Eur J Med Res. 2005;10(11):498-501.
-
106Shah K, Gupta S, Ghosh J, Bajpai J, Maheshwari A. Acute non-ST elevation myocardial infarction following paclitaxel administration for ovarian carcinoma: a case report and review of literature. J Cancer Res Ther. 2012;8(3):442-4.
-
107Douxfils J, Haguet H, Mullier F, Chatelain C, Graux C, Dogne JM. Association Between BCR-ABL Tyrosine Kinase Inhibitors for Chronic Myeloid Leukemia and Cardiovascular Events, Major Molecular Response, and Overall Survival: A Systematic Review and Meta-analysis. JAMA Oncol. 2016;2(5):625-32.
-
108Totzeck M, Mincu RI, Rassaf T. Cardiovascular Adverse Events in Patients With Cancer Treated With Bevacizumab: A Meta-Analysis of More Than 20 000 Patients. J Am Heart Assoc. 2017 Aug 10;6(8):e006278.
-
109Newman JL, Stone JR. Immune checkpoint inhibition alters the inflammatory cell composition of human coronary artery atherosclerosis. Cardiovasc Pathol. 2019 Nov-Dec;43:107148.
-
110Ping Z, Peng Y, Lang H, Xinyong C, Zhiyi Z, Xiaocheng W, et al. Oxidative Stress in Radiation-Induced Cardiotoxicity. Oxid Med Cell Longev. 2020;2020:3579143.
-
111Wang H, Wei J, Zheng Q, Meng L, Xin Y, Yin X, et al. Radiation-induced heart disease: a review of classification, mechanism and prevention. Int J Biol Sci. 2019;15(10):2128-38.
-
112Landes U, Kornowski R, Bental T, Assali A, Vaknin-Assa H, Lev E, et al. Long-term outcomes after percutaneous coronary interventions in cancer survivors. Coron Artery Dis. 2017;28(1):5-10.
-
113Nakatsuma K, Shiomi H, Morimoto T, Watanabe H, Nakagawa Y, Furukawa Y, et al. Influence of a history of cancer on long-term cardiovascular outcomes after coronary stent implantation (an Observation from Coronary Revascularization Demonstrating Outcome Study-Kyoto Registry Cohort-2). Eur Heart J Qual Care Clin Outcomes. 2018;4(3):200-7.
-
114Ganatra S, Sharma A, Levy MS. Re-Evaluating the Safety of Drug-Eluting Stents in Cancer Patients. JACC Cardiovasc Interv. 2017;10(22):2334-7.
-
115Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39(3):213-60.
-
116Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, et al. Defining High Bleeding Risk in Patients Undergoing Percutaneous Coronary Intervention. Circulation. 2019;140(3):240-61.
-
117Iliescu C, Grines CL, Herrmann J, Yang EH, Cilingiroglu M, Charitakis K, et al. SCAI expert consensus statement: Evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (Endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista). Catheter Cardiovasc Interv. 2016;87(5):895-9.
-
118Cameron AC, Touyz RM, Lang NN. Vascular Complications of Cancer Chemotherapy. Can J Cardiol. 2016;32(7):852-62.
-
119Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL. Prognostic importance of Comorbidity in a hospital-based cancer registry. Jama-J Am Med Assoc. 2004;291(20):2441-7.
-
120Boursiquot BC, Zabor EC, Glezerman IG, Jaimes EA. Hypertension and VEGF (Vascular Endothelial Growth Factor) Receptor Tyrosine Kinase Inhibition: Effects on Renal Function. Hypertension 2017.Jul 24.HYPERTENSIONAHA.117.092275 online ahrad of print.
-
121Budolfsen C, Faber J, Grimm D, Kruger M, Bauer J, Wehland M, et al. Tyrosine Kinase Inhibitor-Induced Hypertension: Role of Hypertension as a Biomarker in Cancer Treatment. Curr Vasc Pharmacol. 2019;17(6):618-34.
-
122Li J, Gu J. Cardiovascular Toxicities with Vascular Endothelial Growth Factor Receptor Tyrosine Kinase Inhibitors in Cancer Patients: A Meta-Analysis of 77 Randomized Controlled Trials. Clin Drug Investig. 2018;38(12):1109-23.
-
123Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of Cisplatin nephrotoxicity. Toxins (Basel). 2010;2(11):2490-518.
-
124Stoter G, Koopman A, Vendrik CP, Struyvenberg A, Sleyfer DT, Willemse PH, et al. Ten-year survival and late sequelae in testicular cancer patients treated with cisplatin, vinblastine, and bleomycin. J Clin Oncol. 1989;7(8):1099-104.
-
125Soultati A, Mountzios G, Avgerinou C, Papaxoinis G, Pectasides D, Dimopoulos MA, et al. Endothelial vascular toxicity from chemotherapeutic agents: preclinical evidence and clinical implications. Cancer Treat Rev. 2012;38(15):473-83.
-
126Bendtsen MAF, Grimm D, Bauer J, Wehland M, Wise P, Magnusson NE, et al. Hypertension Caused by Lenvatinib and Everolimus in the Treatment of Metastatic Renal Cell Carcinoma. Int J Mol Sci. 2017;18(8):1736.
-
127Liu Y, Zhou L, Chen Y, Liao B, Ye D, Wang K, et al. Hypertension as a prognostic factor in metastatic renal cell carcinoma treated with tyrosine kinase inhibitors: a systematic review and meta-analysis. BMC Urol. 2019;19(1):49.
-
128Farrugia FA, Martikos G, Tzanetis P, Charalampopoulos A, Misiakos E, Zavras N, et al. Pheochromocytoma, diagnosis and treatment: Review of the literature. Endocr Regal. 2017;51(3):168-81.
-
129Tini G, Sarocchi M, Tocci G, Arboscello E, Ghigliotti G, Novo G, et al. Arterial hypertension in cancer: The elephant in the room. Int J Cardiol 2019;281:133-9.
Publication Dates
-
Publication in this collection
07 Dec 2020 -
Date of issue
Nov 2020