Brazilian Society of Cardiology Guidelines on Unstable Angina and Acute Myocardial Infarction without ST-Segment Elevation – 2021
Brazilian Society of Cardiology Guidelines on Unstable Angina and Acute Myocardial Infarction without ST-Segment Elevation – 2021
Definitions of Recommendations and Evidence
Recommendations
-
Class I: Conclusive evidence, or, failing that, general consensus that the procedure is safe and useful/effective.
-
Class II: Conflicting evidence and/or divergence of opinion about the procedure's safety and usefulness/effectiveness.
-
Class IIa: Evidence/opinion is in favor of the procedure. Most approve of it.
-
Class IIb: The procedure's safety and utility/effectiveness is less well established, opinion is not predominantly in favor of it.
-
Class III: Evidence and/or consensus that the procedure is not useful/effective and, in some cases, is harmful.
Evidence
-
Level A: Data obtained from multiple large randomized studies, concordant and/or robust meta-analysis of randomized clinical studies.
-
Level B: Data obtained from less robust meta-analysis, from a single randomized study or from non-randomized (observational) studies.
-
Level C: Data obtained from expert consensus.
Changes in recommendations
New recommendations
Part 1 – Emergency Assessment and Management
1. Introduction
Acute chest pain is responsible for more than 5% of all emergency department visits and up to 10% of non-trauma related visits. The incidence of chest pain varies between 9 and 19 per 1,000 people/year seen in emergency departments and is involved in up to 40% of hospitalizations. Although most of these patients are discharged with a diagnosis of unspecified or non-cardiac chest pain, about 25% are diagnosed with acute coronary syndrome (ACS).11. Goodacre S, Cross E, Arnold J, Angelini K, Capewell S, Nicholl J. The health care burden of acute chest pain. Heart. 2005;91(2):229-30.,22. Barstow C, Rice M, McDivitt JD. Acute Coronary Syndrome: Diagnostic Evaluation. Am Fam Physician. 2017;95(3):170-7.
The subgroup of patients whose electrocardiography (ECG) results show no ST-segment elevation but whose symptoms or complementary exam results are compatible with a coronary etiology are classified as having non-ST-elevation acute coronary syndromes (NSTE-ACS), the subject of the present guideline. NSTE-ACS can cause significant morbidity and mortality if not promptly and appropriately treated. Since delaying appropriate treatment can result in serious adverse events, it is important to verify the presence of NSTE-ACS by assessing clinical data and complementary exams.33. Gibler WB, Racadio JM, Hirsch AL, Roat TW. Continuum of Care for Acute Coronary Syndrome: Optimizing Treatment for ST-Elevation Myocardial Infarction and Non-St-Elevation Acute Coronary Syndrome. Crit Pathw Cardiol. 2018;17(3):114-38.
2. Definitions
Chest pain is the main symptom in patients with ACS. ECG must be performed and interpreted within the first 10 minutes of medical contact in patients suspected of ACS, and the results may differentiate patients into two groups:
-
ST-elevation ACS: Patients with acute chest pain and persistent ST-segment elevation or new or presumably new left bundle branch block, which is a condition generally related to coronary occlusion and the need for immediate reperfusion.
-
NSTE-ACS: Patients with acute chest pain without persistent ST-segment elevation, whether associated or not with other ECG changes suggestive of some type of myocardial ischemia, including a broad spectrum of severity: transient ST-segment elevation, transient or persistent ST-segment depression, T-wave inversion, other nonspecific T-wave changes (flat or pseudonormalization), and even normal ECG results. This group includes patients with unstable angina (UA), ie, with no changes in myocardial necrosis markers and those with non-ST-segment elevation myocardial infarction (NSTEMI) when there is an increase in myocardial necrosis markers.
A NSTEMI diagnosis is confirmed when there is an acute myocardial injury, which is confirmed by an increase in troponin levels, and the injury is suspected to be caused by ischemia. Based on its pathophysiology and clinical context, acute myocardial infarction (AMI) is classified into several subtypes (Tables 1.1 and 1.2). Increased troponin levels can be secondary to myocardial ischemia, but they can also occur in other clinical situations (Table 1.3).
Myocardial injury and infarction11. Goodacre S, Cross E, Arnold J, Angelini K, Capewell S, Nicholl J. The health care burden of acute chest pain. Heart. 2005;91(2):229-30.
Situations in which there is an increase in myocardial necrosis markers but ischemia is not detected in the clinical picture, ECG or imaging tests should be defined as acute myocardial injury, not AMI. They can be secondary to cardiac causes (such as cardiovascular procedures, myocarditis, arrhythmias, or decompensated HF) or extracardiac causes (such as shock, severe anemia, sepsis and hypoxia).44. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40(3):237-69.
Myocardial injury is often related to a worse prognosis. It is necessary to differentiate between ischemic and nonischemic causes to avoid unnecessary invasive interventions and direct treatment toward other possible etiologies (Table 1.3).
Figure 1.1 summarizes the interpretation of elevated troponin in coronary injury and ischemia scenarios.
Algorithm for interpreting elevated troponin levels. The troponin “curve” indicates elevation greater than 20%
2.1. Myocardial Infarction with Nonobstructive Coronary Arteries (MINOCA)
Cases of AMI without obstructive coronary artery disease (CAD) are classified as myocardial infarction with nonobstructive coronary arteries (MINOCA). Approximately two-thirds of patients with MINOCA have a clinical presentation of NSTEMI.55. Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131(10):861-70. Erratum in: Circulation. 2015;131(19):e475.
According to a document prepared by a European Society of Cardiology working group66. Scalone G, Niccoli G, Crea F. Editor's Choice-Pathophysiology, diagnosis and management of MINOCA: an update. Eur Heart J. Acute Cardiovasc Care. 2019;8(1):54-62. and the Fourth Universal Definition of Myocardial Infarction,44. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40(3):237-69. the diagnostic criteria for MINOCA are: AMI, angiographic documentation without obstructive CAD (atheromatosis with <50% stenosis or normal coronary arteries) or a clinically evident non-coronary cause for acute presentation.
Different pathophysiological mechanisms can cause MINOCA:
-
Dysfunction of epicardial coronary arteries (eg, atherosclerotic plaque rupture, ulceration, cracking, erosion or coronary dissection).
-
Imbalance between oxygen supply and consumption (eg, coronary spasm and coronary embolism).
-
Coronary endothelial dysfunction (eg, microvascular disease).
MINOCA prognosis varies greatly, depending on the underlying mechanisms and associated risk factors, such as age and female gender. Some studies indicate that MINOCA has lower hospital mortality than AMI with obstructive coronary artery disease and similar 1-year mortality to AMI with univascular obstruction. However, when analyzing patients with NSTEMI in the ACUITY study, the MINOCA subgroup had the highest 1-year mortality (4.7% vs. 3.6%), which was linked to an increase in non-cardiac deaths.77. Niccoli G, Camici PG. Myocardial infarction with non-obstructive coronary arteries: what is the prognosis?. Eur Heart J Suppl. 2020;22(Suppl E):E40-5.
8. Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, et al. Contemporary Diagnosis and Management of Patients With Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease: A Scientific Statement From the American Heart Association. Circulation. 2019;139(18):e891-e908.-99. Planer D, Mehran R, Ohman EM, White HD, Newman JD, Xu K, et al. Prognosis of patients with non-ST- segment-elevation myocardial infarction and nonobstructive coronary artery disease: propensity-matched analysis from the Acute Catheterization and Urgent Intervention Triage Strategy trial. Circ Cardiovasc Interv. 2014;7(3):285-93.
The large group of patients presenting with elevated troponin but no coronary obstruction or clinical signs of infarction is classified as TINOCA (troponin-positive nonobstructive coronary arteries), which includes those with ischemic injury (MINOCA) and the other described etiologies of nonischemic myocardial injury (eg, myocarditis or Takotsubo syndrome; see Figure 1.2).
Myocardial infarction with nonobstructive coronary arteries (MINOCA) and troponin-positive nonobstructive coronary arteries (TINOCA): conceptual paradigm. Adapted from Pasupathy et al., 2017.1010. Pasupathy S, Tavella R, Beltrame JF. Myocardial Infarction With Nonobstructive Coronary Arteries (MINOCA): The Past, Present, and Future Management. Circulation. 2017 Apr 18;135(16):1490-3.
2.2. Unstable Angina
UA is defined as myocardial ischemia without myocardial necrosis, ie, with negative biomarkers. During the initial management of ACS, it is often difficult to differentiate UA from NSTEMI on clinical criteria alone (i.e., before levels of myocardial necrosis biomarkers are available), and both conditions should be treated similarly at this stage. Increased troponin sensitivity decreases the percentage of patients diagnosed with UA and increases the percentage of patients with NSTEMI. The prognosis of patients with UA ranges from relatively low risk to high risk. UA classifications based on clinical presentation and prognostic information facilitate therapeutic management, which will be discussed throughout this document.1111. Effects of tissue plasminogen activator and a comparison of early invasive and conservative strategies in unstable angina and non-Q-wave myocardial infarction. Results of the TIMI IIIB Trial. Thrombolysis in Myocardial Ischemia. Circulation. 1994 Apr;89(4):1545-56.
12. Theroux P, Fuster V. Acute coronary syndromes: unstable angina and non-Q-wave myocardial infarction. Circulation. 1998 Mar 31;97(12):1195-206.
13. Zaacks SM, Liebson PR, Calvin JE, Parrillo JE, Klein LW. Unstable angina and non-Q wave myocardial infarction: does the clinical diagnosis have therapeutic implications? J Am Coll Cardiol. 1999 Jan;33(1):107-18.
14. Calvin JE, Klein LW, VandenBerg BJ, Meyer P, Ramirez-Morgen LM, Parrillo JE. Clinical predictors easily obtained at presentation predict resource utilization in unstable angina. Am Heart J. 1998 Sep;136(3):373-81.
15. Kong DF, Blazing MA, O’Connor CM. The health care burden of unstable angina. Cardiol Clin. 1999 May;17(2):247-61.-1616. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(3):267-315.
3. Epidemiology
AMI is the leading cause of death in Brazil and worldwide.1717. Naghavi M, Wang H, Lozano R, Davis A, Liang X, Zhou M, et al; GBD 2013Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117-71. In 2017, according to DATASUS, 7.06% (92,657 patients) of all deaths were caused by AMI. AMI, which accounted for 10.2% of hospitalizations in the Brazilian Unified Health System (SUS), was more prevalent in patients over 50 years of age, accounting for 25% of all hospitalizations).1818. Brasil. Ministério da Saúde. Informação em saúde: Estatísticas vitais. [Citado em 2020 fev 26]. Disponível em: http://datasus.saude.gov.br/).
http://datasus.saude.gov.br/...
In the Brazilian Registry of Acute Coronary Syndromes (BRACE), which evaluated ACS hospitalizations in 72 hospitals, NSTE-ACS was responsible for 45.7%, of which about 2/3 involved AMI and 1/3 UA. The study found generally low use of therapies that impact the prognosis of patients with ACS, including important regional differences. It also developed a performance score, which showed that the greater the adherence to proven treatments, the lower the mortality.1919. Franken M, Giugliano RP, Goodman SG, Baracioloi L, Godoy LC, Furtado RHM, et al. Performance of acute coronary syndrome approaches in Brazil. A report from the BRACE (Brazilian Registry in Acute Coronary syndromEs) [published online ahead of print, 2019 Aug 10]. Eur Heart J Qual Care Clin Outcomes. 2019;qcz045.
4. Pathophysiology
The main pathophysiological characteristic of ACS is the instability of atherosclerotic plaque, involving erosion or rupture and subsequent formation of an occlusive or subocclusive thrombus. Such flow limitations, however, can be due to other mechanisms, such as vasospasm, embolism, or coronary dissection. Other factors may be involved in the pathophysiology of ACS by altering the supply and/or consumption of myocardial oxygen, such as anemia, hypertension, tachycardia, hypertrophic cardiomyopathy, aortic stenosis, etc..2020. Libby P. Mechanisms of Acute Coronary Syndromes and Their Implications for Therapy. N Engl J Med. 2013;368(21):2004-13.
In animals, ischemia has been observed to progress from the subendocardium to the subepicardium. This progression can be delayed when there is efficient collateral irrigation, reduced oxygen consumption in the myocardium, and intermittent flow (generating ischemic preconditioning). Changes in ventricular function due to the progression of myocardial ischemia initially involve diastolic dysfunction, which may be followed or not by systolic dysfunction. A shorter duration of ischemic injury is associated with a smaller area of myocardial necrosis. Several studies have also suggested that restoring perfusion results in some degree of myocardial injury (reperfusion injury), especially in situations of total coronary occlusion.2121. Ibáñez B, Heusch G, Ovize M, Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol.2015; 65(14):1454-71.
5. Initial Assessment
5.1. Initial Approach
Patients with suspected ACS and signs of severity (persistent pain, dyspnea, and palpitations due to potentially severe arrhythmias and syncope) should be referred to emergency departments, ideally monitored while in the ambulance. Patients without signs of severity (listed above) should be instructed to seek out an emergency department that can perform ECG and cardiac biomarker measurement, preferably troponin.
Several tools (scores) have been devised for this purpose and, in conjunction with clinical judgment, can help define which patients could benefit from hospitalization, complementary exams, and specific treatment.
5.2. Diagnosis and Prognostic Assessment
5.2.1. History and Clinical Data
5.2.1.1. Characterization of Chest Pain and Angina
The clinical picture of angina can be defined by four main pain characteristics: location, type, duration, and intensification or relief factors.
-
Location: usually in the chest, close to the sternum. However, it can affect or radiate from the epigastrium to the mandible, the interscapular region and the arms (more commonly to the left, less commonly to both arms or the right arm).
-
Type: the discomfort is usually described as pressure, tightness or weight. Sometimes it involves a feeling of strangulation, compression or burning. It may be accompanied by dyspnea, sweating, nausea, or syncope.
-
Duration: the duration of stable anginal pain is generally short (<10 min); episodes of ≥10 min suggest ACS. However, prolonged continuous pain (hours or days) or ephemeral pain (few seconds) is less likely to indicate ACS.
-
Intensification or relief factors: one important feature of angina is its relationship with physical exertion. Symptoms classically appear or intensify on exertion. In patients with no history of angina, a reduction in the effort threshold required to trigger angina suggests ACS. ACS discomfort does not usually vary with changes in breathing or position.
The clinical history of patients with NSTE-ACS plays an important role in risk stratification. In the presence of angina, patients with NSTE-ACS can present in four ways:
-
Prolonged resting angina (> 20 min).
-
Recent onset angina (class II or III according to Canadian Cardiovascular Society classification): in general, these patients manifest typical symptoms of angina in less than 2 months that begin appearing after minimal exertion.
-
Recent worsening of previously stable angina (crescendo angina). Minimal exertion triggers angina, increased pain intensity and/or duration, changes in pain radiation patterns, changes in response to the use of nitrates.
-
Post-infarction angina.
Some characteristics, antecedents and comorbidities are related to a higher probability of ACS:
-
Advanced age and male sex.
-
Risk factors for atherosclerosis: smoking, diabetes mellitus (DM), dyslipidemia, arterial hypertension and chronic renal failure.
-
Family history of CAD.
-
Previous symptomatic atherosclerosis, such as peripheral arterial obstructive disease, carotid disease, previous coronary disease.
-
Chronic inflammatory diseases, such as lupus or rheumatoid arthritis.
ACS patients may present with atypical symptoms, such as isolated epigastric pain, feelings of gastric fullness, lancinating pain, pleuritic pain or dyspnea. Although ACS typically presents in women and older adults (> 75 years) as angina, atypical presentations are also higher in these groups, as well as in patients with DM, renal failure and dementia.
The Coronary Artery Surgery Study proposed a chest pain classification system,2222. The Principal Investigators of CASS and Their Associates. The National Heart, Lung and Blood Institute Coronary Artery Surgery Study: historical background, design, methods, the registry, the randomized trial, clinical database. Circulation. 1981;63(suppl I):I-1-I-81. which is shown in Table 1.4.
5.2.1.2. NSTE-ACS in Older Adults
Older adults with ACS generally have a different risk profile from younger adults: they have a higher prevalence of hypertension, DM, previous AMI, angina, peripheral vascular disease, stroke, multivessel disease, and HF. Older adults generally wait longer to seek medical care after symptom onset. In NSTE-ACS, instead of pain, patients often have so-called “ischemic-equivalent” symptoms, such as dyspnea, malaise, mental confusion, syncope or pulmonary edema. Older adults have a higher incidence of complications in NSTE-ACS, which implies the need for more intensive treatment. However, especially in adults over 75 years of age, the most appropriate therapy, ie, beta-blockers, acetylsalicylic acid (ASA), anticoagulants and lipid-lowering agents, is not used. Of the 3,318 patients with NSTE-ACS included in the TIMI III study,2323. Stone PH, Thompson B, Anderson HV, Kronenberg MW, Gibson RS, Rogers WJ, et al. Influence of race, sex, and age on management of unstable angina and non-Q-wave myocardial infarction: The TIMI III registry. JAMA. 1996 Apr 10;275(14):1104-12. 828 were over 75 years old. These individuals received anti-ischemic therapy and underwent cardiac catheterization at a lower percentage than younger patients. Although they had more severe and extensive CAD, they less frequently underwent myocardial revascularization procedures and had more adverse events within 6 weeks of onset. According to a national database study, the use of proven efficacious therapies after ACS has increased in the last 15 years, both among the oldest old (age > 80 years) and in younger adults (< 50 years), and this increase has been associated with improved survival in both groups.2424. Nicolau JC, Franci A, Barbosa CJ, Baracioli LM, Franken M, Furtado RH, et al. Influence of proven oral therapies in the very old with acute coronary syndromes: A 15 year experience. Int J Cardiol. 2015 Nov 1;198:213-5.
5.2.1.3. History
5.2.1.3.1. Patients Undergoing Myocardial Revascularization Procedures: Percutaneous Coronary Intervention and/or Myocardial Revascularization Surgery
The recurrence of angina after coronary artery bypass graft (CABG) surgery or percutaneous coronary intervention (PCI) can indicate acute complications, new injuries, stent thrombosis, or restenosis. Chest pain up to 48 hours after percutaneous intervention suggests acute obstruction, transient coronary spasm, non-occlusive thrombus, branch occlusion or distal embolization. Recurrent chest pain approximately 6 months after conventional stenting or later, after drug-eluting stenting, is most likely related to restenosis. On the other hand, angina onset 1 year after stenting is usually related to a new coronary lesion or stent restenosis due to neoaterosclerosis. After a CABG, the early appearance of pain is usually associated with thrombotic obstruction of the graft. In the first 12 months after CABG, the mechanism is usually fibrous intima hyperplasia; after this period, pain is indicative of a new atherosclerotic lesion and/or non-thrombic graft degeneration. The TIMI III registry compared the incidence of death or nonfatal infarction among NSTE-ACS patients with or without previous CABG. Those with previous CABG had higher rates of complications up to 10 days after admission (4.5% vs. 2.8% with or without CABG, respectively) and after 42 days (7.7% vs. 5.1%, respectively), which suggests that this is a higher risk group, mainly due to more extensive atherosclerosis.2525. Kleiman NS, Anderson HV, Rogers WJ, Theroux P, Thompson B, Stone PH. Comparison of outcome of patients with unstable angina and non-Q-wave acute myocardial infarction with and without prior coronary artery bypass grafting (Thrombolysis in Myocardial Ischemia III Registry). Am J Cardiol. 1996 Feb 1;77(4):227-31.
5.2.1.3.2. Risk Factors for Coronary Artery Disease
Some studies have suggested that smokers have a better prognosis, probably due to the fact that they suffer ACS at an earlier age and have less atherosclerotic burden than non-smokers.2626. Barbash GI, Reiner J, White HD, Wilcox RG, Armstrong PW, Sadowski Z, et al. Evaluation of paradoxic beneficial effects of smoking in patients receiving thrombolytic therapy for acute myocardial infarction: mechanism of the “smoker's paradox” from the GUSTO-I trial, with angiographic insights. Global Utilization of Streptokinase and Tissue-Plasminogen Activator for Occluded Coronary Arteries. J Am Coll Cardiol. 1995 Nov 1;26(5):1222-9.,2727. Barbash GI, White HD, Modan M, Diaz R, Hampton JR, Heikkila J, et al. Significance of smoking in patients receiving thrombolytic therapy for acute myocardial infarction. Experience gleaned from the International Tissue Plasminogen Activator/Streptokinase Mortality Trial. Circulation. 1993 Jan;87(1):53-8. On the other hand, Antman et al. showed that having three or more risk factors for CAD (systemic arterial hypertension, DM, dyslipidemia, family history and smoking) is an independent marker of a worse prognosis.2828. Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000 Aug 16;284(7):835-42.
5.2.2. Physical examination
During assessment of individuals with NSTE-ACS, a physical examination can help identify individuals at higher risk (those with signs of severe ventricular dysfunction or mechanical complications) and help in the differential diagnosis of chest pain unrelated to ACS.
As a rule, a normal or slightly modified physical examination is insufficient to stratify patient risk, since even patients with multivessel or left main coronary lesions can appear normal upon physical examination.2929. Braunwald E, Jones RH, Mark DB, Brown J, Brown L, Cheitlin MD, et al. Diagnosing and managing unstable angina. Agency for Health Care Policy and Research. Circulation. 1994 Jul;90(1):613-22.
30. Yeghiazarians Y, Braunstein JB, Askari A, Stone PH. Unstable angina pectoris. N Engl J Med. 2000 Jan 13;342(2):101-14.-3131. Braunwald E, Califf RM, Cannon CP, Fox KA, Fuster V, Gibler WB, et al. Redefining medical treatment in the management of unstable angina. Am J Med. 2000 Jan;108(1):41-53. However, when changes are found in a physical examination, they can be important for categorizing patients as high risk.
Some findings that indicate a poor prognosis include systolic murmur in the mitral focus tachycardia, tachypnea, hypotension, sweating, weak pulse, third heart sound, and pulmonary rales.
Changes in physical examination results allow differential diagnosis between ACS and other causes of chest pain:
-
• Cardiac: pericarditis (pericardial friction), cardiac tamponade (paradoxical pulse), aortic stenosis (aortic systolic murmur), and hypertrophic cardiomyopathy (ejection systolic murmur in the parasternal area that increases with the Valsalva maneuver).
-
• Non-cardiac: aortic dissection (pulse and pressure divergence between the arms and a diastolic murmur of aortic insufficiency), pulmonary embolism/pulmonary infarction (pleural friction), pneumothorax (decreased breath sounds and hyperresonance to percussion), and musculoskeletal symptoms (pain on palpation).
5.2.3. Electrocardiogram
Twelve-lead ECG is the initial diagnostic tool for patients with suspected ACS. Ideally, it should be performed and interpreted during pre-hospital care or within 10 minutes of hospital admission.
About 1% to 6% of patients with NSTE-ACS have normal (non-diagnostic) ECG results at admission. The ECG should be repeated after 15 to 30 minutes, especially in individuals who are still symptomatic. Normal or non-diagnostic ECG results can occur even with left circumflex or right coronary artery occlusion. Thus, additional V3R, V4R, V7, V8, and V9 derivations are recommended to increase the method's sensitivity.
More than 1/3 of patients will have typical ACS changes, such as ST-segment depression, transient ST-segment elevation, and T-wave inversion. Dynamic changes in the ST-segment (depression or elevation) or T-wave inversions during a painful episode that resolve at least partially when the symptoms are relieved are important markers of adverse prognosis, ie, subsequent AMI or death.3232. Bosch X, Theroux P, Pelletier GB, Sanz G, Roy D, Waters D. Clinical and angiographic features and prognostic significance of early postinfarction angina with and without electrocardiographic signs of transient ischemia. Am J Med. 1991 Nov;91(5):493-501. Patients with ST changes in anterior leads often have significant stenosis of the anterior descending coronary artery and are a high-risk group.
ST-segment and T-wave changes are not specific to NSTE-ACS and can occur in several conditions, including: ventricular hypertrophy, pericarditis, myocarditis, early repolarization, electrolyte alteration, shock, metabolic dysfunction, and digitalis effect.
The diagnostic accuracy of an abnormal ECG is increased when previous ECG results are available for comparison.
Transient ST-segment elevation is consistent with Prinzmetal or vasospastic angina. Concomitant ST elevation in the anterior and inferior leads (reflecting extensive ischemia) is associated with an increased risk of sudden death.3333. Gottlieb SO, Weisfeldt ML, Ouyang P, Mellits ED, Gerstenblith G. Silent ischemia predicts infarction and death during 2 year follow-up of unstable angina. J Am Coll Cardiol. 1987 Oct;10(4):756-60.
5.2.3.1. Electrocardiogram Findings and Prognosis
Patients with NSTE-ACS are at high risk of ischemic ECG changes (elevated or depressed ST-segment), atrial fibrillation, and ventricular arrhythmias. These changes imply a worse prognosis.3131. Braunwald E, Califf RM, Cannon CP, Fox KA, Fuster V, Gibler WB, et al. Redefining medical treatment in the management of unstable angina. Am J Med. 2000 Jan;108(1):41-53. In the GUSTO II trial, certain baseline ECG factors in patients with ACS were prognostic of early mortality:3434. Armstrong PW, Fu Y, Chang WC, Topol EJ, Granger CB, Betriu A, et al. Acute coronary syndromes in the GUSTO-IIb trial: prognostic insights and impact of recurrent ischemia. The GUSTO-IIb Investigators. Circulation. 1998 Nov 3;98(18):1860-8. left bundle branch block, left ventricular (LV) hypertrophy or pacemaker rhythm (11.6%); ST-segment depression (8%); ST-segment elevation (7.4%); and normal T-wave inversion or normal ECG (1.2%).
Moreover, in 1,416 patients with ACS, the TIMI III Registry ECG Ancillary Study3535. Cannon CP, McCabe CH, Stone PH, Rogers WJ, Schactman M, Thompson BW, et al. The electrocardiogram predicts one-year outcome of patients with unstable angina and non-Q wave myocardial infarction: results of the TIMI III Registry ECG Ancillary Study. Thrombolysis in Myocardial Ischemia. J Am Coll Cardiol. 1997 Jul;30(1):133-40. found the following ECG presentations: ST-segment deviation > 1 mm (14.3%), left bundle branch block (19%), isolated T-wave inversion (21.9%), or none of these changes (54.9%).
5.2.4. Biomarkers
In patients with ACS, biomarkers are useful for both diagnosis and prognosis. When myocardial cells are injured, their cell membranes lose integrity, intracellular proteins diffuse across the interstitium and into the lymphatic system and capillaries. After a myocardial injury, marker kinetics depend on several factors: intracellular protein degradation, molecule size, regional lymphatic and blood flow, as well as marker clearance rate. Such factors, together with the characteristics of each marker, differentiate each patient's diagnostic AMI performance.3636. Lee TH, Goldman L. Serum enzyme assays in the diagnosis of acute myocardial infarction. Recommendations based on a quantitative analysis. Ann Intern Med. 1986 Aug;105(2):221-33.
In patients with symptoms suggestive of ACS but without diagnosed AMI, cardiac biomarkers are useful to confirm the diagnosis. They also provide important prognostic information, since there is a direct association between elevated serum markers and the risk of cardiac events in the short and medium- term.3737. Newby LK, Christenson RH, Ohman EM, Armstrong PW, Thompson TD, Lee KL, et al. Value of serial troponin T measures for early and late risk stratification in patients with acute coronary syndromes. The GUSTO-IIa Investigators. Circulation. 1998 Nov 3;98(18):1853-9. The necrosis marker results must be available within 60 minutes of collection. If the clinical analysis laboratory cannot provide this, point-of-care technologies should be considered.3838. Amsterdam EA, Kirk JD, Bluemke DA, Diercks DE, Farkouh ME, Garvey JE, et al. Testing of low-risk patients presenting to the emergency department with chest pain. A Scientific Statement From the American Heart Association. Circulation. 2010;122(17):1756-776.
5.2.4.1. Troponins
Troponins are a complex of myofibrillar regulatory proteins in striated cardiac muscle that include troponin T, troponin I, and troponin C. Troponin C is coexpressed in slow-twitch skeletal muscle fibers and is not considered as a specific cardiac marker. In recent decades, immunoassay techniques with specific monoclonal antibodies for cardiac troponin T and cardiac troponin I have been developed. Meta-analyses have shown that the sensitivity and specificity of cardiac troponin I for diagnosing AMI was approximately 90% and 97%, respectively. Cardiac troponins remain elevated for a longer time, up to 7 days after AMI. Troponins are the biomarkers of choice for diagnostic evaluation in patients with suspected AMI since their diagnostic accuracy is higher than that of creatine kinase MB (CK-MB) mass and other biomarkers of myocardial injury. Patients with elevated troponins are at increased risk of cardiac events during the first days of hospitalization and are the NSTE-ACS subgroup that can most benefit from an invasive strategy.3939. Ohman EM, Armstrong PW, Christenson RH, Granger CB, Katus HA, Hamm CW, et al. Cardiac troponin T levels for risk stratification in acute myocardial ischemia. GUSTO IIA Investigators. N Engl J Med. 1996 Oct 31;335(18):1333-41. Although troponins can accurately identify myocardial injury, they cannot identify the mechanism of injury(ies). The mechanisms can include non-coronary etiologies, such as tachyarrhythmias and myocarditis, or non-cardiac conditions, such as sepsis, pulmonary embolism, and renal failure.4040. Jaffe AS, Ravkilde J, Roberts R, Naslund U, Apple FS, Galvani M, et al. It's time for a change to a troponin standard. Circulation. 2000 Sep 12;102(11):1216-20. Thus, in cases where the clinical presentation is not typical of ACS, other causes of cardiac injury related to increased troponins should be considered.
Troponins have recognized value when evaluating patients with ischemic ECG changes or with clinical symptoms suggestive of anginal pain. The major limitation of conventional troponins is their low sensitivity in patients whose time to onset is less than 6 hours. With the introduction of highly sensitive troponin assays, it became possible to detect lower troponin levels in a shorter time after ischemic myocardial injury.4141. Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361(9):858-67.,4242. Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56(2):254-61. The unit used to express the values of conventional troponin is ng/mL, and in highly sensitive troponins, the values can be expressed in ng/L, since their detection power is 10 to 100 times greater than conventional troponins.
Highly sensitive troponins are significantly more sensitive than conventional troponins in ACS diagnosis, improving the diagnostic power for AMI by 61% if collected less than 3 hours after onset and 100% if collected 6 hours after onset.4343. Mueller C. Biomarkers and acute coronary syndromes: an update. Eur Heart J. 2014;35(9):552–6. Due to the highly sensitive troponin assay's increased sensitivity and diagnostic accuracy for AMI, accelerated diagnosis algorithms have been proposed. Thus, the time to diagnosis can be shortened, which means less time in the emergency department and lower costs.4444. Westermann D, Neumann J, Sörensen N,Blankenberg S. High-sensitivity assays for troponin in patients with cardiac disease. Nat Rev Cardiol.2017;14(8):472-83.
45. Stoyanov KM, Hund H, Biener M,Gandowitz J, Riedle C, Lohr J, et al. RAPID-CPU: a prospective study on implementation of the ESC 0/1-hour algorithm and safety of discharge after rule-out of myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2020;9(1):39-51.-4646. Boeddinghaus J, Nestelberger T, Twerenbold R, Wildi K, Badertscher P, Cupa J, et al. Direct Comparison of 4 Very Early Rule-Out Strategies for Acute Myocardial Infarction Using High-Sensitivity Cardiac Troponin I. Circulation. 2017 Apr 25;135(17):1597-11. It is recommended that the investigation algorithm be used within 3 hours (see routine diagnostic flowchart and hospitalization criteria).
5.2.4.2. Creatine Kinase: Isozymes and Isoforms
Before troponins emerged as more accurate biomarkers in AMI diagnosis, CK-MB was the most common biomarker in chest pain protocols. Ideally, CK-MB should be measured by immunoassay for plasma concentration (CK-MB mass) rather than activity. This change in measurement patterns is due, in part, to studies that demonstrated that CK-MB mass has greater sensitivity and specificity for AMI.4747. Mair J, Morandell D, Genser N, Lechleitner P, Dienstl F, Puschendorf B. Equivalent early sensitivities of myoglobin, creatine kinase MB mass, creatine kinase isoform ratios, and cardiac troponins I and T for acute myocardial infarction. Clin Chem. 1995 Sep;41(9):1266-72. CK-MB subforms have emerged as early markers (< 6 hours) of myocardial injury and early inference of AMI severity, according to data from a necropsy study that demonstrated a better correlation between CK-MB mass and AMI size.4848. Costa TN, Strunz CM, Nicolau JC, Gutierrez PS. Comparison of MB fraction of creatine kinase mass and troponin I serum levels with necropsy findings in acute myocardial infarction. Am J Cardiol. 2008 Feb 1;101(3):311-4. However, the main limitation of CK-MB mass is damage to other non-cardiac tissues (false positives), especially after injuries to smooth or skeletal muscle. In about 4% of patients, false-positive results can occur, with positive CK-MB and negative troponin.4949. Lin JC, Apple FS, Murakami M,Luepker RV. Rates of positive cardiac troponin I and creatine kinase MB mass among Patients Hospitalized for Suspected Acute Coronary Syndromes. Clin Chem. 2004;50(2):333–8. In cases where CK-MB is elevated and troponin is normal, both within their kinetic windows, clinical decisions should be based on the troponin results.
5.2.5. Non-Invasive Imaging in the Emergency Department
5.2.5.1. Functional Assessment
Non-invasive exams play an essential role in the diagnosis (especially in patients with normal ECG and biomarker results) and risk stratification of patients with suspected ACS. The choice for each patient – whether ET, myocardial perfusion scintigraphy, cardiac resonance, or coronary computed tomography angiography – will depend on the objectives and clinical questions to be answered.5050. Amsterdam EA, Kirk JD, Diercks DB, Lewis WR, Turnipseed SD. Immediate exercise testing to evaluate low-risk patients presenting to the emergency department with chest pain. J Am Coll Cardiol. 2002;40(2):251-6. 165.
5.2.5.2. Exercise Testing in the Emergency Department
In the emergency department, patients whose chest pain is identified as low or intermediate risk can undergo ET. Normal results indicate a low risk of cardiovascular events, allowing for earlier and safer hospital discharge.5050. Amsterdam EA, Kirk JD, Diercks DB, Lewis WR, Turnipseed SD. Immediate exercise testing to evaluate low-risk patients presenting to the emergency department with chest pain. J Am Coll Cardiol. 2002;40(2):251-6. 165. National and international guidelines recommend ET as the first choice for risk stratification in patients who can exercise since it is a low-cost procedure, is widely available, and has a low frequency of complications.5151. Meneghelo RS, Araújo CG, Stein R, Mastrocolla LE, Albuquerque PF, Serra SM, et al; Sociedade Brasileira de Cardiologia. III Diretrizes da Sociedade Brasileira de Cardiologia sobre teste ergométrico. Arq Bras Cardiol. 2010;95(5 Suppl 1):1-26. However, patients with moderate- to high-risk ACS, acute aortic diseases, pulmonary thromboembolism, myocarditis, or pericarditis must be ruled out since these conditions are absolute contraindications. The testing protocol should be determined according to the patient's clinical condition, with the most recommended being a modified Naughton or Bruce ramp test.
ET indications in ACS (to characterize low risk after initial clinical stratification) include:
-
Baseline ECG and necrosis biomarkers without changes.
-
Absence of symptoms (chest pain or dyspnea).
-
Hemodynamic stability and adequate conditions for physical exertion.
If the ET results are normal and the patient has shown adequate functional capacity, other procedures may not be necessary due to the test's high negative predictive value.5151. Meneghelo RS, Araújo CG, Stein R, Mastrocolla LE, Albuquerque PF, Serra SM, et al; Sociedade Brasileira de Cardiologia. III Diretrizes da Sociedade Brasileira de Cardiologia sobre teste ergométrico. Arq Bras Cardiol. 2010;95(5 Suppl 1):1-26.
5.2.5.3. Echocardiography
Echocardiography is a useful complementary method for evaluating chest pain in emergencies.5252. Peels CH, Visser CA, Kupper AJ, Visser FC, Roos JP. Usefulness of two-dimensional echocardiography for immediate detection of myocardial ischemia in the emergency room. Am J Cardiol. 1990 Mar 15;65(11):687-91.
53. Sabia P, Abbott RD, Afrookteh A, Keller MW, Touchstone DA, Kaul S. Importance of two-dimensional echocardiographic assessment of left ventricular systolic function in patients presenting to the emergency room with cardiac-related symptoms. Circulation. 1991 Oct;84(4):1615-24.-5454. Feigenbaum H. Role of echocardiography in acute myocardial infarction. Am J Cardiol. 1990 Nov 20;66(18):17H-22H. This non-invasive exam provides quick diagnostic information.5555. Parisi AF. The case for echocardiography in acute myocardial infarction. J Am Soc Echocardiogr. 1988 May;1(3):173-8.
56. Reeder GS, Seward JB, Tajik AJ. The role of two-dimensional echocardiography in coronary artery disease: a critical appraisal. Mayo Clin Proc. 1982 Apr;57(4):247-58.
57. Gibson RS, Bishop HL, Stamm RB, Crampton RS, Beller GA, Martin RP. Value of early two dimensional echocardiography in patients with acute myocardial infarction. Am J Cardiol. 1982 Apr 1;49(5):1110-9.
58. Visser CA, Lie KI, Kan G, Meltzer R, Durrer D. Detection and quantification of acute, isolated myocardial infarction by two dimensional echocardiography. Am J Cardiol. 1981 May;47(5):1020-5.-5959. Heger JJ, Weyman AE, Wann LS, Rogers EW, Dillon JC, Feigenbaum H. Cross-sectional echocardiographic analysis of the extent of left ventricular asynergy in acute myocardial infarction. Circulation. 1980 Jun;61(6):1113-8. When performed during an episode of chest pain, a lack of abnormal ventricular segmental contraction indicates a nonischemic cause. Although echocardiography cannot determine whether the segmental change is recent or pre-existing, the presence of segmental contraction abnormalities reinforces the probability of CAD being indicative of infarction, ischemia, or both. However, it can also appear in cases of myocarditis.6060. Ryan T, Vasey CG, Presti CF, O’Donnell JA, Feigenbaum H, Armstrong WF. Exercise echocardiography: detection of coronary artery disease in patients with normal left ventricular wall motion at rest. J Am Coll Cardiol. 1988 May;11(5):993-9.
61. Sawada SG, Ryan T, Conley MJ, Corya BC, Feigenbaum H, Armstrong WF. Prognostic value of a normal exercise echocardiogram. Am Heart J. 1990 Jul;120(1):49-55.-6262. Cheitlin MD, Alpert JS, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, et al. ACC/AHA Guidelines for the Clinical Application of Echocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Clinical Application of Echocardiography). Developed in collaboration with the American Society of Echocardiography. Circulation. 1997 Mar 18;95(6):1686-744.
Other no less important etiologies of chest pain (e.g., aortic dissection, aortic stenosis, hypertrophic cardiomyopathy, and pericardial disease) can also be assessed with echocardiography. Significant coronary heart disease is commonly found in patients with UA. These patients are generally identified by clinical history, and reversible ECG changes can be detected with pain episodes. When the patient's history and ECG are atypical, documenting a segmental contraction abnormality with echocardiography during or immediately after a pain episode strongly suggests the diagnosis.6363. Ersbøll M, Valeur N, Mogensen UM, Andersen MJ, Møller JE, Velazquez EJ, Hassager C,et al. Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol. 2013 Jun 11;61(23):2365-73. Echocardiography also determines the presence and extent of ventricular dysfunction and the presence and severity of valve abnormalities.
Other parameters are also important in determining prognosis in addition to the LV ejection fraction (LVEF) and segmental motility. Ersbǿll et al. prospectively studied patients with infarction and LVEF > 40% within 48 hours of admission. All patients underwent ECG with semi-automated global longitudinal strain (GLS) assessment. Of the 849 patients, 57 (6.7%) had severe cardiac events, and GLS > −14% was associated with a 3-fold increase in the risk of severe events.6363. Ersbøll M, Valeur N, Mogensen UM, Andersen MJ, Møller JE, Velazquez EJ, Hassager C,et al. Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol. 2013 Jun 11;61(23):2365-73.
5.2.5.4. Stress Echocardiography
Stress echocardiography is being increasingly used in emergency departments and soon after hospitalization.6464. Lin SS, Lauer MS, Marwick TH. Risk stratification of patients with medically treated unstable angina using exercise echocardiography. Am J Cardiol. 1998 Sep 15;82(6):720-4. One study analyzed 108 patients who were observed for 4 hours with serial biomarkers and ECG and underwent stress ET or dobutamine stress ECG. Ten patients had positive stress ET results, and 8 had positive stress ECG results. The exams were consistent in 4 patients. All patients whose stress ECG results showed no evidence of ischemia were cardiac event-free at the end of 12 months of follow-up, as were 97% of the patients with negative stress ET results.6565. Colon PJ, III, Guarisco JS, Murgo J, Cheirif J. Utility of stress echocardiography in the triage of patients with atypical chest pain from the emergency department. Am J Cardiol. 1998 Nov 15;82(10):1282-4, A10.
A Brazilian study evaluated 95 patients with low- and moderate-risk UA with echocardiography under dobutamine stress, most of whom were assessed in the first 72 hours of hospitalization. For the clinical outcomes (death, AMI, new hospitalization due to UA and myocardial revascularization procedures), the exam proved to be safe and had an excellent negative predictive value (96%), allowing early discharge without the need for other tests.6666. Markman Filho B, Almeida MC, Markman M, Chaves A, Moretti M,Ramires JA, Cesar LA. Stratifying the risk in unstable angina with dobutamine stress echocardiography. Arq Bras Cardiol. 2006; 87(3):294-9. Using perfusion techniques to perform stress echocardiography can increase diagnostic sensitivity; their applications are discussed in Part 2 of this Guideline.
Because it is an accessible, fast, low-cost, and non-invasive test, echocardiography can provide additional prognostic information about the previously mentioned parameters by assessing global and regional ventricular function and identifying associated valvulopathy. It can be routinely used in the investigation of these patients. Its disadvantages are its limited acoustic window in some patients and reduced sensitivity to dobutamine stress in patients on beta-blockers.
5.2.5.5. Nuclear Cardiology
Several studies have suggested that low-risk results for resting myocardial scintigraphy performed in the emergency department indicate a very low risk of subsequent cardiac events. On the other hand, there is a much greater probability that patients with high-risk myocardial perfusion scintigraphy results will develop AMI, be revascularized (surgery or angioplasty), or have coronary lesions a coronary angiogram.6767. Jain D, Thompson B, Wackers FJ, Zaret BL. Relevance of increased lung thallium uptake on stress imaging in patients with unstable angina and non-Q wave myocardial infarction: results of the Thrombolysis in Myocardial Infarction (TIMI)-IIIB Study. J Am Coll Cardiol. 1997 Aug;30(2):421-9.
68. Geleijnse ML, Elhendy A, van Domburg RT, Cornel JH, Rambaldi R, Salustri A, et al. Cardiac imaging for risk stratification with dobutamine-atropine stress testing in patients with chest pain. Echocardiography, perfusion scintigraphy, or both? Circulation. 1997 Jul 1;96(1):137-47.-6969. Kontos MC, Jesse RL, Schmidt KL, Ornato JP, Tatum JL. Value of acute rest sestamibi perfusion imaging for evaluation of patients admitted to the emergency department with chest pain. J Am Coll Cardiol. 1997 Oct;30(4):976-82. The ERASE trial evaluated care strategies for ACS patients with normal or non-diagnostic ECG, finding admission rates of 54% for patients who underwent MPS and 63% for all others, which suggests that an initial strategy involving resting scintigraphy is an acceptable risk stratifier.7070. Kapetanopoulos A, Heller GV, Selker HP, Ruthazer R, Beshansky JR, Feldman JA, et al. Acute resting myocardial perfusion imaging in patients with diabetes mellitus: results from the Emergency Room Assessment of Sestamibi for Evaluation of Chest Pain (ERASE Chest Pain) trial. J Nucl Cardiol. 2004 Sep;11(5):570-7.
International guidelines recommend rest myocardial perfusion imaging during acute chest pain to stratify risk in patients with suspected ACS who have non-diagnostic ECG results.7171. Hendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA, Pohost GM,et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging. Circulation. 2009 Jun 9;119(22):e561-87;
72. Klocke FJ, Baird MG, Lorell BH, Bateman TM, Messer JV, Berman DS, et al; American College of Cardiology; American Heart Association; American Society for Nuclear Cardiology. American College of Cardiology; American Heart Association Task Force on Practice Guidelines; American Society for Nuclear Cardiology. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the clinical use of cardiac radionuclide imaging). J Am Coll Cardiol. 2003;42(7):1318-33.-7373. Notghi A, Low CS. Myocardial perfusion scintigraphy: past, present and future. Br J Radiol.2011;84(Spec 3):S229-36.
Radiotracer injection:
The main applications of MPS in the first hours after the patient's hospital admission include:
-
Injection of the radiotracer (sestamibi/MIBI or technetium-99m-labeled tetrofosmin, or 99mTc-sestamibi, and 99mTc-tetrofosmin) at rest during a chest pain episode in patients with normal or nonspecific ECG, aiming at a rapid, definitive diagnosis.
-
Injection of the radiotracer at rest in patients without chest pain, normal or nonspecific ECG, and symptom cessation less than 6 hours, but preferably within the previous 2 hours. Wackers et al. found that when the injection was performed in the first 6 hours of pain in ACS patients, the incidence of perfusion abnormalities was 84%, which decreased to 19% when the radiotracer was intravenously administered between 12 and 18 hours after the last pain episode.7474. Wackers FJ, Brown KA, Heller GV, Kontos MC, Tatum JL, Udelson JE, et al. American Society of Nuclear Cardiology position statement on radionuclide imaging in patients with suspected acute ischemic syndromes in the emergency department or chest pain center. J Nucl Cardiol. 2002;9(2):246-50.
Overall, radiotracers can be used to obtain resting perfusion images for acute chest pain assessment without formal contraindications, and most patients well tolerate them. MPS with physical or pharmacological stress in low- or intermediate-risk ACS patients is recommended after stabilizing the acute condition and is usually performed during hospitalization. Stable clinical and hemodynamic conditions are paramount in both situations. The limitations of this method are its availability and cost.
5.2.5.6. Anatomical Assessment: Coronary Computed Tomography Angiography
In recent years, coronary computed tomography angiography (CCTA) has been increasingly used to assess patients with suspected obstructive coronary disease. It has been demonstrated that the method is more accurate in diagnosing luminal stenosis than invasive coronary angiography, especially its high negative predictive value.7575. Budoff MJ, Dowe D, Jollis JG,Gitter M, Sutherland J, Halamert E, et al. iagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52(21):1724-32.
76. Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Gottlieb NH, Clouse ME, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 2008;359(22):2324-36.-7777. Meijboom WB, Meijs MF, Schuijf JD, Cramer MJ, Mollet NR, van Mieghem CA, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52(25):2135-44. Several studies in different clinical situations have also shown the prognostic value of CCTA regarding the presence and extent of obstructive and non-obstructive CAD, which can help with decision-making.7878. Hoffmann U, Ferencik M, Udelson JE, Picard MH, Truong QA, Patel MR, et al.; PROMISE Investigators. Prognostic Value of Noninvasive Cardiovascular Testing in Patients With Stable Chest Pain: Insights From the PROMISE Trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation. 2017;135(24):2320-32.,7979. Collet C, Onuma Y, Andreini D, Sonck J, Pompilio G, Mushtaq S,et al. Coronary computed tomography angiography for heart team decision-making in multivessel coronary artery disease. Eur Heart J. 2018 Nov 1;39(41):3689-98.
Three large multicenter randomized controlled trials have evaluated CCTA for chest pain in emergency departments, including a total of more than 3,000 patients. The multicenter CT-STAT study randomized 699 patients with low-risk chest pain to stratification with CCTA or rest-stress myocardial scintigraphy.8080. Goldstein JA, Chinnaiyan KM, Abidov A, Achenbach S, Berman DS, Hayes SW,et al. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol. 2011;58(14):1414-22. The CCTA strategy reduced diagnosis time by 54% and hospitalization costs by 38%, with no difference in the rate of adverse events between the two methods. The ACRIN-PA study primarily aimed at assessing the safety of CCTA for evaluating patients with low- and intermediate-risk chest pain compared to the traditional approach.8181. Litt HI, Gatsonis C, Snyder B, et al. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med. 2012;366(15):1393-403. None of the patients with negative CCTA had the primary outcome (cardiac death or infarction) in the first 30 days after admission. Also, patients in the CCTA group had a higher rate of discharge from emergency departments (49.6% vs. 22.7%) and fewer days of hospitalization (18 hours vs. 24.8 hours, p < 0.001), with no significant differences in the incidence of coronary angiography or revascularization in the first 30 days after admission. Finally, the ROMICAT II study evaluated the emergency department length of stay and hospital costs in similar groups of patients.8282. Hoffmann U, Truong QA, Schoenfeld DA, Chou ET, Woodard PK, Nagurney JT, et al. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med 2012;367(4):299-308. It included 1000 patients with an average age of 54 years (46% female). The length of hospital stay was significantly shorter in patients stratified by CCTA than the traditionally-assessed group (23.2 ± 37 hours vs. 30.8 ± 28 hours; p = 0.0002). The time until an ACS diagnosis was also shorter in the CCTA group (17.2 ± 24.6 hours vs. 27.2 ± 19.5 hours; p < 0.0001). There were no significant safety differences between the groups. In the CCTA group, there was a substantial increase in patients discharged directly from the emergency department (46.7% vs. 12.4%; p = 0.001), but significantly more diagnostic tests were used in this group (97% vs. 82%, p < 0.001). Despite the higher cost associated with CCTA and a tendency toward more catheterizations and revascularizations, the overall costs were similar between the two groups (p = 0.65).
A subsequently published meta-analysis confirmed that using CCTA to assess patients with acute chest pain is associated with reduced cost and length of hospital stay, with an apparent increase in invasive angiographies and myocardial revascularizations compared to traditional approaches.8383. Hulten E, Pickett C, Bittencourt MS,Villines Tc, Petrillo S, Di Carlo MF, et al. Outcomes After Coronary Computed Tomography Angiography in the Emergency Department: A Systematic Review and Meta-Analysis of Randomized, Controlled Trials. J Am Coll Cardiol. 2013;61(8):880-92.
5.2.5.6.1. Triple Rule-out
CCTA can be used in the emergency department to visualize the coronary arteries and obtain information about the aorta and pulmonary arteries, allowing assessment for acute aortic syndromes, pulmonary thromboembolism, or other thoracic changes that may be differential diagnoses of ACS (pneumonia and trauma).8484. Schertler T, Frauenfelder T, Stolzmann P, Scheffel H, Desbiolles L, Marincek B, Kaplan V, Kucher N, Alkadhi H. Triple rule-out CT in patients with suspicion of acute pulmonary embolism: findings and accuracy. Acad Radiol. 2009 Jun;16(6):708-17.,8585. Takakuwa KM, Halpern EJ, Shofer FS. A time and imaging cost analysis of low-risk ED observation patients: a conservative 64-section computed tomography coronary angiography “triple rule-out” compared to nuclear stress test strategy. Am J Emerg Med. 2011 Feb;29(2):187-95. Through specific acquisition protocols, all of this information can be obtained in a single exam. This approach is called the triple rule-out, but it should only be used in specific situations where clinical evaluation cannot direct the diagnosis.8686. Sara L, Szarf G, Tachibana A, Shiozaki AA, Villa AV, de Oliveira AC, de Albuquerque AS, et al. II Guidelines on Cardiovascular Magnetic Resonance and Computed Tomography of the Brazilian Society of Cardiology and the Brazilian College of Radiology. Arq Bras Cardiol. 2014 Dec;103(6 Suppl 3):1-86.
In summary, CCTA is a safe and efficient strategy for evaluating patients with acute low- and intermediate-risk chest pain in emergency departments, reducing the time until correct diagnosis and length of hospital stay.8787. Goehler A, Mayrhofer T, Pursnani A, Ferencik M, Lumish HS, Barth C, et al. Long-term health outcomes and cost-effectiveness of coronary CT angiography in patients with suspicion for acute coronary syndrome. J Cardiovasc Comput Tomogr. 2020 Jan-Feb;14(1):44-54. The method's disadvantages include the use of ionizing radiation, its high cost, the need for iodinated contrast media, its limited availability in Brazil, and the fact that it cannot be used in patients with heart rates above 80 beats per minute or who cannot use beta-blockers.
5.3. Risk Stratification
5.3.1. Risk Stratification of Ischemic Cardiovascular Events
Using the TIMI IIB database, Antman et al. found the following independent markers of worse prognosis in patients with NSTE-ACS (“TIMI group risk score”): age ≥65 years, elevated biochemical markers, ST-segment depression ≥0.5 mm, ASA use in the 7 days prior to symptom onset, three or more traditional risk factors for CAD (hypertension, hypercholesterolemia, DM, smoking, or family history), known CAD, and recent severe angina (< 24 hours).2828. Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000 Aug 16;284(7):835-42. With one point for each item, a score of 0 to 2 is classified as low risk, 3 to 4 as intermediate risk, and 5 to 7 as high risk. This risk score has been validated in other NSTE-ACS studies. An association has been found between each marker and a higher incidence of events (death, reinfarction, and recurrent ischemia requiring revascularization) and higher risk scores. (Figure 1.3).
Due to its good discriminatory power, the Global Registry of Acute Coronary Events (GRACE) risk score allows more accurate stratification at both hospital admission and discharge (Figure 1.4). However, it is complex, requiring a personal computer or digital device to calculate the risk.8888. Fox KA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, Van der Wef F, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ. 2006;333(7578):1091. The original GRACE score provides an estimate of in-hospital death or AMI and death 6 months after discharge. The score was later validated for risk estimation at 1 and 3 years. Eight prognostic variables of hospital mortality have been identified for this score, and the total score for a given patient is the sum of each variable:
-
Age in years - ranging from 0 (< 30) to 100 points (> 90).
-
Heart rate (bpm) - ranging from 0 (< 50) to 46 points (> 200).
-
Systolic blood pressure (mmHg) - ranging from 0 (> 200) to 58 points (< 80).
-
Creatinine levels (mg/dL) - ranging from 1 (< 0.40) to 28 points (> 4.0).
-
Heart failure (Killip class) - ranging from 0 (class I) to 59 points (class IV).
-
Cardiac arrest at admission - ranging from 0 (no) to 39 points (yes).
-
ST-segment deviation - ranging from 0 (no) to 28 points (yes).
-
Increased levels of cardiac injury biomarkers - ranging from 0 points (no) to 14 points (yes).
The incidence of hospital death is ≤ 1% for low-risk patients (total scores ≤ 108), between 1% and 3% for intermediate-risk scores (between 109 and 140), and over 3% for high-risk patients (> 140).8989. Granger CB, Goldberg RJ, Dabbous O, et al. Predictors of hospital mortality in the Global Registryof Acute Coronary Events. Arch Intern Med. 2003;163(19):2345-53. A new version of the GRACE score (GRACE 2.0), developed using the same predictor variables for treatment outcome, has expanded the risk estimates for in-hospital death to 6 months, 1 year, and 3 years risk of death or AMI to 1 year.9090. Fox KAA, FitzGerald G, Puymirat E,Huang W, Carruthers K, Simon T, et al. Should patients with acute coronary disease be stratified for management according to their risk? Derivation, external validation and outcomes using the updated GRACE risk score. BMJ Open. 2014;4(2):e004425. Table 1.5 shows the risk stratification based on clinical, ECG, and laboratory variables.
Risk stratification for death or infarction in patients with acute ischemic syndrome without ST-segment elevation9191. Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, et al. ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina). J Am Coll Cardiol. 2000 Sep;36(3):970-1062.
The HEART score (discussed in item 5.4) assesses the risk of a major cardiac event (infarction, revascularization, or death) 6 weeks after initial presentation in patients with chest pain.
5.3.2. Bleeding Risk Stratification
Bleeding is associated with an adverse prognosis in NSTE-ACS, and every effort should be made to reduce it. Certain variables may help classify patients at different risk levels for major bleeding during hospitalization. Bleeding risk scores have been developed based on cohort studiesand ACS clinical studies. The CRUSADE score (www.crusadebleedingscocre.org) was developed from a cohort of 71,277 patients and subsequently validated in a cohort of 17,857 patients9292. Subherwal S, Bach RG, Chen AY, Gage BF, Rao SV, Newby LK,et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation. 2009;119(14):1873–82. (Table 1.6). In a Brazilian population, it predicted not only bleeding but in-hospital mortality (area under the ROC curve = 0.753, p < 0.001).9393. Nicolau JC, Moreira HG, Baracioli LM, Serrano CV Jr, Lima FG, Franken M, et al. The bleeding risk score as a mortality predictor in patients with acute coronary syndrome. Arq Bras Cardiol. 2013 Dec;101(6):511-8. The rate of major bleeding gradually increased as the bleeding risk score increased. By incorporating admission and treatment variables, this score's accuracy for estimating bleeding risk is relatively high. Although age is not listed among the prognostic factors in this score, it is included in the creatinine clearance calculations.
CRUSADE bleeding risk score (reference b) | Algorithm used to determine the CRUSADE risk score for major in-hospital bleeding
Another score was derived from the ACUITY and HORIZONS studies. Six independent variables (female sex, advanced age, elevated serum creatinine, elevated white blood cell count, anemia, ACS with or without ST elevation) and one treatment-related variable (heparin and GP IIb/IIIa inhibitor use instead of bivalirudin alone) were identified (Table 1.7). This score identified patients at increased risk of bleeding (unrelated to CABG) and mortality after 1 year.9494. Mehran R, Pocock SJ, Nikolsky E, Clayton T, Dangas GD, Kirtane AJ,et al. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol. 2010;55(23):2556–66.
Bleeding risk score proposed by Mehran et al.9494. Mehran R, Pocock SJ, Nikolsky E, Clayton T, Dangas GD, Kirtane AJ,et al. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol. 2010;55(23):2556–66. Algorithm used to determine the risk of major in-hospital bleeding
Both scores were derived from cohorts in which femoral access was predominantly or exclusively used. Their prognostic value may be reduced regarding radial access.9595. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2020 Aug 29:ehaa575.
Finally, the authors point out that no score (either for ischemic events or for hemorrhagic events) should replace clinical evaluation. Rather, they are merely tools to help doctors in their decision-making process.
5.4. Emergency Diagnostic Flowchart and Hospitalization Criteria
It is essential to develop a diagnosis flowchart for patients with chest pain, determining the criteria for early discharge and hospitalization. This can identify low-risk patients to be investigated in an outpatient setting, as well as more serious cardiac conditions that require hospitalization.
Emergency department chest pain screening is based on a brief clinical history, physical examination, 12-lead ECG within 10 minutes of arrival, and biomarker measurement. This assessment protocol is mainly aimed at early identification of high-risk patients who require hospitalization or urgent transfer for hemodynamic monitoring.
To avoid premature discharge, these patients should remain monitored in an environment where an accelerated diagnostic protocol can be used. This protocol involves serial ECG and highly sensitive troponin measurement upon arrival at the emergency department and 3 hours later. In addition to this protocol, risk scores that include demographic data, symptoms, ECG findings and biomarkers are important tools for assessing chest pain patients in the emergency department. The most commonly-used scores (HEART, ADAPT, and EDACS)9696. Six AJ, Backaus BE, Kelder SC, Chest pain in the emergency room: value of the Heart score. Neth Heart J.2008;16(6):191-6.
97. Than M, Cullen L, Aldous S, Goodacre S, Frampton CM, Troughton R, et al. 2-Hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: the ADAPT trial. J Am Coll Cardiol. 2012;59(23):2091-8.-9898. Than M,Flaws D, Sanders S, Doust J, Glasziou P, Kline J, et al. Development and validation of the Emergency Department Assessment of Chest pain Score and 2 h accelerated diagnostic protocol. Emerg Med Australas.2014;26(1):34-44. are summarized in Figure 1.3.
Greenslade et al. compared the effectiveness of these scores in relation to premature discharge from the emergency department, finding that all scores were effective and had very high sensitivity.9999. Greenslade JH et al. Diagnostic Accuracy of a New High-Sensitivity Troponin I Assay and Five Accelerated Diagnostic Pathways for Ruling Out Acute Myocardial Infarction and Acute Coronary Syndrome. Ann Emerg Med. 2018;71(4):439-51.e3.
The HEART score was developed using conventional troponin as a biomarker. However, retrospective studies that used highly sensitive troponin found results similar to those of validation studies.100100. Santi L, Farina G, Gramenzi A, Trevisani F, Baccini M, Bernardi M, et al. The HEART score with high-sensitive troponin T at presentation: ruling out patients with chest pain in the emergency room. Intern Emerg Med. 2017;12(3):357-64.,101101. Carlton EW, Cullen L, Than M, Gamble J, Khattab A, Greaves K. A novel diagnostic protocol to identify patients suitable for discharge after a single high-sensitivity troponin. Heart. 2015;101(13):1041-6. No studies have validated this tool for care outside the hospital. However, an ongoing study (ARTICA) aims to validate this score for pre-hospital care.102102. Aarts GWA, Camaro C, van Geuns RJ, et al. Acute rule-out of non-ST-segment elevation acute coronary syndrome in the (pre) hospital setting by HEART score assessment and a single point-of-care troponin: rationale and design of the ARTICA randomised trial. BMJ Open. 2020;10(2): e034403.
A HEART score from 0 to 3 identifies 35% to 46% of patients at low risk, showing high sensitivity and negative predictive value, while patients with a score of 7 to 10 are at high risk, with an event rate > 50% in 6 weeks.103103. Backus BE, Six AJ, Kelder JC, Mast TP, van den Akker F, Mast EG, Monnink SH, van Tooren RM, Doevendans PA. Chest pain in the emergency room: a multicenter validation of the HEART Score. Crit Pathw Cardiol. 2010 Sep;9(3):164-9.
104. Backus BE, Six AL, Kelder SC,Bosschaert EG, Mast A, Mosterd RF, et al. A prospective validation of the Heart score for chest pain patients at the emergency departament. Int J Cardiol. 2013; 168:2153-8.
105. Six Aj, Cullen L,Backus BE, Greenslade J, Parsonage W, Aldous S, et al. The HEART score for the assessment of patients with chest pain in the emergency department: a multinational validation study. Crit Pahtw Cardiol. 2013;12(3):121-6.-106106. Van Den Berg P, Body R. The HEART score for early rule out of acute coronary syndromes in the emergency department: a systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care. 2018; 7(2): 111–9. The HEART score could better distinguish low-risk patients for major cardiac events than the GRACE and TIMI scores, with a lower loss rate and greater accuracy for initial risk stratification in the emergency department.107107. Poldervaart JM, Langedijk M, Backus BE, Dekker IMC, Six AJ, Doevendans PA, Hoes AW, Reitsma JB. Comparison of the GRACE, HEART and TIMI score to predict major adverse cardiac events in chest pain patients at the emergency department. Int J Cardiol. 2017 Jan 15;227:656-61.,108108. Sakamoto JT, Liu N, Koh ZX, Fung NX, Heldeweg ML, Ng JC. Comparing HEART, TIMI, and GRACE scores for prediction of 30-day major adverse cardiac events in high acuity chest pain patients in the emergency department. Int J Cardiol. 2016 Oct 15;221:759-64.
The growing joint use of clinical data, ECG, highly sensitive troponin, and the systematized use of scores has amplified the effectiveness of diagnostic assessment protocols and the risk stratification of chest pain in the emergency department, which promotes safe early discharge and reduction of additional exams within 30 days.109109. Mahler SA, Riley RF, Hiestand BC, Russell GB, Hoekstra JW, Lefebvre CW, et al. The HEART Pathway randomized trial: identifying emergency department patients with acute chest pain for early discharge. Circ Cardiovasc Qual Outcomes. 2015;8(2):195-203.
A randomized study that compared emergency care HEART scores with chest pain found a high negative predictive value for major cardiovascular events in the first year, with no differences observed regarding hospitalization or readmission to the emergency department.110110. Stopyra JP, Riley RF, Hiestand BC, Russell G, Holkstra JW, Lefebvre CW, et al. The HEART Pathway Randomized Controlled Trial One-year Outcomes. Acad Emerg Med. 2019;26(1):41-50.
Thus, the impact of additional routine imaging exams on low-risk patients has been reconsidered because, despite the reduced diagnosis time, current evidence has shown that these additional tests have no verified benefit regarding the occurrence of AMI or relevant clinical events.111111. Reinhardt SW, Lin CJ, Novak E, Brown DL. Noninvasive Cardiac Testing vs Clinical Evaluation Alone in Acute Chest Pain: A Secondary Analysis of the ROMICAT-II Randomized Clinical Trial. JAMA Intern Med. 2018;178(2):212-9.
5.5. Nurses and the Chest Pain Protocol
When performed by qualified nurses, hospital screening improves the identification of high-risk patients and reduces door-to-ECG time.112112. O’Neill L, Smith K, Currie P,Elder Dhj, Wei J, Lang CC. et al. Nurse-led Early Triage (NET) study of chest pain patients: a long term evaluation study of a service development aimed at improving the management of patients with non-ST-elevation acute coronary syndromes. Eur J Cardiovasc Nurs. 2014; 13(3):253–60. It has been shown that, in rural hospitals, emergency nurses have a high level of accuracy, which reduces waiting time and length of stay without compromising patient safety.113113. Roche TE, Gardner G, Jack L. The effectiveness of emergency nurse practitioner service in the management of patients presenting to rural hospitals with chest pain: a multisite prospective longitudinal nested cohort study. BMC Health Serv Res. 2017; 17(1): 445. Thus, nurses should be actively encouraged to participate in the screening of patients with chest pain, and the multidisciplinary team should receive continuing training about patients with chest pain.
A flowchart for emergency department treatment of patients with chest pain is shown in Figure 1.5.
Flowchart of routine diagnosis of the patient with acute chest pain in the Emergency Department. HS: highly sensitive; TTE: transthoracic echocardiogram.
6. Emergency Procedures After Risk Stratification
6.1. Indications for Early Invasive Strategy
An invasive strategy is indicated for patients with a very high risk of death (Table 1.8). This patient profile is not widely represented in randomized studies. Coronary intervention within 2 hours is indicated for these patients. When interventional cardiology service is not available, these patients should ideally be transferred to other centers.
6.2. Initial Treatment (Emergency Department/Ambulance)
6.2.1. Supplemental Oxygen
Hypoxemia and myocardial ischemia can occur due to changes in the ventilation-perfusion ratio secondary to pulmonary arteriovenous shunting (due to an increase in LV end-diastolic pressure) and interstitial and/or pulmonary alveolar edema. Hypoxemia worsens myocardial ischemia, increasing myocardial injury. O2 saturation (SaO2) should be measured using digital oximetry in pre-hospital and/or ambulance care for early diagnosis of hypoxemia.
Supplemental oxygen therapy is indicated for patients with AMI who present hypoxia with SaO2 <90% or clinical signs of respiratory distress.114114. Stub D, Smith K, Bernard S, Nehme Z, Stephenson M, Bray JE, eet al. Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation 2015;131(24):2143—50. However, in a randomized study of more than 6000 patients, oxygen therapy in patients with suspected ACS and SaO2 ≥90% was not associated with reduced mortality or other cardiovascular outcomes in 1 year of follow-up. 115115. Hofmann R, James SK, Jernberg T, Lindahl B, Erlinge D, Witt N,et al. Oxygen Therapy in Suspected Acute Myocardial Infarction. N Engl J Med. 2017 Sep 28;377(13):1240-9.
Oxygen therapy should not eliminate hypoxic respiratory stimulation when there is a chronic obstructive pulmonary disease or other causes of hypercapnia. Patients with pulmonary congestion, cyanosis, arterial hypoxemia, or respiratory failure should receive oxygen supplementation and be carefully monitored with serial blood gas analysis. Non-invasive ventilatory support should be used in certain cases with signs of respiratory failure, persistent pulmonary congestion, and hypoxemia. Treatment should progress to invasive ventilation in the event of circulatory shock, non-invasive ventilatory support failure, and in unstable patients indicated for urgent myocardial revascularization (PCI or CABG). The unnecessary administration of oxygen for a prolonged period can cause systemic vasoconstriction and can even be harmful.
6.2.2. Analgesia and Sedation
The precordial pain and anxiety usually present in ACS lead to hyperactivity of the sympathetic nervous system. This hyperadrenergic state, in addition to increasing myocardial oxygen consumption, predisposes patients to atrial and ventricular tachyarrhythmias. Initial antianginal therapy with beta-blockers and nitrates should be performed as long as there are no contraindications, such as cardiogenic shock and/or hypotension. Morphine sulfate may be used in refractory cases or when there is a contraindication to nitrates or beta-blockers. The therapy should be administered intravenously, at a dose of 2 to 4 mg (diluted) every 5 minutes, up to a maximum of 25 mg. The low dosage is to avoid adverse effects such as hypotension and respiratory depression. Morphine derivatives should be avoided, except in cases of hypersensitivity, for which meperidine sulfate can be used in fractional doses of 20 to 50 mg intravenously.
Despite its efficacy in controlling angina, there is evidence that morphine reduces the antiplatelet effect of P2Y12 inhibitors, such as clopidogrel,116116. Hobl EL, Stimpfl T, Ebner J, Schoergenjofer C, Derhaschnig U, Plassman RS, et al. Morphine decreases clopidogrel concentrations and effects: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol. 2014;63(7):630–5. and more potent antiplatelet drugs, such as prasugrel and ticagrelor.117117. Thomas MR, Morton AC, Hossain R, Chen b, Luo L, Shahari NN, et al. Morphine delays the onset of action of prasugrel in patients with prior history of ST-elevation myocardial infarction. Thromb Haemost. 2016; 116(7):96–102.,118118. Kubica J, Adamski P, Ostrowska M, Sikora J, Kubica JM, Srooka WD, et al. Morphine delays and attenuates ticagrelor exposure and action in patients with myocardial infarction: the randomized, double-blind, placebo-controlled IMPRESSION trial. Eur Heart J. 2016;37(3):245-52.
Regarding clinical events, a subanalysis of the CRUSADE119119. Meine TJ, Roe MT, Chen AY, Patel MR, Washam JB, Ohman EM, et al; CRUSADE Investigators. Association of intravenous morphine use and outcomes in acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative. Am Heart J. 2005;149(6):1043-9. registry and, more recently, a meta-analysis that included almost 70,000 patients found that early routine use of morphine may be associated with increased in-hospital mortality and major cardiovascular events.120120. Duarte GS, Nunes-Ferreira A, Rodrigues FB, Pinto FJ, Ferreira JJ, Costa J, et al. Morphine in acute coronary syndrome: systematic review and meta-analysis. BMJ Open. 2019;9(3):e025232. In a post-hoc analysis of the EARLY-ACS trial, it was found that morphine was associated with a greater risk of early ischemic events when used concomitantly with clopidogrel pretreatment. However, in the group of patients who did not receive clopidogrel before catheterization, morphine did not worsen the clinical outcome.121121. Furtado RHM, Nicolau JC, Guo J, Im K, White JA, Sabatine MS, et al Morphine and Cardiovascular Outcomes Among Patients With Non-ST-Segment Elevation Acute Coronary Syndromes Undergoing Coronary Angiography. J Am Coll Cardiol. 2020 Jan 28;75(3):289-300.
Although the routine use of anxiolytics has become common practice in Brazil, it often seems excessive and should be reserved for special situations. A randomized, double-blind clinical trial with 131 male AMI patients found that the degree of anxiety, blood pressure, heart rate, and precordial discomfort did not differ between diazepam and placebo groups122122. Dixon RA, Edwards IR, Pilcher J. Diazepam in immediate post-myocardial infarct period. A double blind trial. Br Heart J.1980 May;43(5):535-40.. Diazepine derivatives have been the most commonly used drug in this indication.
Nonsteroidal anti-inflammatory drugs (NSAIDs) should not be used (except ASA) to control pain in AMI patients since they increase the risk of major cardiovascular events. NSAID use should be suspended in patients who used them prior to hospitalization due to the worsening cardiovascular prognosis associated with elevated blood pressure, the risk of acute kidney injury, and blood hyperviscosity.123123. Gislason GH, Jacobsen S, Rasmussen JN,Rasmussen S, Bucch P, Fribens J, et al. Risk of death or reinfarction associated with the use of selective cyclooxygenase-2 inhibitors and nonselective nonsteroidal antiinflammatory drugs after acute myocardial infarction. Circulation. 2006;113(25):2906–13.
6.2.3. Glycemic Control
More than 30% of AMI patients have DM or are unaware of their diagnosis. These individuals have higher bleeding rates and a worse 30-day prognosis than patients with normal blood glucose levels.124124. Conaway DG, O’Keefe JH, Reid KJ, Spertus J. Frequency of undiagnosed diabetes mellitus in patients with acute coronary syndrome. Am J Cardiol. 2005;96(3):363–5.
125. Goyal A, Mahaffey KW, Garg J, Nicolau JC, Hochman JS, Weaver WD, Theroux P, Oliveira GB, Todaro TG, Mojcik CF, Armstrong PW, Granger CB. Prognostic significance of the change in glucose level in the first 24 h after acute myocardial infarction: results from the CARDINAL study. Eur Heart J. 2006 Jun;27(11):1289-97.
126. Nicolau JC, Serrano CV Jr, Giraldez RR, Baracioli LM, Moreira HG, Lima F, et al. In patients with acute myocardial infarction, the impact of hyperglycemia as a risk factor for mortality is not homogeneous across age-groups. Diabetes Care. 2012 Jan;35(1):150-2.-127127. Finfer S, Chittock DR, Su SY, Bleir D, Foster D, Dhingra V, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–97. Thus, glycemic control protocols should be used for AMI patients with significant hyperglycemia (> 180 mg/dL). This therapy aims to reduce blood glucose levels and avoid hypoglycemic episodes (< 70 mg/dL), which can cause several harmful effects, including the expansion of the AMI area. In patients at higher risk of hypoglycemia, such as older adults, those with chronic kidney disease, and those with residual effects from oral and/or fasting hypoglycemic agents, glycemic control should be adjusted to tolerate slightly higher blood glucose levels and thus prevent hypoglycemia.
6.2.4. Anti-ischemic Therapy
The goal of anti-ischemic therapy is to reduce oxygen consumption (by decreasing the heart rate, blood pressure, and myocardial contractility) or to increase oxygen supply (by administering oxygen or promoting vasodilation).
6.2.4.1. Nitrates
The therapeutic benefits of nitrates are related to their effects on peripheral and coronary circulation. Their vasodilator effect, which decreases venous return to the heart and LV end-diastolic volume, reduces oxygen consumption by the myocardium. Additionally, they have vasodilatory effects on coronary arteries (whether normal or atherosclerotic) and redirect intracoronary flow, with increased collateral circulation and platelet aggregation inhibition. In addition to their symptomatic effects, nitrates reduce pulmonary congestion, mainly by lowering systemic venous return.
No controlled clinical studies have tested nitrates' effects on clinical outcomes and mortality in NSTE-ACS, although their use is universally accepted. UA studies that have evaluated them have been small and observational.128128. DePace NL, Herling IM, Kotler MN, Hakki AH, Spielman SR, Segal BL. Intravenous nitroglycerin for rest angina. Potential pathophysiologic mechanisms of action. Arch Intern Med. 1982 Oct;142(10):1806-9.
129. Kaplan K, Davison R, Parker M, Przybylek J, Teagarden JR, Lesch M. Intravenous nitroglycerin for the treatment of angina at rest unresponsive to standard nitrate therapy. Am J Cardiol.1983 Mar 1;51(5):694-8.
130. Roubin GS, Harris PJ, Eckhardt I, Hensley W, Kelly DT. Intravenous nitroglycerine in refractory unstable angina pectoris. Aust N Z J Med. 1982 Dec;12(6):598-602.
131. Curfman GD, Heinsimer JA, Lozner EC, Fung HL. Intravenous nitroglycerin in the treatment of spontaneous angina pectoris: a prospective, randomized trial. Circulation. 1983 Feb;67(2):276-82.-132132. Dellborg M, Gustafsson G, Swedberg K. Buccal versus intravenous nitroglycerin in unstable angina pectoris. Eur J Clin Pharmacol. 1991;41(1):5-9. Nitrates are associated with the improvement of both anginal and congestive symptoms, although they have no impact on mortality, infarction, or the need for revascularization.
Treatment is initiated in the emergency department, with sublingual administration (nitroglycerin, mononitrate, or isosorbide dinitrate). If rapid pain relief does not occur, these patients may benefit from intravenous administration (nitroglycerin and isosorbide mononitrate are available in Brazil).
Nitrates are contraindicated in the presence of significant arterial hypotension (systolic blood pressure [SBP] < 100 mmHg), the use of sildenafil in the last 24 hours, or the use of tadalafil in the previous 48 hours. A common side effect is a headache. The sublingual use of nitroglycerin (0.4 mg tablet), isosorbide dinitrate (5 mg tablet), or isosorbide mononitrate (5 mg tablet) should not exceed three tablets, administered at 5-min intervals. Intravenous nitroglycerin is used at a dose of 10 µg/min in increments of 10 µg every 5 min until symptoms improve, blood pressure is reduced (the drop in SBP should not exceed 30% or reach < 110 mmHg), or the heart rate increases (> 10% of baseline). Tolerance to the hemodynamic effects of this medication can be expected after the first 24 hours of use. Intravenous treatment should continue 24 to 48 hours after the last angina attack and should be suspended gradually.
6.2.4.2. Beta-blockers
As with nitrates, few controlled clinical trials have been conducted on beta-blockers in UA. Evidence for their beneficial effects is based on their mechanism of action, the results of small controlled clinical trials, and the extrapolation of results from studies on stable angina and STEMI. Beta-blockers competitively inhibit the effects of circulating catecholamines. In UA, their benefits are related to their action on beta-1 receptors. They decrease heart rate, blood pressure, and myocardial contractility, causing reduced myocardial oxygen consumption. Despite the lack of large-scale randomized trials evaluating their action on hardclinical outcomes, such as mortality in patients with NSTE-ACS, these drugs, together with nitrates, are considered agents of the first choice for ACS treatment the emergency department in patients without contraindications. Only a few small studies have compared beta-blockers with placebo in UA.133133. Gottlieb SO, Weisfeldt ML, Ouyang P, Achuff SC, Baughman KL, Traill TA, et al. Effect of the addition of propranolol to therapy with nifedipine for unstable angina pectoris: a randomized, double-blind, placebo-controlled trial. Circulation. 1986 Feb;73(2):331-7.
134. Telford AM, Wilson C. Trial of heparin versus atenolol in prevention of myocardial infarction in intermediate coronary syndrome. Lancet. 1981 Jun 6;1(8232):1225-8.-135135. Lubsen J, Tijssen JG. Efficacy of nifedipine and metoprolol in the early treatment of unstable angina in the coronary care unit: findings from the Holland Interuniversity Nifedipine/metoprolol Trial (HINT). Am J Cardiol. 1987 Jul 15;60(2):18A-25A. Although no mortality-reducing effect was found, a meta-analysis of five small studies 136136. Yusuf S, Wittes J, Friedman L. Overview of results of randomized clinical trials in heart disease. II. Unstable angina, heart failure, primary prevention with aspirin, and risk factor modification. JAMA. 1988 Oct 21;260(15):2259-63. on beta-blocker therapy in 4,700 UA patients showed a 13% reduction in the relative risk of progression to AMI. A Brazilian study in patients with NSTE-ACS found a significant association between oral beta-blocker therapy in the first 24 hours of hospitalization and lower in-hospital mortality, regardless of LV dysfunction.137137. Nicolau JC, Furtado RHM, Baracioli LM, Lara LM, Dalçóquio TF, Scanavini Junior MA, et al. The Use of Oral Beta-Blockers and Clinical Outcomes in Patients with Non-ST-Segment Elevation Acute Coronary Syndromes: a Long-Term Follow-Up Study. Cardiovasc Drugs Ther. 2018 Oct;32(5):435-42.
Despite involving patients with STEMI, the COMMIT trial 138138. Chen ZM, Pan HC, Chen YP, Peto R, Collins R, Jiang LX, et al. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005 Nov 5;366(9497):1622-32. suggested that the routine use of a high intravenous dose of beta-blocker followed by oral administration could increase the incidence of cardiogenic shock, mainly when used in the first 24 to 48 hours or in patients with LV dysfunction or risk factors for cardiogenic shock (age over 70 years, heart rate > 110 bpm/min or systolic pressure < 120 mmHg). Although no randomized clinical trial specifically for a population with NSTE-ACS has been conducted, a database study with more than 20,000 patients found results similar to the COMMIT study.139139. Kontos MC, Diercks DB, Ho PM, Wang TY, Chen AY, Roe MT. Treatment and outcomes in patients with myocardial infarction treated with acute β-blocker therapy: results from the American College of Cardiology's NCDR(®). Am Heart J. 2011 May;161(5):864-70.
Thus, the routine use of oral beta-blockers is recommended in patients without contraindications (Part 2 of this Guideline provides further details). If the patient has persistent ischemic pain and/or tachycardia (non-compensatory), the intravenous formulation can be used. Various therapeutic regimens can be used depending on the selected beta-blocker. There is no evidence that one beta-blocker is superior to others. Beta-blockers (carvedilol, bisoprolol, and metoprolol succinate) are highly recommended for patients who develop ventricular dysfunction (LVEF <40%) without pulmonary congestion.
The following is a dosage list for metoprolol and atenolol, the most commonly used beta-blockers in Brazil:
Metoprolol: Intravenous – 5 mg (1 to 2 min) every 5 min until a maximum dose of 15 mg is reached.
Oral - 50 to 100 mg every 12 hours, beginning 15 min after the last intravenous dose.
Atenolol: Intravenous – 5 mg (1 to 2 min) every 5 min until a maximum dose of 10 mg is reached.
Oral - 25 to 50 mg every 12 hours, beginning 15 min after the last intravenous dose.
During intravenous administration, careful monitoring of heart rate, blood pressure, ECG, and pulmonary auscultation should be performed.
Beta-blockers should not be used during acute intoxication in patients with symptoms of vasospasm due to cocaine use. In patients with asthma or chronic obstructive pulmonary disease, beta-blockers are contraindicated only in the presence of bronchospasm; in such cases, beta-1 selective beta-blockers are recommended.
6.2.5. Initial Antithrombotic Therapy (Ambulance/Emergency Department)
6.2.5.1. Oral Antiplatelet Therapy
The phenomenon of coronary atherosclerotic plaque instability, a recognized mechanism of ACS, results from rupture or erosion, with subsequent platelet activation and thrombosis.140140. Crea F, Libby P. Acute Coronary Syndromes: The Way Forward From Mechanisms to Precision Treatment. Circulation. 2017 Sep 19;136(12):115566.
This concept has guided advances in antiplatelet therapy and the current idea of dual therapy with ASA and P2Y12 inhibitors in patients with ACS, determining which should receive invasive strategy and the initially proposed clinical treatment.141141. Bhatt DL, Hulot JS, Moliterno DJ, Harrington RA. Antiplatelet and anticoagulation therapy for acute coronary syndromes. Circ Res. 2014;114(12):1929-43.
The characteristics of and scientific evidence for antiplatelet drugs are described in detail in Part 2. However, a critical decision in the emergency department is for the time for beginning P2Y12 inhibitors, particularly in patients undergoing early invasive strategy with cardiac catheterization and PCI. The term "pre-treatment" generally refers to administering P2Y12 inhibitors before determining the coronary anatomy, either in the ambulance or the emergency department.
6.2.5.1.1. The Basics of Pretreatment with P2Y12 Inhibitors
The goal of pre-treatment with a second potent antiplatelet agent in NSTE-ACS is to achieve effective platelet inhibition and prevent further aggregation, which would prepare the patient for invasive strategy, potentially reducing the extent of thrombosis, as well as the risk of recurrent AMI and PCI-related complications, such as stent thrombosis and periprocedural infarction.142142. Lüscher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation. 2007 Feb 27;115(8):1051-8.
In contrast, pre-treatment involves an increased risk of bleeding, associated or not with coronary artery bypass surgery. This factor is associated with a relevant increase in morbidity and mortality in ACS.143143. Bellemain-Appaix A, Kerneis M, O’Connor SA, Silvain J, Cucherat M, Beygui F, et al. Reappraisal of thienopyridine pretreatment in patients with non-ST elevation acute coronary syndrome: a systematic review and meta-analysis. BMJ.2014;349:g6269.
144. Golwala H, Bhatt DL. The Timing of P2Y12 Inhibitor Initiation in the Treatment of ACS? Does the Evidence Exist in This Era? Prog Cardiovasc Dis. 2018 Jan-Feb;60(4-5):471-7.-145145. Dworeck C, Redfors B, Angerås O, Haraldsson I, Odenstedt J, Ioanes D, et al. Association of Pretreatment With P2Y12 Receptor Antagonists Preceding Percutaneous Coronary Intervention in Non–ST-Segment Elevation Acute Coronary Syndromes With Outcomes. JAMA Netw Open. 2020;3(10):e2018735.
One important issue is determining the minimum time required for clinically useful action, knowing that even the most potent oral antiplatelet agents need at least 30 to 60 minutes for effective platelet inhibition, thus providing "appropriate pretreatment" before catheterization and PCI.146146. Wiviott SD, Trenk D, Frelinger AL, O’Donoghue M, Neumann FJ, Michelson AD, et al. PRINCIPLE-TIMI 44 Investigators. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation. 2007 Dec 18;116(25):2923-32.
147. Gurbel PA1, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009 Dec 22;120(25):2577-85.
148. Bonello L, Laine M, Camoin-Jau L, Noirot F, Guieu R, Dignet-George F, et al. Onset of optimal P2Y12-ADP receptor blockade after ticagrelor and prasugrel intake in Non-ST elevation acute coronary syndrome. Thromb Haemost. 2015;114(4):702-7.-149149. Singh S, Singh M, Grewal N, Khosla S. Comparative Efficacy and Safety of Prasugrel, Ticagrelor, and Standard-Dose and High-Dose Clopidogrel in Patients Undergoing Percutaneous Coronary Intervention: A Network Meta-analysis. Am J Ther. 2016;23(1):e52-e62.
Following pretreatment with P2Y12 inhibitors, a second antiplatelet agent was used in the CURE (clopidogrel)150150. Yusuf S, Bijsterveld N, Moons A. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation: the clopidogrel in unstable angina to prevent recurrent events trial investigators. New Engl J Med. 2001; 345(7):494–502.,151151. Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358(9281):527-33. and PLATO (ticagrelor) trials;152152. Wallentin L, Becker RC, Budaj A, Cannon CP, Emmanuelson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(1):1045-57.,153153. Cannon CP, Harrington RA, James S, Ardissino D, Becker RC, Emanuelsson H et al. PLATelet inhibition and patient Outcomes (PLATO) investigators. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet. 2010; 375(9791): 283–93. in the TRITON trial, prasugrel was only administered after the coronary anatomy had been determined.154154. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007; 357(20): 2001-15.
6.2.5.1.2. Comparative Studies on Pretreatment vs. Second Antiplatelet Use in the Catheterization Unit
The ARMYDA-5 PRELOAD trial assessed clopidogrel pretreatment in NSTE-ACS (n = 409) and stable angina patients and found no reduction in adverse cardiovascular events in either group.155155. Di Sciascio MG, Patti G, Pasceri V, Gatto L. Effectiveness of in-laboratory high-dose clopidogrel loading versus routine pre-load in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol.2010;56(7):550-7.
The CREDO trial was designed to assess the benefits of pretreatment with clopidogrel and ASA in patients undergoing PCI. In this controlled trial, 2116 patients (39% with ACS and 61% with stable CAD) received 300 mg of clopidogrel (n = 1053) or placebo (n = 1063) 3 to 24 hours before PCI. The average time between the loading dose of clopidogrel and PCI was 9.8 hours, with 51% and 49% of the patients receiving the loading dose 3 to 6 hours and 6 to 24 hours before PCI, respectively. No significant reduction was found in the combined outcome (death, infarction, or urgent revascularization of the target vessel) in 28 days. However, when analyzed according to treatment time (3 to 6 hours, 6 to 12 hours, or 12 to 24 hours before PCI), a significant interaction was observed: clopidogrel pretreatment < 6 hours before PCI had no effect, but treatment 6 to 12 hours and 12 to 24 hours before PCI resulted in 35.5% and 40.1% relative reductions in the combined final outcome, respectively. These findings support the hypothesis that clopidogrel requires more time to act when used as a pretreatment.156156. Steinhubl SR, Berger PB, Mann JT 3rd, Fry ET, Delago A, Wilmer CH, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288(19):2411-20.
The ACCOAST clinical trial (n = 4,033) was a randomized, multicenter controlled study that compared administration of 60 mg of prasugrel in the catheterization room at the time of PCI versuspretreatment with 30 mg of prasugrel and a second 30 mg dose during coronary angiography within 2 to 48 hours after randomization.157157. Montalescot G, Bolognese L, Dudek D, Goldstein P, Hamm C, Tanguay J-F, ten Berg JM, et al. Pretreatment with prasugrel in non–ST-segment elevation acute coronary syndromes. N Engl J Med. 369(11):999–1010. The primary composite endpoint was death from cardiovascular causes, AMI, stroke, urgent revascularization, or rescue therapy with GP IIb/IIIa inhibitors by the seventh day. PCI was performed in 69% of the patients, a mean of 4.3 hours after the initial loading dose. Only 9% of the patients had a GRACE score > 140. The incidence of the primary endpoint at seven at 30 days did not differ significantly between the pretreatment and placebo groups (10.0% vs. 9.8%, hazard ratio [HR] 1.02, 95% CI 0.84-1.25, p = 0.81). A higher rate of major bleeding (related or not to CABG) was found in the pretreatment group (HR, 1.90; 95% CI, 1.19-3.02; p = 0.006). The rates of major bleeding (TIMI) or life-threatening bleeding unrelated to CABG were 3 and 6 times higher in the pretreatment and placebo groups, respectively. Half of all episodes were related to urgent revascularization surgery, and half of the major bleeding events unrelated to CABG were caused by vascular bleeding at the access site.
The DUBIUS randomized clinical trial evaluated 1,449 NSTE-ACS patients whose coronary angiography was scheduled within 72 hours, comparing pretreatment with ticagrelor versus. a second antiplatelet agent, either prasugrel or ticagrelor (randomization 1:1), administered in the hemodynamics unit in patients indicated for PCI.158158. Tarantini G, Mojoli M, Varbella F,Caporale R, Riggatieri S, Condo G, et al. Timing of Oral P2Y12 Inhibitor Administration in Non-ST Elevation Acute Coronary Syndrome. J Am Coll Cardiol. 2020;S0735-1097(20)36444-5. The population had a moderate ischemic risk profile (mean GRACE score = 122) and did not have a high hemorrhagic risk (mean CRUSADE score = 22). Pre-specified interim futility analysis guided early interruption of the study. After 30 days, the primary outcome, i.e., death due to vascular causes, non-fatal infarction or non-fatal stroke, and hemorrhagic phenomena (Bleeding Academic Research Consortium [BARC] types 3, 4, and 5), did not differ significantly between the groups (2.9% and 3.3%, respectively, for no pretreatment vs. pretreatment). This finding was consistent in the subgroup that underwent PCI (72% of the population); the meantime until PCI was 23.3 hours, which was notably longer than the ACCOAST study (4 hours). The findings, however, were similar to those who underwent coronary angiography after 24 hours. An original strategy of this study was that the non-pretreatment group still received ticagrelor, although a drug comparison could not be performed. Despite the analysis limitations due to early interruption, pretreatment in NSTE-ACS with invasive handling showed no benefits, although the results indicate that an individualized choice of drugs is possible in the hemodynamics unit.
6.2.5.1.3. Pretreatment Data in Meta-analysis
A meta-analysis that included 16 studies, including 61,517 patients with ACS with or without ST-segment elevation, found a significantly lower incidence of major adverse cardiac events in the subgroup with NSTE-ACS allocated to pretreatment with clopidogrel (OR = 0.83; 95% CI 0.71- 0.96, p = 0.01), and no increase in major bleeding in patients who underwent PCI within 48 hours.159159. Nairooz R, Valgimigli M, Rochlani Y, Pothineni NV, Raina S, Sardar P, et al. Meta-analysis of clopidogrel pretreatment in acute coronary syndrome patients undergoing invasive strategy. Int J Cardiol. 2017 Feb 15;229:82-9.
Another meta-analysis of 23 studies and 60,907 patients aimed to compare the efficacy and safety of P2Y12 inhibitors (clopidogrel, ticagrelor, and prasugrel) administered at two different periods compared to PCI: early (< 2 hours pre-PCI) vs. late (> 2 hours pre-PCI or post-PCI). In studies that included patients with NSTE-ACS pretreatment with ticagrelor or prasugrel showed no benefit, but prasugrel increased the risk of bleeding in this population.160160. Komosa A, Lesiak M, Krasiński Z, Grygier M, Siniawski A, Skorupski W, et al. Optimal Timing of P2Y12 Inhibitor Loading in Patients Undergoing PCI: A Meta-Analysis. Thromb Haemost. 2019 Jun;119(6):1000-20.
As previously explained, the routine use of a second oral antiplatelet agent in the initial treatment of NSTE-ACS has shown no clear benefits, especially for invasive strategies in emergencies, where the drug can be determined in the catheterization room. However, pretreatment can be considered in patients without a critical hemorrhagic risk, with moderate or high ischemic risk, when a coronary angiography has been scheduled, or during initial conservative treatment in full anti-ischemic therapy.
The challenge of balancing ischemic and bleeding risks requires a personalized approach to the use and type of second antiplatelet. The patient's clinical characteristics, the availability of coronary angiography, efficacy and safety data, and the pharmacodynamic properties of P2Y12 inhibitors available in Brazil must be considered. However, it should be pointed out that prasugrel is contraindicated for pretreatment when the coronary anatomy has not yet been determined. Although any P2Y12 inhibitor can be used in the catheterization room, in the absence of contraindications, prasugrel or ticagrelor are preferred due to their rapid onset and greater antithrombotic efficacy. Clopidogrel can be used when there is a very high hemorrhagic risk (Figure 1.6).
Initial antithrombotic therapy in non-ST-elevation acute coronary syndromes. CC: cardiac catheterization; HU: hemodynamics unit; SC: subcutaneously; UFH: unfractionated heparina.
6.2.5.2. Glycoprotein IIb/IIIa Inhibitors
Activation of platelet surface receptors, called GP IIb/IIIa, is the final mechanism of platelet aggregation. It results from a morphological alteration in the receptor, which increases its affinity to bind to fibrinogen, a protein that acts as a bridge between two platelets. These drugs have been used in clinical situations that have great potential for platelet activation, such as complex PCIs and thrombotic complications, e.g., no-reflow after angioplasty.
Abciximab is a monoclonal antibody that acts as a non-competitive and irreversible GP IIb/IIIa inhibitor. It has a short plasma half-life of 5 to 10 minutes, and its biological half-life is 6 to 12 hours after injection of an isolated bolus. These therapeutic doses block 80% to 90% of surface receptors. Fifty percent of these receptors still remain blocked one week after use. The recommended dose is 0.25 mg/kg in a bolus, followed by 0. 25µg/kg for 12 hours.
Tirofiban, a synthetic derivative, is a non-peptide, small molecule GP IIb/IIIa inhibitor whose molecular structure includes the RGD sequence (arginine-glycine-aspartate), an integrin recognition site present in adhesive proteins of fibrinogen, von Willebrand factor, vetronectin, etc. Tirofiban also acts competitively at GP IIb/IIIa receptors, preventing them from binding to fibrinogen. The recommended dose is 0.4 µg/kg/min for 30 min, followed by a maintenance dose of 0.11 µg/kg/min for 12 to 24 hours after the procedure (maximum 96 hours). If the first administration is in the catheterization room, it should begin at a dose of 1010 µg/kg, administered in a bolus in 3 minutes, followed by 0.15 µg/kg/min.
The efficacy of abciximab and tirofiban is comparable when a large bolus of tirofiban (25 µg/kg) is injected. However, abciximab is superior to tirofiban when used with a normal bolus of 10g/kg.161161. Topol EJ, Moliterno DJ, Herrmann HC, Powers ER, Grines CL, Cohen DJ, et al. the TARGET Investigators. Comparison of two platelet glycoprotein IIb/IIIa inhibitors, tirofiban and abciximab, for the prevention of ischemic events with percutaneous coronary revascularization. N Engl J Med. 2001;344(25):1888–94.,162162. Valgimigli M, Biondi-Zoccai G, Tebaldi M, van't Hof AWJ, Campo G, Hamm C, et al.T irofiban as adjunctive therapy for acute coronary syndromes and percutaneous coronary intervention: a meta-analysis of randomized trials. Eur Heart J. 2010;31(1):35-49.
Generally speaking, GP IIb/IIIa inhibitors tend to increase bleeding risk, and thrombocytopenia, although rare, is a non-negligible complication.
In patients with NSTE-ACS who undergo essentially "conservative" strategies (i.e., without early intervention), the use of GP IIb/IIa inhibitors is based on studies in which, in addition to heparinization, platelet activation was systematically antagonized only by ASA.163163. Cannon CP, Weintraub WS, Demopoulos LA, Vicari R, Frey MJ, Lakkis N, et al. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med 2001 Jun 21;344(25):1879-87.
164. Novel dosing regimen of eptifibatide in planned coronary stent implantation (ESPRIT): a randomised, placebo-controlled trial. Lancet 2000 Dec 16;356(9247):2037-44.
165. Platelet Receptor Inhibition in Ischemic Syndrome Management (PRISM) Study Investigators. A comparison of aspirin plus tirofiban with aspirin plus heparin for unstable angina. N Engl J Med. 1998 May 21;338(21):1498-505.
166. International, randomized, controlled trial of lamifiban (a platelet glycoprotein IIb/IIIa inhibitor), heparin, or both in unstable angina. The PARAGON Investigators. Platelet IIb/IIIa Antagonism for the Reduction of Acute coronary syndrome events in a Global Organization Network. Circulation. 1998 Jun 23;97(24):2386-95.
167. Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy (PURSUIT) Trial Investigators. Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. N Engl J Med. 1998 Aug 13;339(7):436-43.
168. Mukherjee D, Mahaffey KW, Moliterno DJ, Harrington RA, Yadav JS, Pieper KS, et al. Promise of combined low-molecular-weight heparin and platelet glycoprotein IIb/IIIa inhibition: results from Platelet IIb/IIIa Antagonist for the Reduction of Acute coronary syndrome events in a Global Organization Network B (PARAGON B). Am Heart J. 2002 Dec;144(6):995-1002.
169. Simoons ML. Effect of glycoprotein IIb/IIIa receptor blocker abciximab on outcome in patients with acute coronary syndromes without early coronary revascularisation: the GUSTO IV-ACS randomised trial. Lancet. 2001 Jun 16;357(9272):1915-24.-170170. Boersma E, Harrington RA, Moliterno DJ, White H, Theroux P, Van de WF, et al. Platelet glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: a meta-analysis of all major randomised clinical trials. Lancet. 2002 Jan 19;359(9302):189-98. A meta-analysis of > 30,000 patients found a 9% reduction in the relative risk of death or infarction at 30 days of follow-up (p = 0.015) with beta-blockers,169169. Simoons ML. Effect of glycoprotein IIb/IIIa receptor blocker abciximab on outcome in patients with acute coronary syndromes without early coronary revascularisation: the GUSTO IV-ACS randomised trial. Lancet. 2001 Jun 16;357(9272):1915-24. although the benefits were restricted to higher-risk patients, mainly with elevated troponin and/or depressed ST-segment and/or undergoing PCI (24% at 30 days of follow-up).
Several studies have tested the role of GP IIb/IIIa inhibitors in patients undergoing PCI.171171. EPIC Investigators. Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. N Engl J Med. 1994 Apr 7;330(14):956-61.
172. Randomised placebo-controlled trial of abciximab before and during coronary intervention in refractory unstable angina: the CAPTURE Study. Lancet. 1997 May 17;349(9063):1429-35. Erratum in: Lancet 1997 Sep 6;350(9079):744.
173. Platelet glycoprotein IIb/IIIa receptor blockade and low-dose heparin during percutaneous coronary revascularization. The EPILOG Investigators. N Engl J Med. 1997 Jun 12;336(24):1689-96.
174. EPISTENT Investigators. Randomised placebo-controlled and balloon-angioplasty-controlled trial to assess safety of coronary stenting with use of platelet glycoprotein-IIb/IIIa blockade. Lancet. 1998 Jul 11;352(9122):87-92.
175. Randomised placebo-controlled trial of effect of eptifibatide on complications of percutaneous coronary intervention: IMPACT-II. Integrilin to Minimise Platelet Aggregation and Coronary Thrombosis-II. Lancet. 1997 May 17;349(9063):1422-8.
176. Effects of platelet glycoprotein IIb/IIIa blockade with tirofiban on adverse cardiac events in patients with unstable angina or acute myocardial infarction undergoing coronary angioplasty. The RESTORE Investigators. Randomized Efficacy Study of Tirofiban for Outcomes and REstenosis. Circulation. 1997 Sep 2;96(5):1445-53.-177177. O'shea JC, Hafley GE, Greenberg S, Hasselblad V, Lorenz TJ, Kitt MM, et al. Platelet glycoprotein IIb/IIIa integrin blockade with eptifibatide in coronary stent intervention: the ESPRIT trial: a randomized controlled trial. JAMA. 2001 May 16;285(19):2468-73. The results of these studies are more homogeneous, invariably demonstrating benefits, although there is a significant increase in bleeding. It should be noted that these studies did not involve routine use of thienopyridine upon patient arrival at the hospital. A meta-analysis of studies that analyzed the role of GP IIb/IIIa inhibitors in ACS patients with or without ST elevation found, in addition to significant decreases in death and (re) infarction (p <0.0001), a 21% decrease (95% CI: 0.64-0.67) in the relative risk of death at six months of follow-up.178178. Karvouni E, Katritsis DG, Ioannidis JP. Intravenous glycoprotein IIb/IIIa receptor antagonists reduce mortality after percutaneous coronary interventions. J Am Coll Cardiol. 2003 Jan 1;41(1):26-32.
The ISAR-REACT-2 trial involved 2,022 patients with NSTE-ACS who received dual antiplatelet therapy (DAPT) with ASA + clopidogrel, adding abciximab was compared to placebo. Abciximab was associated with a lower incidence of the composite outcome, including death, AMI, and urgent revascularization (risk ratio [RR] 0.75; CI 0.58-0.97; p = 0.03), and no significant increase in serious or non-serious hemorrhagic outcomes. It should be pointed out that abciximab only benefitted individuals with troponin elevation.179179. Kastrati A, Mehilli J, Neumann FJ, Dotzer F, Ten BJ, Bollwein H, et al. Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: the ISAR-REACT 2 randomized trial. JAMA. 2006 Apr 5;295(13):1531-8.
The ACUITY-Timing trial included 9207 patients with NSTE-ACS (98% with pre-angiography ASA and 65% with pre-angiography P2Y12 inhibitor) and investigated the best time to administer GP IIb/IIIa inhibitors, randomizing patients to the routine early tirofiban or eptifibatide in the emergency room before coronariography versus the selective use at the cath lab whenPCI is about to be performed. It was found that the routine use of GP IIb/IIIa inhibitors did not reduce ischemic outcomes, and the incidence of significant bleeding increased significantly (RR = 0.80, p < 0.001).180180. Stone GW, Bertrand ME, Moses JW, Ohman EM, Lincoff AM, Ware JH, et al. Routine upstream initiation vs deferred selective use of glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: the ACUITY Timing trial. JAMA. 2007 Feb 14;297(6):591-602.
Similar results were found in the EARLY-ACS study, in which 9,492 patients with NSTE-ACS were randomized to platelet anti-aggregation with eptifibatide before catheterization vs. selected use before angioplasty (based on angiographic aspects). Again, the routine use of GP IIb/IIIa inhibitors did not reduce ischemic complications and increased the incidence of bleeding, as well as the need for transfusions.181181. Giugliano RP, White JA, Bode C, Armstrong Pw, Montalescot G, Lewis BS, et al. et al. Early versus delayed, provisional eptifibatide in acute coronary syndromes. N Engl J Med 2009;360(21):2176 –90.
Based on this body of evidence, the use of GP IIb/IIIa inhibitors as a third antiplatelet agent should be reserved for patients who do not have a high risk of hemorrhaging but are at high ischemic/thrombotic risk according to clinical and angiographic criteria.
6.2.5.3. Anticoagulants
Anticoagulant therapy should be administered as quickly as possible to all patients with NSTE-ACS since the use of these compounds reduces the incidence of AMI and death when used within hours of diagnosis.182182. Eikelboom JW, Anand SS, Malmberg K, Weitz JI, Ginsberg JS, Yusuf S. Unfractionated heparin and low-molecular-weight heparin in acute coronary syndrome without ST elevation: a meta-analysis. Lancet. 2000 Jun 3;355(9219):1936-42.
The choice and timing of the anticoagulant are determined by the treatment strategy (invasive or conservative), the severity of the clinical presentation, and the particularities of each hospital department. In patients with an initial conservative treatment, enoxaparin or fondaparinux is preferential. The recommended doses are shown in Table 1.9. Enoxaparin should not be administered to patients with creatinine clearance < 15 mL/min, and fondaparinux should not be administered to patients with creatinine clearance < 20 mL/min. For patients whose clearance is between 15 and 30 mL/min and in obese patients (BMI > 30 kg/m² or weight > 100 kg), the enoxaparin usage guidelines recommended anti-factor Xa monitoring to minimize the risk of excessive doses and severe bleeding.183183. Spinler SA, Mahaffey KW, Gallup D, Levine GM, Ferguson JJ, Rao SV,et al. Relationship between renal function and outcomes in high-risk patients with non-ST-segment elevation acute coronary syndromes: results from SYNERGY. Int J Cardiol. 2010;144(1):36-41.
184. Bazinet A, Almanric K, Brunet C,Martineau J, Turcotte I, Caron S, et al. Dosage of enoxaparin among obese and renal impairment patients. Thromb Res. 2005;116(1):41-50.-185185. Garcia DA, Baglin TP, Weitz JI, Samama MM. Parenteral anticoagulants: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 May;141(2 Suppl):e24S-e43S.
In morbidly obese patients, pharmacodynamic studies and observational data identified a frequent need for dose adjustment according to anti-factor Xa level to achieve the therapeutic goal (BMI > 40 kg/m²), which suggests the usefulness of monitoring when available.186186. Lalama JT, Feeney ME, Vandiver JW, Beavers KD, Walter LN, McClintic JR. Assessing an enoxaparin dosing protocol in morbidly obese patients. J Thromb Thrombolysis. 2015;39(4):516-21.,187187. Lee YR, Palmere PJ, Burton CE, Benavides TM. Stratifying Therapeutic Enoxaparin Dose in Morbidly Obese Patients by BMI Class: A Retrospective Cohort Study. Clin Drug Investig. 2020;40(1):33-40.
However, in the CRUSADE registry, which assessed obese patients with ACS, a standard dose of enoxaparin (1 mg/kg/weight) in patients > 150 kg was associated with a greater risk of bleeding than in a subgroup of patients between 120 and 150 kg. (11.4% vs. 5.6%, p < 0.001). UFH is an alternative for these patients.188188. Spinler SA, Ou FS, Roe MT, Gibler WB, Ohman EM, Pollack CV,et al. Weight-based dosing of enoxaparin in obese patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE initiative. Pharmacotherapy. 2009 Jun;29(6):631-8.,189189. Chua D, Tataru A. Enoxaparin Dosing for Acute Coronary Syndromes in Obese Patients-Should There be a Maximum Dose. J Cardio Cardiovasc Med. 2016;1:002.
Enoxaparin, fondaparinux, and UFH are options for invasive strategy. Despite the fact that enoxaparin and UFH had similar efficacy in the SINERGY study, for very high-risk patients who require immediate catheterization, using UFH in the emergency department or, preferably, in the catheterization room can prevent crossing over from enoxaparin to UFH and the greater hemorrhagic risk that results from this practice.190190. White HD, Kleiman NS, Mahaffey KW, Lokhnygina Y, Pieper K, Cheswell K. et al. Efficacy and safety of enoxaparin compared with unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention in the Superior Yield of the New Strategy of Enoxaparin, Revascularization and Glycoprotein IIb/IIIa Inhibitors (SYNERGY) trial [published correction appears in Am Heart J. 2007 Feb;153(2):327]. Am Heart J. 2006;152(6):1042-50.
For other patients, the choice should be guided by the patient's ischemic and hemorrhagic risk and the department's own experience.
Enoxaparin is preferred to UFH because systematic review data from randomized trials across the ACS spectrum show that enoxaparin is more effective than UFH at preventing the composite outcome of all-cause mortality or severe bleeding in patients with NSTE-ACS who undergo PCI.191191. Silvain J, Beygui F, Barthelemy O, Pollack C, Jr., Cohen M, Zeymer U, et al. Efficacy and safety of enoxaparin versus unfractionated heparin during percutaneous coronary intervention: systematic review and meta-analysis. BMJ. 2012;344:e553.
192. Petersen JL, Mahaffey KW, Hasselblad V, Antman EM, Cohen M, Goodman SG, et al. Efficacy and bleeding complications among patients randomized to enoxaparin or unfractionated heparin for antithrombin therapy in non-ST-segment elevation acute coronary syndromes: a systematic overview. JAMA. 2004; 292(1): 89–96.-193193. Califf RM, Petersen JL, Hasselblad V, Mahaffey KW, Ferguson JJ. A perspective on trials comparing enoxaparin and unfractionated heparin in the treatment of non-ST-elevation acute coronary syndromes. Am Heart J. 2005 Apr;149(4 Suppl):S91-9.
Fondaparinux is a safe option for non-invasive treatment. If catheterization is subsequently indicated, UFH is recommended during the procedure due to the risk of catheter thrombosis.194194. Yusuf S, Mehta SR, Chrolavicius S, Afzal R, Pogue J, Granger CB, et al. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med. 2006 Apr 6;354(14):1464-76.,195195. Karthikeyan G, Mehta SR, Eikelboom JW. Fondaparinux in the treatment of acute coronary syndromes: evidence from OASIS 5 and 6. Expert Rev Cardiovasc Ther. 2009 Mar;7(3):241-9.
Patients who have previously used direct-acting oral anticoagulants (DOAC) in ACS should be handled with caution to avoid increasing the risk of hemorrhage. There is no evidence that early parenteral anticoagulation or PCI can be performed. In urgent situations, PCI should be performed regardless of when the last dose of DOAC was administered. The procedure should be postponed in patients with lower ischemic risk. In patients with normal renal function (creatinine clearance > 50 mL/min), the effect of DOAC is reduced 24 hours after the last dose. In patients with renal dysfunction, this period is 48 hours. Thus, the patient can undergo PCI with less risk of bleeding. Parenteral anticoagulation is indicated in patients who undergo emergency PCI regardless of when the last dose of DOAC was administered.196196. Serrano Jr. CV, Soeiro AM, Leal TCAT, Godoy LC, Biselli B, Hata LA et al. Posicionamento sobre Antiagregantes Plaquetários e Anticoagulantes em Cardiologia – 2019. Arq Bras Cardiol. 2019; 113(1):111-34.
For hemodynamic/PCI examinations, it has been shown that the radial approach is the most effective strategy to reduce bleeding, resulting in reduced mortality in patients with ACS.197197. Mason PJ, Shah B, Tamis-Holland JE,Bitt JA, Cohen MG, Safirstein J, et al. An Update on Radial Artery Access and Best Practices for Transradial Coronary Angiography and Intervention in Acute Coronary Syndrome: A Scientific Statement From the American Heart Association. Circ Cardiovasc Interv. 2018;11(9):e000035. doi:10.1161/HCV.0000000000000035
https://doi.org/10.1161/HCV.000000000000...
Part 2 – Treatment During Hospitalization
1. Hospitalization and Coronary Care Unit Discharge
Patients with intermediate- and high-risk NSTE-ACS should remain hospitalized in a coronary care unit whenever possible until the definitive treatment is implemented. After the PCI is completed, the patient must return to the coronary care unit and remain for 12 to 24 hours if there are no complications. Post-PCI complications are serious outcomes, such as occlusion of the target vessel, the need for emergency revascularization surgery or a new unscheduled PCI, recurrent angina, and death. When a CABG procedure is indicated, patients should remain hospitalized (ICU or Coronary Unit) until surgery is performed. When intravenous drug treatment is indicated, patients should remain in the coronary care unit until they are stable and can be discharged and prescribed oral medications.
2. Nitrates
No controlled clinical trials have tested the effects of nitrates on clinical outcomes and mortality in UA, although their use is universally accepted; only small and observational studies have investigated them.128128. DePace NL, Herling IM, Kotler MN, Hakki AH, Spielman SR, Segal BL. Intravenous nitroglycerin for rest angina. Potential pathophysiologic mechanisms of action. Arch Intern Med. 1982 Oct;142(10):1806-9.
129. Kaplan K, Davison R, Parker M, Przybylek J, Teagarden JR, Lesch M. Intravenous nitroglycerin for the treatment of angina at rest unresponsive to standard nitrate therapy. Am J Cardiol.1983 Mar 1;51(5):694-8.-130130. Roubin GS, Harris PJ, Eckhardt I, Hensley W, Kelly DT. Intravenous nitroglycerine in refractory unstable angina pectoris. Aust N Z J Med. 1982 Dec;12(6):598-602.,198198. Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganisats TG, Holmes DR, et al. 2014 AHA/ACC Guideline for the management of patients with non-ST-elevation acute coronary syndromes. Circulation. 2014;130(25):e344-e426. Borzak et al. evaluated the effects of pre and in-hospital antianginal medication (calcium antagonists, beta-blockers, and nitrates) on clinical outcomes, mortality, and non-fatal myocardial infarction in 410 patients. No benefits were found. Multivariate analysis also showed no correlation with the incidence of death, nonfatal infarction, or recurrent angina.199199. Borzak S, Cannon C P, Kraft PL, Douthat L, Becker R C, Palmeri S T et al. Effects of Prior Aspirin and Anti-Ischemic Therapy on Outcome of Patients With Unstable Angina. Am J Cardiol.1998;81(6):678-81.
3. Beta-blockers
Clinical experience with beta-blockers is limited to small controlled clinical studies whose results are extrapolated to patients with UA and STE-ACS. Despite the lack of large-scale randomized studies, beta-blockers, such as nitrates, are widely used. Further details about this drug class are provided in Part 1.
4. Calcium Channel Antagonists
Calcium channel antagonists are a heterogeneous class of drugs, some of which act as negative regulators of chronotropism and coronary vasodilators. Their anti-ischemic action occurs by reducing calcium influx through the cell membrane, which reduces myocardial contractility, vascular tone, atrioventricular (AV) conduction, and sinus node activity.
These agents differ in terms of their ability to produce arterial vasodilation, reduce myocardial contractility, and delay AV conduction. Their beneficial effects in NSTE-ACS are due to a combination of decreased myocardial oxygen consumption, afterload, myocardial contractility, and heart rate, in addition to increased oxygen supply to the myocardium due to coronary vasodilation. Coronary vasodilation among different agents is similar. Dihydropyridines cause greater peripheral arterial vasodilation and tend to produce reflex tachycardia (more evident with short-acting nifedipine); verapamil and diltiazem tend to cause bradycardia by reducing chronotropism and dromotropism, which can lead to atrioventricular block (most evident with verapamil). These drugs should generally be avoided in patients with impaired LV function and/or changes in atrioventricular conduction. Water retention is another side effect that usually appears only after prolonged use.
Calcium channel antagonists are as effective as beta-blockers for controlling symptoms.200200. Theroux P, Taeymans Y, Morissette D, Bosch X, Pelletier GB, Waters DD. A randomized study comparing propranolol and diltiazem in the treatment of unstable angina. J Am Coll Cardiol. 1985;5(3):717-22.,201201. Parodi O, Simonetti I, Michelassi C, Carpeggiani C, Biagini A, L’Abbate A, et al. Comparison of verapamil and propranolol therapy for angina pectoris at rest: a randomized, multiple-crossover, controlled trial in the coronary care unit. Am J Cardiol. 1986;57(11):899-906. However, they do not reduce the incidence of refractory angina, infarction, or death; on the contrary, a meta-analysis suggests that they increase the incidence of these complications.202202. Held PH, Yusuf S, Furberg CD. Calcium channel blockers in acute myocardial infarction and unstable angina: an overview. BMJ. 1989;299(6709):1187-92. So far, only first-generation representatives have been assessed in UA. Although these deleterious results were observed in all tested classes of calcium antagonists 135135. Lubsen J, Tijssen JG. Efficacy of nifedipine and metoprolol in the early treatment of unstable angina in the coronary care unit: findings from the Holland Interuniversity Nifedipine/metoprolol Trial (HINT). Am J Cardiol. 1987 Jul 15;60(2):18A-25A.,136136. Yusuf S, Wittes J, Friedman L. Overview of results of randomized clinical trials in heart disease. II. Unstable angina, heart failure, primary prevention with aspirin, and risk factor modification. JAMA. 1988 Oct 21;260(15):2259-63.,203203. Smith NL Reiber GE, Psaty BM, Heckbert SR, Siscovick DS, Ritchie JL, et al. Health outcomes associated with beta-blocker and diltiazem treatment of unstable angina. J Am Coll Cardiol. 1998;32(5):1305-11., conclusive data on dihydropyridines do not exist. On the other hand, in patients with NSTEMI, there is evidence that diltiazem and verapamil (which are not associated with induced tachycardia) may have a protective effect.204204. Yusuf S, Held P, Furberg C. Update of effects of calcium antagonists in myocardial infarction or angina in light of the second Danish Verapamil Infarction Trial (DAVIT-II) and other recent studies. Am J Cardiol. 1991;67(15):1295-7.,205205. Boden WE, van Gilst WH, Scheldewaert RG, Starkey IR, Carlier MF, Julian DG, et al. Diltiazem in acute myocardial infarction treated with thrombolytic agents: a randomised placebo-controlled trial. Incomplete Infarction Trial of European Research Collaborators Evaluating Prognosis post-Thrombolysis (INTERCEPT). Lancet. 2000;355(9217):1751-6. These agents can be used to control refractory ischemic symptoms in patients already using nitrates and beta-blockers in appropriate doses or in patients who cannot tolerate these drugs (especially in cases of contraindication), or even in cases of variant angina of Prinzmetal. However, the routine use of calcium channel antagonists is not recommended, and fast-acting nifedipine is particularly contraindicated.
5. Antiplatelet Drugs
5.1. Acetylsalicylic acid
ASA is the antiplatelet drug of choice and should always be prescribed, except in cases of intolerance or adverse events.206206. De Luca G, Verdoia M, Binda G, Schaffer A, Suryapranata H, Marino P. Aspirin desensitization in patients under-going planned or urgent coronary stent implantation: a single-center experience. Int J Cardiol. 2013;167(2):561-3.
207. Rossini R, Angiolillo DJ, Musumeci G, Scuri P, Invernizzi P, Bass TA, et al. Aspirin desensitization in patients undergoing percutaneous coronary interventions with stent implantation. Am J Cardiol. 2008;101(6):786-9.
208. Page NA, Schroeder WS. Rapid desensitization protocols for patients with cardiovascular disease and aspirin hypersensitivity in an era of dual antiplatelet therapy. Ann Pharmacother. 2007;41(1):61-7.-209209. McMullan KL, Wedner HJ. Safety of aspirin desensitization in patients with reported aspirin allergy and cardiovascular disease. Clin Cardiol. 2013;36(1):25-30. Its importance in NSTE-ACS treatment is based on studies published in the 1980s,210210. Risk of myocardial infarction and death during treatment with low dose aspirin and intravenous heparin in men with unstable coronary artery disease. The RISC Group. Lancet. 1990;336(8719):827-30.,211211. Theroux P, Ouimet H, McCans J, Latour JG, Joly P, Levy G, et al. Aspirin, heparin, or both to treat acute unstable angina. N Engl J Med. 1988;319(17):1105-11. which, for the most part, had relatively small samples and a low outcome incidence. However, in general, they found that ASA clearly reduced non-fatal AMI and mortality in the short and medium terms.
The recommended dosage for ASA is 150 to 300 mg for the loading dose, followed by 75 to 100 mg daily for the maintenance dose. In one of its arms, the CURRENT OASIS-7 trial tested a high maintenance dose of ASA in patients with ACS (about 70% of patients without ST elevation). There was no difference between the normal (75 to 100 mg per day) and high maintenance doses (300 to 325 mg/day) for serious cardiovascular events (mortality, non-fatal AMI, or stroke, p = 0.61). There was also no difference regarding the occurrence of severe bleeding (p = 0.90).212212. Mehta SR, Tanguay JF, Eikelboom JW, JollySS, J oyner CD, Granger CB, et al; CURRENT-OASIS 7 trial investigators. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376(9748):1233-43.
5.2. P2Y12 Inhibitors
Thienopyridine derivatives (clopidogrel and prasugrel) are platelet-activating antagonistic drugs mediated by adenosine diphosphate (ADP). They irreversibly inhibit P2Y12 platelet receptors, reduce the level of circulating fibrinogen, and partially block GP IIb/IIIa receptors, impeding binding to fibrinogen and von Willebrand factor. In addition to ticagrelor (derived from cyclopentyltriazolopyrimidine, a non-thienopyridine agent), a reversible inhibitor of ADP-induced platelet aggregation, these are the three platelet receptor P2Y12 inhibitors currently available in Brazil and should preferably be used in association with ASA.
5.2.1. Clopidogrel
CURE, the first large clinical trial to test the role of clopidogrel in addition to ASA in NSTE-ACS, followed more than 12,000 patients for 3 to 12 months (average of 9 months). At the end of follow-up, there was a 20% reduction (RR 0.80; 95% CI 0.72-0.89; p = 0.00005) in the incidence of events (cardiovascular death, AMI or stroke) in the clopidogrel group + ASA vs. the placebo + ASA group, at the expense of increased bleeding incidence in the clopidogrel + ASA group (RR 1.38, p = 0.001).213213. Yusuf S, Mehta SR, Zhao F, Gersh BJ, Commerford PJ, Blumenthal M, et al; Clopidogrel in Unstable angina to prevent Recurrent Events Trial Investigators. Early and late effects of clopidogrel in patients with acute coronary syndromes. Circulation. 2003;107(7):966-72. The beneficial effects of DAPT occurred in patients of all risk levels. Other CURE analyses demonstrated that DAPT would benefit both surgical myocardial revascularization patients and patients receiving isolated drug treatment.214214. Peters RJ, Mehta SR, Fox KA, Zhao F, Lewis BS, Kopecky SL, et al; Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) Trial Investigators. Effects of aspirin dose when used alone or in combination with clopidogrel in patients with acute coronary syndromes: observations from the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) study. Circulation. 2003;108(14):1682-7.
Based on ACS studies and a study that focused primarily on PCI215215. Leon MB, Baim DS, Popma JJ, Gordon PC, Cutlip DE, Ho KK, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med.1998;339(23):1665-71., DAPT becomes imperative when NSTE-ACS patients are treated with PCI. In the context of ACS, the CURE sub-study (PCI-CURE),151151. Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358(9281):527-33. , and the CREDO study have been developed.156156. Steinhubl SR, Berger PB, Mann JT 3rd, Fry ET, Delago A, Wilmer CH, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288(19):2411-20.
Due to clopidogrel's limitations regarding metabolism and drug interactions, a double dose was also tested in the CURRENT OASIS-7 trial.216216. Mehta SR, Bassand JP, Chrolavicius S, Diaz R, Eikelboom JW, Fox KA, et al; CURRENT-OASIS 7 Investigators. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med. 2010;363(10):930-42. Erratum in: N Engl J Med. 2010;363(16):1585. In this study, 25,086 invasive strategy ACS patients (71% without ST elevation) were randomized to receive a double dose of clopidogrel (600 mg loading dose followed by 150 mg daily for 6 days and 75 mg daily after the first week) or the usual dosage (300 mg loading dose followed by 75 mg daily). At the end of 30 days, there was no significant difference in the primary outcome (cardiovascular death, non-fatal AMI, or stroke) between groups (HR 0.94; 4.2% vs. 4.4%, p = 0.30). However, the double dose was associated with a significant increase in major bleeding (HR 1.24; 2.5% vs. 2%, p = 0.01). In a pre-specified analysis,212212. Mehta SR, Tanguay JF, Eikelboom JW, JollySS, J oyner CD, Granger CB, et al; CURRENT-OASIS 7 trial investigators. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376(9748):1233-43. considering only patients treated with PCI (n = 17,263), there was a 14% reduction in the primary composite outcome at 30 days (HR 0.86; 3.9% vs. 4.5 %, p = 0.039, number needed to treat = 167), in addition to a significant reduction in definite stent thrombosis (HR 0.54; 0.7% vs. 1.3%, p = 0.0001), although there was a higher incidence of severe bleeding (HR 1.41; 1.6% vs. 1.1%, p = 0.009, number needed to harm = 200). Considering the high number needed to treat, a cautious assessment of the patient's prior bleeding risk is recommended when determining the dose.
Patients who do not reach the expected level of platelet inhibition with clopidogrel are referred to as having a “poor response” (or “resistance”). This is identified byin vitro laboratory tests that quantify the intensity of platelet aggregation mediated by ADP. Poor clopidogrel response has been consistently associated with a higher incidence of thrombotic events, especially in patients undergoing PCI with stenting.217217. Breet NJ, van Werkum JW, Bouman HJ, Kelder JC, Ruven HJ, Bal ET, et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA. 2010;303(8):754-62. Erratum in: JAMA. 2010;303(13):1257. JAMA. 2011;305(21):2174. JAMA. 2011;305(21):2172-3.
218. Buonamici P, Marcucci R, Migliorini A, Gensini GF, Santini A, Paniccia R, et al. Impact of platelet reactivity after clopidogrel administration on drugeluting stent thrombosis. J Am Coll Cardiol. 2007; 49(24): 2312-7.-219219. Price MJ, Endemann S, Gollapudi RR, Valencia R, Stinis CT, Levisay JP, et al. Prognostic significance of postclopidogrel platelet reactivity assessed by a point-of-care assay on thrombotic events after drug-eluting stent implantation. Eur Heart J. 2008;29(8):992-1000. Currently, three main factors are related to poor clopidogrel response: 1) genetic variability, characterized by polymorphisms associated with cytochrome P450 enzymes involved in the hepatic metabolization process, notably CYP2C19;220220. Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a metaanalysis. JAMA. 2010;304(16):1821-30. 2) changes in intestinal absorption related to P-GP expression in intestinal epithelial cells;221221. Taubert D, von Beckerath N, Grimberg G, Lazar A, Jung N, Goeser T, et al. Impact of P-glycoprotein on clopidogrel absorption. Clin PharmacolTher. 2006;80(5):486-501. 3) concomitant use of other drugs that could interfere with liver metabolism mediated by cytochrome P450 enzymes, such as ketoconazole (which inhibits cytochrome P450 and reduces the action of clopidogrel) and rifampicin (which stimulates cytochrome P450 and enhances the action of clopidogrel).
Several in vitro studies have indicated that platelet inhibition is reduced when proton pump inhibitors are associated with clopidogrel, which could be especially frequent with omeprazole.222222. Angiolillo DJ, Gibson CM, Cheng S, Ollier C, Nicolas O, Bergougnan L, et al. Differential effects of omeprazole and pantoprazole on the pharmacodynamics and pharmacokinetics of clopidogrel in healthy subjects: randomized, placebo-controlled, crossover comparison studies. Clin PharmacolTher. 2011;89(1): 65-74.
223. Cuisset T, Frere C, Quilici J, Poyet R, Gaborit B, Bali L, et al. Comparison of omeprazole and pantoprazole influence on a high 150-mg clopidogrel maintenance dose the PACA (Proton Pump Inhibitors And Clopidogrel Association) prospective randomized study. J Am Coll Cardiol. 2009;54(13):1149-53.
224. Frelinger AL 3rd, Lee RD, Mulford DJ, Wu J, Nudurupati S, Nigam A, et al. A randomized, 2-period, crossover design study to assess the effects of dexlansoprazole, lansoprazole, esomeprazole, and omeprazole on the steady-state pharmacokinetics and pharmacodynamics of clopidogrel in healthy volunteers. J Am Coll Cardiol. 2012;59(14):1304-11. Erratum in J Am Coll Cardiol. 2012;60(6):566-7.-225225. Gilard M, Arnaud B, Cornily JC, Le Gal G, Lacut K, Le Calvez G, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (OmeprazoleCLopidogrel Aspirin) study. J Am Coll Cardiol. 2008;51(3):256-60. However, studies analyzing ischemic events showed conflicting results,226226. Ho PM, Maddox TM, Wang L, Fihn SD, Jesse RL, Peterson ED, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009;301(9):937-44.
227. Juurlink DN, Gomes T, Ko DT, Szmitko PE, Austin PC, Tu JV, et al. A population-based study of the drug interaction between proton pumpinhibitors and clopidogrel. CMAJ. 2009;180(7):713-8.-228228. Nicolau JC, Bhatt DL, Roe MT, Lokhnygina Y, Neely B, Corbalán R, et al. TRILOGY ACS investigators. Concomitant proton-pump inhibitor use, platelet activity, and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel and managed without revascularization: insights from the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes trial. Am Heart J. 2015;170(4):683-694., as well as an association between increased ischemic events and the use of clopidogrel + proton pump inhibitors. A subanalysis of the TRITON trial found no association,229229. O’Donoghue ML, Braunwald E, Antman EM, Murphy SA, Bates ER, Rozenman Y, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: na analysis of two randomised trials. Lancet. 2009;374(9694):989-97., and a similar subanalysis of the PLATO trial230230. Goodman SG, Clare R, Pieper KS, Nicolau JC, Storey RF, Cantor WJ, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation. 2012;125(8):978-86. found a greater incidence of ischemic events in both the clopidogrel and ticagrelor groups when taken with proton pump inhibitors. More recently, the TRILOGY trial found that among ACS patients who did not undergo myocardial revascularization, proton pump inhibitors did not affect the antiplatelet response in the prasugrel or clopidogrel groups although they were associated with a lower incidence of infarction in the prasugrel group.228228. Nicolau JC, Bhatt DL, Roe MT, Lokhnygina Y, Neely B, Corbalán R, et al. TRILOGY ACS investigators. Concomitant proton-pump inhibitor use, platelet activity, and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel and managed without revascularization: insights from the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes trial. Am Heart J. 2015;170(4):683-694.
COGENT, the only randomized clinical trial that has directly tested such a hypothesis,231231. Bhatt DL, Cryer BL, Contant CF, Cohen M, Lanas A, Schnitzer TJ, et al; COGENT Investigators. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363(20):1909-17. evaluated 3761 patients indicated for DAPT for at least 12 months (clopidogrel + omeprazole group vs. a clopidogrel + placebo group). However, this study was stopped early due to funding issues and an insufficient number of patients for follow-up (i.e., a very small number of events were obtained), which significantly compromised its statistical power. In any case, until the study was interrupted, there was no significant difference in the incidence of ischemic events (4.9% in the clopidogrel + omeprazole group vs. 5.7% in the clopidogrel + placebo group, p = 0.96). As expected, a higher incidence of digestive bleeding was observed in the placebo group (2.9% vs. 1.1%, p < 0.001). Thus, considerable research suggests that, in principle, using PPIs (mainly omeprazole) in conjunction with clopidogrel should be avoided. Patients at increased risk of gastrointestinal bleeding (history of gastrointestinal bleeding, diagnosed peptic ulcer, Helicobacter pylori infection, age ≥ 65 years, and concomitant use of anticoagulants or steroids) may receive H2-receptor blockers (e.g., ranitidine). If a PPI is necessary, pantoprazole is suggested, whose metabolism via cytochrome P450 is less pronounced.
Therefore, clopidogrel is indicated in NSTE-ACS when there is a moderate to high risk of new ischemic events. Administration consists of a loading dose of 300 mg and a maintenance dose of 75 mg daily. In patients undergoing PCI and with a low risk of bleeding, a loading dose of 600 mg, with a maintenance dose of 150 mg in the first 7 days and 75 mg/day afterward, can be considered. The drug must be used for 12 months, regardless of the treatment received (clinical, percutaneous, or surgical). When elective surgical revascularization is indicated, clopidogrel should be discontinued at least 5 days before the procedure due to the risk of bleeding. Since there is no evidence that adjusting antiplatelet therapy by assessing platelet aggregability is superior to standard antiplatelet therapy, this strategy should only be used in special situations.232232. Price MJ, Berger PB, Teirstein PS, Tanguay JF, Angiolillo DJ, Spriggs D, et al; GRAVITAS Investigators. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305(11):1097-105. Erratum in JAMA. 2011 Jun 1;305(21);2174.-233233. Collet JP, Cuisset T, Rangé G, Cayla G, Elhadad S, Pouillot C, et al; ARCTICInvestigators. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med. 2012;367(22):2100-9. Due to clopidogrel's limitations, new drugs have been developed to obtain faster, more effective, andd more consistent platelet aggregation inhibition.
5.2.2. Prasugrel
Prasugrel is a newer generation thienopyridine that fulfills these objectives, due basically to the fact that its metabolism is simpler than clopidogrel, involving only one phase of hepatic metabolism. As a consequence, its active metabolite reaches the plasma peak in just 30 minutes, in addition to having less interaction with medications metabolized by cytochrome P450.234234. Jakubowski JA, Winters KJ, Naganuma H, Wallentin L. Prasugrel: a novel thienopyridine antiplatelet agent: a review of preclinical and clinical studies and the mechanistic basis for its distinct antiplatelet profile. Cardiovasc Drug Rev. 2007;25(4):357-74.
The TRITON trial included 13,608 ACS patients who had not recently used thienopyridine and had defined coronary anatomy (in patients with NSTE-ACS) and scheduled PCI. Of these patients, 74% presented with NSTE-ACS and a moderate or high risk of ischemic/thrombotic complications. Patients who were thrombocytopenic, anemic, or at high risk of bleeding were excluded. The patients were randomized to clopidogrel (300 mg loading and 75 mg daily maintenance) or prasugrel (60 mg loading and 10 mg daily maintenance) after coronary angiography and PCI indication, with a mean follow-up of 14.5 months. The primary efficacy endpoint, cardiovascular death, (re)infarction, and stroke, was 19% lower in the prasugrel group (RR 0.81; 12.1% vs. 9.9%, p < 0.001) than the clopidogrel group. Regarding secondary efficacy outcomes, the prasugrel group had a 24% reduction in AMI (RR 0.76; 9.5% vs. 7.3%, p < 0.001), a 34% reduction in urgent revascularization (RR 0.76; 3.7% vs. 2.5%, p < 0.001) and a 52% reduction in stent thrombosis(RR 0.48; 2.4% vs. 1.1%, p < 0.001).
In the prasugrel group, the safety outcome analysis showed a 32% increase in the incidence of major bleeding (according to the TIMI criterion) unrelated to revascularization surgery (RR 1.32; 1.8% vs. 2.4%, p = 0.03) and a 52% increase in life-threatening bleeding (RR 1.52; 0.9% vs. 1.4%, p = 0.01), in addition to a significant increase in fatal bleeding (RR 4.1; 0.1% vs. 0.4%, p < 0.002).
Overall, prasugrel had lower net benefits than clopidogrel in individuals with previous stroke or TIA (RR 1.54, p = 0.04), was neutral in individuals > 75 years of age or weighing < 60, kg and had higher net benefits in individuals < 75 years of age and weighing > 60 kg without previous stroke or TIA, (RR 0.8; p < 0.001).154154. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007; 357(20): 2001-15.
Applying the new AMI classification to the TRITON-TIMI results, Morrow et al. demonstrated that prasugrel was more effective than clopidogrel for reducing various types of infarction.235235. Morrow DA, Wiviott SD, White HD, Nicolau JC, Bramucci E, Murphy SA, et al. Effect of the novel thienopyridine prasugrel compared with clopidogrel on spontaneous and procedural myocardial infarction in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction 38: an application of the classification system from the universal definition of myocardial infarction. Circulation. 2009;119(21):2758-64. Study sub-analyses showed favorable results to prasugrel in diabetic patients236236. Wiviott SD, Braunwald E, Angiolillo DJ, Meisel S, Dalby AJ, Verheugt FW, et al; TRITON-TIMI 38 Investigators. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-Thrombolysis in Myocardial Infarction 38. Circulation. 2008;118(16):1626-36. and in those who underwent myocardial revascularization surgery.237237. Smith PK, Goodnough LT, Levy JH, Poston RS, Short MA, Weerakkody GJ, et al. Mortality benefit with prasugrel in the TRITON-TIMI 38 coronary artery bypass grafting cohort: risk-adjusted retrospective data analysis. J Am Coll Cardiol. 2012,31;60(5):388-96.
Prasugrel was also evaluated in the TRILOGY trial, which included 9326 patients with NSTE-ACS and an additional risk factor (minimum age of 60, DM, history of AMI, PCI or myocardial revascularization), who did or did not undergo coronary angiography but were specifically indicated for clinical treatment (without myocardial revascularization after the index event for study entry). The patients were randomized to clopidogrel (300 mg loading dose with a 75 mg daily maintenance dose) or prasugrel (30 mg loading dose with a 10 mg daily maintenance dose if aged < 75 years, or 5 mg daily if aged ≥ 75 years or weighing < 60 kg). The patients were followed up for an average of 17 months, and the medication was used for an average of 15 months.238238. Gurbel PA, Erlinge D, Ohman EM, Neely B, Neely M, Goodman SG, et al. Platelet function during extended prasugrel and clopidogrel therapy for patients with ACS treated without revascularization: the TRILOGY ACS platelet function substudy. JAMA. 2012;308(17):1785-94.
Regarding the primary efficacy endpoint (cardiovascular death, AMI, or stroke), there was no significant difference between the prasugrel and clopidogrel groups (p = 0.21). There were also no significant differences between groups regarding the main safety outcomes (severe or life-threatening bleeding by the GUSTO criterion or severe bleeding by the TIMI criterion).
Finally, in the TRILOGY trial, approximately one-third of the patients underwent platelet aggregation analysis and, despite superior platelet aggregation inhibition in the prasugrel group, a significant association between platelet reactivity and ischemic/thrombotic events could not be determined.238238. Gurbel PA, Erlinge D, Ohman EM, Neely B, Neely M, Goodman SG, et al. Platelet function during extended prasugrel and clopidogrel therapy for patients with ACS treated without revascularization: the TRILOGY ACS platelet function substudy. JAMA. 2012;308(17):1785-94.
As with clopidogrel, if indicated, prasugrel should, in principle, be taken for 12 months. Prasugrel should be suspended at least 7 days before elective myocardial revascularization.
5.2.3. Ticagrelor
Ticagrelor also blocks ADP-induced platelet aggregation through P2Y12 inhibition, although it does not belong to the thienopyridine class. Ticagrelor is a cyclopentyltriazolopyrimidine with a half-life of approximately 12 hours and, unlike thienopyridines, its inhibition of P2Y12 receptors is reversible and does not depend on hepatic metabolism to initiate its action. With such characteristics, ticagrelor has a more intense, rapid, and consistent antiplatelet effect than clopidogrel.239239. Ohman J, Kudira R, Albinsson S, Olde B, Erlinge D. Ticagrelor induces adenosine triphosphate release from human red blood cells. Biochem Biophys Res Commun. 2012;418(4):754-8.
In the PLATO trial,152152. Wallentin L, Becker RC, Budaj A, Cannon CP, Emmanuelson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(1):1045-57. approximately 18,000 patients admitted for intermediate- and high-risk ACS within 24 hours of symptom onset were randomized to receive ticagrelor (180 mg loading dose followed by a maintenance dose of 90 mg every 12 hours) or clopidogrel (300 mg loading dose followed by a maintenance dose of 75 mg daily). Patients who underwent PCI received an additional dose of 90 mg of ticagrelor and could, at the discretion of the investigator, receive an additional dose of 300 mg of clopidogrel. All patients, except when contraindicated, received ASA, and the study medication was continued for 12 months, regardless of the treatment strategy (PCI, surgical revascularization, or clinical treatment alone). In patients indicated for CABG, clopidogrel was discontinued 5 days before the procedure, while ticagrelor was discontinued 24 to 72 hours prior to surgery. The primary efficacy endpoint was the composite of death from vascular causes, non-fatal AMI or stroke in 12 months, while the primary safety endpoint was severe bleeding according to the study's own criterion. In the total sample, the prevalence of NSTE-ACS was approximately 60%, of whom approximately 43% had AMI without ST elevation, and 17% had intermediate- or high-risk UA. The median patient age was 62 years, with 15% older than 75 years. Approximately 64% underwent PCI, and 10% underwent CABG, with the remainder receiving clinical treatment alone. At the end of the study, the ticagrelor group had a significant reduction (16%) in the primary efficacy outcome (RR 0.84; 9.8% vs. 11.7%, p < 0.001). In separate analyses of composite outcome components (main secondary goals), there were significant reductions in the incidence of AMI (RR 0.84; 5.8% vs. 6.9 %, p = 0.005) and death from vascular causes (RR 0.79; 4.0% vs. 5.1%, p < 0.001), with no significant differences in stroke (p = 0.22). Regarding the primary safety outcome, there was no significant difference in the incidence of severe bleeding according to the study's criterion (HR = 1.04; p = 0.43) and the TIMI criterion (HR = 1.03, p = 0.57). Although there was no difference in the incidence of fatal bleeding (HR = 0.87; p = 0.66) or in the need for transfusions (HR = 1; p = 0.96), ticagrelor led to a slight but significant overall increase in fatal intracranial bleeding (0.1 vs. 0.01; p = 0.02) and major bleeding unrelated to CABG (4.5% vs. 3.8%, p = 0.03). There was a higher incidence of other adverse effects in the ticagrelor group. There was a significant increase in the occurrence of dyspnea (RR 1.84; 13.8% vs. 7.8%, p < 0.001), which, in general, was transient and led to drug cessation in less than 1% of patients. It is debated whether this side effect could be explained by increased plasma adenosine levels.240240. Bonello L, Laine M, Kipson N, Mancini J, Helal O, Fromonot J, et al. Ticagrelor increases adenosine plasma concentration in patients with an acute coronary syndrome. J Am Coll Cardiol 2014;63(9):872-7.
241. Orme RC, Parker WAE, Thomas MR, Judge HM, Baster K, Sumaya W, et.al. Study of Two Dose Regimens of Ticagrelor Compared with Clopidogrel in Patients Undergoing Percutaneous Coronary Intervention for Stable Coronary Artery Disease (STEEL-PCI). Circulation 2018;138(13):1290-300.-242242. Ortega-Paz L, Brugaletta S, Ariotti S, Akkerhuis KM, Karagiannis A, Windecker S, et al. HI-TECH investigators. Adenosine and Ticagrelor Plasma Levels in Patients With and Without Ticagrelor-Related Dyspnea. Circulation 2018;138(6):646-48. There was also an increase in the incidence of transient bradycardia, with a significant increase in the ventricular pauses > 3 seconds in the first 7 days of medication use (5.8% vs. 3.6%, p = 0.01). However, this effect lost significance after 30 days of medication use (2.1% vs. 1.7%, p = 0.52).243243. Scirica BM, Cannon CP, Emanuelsson H, Michelson EL, Harrington RA, Husted S, et al.; PLATO Investigators. The incidence of bradyarrhythmias an clinical bradyarrhythmic events in patients with acute coronary syndromes treated with ticagrelor or clopidogrel in the PLATO (Platelet Inhibition and Patient Outcomes) trial: results of the continuous electrocardiographic assessment substudy. J Am Coll Cardiol. 2011;57(19):1908-16. There was no overall difference between the groups regarding the need for pacemaker implantation or the occurrence of syncope or heart block.243243. Scirica BM, Cannon CP, Emanuelsson H, Michelson EL, Harrington RA, Husted S, et al.; PLATO Investigators. The incidence of bradyarrhythmias an clinical bradyarrhythmic events in patients with acute coronary syndromes treated with ticagrelor or clopidogrel in the PLATO (Platelet Inhibition and Patient Outcomes) trial: results of the continuous electrocardiographic assessment substudy. J Am Coll Cardiol. 2011;57(19):1908-16. There was an increase in creatinine (10% vs. 8%) and uric acid levels (14% vs. 7%), which reverted 1 month after the end of treatment.244244. Nilsen DW. Potential benefits of ticagrelor beyond platelet inhibition. Cardiology. 2013;125(1):31-3.
The PLATO database provides data on several pre-specified subgroups, including diabetics245245. ames S, Angiolillo DJ, Cornel JH, Erlinge D, Husted S, Kontny F, et al; PLATO Study Group. Ticagrelor vs. clopidogrel in patients with acute coronary syndromes and diabetes: a substudy from the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J. 2010;31(24):3006-16. chronic kidney disease,246246. James S, Budaj A, Aylward P, Buck KK, Cannon CP, Cornel JH, et al. Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: results from the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2010;122(11):1056-67. previous stroke,247247. James SK, Storey RF, Khurmi NS, Husted S, Keltai M, Mahaffey KW, et al; PLATO Study Group. Ticagrelor versus clopidogrel in patients with acute coronary syndromes and a history of stroke or transient ischemic attack. Circulation. 2012;125(23):2914-21. PPI use,230230. Goodman SG, Clare R, Pieper KS, Nicolau JC, Storey RF, Cantor WJ, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation. 2012;125(8):978-86. initial invasive or conservative strategy,248248. Cannon CP, Harrington RA, James S, Ardissino D, Becker RC, Emanuelsson H, et al; PLATelet inhibition and patient Outcomes Investigators. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet. 2010;375(9711):283-93. surgical myocardial revascularizations,249249. Held C, Asenblad N, Bassand JP, Becker RC, Cannon CP, Claeys MJ, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes undergoing coronary artery bypass surgery: results from the PLATO (Platelet Inhibition and Patient Outcomes) trial. J Am Coll Cardiol. 2011;57(6):672-84. the recurrence of events,250250. Kohli P, Wallentin L, Reyes E, Horrow J, Husted S, Angiolillo DJ, et al. Reduction in first and recurrent cardiovascular events with ticagrelor compared with clopidogrel in the PLATO Study. Circulation. 2013;127(6):673-80. cost-effectivenesses,251251. Nikolic E, Janzon M, Hauch O, Wallentin L, Henriksson M; PLATO Health Economic Substudy Group. Cost-effectivenessof treating acute coronary syndrome patients with ticagrelor for 12 months: results from the PLATO study. Eur Heart J. 2013;34(3):220-8., etc. In summary, ticagrelor appears to be cost-effective, and the sub-analysis results were very close to those of the original publication, which included the entire population. The drug must be suspended at least 3 days prior to elective myocardial revascularization surgery.
More recently, ISAR-REACT 5, a multicenter, non-blinded randomized trial, compared the use of ticagrelor (180 mg at hospital arrival followed by 90 mg twice a day) vs. prasugrel (60 mg in the hemodynamics unit followed by 10 mg or 5 mg for patients ≥ 75 years of age or < 60 kg, respectively) in 4018 patients with ACS indicated for early invasive treatment (41.1% of AMI with supra and 58.9% of NSTE-ACS). The primary outcome (death, AMI, or stroke within 1 year of follow-up) occurred in 184 patients (9.3%) in the ticagrelor group and in 137 patients (6.9%) in the prasugrel group (HR 1.36; 95% CI, 1.09 to 1.70; p = 0.006). Major bleeding, the safety outcome, was similar in both groups: 5.4% in the ticagrelor group vs. 4.8% in the prasugrel group (HR 1.12; 95% CI, 0.83 to 1.51; p = 0.46). However, some study limitations should be pointed out: the study was open (not blinded); 90% of the patient contacts were made by telephone (83%) or letter (7%); neither medication was dispensed by the study (patients acquired them on their own); invasive stratification in NSTE-ACS was determined very quickly, which is not our practice; in the prasugrel group, the primary outcome occurred less frequently (6.9%) than in the TRITON TIMI 38 trial (9.9%) and well below what was estimated for this group (12.9%) in the sample calculation.252252. Schüpke S, Neumann FJ, Menichelli M, Mayer K, Bernlochner I, Wöhrle J, et al., ISAR-REACT 5 Trial Investigators Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes N Engl J Med 2019;381(16):1524-34.
5.3. Adjusting Antiplatelet Therapy According to Platelet Aggregation Tests or Genetic Tests
Three randomized clinical studies have sought to demonstrate the clinical impact of antiplatelet therapies in patients with poor response to clopidogrel. The GRAVITAS trial232232. Price MJ, Berger PB, Teirstein PS, Tanguay JF, Angiolillo DJ, Spriggs D, et al; GRAVITAS Investigators. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305(11):1097-105. Erratum in JAMA. 2011 Jun 1;305(21);2174. tested whether a high dose of clopidogrel (600 mg loading dose and 150 mg/day maintenance dose) would prevent cardiovascular events after PCI and drug-eluting stenting better than standard therapy (75 mg/day without a loading dose). Patients were selected according to high platelet reactivity to clopidogrel, which was assessed with the VerifyNow® P2Y12 assay. Of the 5429 patients initially assessed, 2214 (40.8%) had platelet hyperreactivity (via ADP) and were included in the study (40% diagnosed with NSTE-ACS). Despite a 22% overall reduction in the number of patients with continued platelet hyperreactivity (P2Y12 reaction unit value ≥ 230), treatment with a high dose of clopidogrel did not reduce the risk of death from cardiovascular causes, non-fatal AMI, or stent thrombosis in 6 months after PCI (HR 1.01, 95% CI 0.58 to 1.76, p = 0.97). There was also no increase in severe bleeding between groups. In the ARCTIC study,233233. Collet JP, Cuisset T, Rangé G, Cayla G, Elhadad S, Pouillot C, et al; ARCTICInvestigators. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med. 2012;367(22):2100-9. 2440 patients with scheduled PCI and drug-eluting stent implantation (27% with NSTE-ACS) were randomized to two different platelet anti-aggregation strategies: a conventional strategy with ASA and P2Y12 inhibitors used at normal dosages and a monitored strategy in which the doses were adjusted according to the results of platelet aggregability determined through the VerifyNow® aspirin system and P2Y12 assay. Randomization, assessment of platelet reactivity, and intervention, when indicated in the monitored therapy group, were performed prior to the scheduled stent implantation procedure. The incidence of platelet hyperreactivity in clopidogrel patients was 34.5%, and resistance to ASA was observed in 7.6%. In the monitored therapy group, a new platelet function assessment was performed 2 and 4 weeks after stent implantation, and new therapeutic adjustments were made when necessary. In this new assessment, there was a significant reduction (15.6% vs. 34.5% [i.e., approximately 50%], p <0.001) in the number of patients with platelet hyperreactivity at the time of PCI, defined as a P2Y12 reaction unit value ≥ 235 or ≤ 15% platelet inhibition compared to control values. However, the primary outcome (all-cause mortality, AMI, stroke, TIA, emergency coronary revascularization, or stent thrombosis within 1 year) was 34.6% in patients who received monitored therapy and 31.1% in those treated conventionally (HR 1.13; 95% CI 0.98 to 1.29; p = 0.10). The rate of serious bleeding events also did not differ significantly between groups. In the ANTARTIC trial, older adults (age ≥ 75 years) undergoing PCI after ACS were randomized to a strategy guided by platelet aggregation assays vs. a conventional strategy. All patients were initially treated with prasugrel 5 mg and, in the guided therapy group, the dose could be increased to 10 mg in case of inadequate platelet inhibition (defined as P2Y12 reaction unit ≥ 208) or to 75 mg clopidogrel in case of excessive platelet inhibition (defined as P2Y12 reaction unit ≤ 85). The outcome consisted of cardiovascular death, stroke, infarction, urgent revascularization, stent thrombosis, or clinically relevant bleeding in 12 months (HR 1.00, 95% CI 0.78 to 1.29).253253. Cayla G, Cuisset T, Silvain J, Leclercq F, Manzo-Silberman S, Saint-Etienne C, et al.ANTARCTIC investigators. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): an open-label, blinded-endpoint, randomised controlled superiority trial. Lancet. 2016;388(10055):2015-22. Thus, antiplatelet therapy guided by aggregability tests should only be used in selected cases.
Regarding genetic tests, the POPular Genetics trial tested therapy guided by cytochrome 2C19 polymorphism testing. In this study, 2488 patients with ACS were randomized to a standard strategy with ticagrelor or prasugrel vs. a personalized strategy in which patients without polymorphisms (which leads to a loss of 2C19 function and, thus, have normal clopidogrel metabolism) received clopidogrel instead of the other two antiplatelet agents. The personalized strategy was not inferior to the conventional one for the composite outcome (death, infarction, stroke, stent thrombosis, or major bleeding), and it reduced major bleeding (HR 0.78; 95% CI 0.61 to 0.98; p = 0.04).254254. Claassens DMF, Vos GJA, Bergmeijer TO, Hermanides RS, van ‘t Hof AWJ, van der Harst P, et al. A Genotype-Guided Strategy for Oral P2Y 12 Inhibitors in Primary PCI. N Engl J Med. 2019;381(17):1621-31. The TAILOR PCI study included patients undergoing PCI (approximately 80% with ACS and 60% with NSTE-ACS) and assessed a therapy strategy guided by genotyping. This strategy was not superior to conventional treatment for reducing the composite outcome of cardiovascular death, infarction, stroke, stent thrombosis, or severe recurrent ischemia (HR 0.66; 95% CI 0.43-1.02; p = 0.06) in patients carrying the allele that leads to loss of 2C19 function (approximately 35% of included patients).255255. Pereira NL, Farkouh ME, So D. Effect of Genotype-Guided Oral P2Y12 Inhibitor Selection vs Conventional Clopidogrel Therapy on Ischemic Outcomes After Percutaneous Coronary Intervention: The TAILOR-PCI Randomized Clinical Trial JAMA. 2020;324(8):761-77 Further studies, including cost-effectiveness analyses, are needed to confirm these results before a final decision is made about the routine use of genotyping.
6. Antithrombotic Therapy
6.1. Unfractionated Heparin
UFH was the first injectable anticoagulant to be synthesized. Heparin is a sulfated polysaccharide with a molecular weight range of 3000 to 30,000 Da (mean 15,000 Da). Its action is due to the inactivation of thrombin and activated factor X (factor Xa) through an antithrombin-dependent mechanism. Heparin binds to antithrombin through a high-affinity pentasaccharide and, to inhibit thrombin, it binds to both antithrombin and the coagulation enzyme. By inactivating thrombin, prevents fibrin formation and inhibits thrombin-induced activation of platelets and factors V and VIII. Its variable pharmacological response occurs through independent binding of heparin antithrombin to plasma proteins and through the proteins released by platelets and endothelial cells, causing heparin resistance. For this reason, it is important to monitor thromboplastin activation time. Due to its short half-life, UFH should be administered in a continuous intravenous infusion to ensure stable levels of anticoagulation.256256. Hirsh J, Warkentin TE, Shaughnessy SG, Annand SS. Heparin and Low-Molecular-Weight Heparin, Mechanisms of Action, Pharmacokinetics, Dosing, Monitoring, Efficacy, and Safety. Chest. 2001; 119(1 Suppl):64S-94S.,257257. Hirsh J, Anand SS, Halperin JL, Fuster V. Mechanism of Action and Pharmacology of Unfractionated Heparin. Arterioscler Thromb Vasc Biol. 2001;21(7):1094-96.
The evidence for using UFH in NSTEMI treatment comes from randomized studies and meta-analyses. In a meta-analysis of six randomized studies, Eikelboom et al. found that UFH or LMWH reduces major cardiovascular outcomes in ACS.182182. Eikelboom JW, Anand SS, Malmberg K, Weitz JI, Ginsberg JS, Yusuf S. Unfractionated heparin and low-molecular-weight heparin in acute coronary syndrome without ST elevation: a meta-analysis. Lancet. 2000 Jun 3;355(9219):1936-42. UFH is a widely used anticoagulant in NSTEMI when cardiac catheterization is performed within the first 2 hours of arrival at the hospital, despite consistent evidence of a higher risk of bleeding compared to other antithrombotics.191191. Silvain J, Beygui F, Barthelemy O, Pollack C, Jr., Cohen M, Zeymer U, et al. Efficacy and safety of enoxaparin versus unfractionated heparin during percutaneous coronary intervention: systematic review and meta-analysis. BMJ. 2012;344:e553.
The proposed therapeutic regimen for UFH should be adjusted to patient body weight: an initial dose of 60 units/kg should be administered intravenously, followed by an infusion of 12 units/kg/hour. Anticoagulation intensity is adjusted by monitoring thromboplastin activation time. The first blood sample must be collected in the third hour of infusion and, subsequently, every 6 hours until the target range is reached, after which it can be collected every 24 hours. The therapeutic range is narrow, so thromboplastin activation time values should be maintained between 1.5 and 2.0 times the control values in seconds.198198. Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganisats TG, Holmes DR, et al. 2014 AHA/ACC Guideline for the management of patients with non-ST-elevation acute coronary syndromes. Circulation. 2014;130(25):e344-e426.
During PCI, the UFH dose should keep the activated clotting time between 250 and 350 seconds. Dose titration should be guided by body weight. The administration of a bolus of 70-100 IU/kg of UFH is sufficient for adequate anticoagulation. With the concomitant use of GP IIb/IIIa inhibitors, the initial dose should be 60-70 IU/kg.258258. Lee MS, Wali AU, Menon V, Berkowitz SD, Thompson TD, Califf RM, et al. The determinants of activated partial thromboplastin time, relation of activated partial thromboplastin time to clinical outcomes, and optimal dosing regimens for heparin treated patients with acute coronary syndromes: are view of GUSTO-IIb. J Thromb Thrombolysis 2002;14(2):91–101.
The most frequent side effects of UFH are major or minor bleeding, which occurs mainly when the clotting time is above the therapeutic range. UFH can trigger an intense immune reaction, heparin-induced thrombocytopenia, a potentially fatal clinical condition that concomitantly induces bleeding and thrombosis.258258. Lee MS, Wali AU, Menon V, Berkowitz SD, Thompson TD, Califf RM, et al. The determinants of activated partial thromboplastin time, relation of activated partial thromboplastin time to clinical outcomes, and optimal dosing regimens for heparin treated patients with acute coronary syndromes: are view of GUSTO-IIb. J Thromb Thrombolysis 2002;14(2):91–101.
During life-threatening bleeding or when emergency surgery is required, an antidote must be used to neutralize the anticoagulant effect. Protamine sulfate is used to inactivate the effects of UFH. Since the half-life of UFH is approximately 1 to 1.5 hours, the protamine dose should be based on the total UFH dose administered in the previous 2 to 3 hours. Approximately 1 mg neutralizes 100 IU of UFH. A slow intravenous infusion is recommended to avoid hypotension and bradycardia.258258. Lee MS, Wali AU, Menon V, Berkowitz SD, Thompson TD, Califf RM, et al. The determinants of activated partial thromboplastin time, relation of activated partial thromboplastin time to clinical outcomes, and optimal dosing regimens for heparin treated patients with acute coronary syndromes: are view of GUSTO-IIb. J Thromb Thrombolysis 2002;14(2):91–101.
6.2. Low-Molecular-Weight Heparins
During investigations into the structure of conventional heparin (UFH), it was found that its polysaccharide chains can undergo depolymerization through various physical and chemical processes to obtain heterogeneous compounds with lower molecular weight, which are called generic fractionated or LMWH.256256. Hirsh J, Warkentin TE, Shaughnessy SG, Annand SS. Heparin and Low-Molecular-Weight Heparin, Mechanisms of Action, Pharmacokinetics, Dosing, Monitoring, Efficacy, and Safety. Chest. 2001; 119(1 Suppl):64S-94S.,257257. Hirsh J, Anand SS, Halperin JL, Fuster V. Mechanism of Action and Pharmacology of Unfractionated Heparin. Arterioscler Thromb Vasc Biol. 2001;21(7):1094-96.
LMWH consist of short polysaccharide chains, which result in a more predictable anticoagulant effect than UFH. LMWH has several potential advantages over UFH:257257. Hirsh J, Anand SS, Halperin JL, Fuster V. Mechanism of Action and Pharmacology of Unfractionated Heparin. Arterioscler Thromb Vasc Biol. 2001;21(7):1094-96.
-
Greater anti-factor Xa activity than factor IIa activity. More effective inhibition of thrombin production.
-
Greater inhibition than UFH in the tissue factor pathway.
-
Less frequently induce thrombocytopenia.
-
Subcutaneous administration due to their great bioavailability.
-
Predictable anticoagulation due to less binding to plasma proteins.
-
Plasma level monitoring is unnecessary.
Fraxiparin, dalteparin, and enoxaparin are examples of LMWH that have been tested in ACS. Enoxaparin remains the drug of choice in this context due to the larger body of experimental data. Previous studies have shown that fraxiparin and nadroparin are similar to UFH.
Two studies, TIMI IIB, and ESSENCE have compared enoxaparin with UFH for clinical efficacy and safety in patients with UA and NSTEMI. In summary, they showed for the first time that LMWH (in this case, enoxaparin) was superior to UFH and that enoxaparin provides no additional benefits after the hospitalization phase (TIMI IIB). During the outpatient phase, major bleeding occurred in 1.5% of the group treated with placebo and 2.9% in the group treated with enoxaparin (p = 0.021).259259. AntmanEM, McCabe CH, Gurfinkel EP, Turpie AG, Bernink PJ, Salein D, et al. Enoxaparin prevents death and cardiac Ischemic events in unstable angina/non-Q-wave myocardial infarction: results of the Thrombolysis in Myocardial Infarction (TIMI) 11B trial. Circulation. 1999;100(15):1593–601.,260260. Cohen M, Demers C, Gurfinkel EP, Turpie AGG, Fromell GJ, Goodman S, et al. A Comparison of Low-Molecular-Weight Heparin with Unfractionated Heparin for Unstable Coronary Artery Disease. N Engl J Med. 1997;337(7),447-52. Perhaps, more importantly, a pooled analysis of the two studies showed that the enoxaparin group had significantly fewer “hard” events, i.e., death or (re)infarction, than UFH, with the advantages still evident 1 year after the initial treatment.261261. Antman EM, Cohen M, Radley D, McCabe C, Rush J, Premmereur J, Braunwald E. Assessment of the Treatment Effect of Enoxaparin for Unstable angina/non-Q-wave Myocardial Infarction. TIMI 11B-ESSENCE Meta-Analysis. Circulation. 1999;100(15), 1602–8.
The SINERGY study compared enoxaparin to UFH in patients with high-risk NSTE-ACS. A total of 10,027 patients were randomized, and the primary outcome was a composite of cardiovascular death, AMI, stroke, and urgent revascularization. In the enoxaparin arm, the event rate was 14.0% (696/4993), while in the UFH arm, it was 14.5% (722/4985) (RR, 0.96; 95% CI, 0.86-1.06). Regarding safety, there was a statistically significant increase in major bleeding with enoxaparin (9.1% vs. 7.6%, p = 0.008), (TIMI criteria) and a non-significant increase in severe bleeding (GUSTO criteria) (2.7% vs. 2.2%, p = 0.08) and blood transfusions (17.0% vs. 16.0%, p = 0.16). The authors' conclusion was that enoxaparin was neither superior to UFH nor inferior to high-risk treatment for NSTE-ACS).262262. Ferguson JJ, Califf RM, Antman EM, Cohen M, Grines CL, Goodman S, et al. Enoxaparin VS unfractionated heparin in high risk patients with non-ST-segment elevation acute coronary syndromes managed with na intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA. 2004;292(1):45–54. Enoxaparin is a safe and effective alternative to UFH. The advantage of subcutaneous use and the fact that it does not require routine anticoagulation monitoring should be considered in light of the modest excess of major bleeding. Another consideration from an unspecified analysis was that switching classes of anticoagulants during the hospital phase of patients with ACS should be avoided.262262. Ferguson JJ, Califf RM, Antman EM, Cohen M, Grines CL, Goodman S, et al. Enoxaparin VS unfractionated heparin in high risk patients with non-ST-segment elevation acute coronary syndromes managed with na intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA. 2004;292(1):45–54.
A 1 mg/kg dose of enoxaparin should be administered subcutaneously twice a day. Beginning at 75 years of age, the dose should be reduced to 0.75 mg/kg twice a day. In patients with an estimated glomerular filtration rate ≤ 30 mL/min/1.73 m², the dose should be reduced by half, and then 1 mg/kg should be administered subcutaneously once a day. When the estimated glomerular filtration rate reaches 15 mL/min/1.73 m², enoxaparin should be avoided. UFH is an alternative for this condition since its metabolism is exclusively hepatic. In patients pretreated with enoxaparin, using additional enoxaparin during PCI is not recommended if the last subcutaneous enoxaparin injection was administered 8 hours before the procedure. An additional 0.3 mg/kg intravenous bolus is recommended if the last subcutaneous dose of enoxaparin was administered ≥ 8 hours before angioplasty.263263. Collet JP, Montalescot G, Lison L, Choussat R, Ankri A, Drobinski G, et al. Percutaneous coronary intervention after subcutaneous enoxaparin pretreatment in patients with unstable angina pectoris. Circulation. 2001;103(5):658–63.
6.3. Fondaparinux
Fondaparinux is a synthetic pentasaccharide that consists of a sequence of sugars (A B C D F) - the smallest sequence capable of directly binding to thrombin. This discovery was a milestone in the pharmacological development of heparins. It provided the tools to understand the biology of this class of drugs and advance its pharmacology. The development of fondaparinux showed that selective and direct inhibition of factor Xa promotes an important antithrombotic effect, which opened the way for new direct oral anticoagulants.264264. Petitou M, Casu B, Lindahl U. 1976-1983, a critical period in the history of heparin: the discovery of the antithrombin binding site. Biochimie. 2003;85(12):83–9. Synthetic pentasaccharide, which selectively binds to thrombin, leads to factor Xa inhibition. Due to its discreet interaction with plasma components, it has predictable anticoagulant action and little individual variability. It has good bioavailability, which favors subcutaneous administration. It reaches its plasma peak quickly (2 hours) and has a long half-life (17 hours), allowing the use of a single daily dose. Fondaparinux is eliminated exclusively by the kidneys, which requires renal monitoring. When the estimated glomerular filtration rate is < 30/mL/1.73 m², its use should be suspended.265265. Holbrook A, Schulman S, Witt DM, Vandnivick PO, Fish J, Kovacs MJ, et al. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e152S–e184S.
The OASIS-5 trial tested the efficacy and safety of fondaparinux vs. enoxaparin in patients with NSTE-ACS. It was designed to assess whether fondaparinux would continue the anti-ischemic benefits of enoxaparin while reducing bleeding in patients with ACS. A total of 20,078 patients with NSTE-ACS who received fondaparinux (2.5 mg/day) or enoxaparin (1 mg/kg twice a day) were randomized for an average exposure of 6 days. The primary composite efficacy outcome was cardiovascular death, AMI, or refractory ischemia on a ninth day, while major bleeding was the primary safety outcome. A combination of these outcomes was considered the net clinical benefit. The patients were followed for up to 6 months, and the composite efficacy outcome was similar in both groups (579 events with fondaparinux [5.8%] vs. 573 with enoxaparin [5.7%]; RR: 1.01 in the fondaparinux group; 95 % CI 0.90-1.13; p for non-inferiority = 0.007).194194. Yusuf S, Mehta SR, Chrolavicius S, Afzal R, Pogue J, Granger CB, et al. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med. 2006 Apr 6;354(14):1464-76. Further sub-analyses of the study were published, the most important of which analyzed the 12,715 patients who underwent angiography during hospitalization (6238 treated with PCI). During the course of OASIS 5 (after reports of catheter thrombosis from some centers), the protocol was amended, requiring the hemodynamic catheter to be washed with 200 IU of UFH before the procedure. Among the patients who underwent PCI, the efficacy of fondaparinux was similar to enoxaparin for the primary outcome in 9 days (6.2% vs. 6.3%; RR 1.09; p = 0.79), with a lower incidence of severe bleeding (10.4% vs. 8.2%; RR = 0.78; p = 0.004). However, there was a higher incidence of catheter thrombosis (0.4% vs. 0.9%), and this complication was associated with higher rates of stroke and AMI.266266. Zhang Y, Zhang M, Tan L, Pan N, Zhang L. The clinical use of Fondaparinux: A synthetic heparin pentasaccharide. Prog Mol Biol Transl Sci. 2019;163:41–53.,267267. Mehta SR, Granger CB, Eikelboom JW, Bassand JP, Wallentin L,Faxon DP, et al. Efficacy and Safety of Fondaparinux Versus Enoxaparin in Patients With Acute Coronary Syndromes Undergoing Percutaneous Coronary Intervention: Results From the OASIS-5 Trial. J Am Coll Cardiol 2007;50(18):1742-51
7. Associating Oral Antiplatelets and Anticoagulants in Special Circumstances
In recent years, four DOACs have been studied regarding atrial fibrillation in patients undergoing angioplasty and have become viable alternatives to warfarin.
Clinical studies that tested DOACs in patients with CAD (chronic and acute) associated with atrial fibrillation found the following results in comparison to triple therapy (ASA + clopidogrel + warfarin):
In the PIONEER AF-PCI trial, 15 mg rivaroxaban (reduced to 10 mg in patients with creatinine clearance between 30-50 mL/min) + P2Y12 inhibitors (clopidogrel/ticagrelor) for 12 months was associated with significantly lower bleeding rates (primary goal), and the efficacy component (cardiovascular death, AMI, and stroke) was similar (secondary outcome).268268. Gibson CM, Mehran R, Bode C, Halpecin J, Verheugt FW, Goose P, Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375(25):2423–34.
In the REDUAL PCI trial, dabigatran 110 mg or 150 mg twice a day + P2Y12 inhibitors (clopidogrel/ticagrelor) for 12 months was associated with significantly lower bleeding rates (primary outcome), with a significant p-value for non-inferiority in both dabigatran groups but a significant p-value for superiority only in the 110 mg dabigatran group; dual therapy (both dabigatran groups) was similar to triple therapy for the composite outcome of thromboembolic events.269269. Cannon CP, Bhatt DL, Oldgren J, Thomas L, Ansell J, Fonarow GC, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377(25):1513–24.
• In the AUGUSTUS trial, apixaban 5 mg twice a day (or 2.5 mg twice a day was used if two of the following three conditions were present: age > 80 years, weight < 60 kg or serum creatinine > 1.5 mg/dl) + P2Y12 inhibitors (clopidogrel) was associated with a lower bleeding rate (primary outcome) and a lower rate of death or hospitalization (secondary outcome), with no significant difference in the incidence of ischemic events. It should be pointed out that ACS patients without PCI were also included in the study.270270. Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, et al Antithrombotic Therapy After Acute Coronary Syndrome or PCI in Atrial Fibrillation. N Engl J Med. 2019;380(16):1509-24.
• In the ENTRUST AF-PCI trial, edoxaban 60 mg in association with P2Y12 inhibitors (n = 751) was used in one group, while warfarin was combined with DAPT (ASA + P2Y12 inhibitors) was used in the other group, totaling 1,506 patients. The primary outcome was major bleeding, (HR = 0.83; 95% CI 0.65-1.05; p = 0.0010 for non-inferiority and p = 0.1154 for superiority). There was no significant difference for the secondary outcome (cardiovascular death, stroke, systemic embolism, AMI, or stent thrombosis).271271. Vranckx P, Valgimigli M, Eckardt L, Tijsssen J, Lewatter T, Gargiuo G, et al. Edoxaban-based Versus Vitamin K Antagonist-Based Antithrombotic Regimen After Successful Coronary Stenting in Patients With Atrial Fibrillation (ENTRUST-AF PCI): A Randomised, Open-Label, Phase 3b Trial. Lancet, 2019;394 (10206), 1335-43.
In these studies, about half of the patients had ACS, and clopidogrel was much more frequently used than ticagrelor as a P2Y12 inhibitor. The safety outcome (bleeding) was always the primary objective. Although they had different designs, these studies showed that DOACs were safe compared to warfarin when associated with antiplatelet agents. However, there were no significant differences in ischemic (i.e., secondary) outcomes.272272. Lopes RD, Hong H, Harskamp RE, Bhatt DL, Mehran R, Cannon CP, et al. Optimal Antithrombotic Regimens for Patients with Atrial Fibrillation Undergoing Percutaneous Coronary Intervention: an Updated Network Meta-analysis. JAMA Cardiol. 2020;5(5):582-589. The total time for triple therapy, either with DOACs or warfarin, and for suspending ASA, should be determined according to the risk of ischemic and hemorrhagic events 273273. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al ESC Scientific Document Group, 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2020 Aug 20 ehaa575 [online]
8. Hypolipidemic Drugs
Several studies have demonstrated the benefits of cholesterol-lowering therapies in secondary prevention.274274. Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, et.al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–15042.,275275. Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001-9. In high-risk patients, the benefits are independent of baseline LDL-C levels.276276. Heart Protection Collaborative Group. MRC/BHF Heart Protection Study of cholsterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7-22. A study by Dondo et al. with more than 389,000 NSTE-ACS patients and a mean follow-up of 2.2 years found that non-adherence to statin treatment was one of the most important factors in reduced survival. If the guidelines were applied and followed correctly,i.e., adherence to statin use, coronary angiography when indicated, cardiovascular rehabilitation, and smoking cessation, 28.9% of the associated deaths could be prevented.277277. Dondo TB, Hall M, Timmis AD, Gilthorpe MS, Alabas AO, Batin PD, et al. Excess Mortality and Guideline-Indicated Care Following non-ST-elevation Myocardial Infarction. Eur Heart J Acute Cardiovasc Care, 2107;6 (5), 412-20.
Although lipid targets vary in different guidelines, a more rigorous strategy to reduce LDL levels has recently been recommended. The 2017 Brazilian Guideline on Dyslipidemia and the Prevention of Atherosclerosis recommended LDL levels < 50 mg/dL and non-HDL levels < 80 mg/dL in very high-risk patients.278278. Faludi AA, Izar MCO, Saraiva JFK, Bianco HT, Chacra APM, Bertoluci MC et al. Diretriz brasileira baseada em evidências sobre prevenção de doenças cardiovasculares em pacientes com diabetes: posicionamento da Sociedade Brasileira de Diabetes (SBD), da Sociedade Brasileira de Cardiologia (SBC) e da Sociedade Brasileira de Endocrinologia e Metabologia (SBEM). Arq Bras Cardiol. 2017; 109(6 Supl.1):1-31 When large doses of statins cannot be tolerated, association with ezetimibe is an alternative.279279. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et.al. IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-97.
It is assumed that lipid-lowering therapy should ideally begin during hospitalization, provided there is no contraindication. The SECURE-PCI trial evaluated an 80 mg loading dose of atorvastatin before coronary angiography in 4,191 ACS patients indicated for invasive strategy. The loading dose did not reduce major cardiovascular outcomes in 30 days compared to the placebo (HR 0.88; 95% CI 0.69 to 1.11; p = 0.27), although the subgroup of patients who underwent PCI may have benefitted (HR 0.72; 95% CI 0.54 to 0.96; p = 0.02).280280. Berwanger O, Santucci EV, de Barros e Silva PGM, Jesuíno IA, Damiani LP, Barbosa LM, et al. Effect of Loading Dose of Atorvastatin Prior to Planned Percutaneous Coronary Intervention on Major Adverse Cardiovascular Events in Acute Coronary Syndrome. JAMA. 2018;319(13):1331-40.
9. Renin-Angiotensin-Aldosterone System Inhibitors
This group of drugs is very important for treating high blood pressure, HF, and some groups of patients with CAD. There is no conclusive evidence that the early use of these drugs benefits patients with NSTE-ACS, although some studies have suggested that they may be useful in the chronic phase after an acute episode. The HOPE trial281281. Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of na angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342(3):145-53. Erratum in N Engl J Med. 2000;342(10):748 Erratum: N Engl J Med. 2000;342(18):1376. included patients at high risk of cardiovascular events, often with major atherosclerotic arterial disease (usually reaching the coronary region). Regardless of the stage, ramipril (target dose of 10 mg/day) had long-term benefits: in 5 years of follow-up, there was a 26% reduction in the relative risk of death (p < 0.001), a 20% reduction in infarction, (p < 0.001), and a 32% reduction in stroke (p < 0.001). Similar results have also been demonstrated for perindopril in chronic coronary disease patients.282282. Fox KM; EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet. 2003; 362(9386):782-8. The benefits are greater in patients with LV dysfunction and previous AMI, including improved cardiac remodeling and LV function and decreased progression to HF.283283. Hoedemaker NPG, Damman P, Ottervanger JP, Dambrink JHE, Gosselink ATM, Kedhi E, et.al. Trends in Optimal Medical Therapy Prescription and Mortality After Admission for Acute Coronary Syndrome: A 9-year Experience in a Real-World Setting. Eur Heart J Cardiovasc Pharmacother,2018; 4(2):102-10.
The aim of treatment is to reach the highest tolerable dose of medications: captopril 50 mg every 8 hours (which can later be replaced by ramipril 10 mg/day or enalapril 20 mg/day every 12 hours), losartan 50 mg every 12 hours, or valsartan 320 mg/day.
Regarding aldosterone antagonists, the EPHESUS trial found lower mortality with eplerenone (not available in Brazil) in patients with AMI (with or without ST elevation) and LV dysfunction who showed symptoms of HF or DM (RR 0.85; 95% CI 0.75-0.96; p = 0.008). The benefits were already evident in the first 30 days of treatment.284284. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003 Apr 3;348(14):1309-21. Erratum in: N Engl J Med. 2003; 348(22):2271.,285285. Carillo S, Zhang Y, Fay R, Angioi M, Vincent J, Sutradhor SC, et al. Heart failure with systolic dysfunction complicating acute myocardial infarction - differential outcomes but similar eplerenone efficacy by ST-segment or non-ST-segment elevation: A post hoc substudy of the EPHESUS trial. Arch Cardiovasc Dis. 2014 Mar;107(3):149-57. The RALES trial had previously found lower mortality in patients with chronic HF and LV ejection fraction (LVEF) ≤ 35%.286286. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999 Sep 2;341(10):709-17. Thus, a target dose of 25 mg of spironolactone (an aldosterone antagonist available in Brazil) once a day (which can be increased to 50 mg after 8 weeks of treatment if there are signs of HF progression without hyperkalemia). Aldosterone blockers are contraindicated in patients with significant renal dysfunction (Cr > 2.5 mg/dL) or potassium levels > 5.5 mEq/L. Potassium levels and renal function must be carefully monitored, especially when aldosterone blockers are used concomitantly with angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers.
10. Complementary Methods of Risk Stratification
In patients with ACS, risk stratification must be a continuous process, from the initial clinical evaluation to the previously discussed subsidiary exams to the complementary methods described below.
11. Non-Invasive Tests for Ischemia Diagnosis and Prognostic Assessment
Functional tests are indicated for assessing myocardial ischemia and, in some cases, are essential for decision-making, especially for the diagnosis and prognosis of intermediate- and high-risk patients. Non-invasive prognostic methods are more common in the conservative therapeutic strategy.
11.1. Exercise Testing
During the hospitalization phase, ET is used to determine the residual degree of ischemia and assess cardiac performance. Residual ischemia is estimated by ST-segment changes during exercise or the recovery phase and by anginal pain. Cardiac performance is assessed through blood pressure and double product. Cardiac performance and autonomic response related to heart rate reduction during the recovery phase have shown a better correlation with mortality than other parameters and have a very high value (98% to 100%), although their positive predictive value is modest (approximately 50%), with abnormal ET results being uncommon in patients indicated for this stratified procedure in chest pain units.287287. Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart AssociationTask Force on Practice Guidelines (Committee to Update the 1997 ExerciseTesting Guidelines). Circulation. 2002;106(14):1883-92.
288. Gibler WB, Cannon CP, Blomkalns AL, Char DM, Drew BJ, Hollander JE, et al; American Heart Association Council on Clinical Cardiology (Subcommittee on Acute Cardiac Care); Council on Cardiovascular Nursing, and Quality of Care and Outcomes Research Interdisciplinary Working Group; Society of Chest Pain Centers. Practical implementation of the guidelines for unstable angina/non-ST-segment elevation myocardial infarction in the emergency department: a scientific statement from the American Heart Association Councilon Clinical Cardiology (Subcommittee on Acute Cardiac Care), Councilon Cardiovascular Nursing, and Quality of Care and Outcomes Research Interdisciplinary Working Group,in Collaboration With the Society of Chest Pain Centers. Circulation. 2005;111(20):2699-710.-289289. Amsterdam EA, Kirk JD, Diercks DB, Lewis WR, Turnipseed SD. Early exercise testing in the management of low risk patients in chest pain centers. Prog Cardiovasc Dis. 2004;46(5):438-52.
In multivariate regression analysis, the main independent predictors of event-free survival (death and AMI) in 1 year were the number of leads with depressed ST-segment and the maximum load achieved. Regarding safety, there is a 0.5% incidence of complications (death or AMI) in stabilized patients within 24 hours of ET.290290. Mark DB, Hlatky MA, Harrell FE Jr, Lee KL, Califf RM, Pryor DB. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med. 1987;106(6):793-800.
291. Stein RA, Chaitman BR, Balady GJ, Fleg JL, Limacher MC, Pina IL, et al. Safety and utility of exercise testing in emergency room chest pain centers: an advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association. Circulation. 2000;102(12):1463-7.-292292. Larsson H, Areskog M, Areskog NH, Nylander E, Nyman I, Swahn E, et al. Should the exercise test (ET) be performed at discharge or one month later after an episode of unstable angina or non-Q-wave myocardial infarction? Int J Card Imaging. 1991;7(1):7-14.
ET is recommended for estimating prognosis and assisting clinical decision-making in intermediate-risk patients 24 to 48 hours after complete clinical stabilization (hemodynamic stability, no active clinical or electrocardiographic ischemia, no new Q waves, no clinical signs of HF, and normal myocardial injury markers), provided the patient is able to exercise.5151. Meneghelo RS, Araújo CG, Stein R, Mastrocolla LE, Albuquerque PF, Serra SM, et al; Sociedade Brasileira de Cardiologia. III Diretrizes da Sociedade Brasileira de Cardiologia sobre teste ergométrico. Arq Bras Cardiol. 2010;95(5 Suppl 1):1-26.,198198. Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganisats TG, Holmes DR, et al. 2014 AHA/ACC Guideline for the management of patients with non-ST-elevation acute coronary syndromes. Circulation. 2014;130(25):e344-e426.
Thus, ET as an early strategy (< 48 hours) is formally contraindicated in high-risk patients. However, 48 hours after full stabilization of the clinical condition, even during hospitalization, ET may be indicated in patients undergoing coronary angiography, when a functional assessment of a known lesion is required or the risk level must be determined before hospital discharge, as well as to properly guide cardiac rehabilitation programs.293293. Hermann LK, Weingart SD, Duvall WL, Henzlova MJ. The limited utility of routine cardiac stress testing in emergency department chest pain patients younger than 40 years. Ann Emerg Med. 2009;54(1):12-6.,294294. Natsui S, Sun BC, Shen E, Wu YL, Redberg RF, Lee MS, et al. Evaluation of Outpatient Cardiac Stress Testing After Emergency Department Encounters for Suspected Acute Coronary Syndrome. Ann Emerg Med. 2019 Aug;74(2):216-23.
11.2. Echocardiographic Methods (Ischemia, Viability, Stress, Contrast Microbubbles, Strain, etc.)
11.2.1. Transthoracic Echocardiogram
A new analysis of LV contractile changes may, in fact, help determine the diagnosis and prognosis of ACS.295295. Sabia P, Afrookteh A, Touchstone DA, Keller MW, Esquivel L, Kaul S. Value of regional wall motion abnormality in the emergency room diagnosis of acute myocardial infarction: a prospective study using two-dimensional echocardiography. Circulation. 1991;84(3 Suppl):I85-92.
296. Stein JH, Neumann A, Preston LM, VandenBerg BJ, Parrillo JE, Calvin JE, et al. Improved risk stratification in unstable angina: identification of patients at low risk for in hospital cardiac events by admission echocardiography. Clin Cardiol. 1998;21(10):725-30.
297. Horowitz RS, Morganroth J, Parrotto C, Chen CC, Soffer J, Pauletto FJ. Immediate diagnosis of acute myocardial infarction by two-dimensional echocardiography. Circulation. 1982;65(2):323-9.
298. Kontos MC, Arrowood JA, Paulsen WH, Nixon JV. Early echocardiography can predict cardiac events in emergency department patients with chest pain. Ann Emerg Med. 1998;31(5):550-7.-299299. Barberato SH, Romano MMD, Beck ALS, Rodrigues ACT, Almeida ALC, Assunção BMBL, et al. Position Statement on Indications of Echocardiography in Adults - 2019. Arq Bras Cardiol. 2019; 113(1):135-81. The wall motion score index is the reference parameter for expressing LV segmental function, and its normal value is 1 (the LV is divided into 16 or 17 segments that are classified based on systolic thickening); values between 1 and 1.6 show a mild contractile change, while wall motion score index values > 1.6 indicate greater involvement and worse prognosis. Obviously, a lack of segmental contractility changes in resting transthoracic echocardiogram results does not exclude CAD.300300. Campos FO, Zielinsky P, Ortiz J, Maciel BC, Andrade JL, Mathias W Jr, et al; Brazilian Society of Cardiology. [Guideline for indication and utilization of echocardiography in clinical practice]. Arq Bras Cardiol. 2004;82(Suppl 2):11-34.
It is worth remembering that ventricular function assessment by transthoracic echocardiogram during the acute phase of ischemia could be compromised by myocardial stunning. After 2 weeks, marked improvement in ventricular function may occur.301301. Atici A, Ali Barman H, Durmaz E, Demir K, Cakmak R, Tugrul S, et al. Predictive value of global and territorial longitudinal strain imaging in detecting significant coronary artery disease inpatients with myocardial infarction without persistent ST-segment elevation. Echocardiography. 2019; 36(3):425-622.
Through new technologies in echocardiography, such as 2D speckle-tracking, it has become possible, by analyzing the global longitudinal strain (GLS) of the LV walls, to obtain an early diagnosis of ischemic changes in patients with troponin changes but no ECG or resting echocardiogram changes (25% to 75% of patients with ACS have normal echocardiogram results).302302. Solomon SD, Glynn RJ, Greaves S, Ajani U, Rouleau JL, Menapace RF, et al. Recovery of venricular function after myocardial infarction in the reperfusion era: The healing and early after local therapy study. Ann Intern Med. 2001; 134(6): 451 - 8.
GLS (when < 16.5%) can complement existing diagnostic algorithms and act as an early marker of ischemia.303303. Liou K, Negishi K, Ho S, Russell EA, Cranney G, Ooi SY. Detectionof Obstructive Coronary Artery Disease Using Peak Systolic Global Longitudinal Strain Derived by Two-Dimensional Speckle-Tracking: A Systematic Review and Meta-Analysis. J Am Soc Echocardiogr.2016;29(8):724-35 e4. A recent publication proposes that GLS can help determine candidates for invasive strategy.304304. Marques-Alves P, Espirito-Santo N, Baptista R, Teixeira R, Martins R, Gonçalvrs F, et al. Two-dimensional speckle-tracking global longitudinal strain in high-sensitivity troponin-negative low-risk patients with unstable angina: a “resting ischemia test”? Int J Cardiovasc Imaging. 2017; 34(4):561-8. Small centers have routinely used GLS in the emergency department, and changes in GLS patterns (< 18%) have been reported even before changes in troponin.305305. Dahlslett T, Karlsen S, Grenne B, Eek C, Sjoli B, Skustad H, et al. Early assessment of strain echocardiography can accurately exclude significant coronary artery stenosis in suspected non-ST-segment elevation acute coronary syndrome. J Am Soc Echocardiogr. 2014;27(5):512–9.
In special circumstances with a suboptimal acoustic window, a transthoracic echocardiogram should be complemented with microbubble contrast echocardiography to better determine the endocardial borders or evaluate any myocardial perfusion defects.306306. Porter TR, Mulvagh SL, Abdelmoneim SS, Becher H, Belcik JT, Bierig M, et.al. Clinical Applications of Ultrasonic Enhancing Agents in Echocardiography: 2018 American Society of Echocardiography Guidelines Update. J Am Soc Echocardiogr. 2018; 31(3):241-74.
11.2.2. Stress Echocardiography
Stress echocardiography can detect transient regional contraction abnormalities, which are indicative of exercise-induced and pharmacologically-induced ischemia.307307. Pellikka PA, Olson AA, Chaudhry FA, Chen MH, Marshall JE, Porter TR, Sawada SG, Guidelines for Performance, Interpretation, and Application of Stress Echocardiography in Ischemic Heart Disease: From the American Society of Echocardiography. J Am Soc Echocardiogr.2020;33(1):1-41. The stress echocardiogram is an independent predictor of cardiovascular death; adding value to other methods, it can prevent cardiac catheterization. It is recommended for risk stratification in chest pain units, especially when the diagnosis cannot be determined through ECG and the ET is submaximal, cannot be performed, or is inconclusive, given that the pain is relieved for at least 24 hours in low- to moderate-risk patients and no ischemic changes or necrosis markers are evident in the ECG.307307. Pellikka PA, Olson AA, Chaudhry FA, Chen MH, Marshall JE, Porter TR, Sawada SG, Guidelines for Performance, Interpretation, and Application of Stress Echocardiography in Ischemic Heart Disease: From the American Society of Echocardiography. J Am Soc Echocardiogr.2020;33(1):1-41.,308308. Sicari R, Landi P, Picano E, Pirelli S, Chiaranda G, Previtali M, et al. Exercise-electrocardiography and/or pharmacological stress echocardiography for non-invasive risk stratification early after uncomplicated myocardial infarction: a prospective international large scale multicentre study. Eur Heart J. 2002;23(13):1030-7.
An improved segmental contraction in dyssynergic areas after initial dobutamine doses (5 to 10 g/kg/min) identifies myocardial viability in regions "stunned" by prior ischemia.307307. Pellikka PA, Olson AA, Chaudhry FA, Chen MH, Marshall JE, Porter TR, Sawada SG, Guidelines for Performance, Interpretation, and Application of Stress Echocardiography in Ischemic Heart Disease: From the American Society of Echocardiography. J Am Soc Echocardiogr.2020;33(1):1-41.
308. Sicari R, Landi P, Picano E, Pirelli S, Chiaranda G, Previtali M, et al. Exercise-electrocardiography and/or pharmacological stress echocardiography for non-invasive risk stratification early after uncomplicated myocardial infarction: a prospective international large scale multicentre study. Eur Heart J. 2002;23(13):1030-7.-309309. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949-3003.
11.2.3. Echocardiographic Assessment of Myocardial Perfusion
In patients with acute chest pain and non-diagnostic ECG, contrast echocardiography increases sensitivity for ACS diagnosis.310310. Picano E, Mathias W Jr, Pingitore A, Bigi R, Previtali M. Safety and tolerability of dobutamine-atropine stress echocardiography: a prospective, multicentre study. Echo Dobutamine International Cooperative Study Group. Lancet. 1994;344(8931):1190-2. Patients with normal perfusion and myocardial function at rest have a good prognosis, while perfusion defects at rest indicate a high-risk ACS subgroup.306306. Porter TR, Mulvagh SL, Abdelmoneim SS, Becher H, Belcik JT, Bierig M, et.al. Clinical Applications of Ultrasonic Enhancing Agents in Echocardiography: 2018 American Society of Echocardiography Guidelines Update. J Am Soc Echocardiogr. 2018; 31(3):241-74.,311311. Caldas MA, Tsutsui JM, Kowatsch I, Andrade JL, Nicolau JC, Ramires JF, et al. Value of myocardial contrast echocardiography for predicting left ventricular remodeling and segmental functional recovery after anterior wall acute myocardial infarction. J Am Soc Echocardiogr. 2004;17(9):923-32.
Echocardiographic contrast agents are solutions that contain gas microbubbles with size of red blood cells, whose interface with the liquid medium is highly refractive, which improves the echocardiographic signal of the medium. Myocardial microcirculation can be assessed at the bedside with this method since microbubbles are microvascular markers that behave like red blood cells, i.e.,i.e.hey do not reach areas of microvascular obstruction. Thus, infarcted areas do not show this contrast and are easily detected by echocardiography.306306. Porter TR, Mulvagh SL, Abdelmoneim SS, Becher H, Belcik JT, Bierig M, et.al. Clinical Applications of Ultrasonic Enhancing Agents in Echocardiography: 2018 American Society of Echocardiography Guidelines Update. J Am Soc Echocardiogr. 2018; 31(3):241-74. Through this type of echocardiography, changes in LV segmental contraction and myocardial perfusion are obtained simultaneously and instantly.312312. Mathias W Jr, Arruda AL, Andrade JL, Filho OC, Porter TR. Endocardial borderdeline action during dobutamine infusion using contrast echocardiography. Echocardiography. 2002;19(2):109-14.
Echocardiographic contrast allows a better definition of the endocardial borders, allowing an adequate assessment of myocardial thickening and global and segmental contractile functions of the LV at rest and under stress. In addition, contrast agents allow more accurate measurement of ventricular volumes and LVEF, especially in cases of suboptimal images, and have proven useful in determining anatomical changes.306306. Porter TR, Mulvagh SL, Abdelmoneim SS, Becher H, Belcik JT, Bierig M, et.al. Clinical Applications of Ultrasonic Enhancing Agents in Echocardiography: 2018 American Society of Echocardiography Guidelines Update. J Am Soc Echocardiogr. 2018; 31(3):241-74.,313313. Porter TR, Abdelmoneim S, Belcik JT, McCulloch ML, Mulvagh SL, Olson JJ, et al. Guidelines for the cardiac sonographer in the performance of contrast echocardiography: a focused update from the American Society of Echocardiography. J Am Soc Echocardiogr. 2014;27(8):797-810.
Extensive transient regional perfusion defects during stress are indicative of severe CAD. An improved segmental contraction in dyssynergic areas with perfusion during the initial dobutamine infusion (5 to 10 µg/kg/min) identifies myocardial viability in regions "stunned" by prior ischemia.
In summary, in patients with chest pain treated at the emergency department, contrast echocardiography at rest adds diagnostic and prognostic data. Complete filling of the myocardium in contrast (perfusion) within 4 seconds identifies patients with normal motility and perfusion and, therefore, of low risk. Normal perfusion but a segmental change in wall movement indicates a stunned myocardium, while a fixed perfusion defect shows that the entire area is at risk during acute ischemic injury.314314. Tsutsui JM, Elhendy A, Xie F, O’Leary EL, McGrain AC, Porter TR. Safety of dobutamine stress real-time myocardial contrast echocardiography. J Am Coll Cardiol. 2005;45(8):1235-42.,315315. Tsutsui JM, Xie F, O’Leary EL, Elhendy A, Anderson JR, McGrain AC, et al. Diagnostic accuracy and prognostic value of dobutamine stress myocardial contrast echocardiography in patients with suspected acute coronary syndromes. Echocardiography. 2005;22(6):487-95. In patients with AMI, stress echocardiography with myocardial contrast not only identifies the areas of infarction or stunning at rest but can identify ischemic areas at a distance by analyzing the transient changes in myocardial motility and perfusion.
11.2.3.1. Echocardiographic Assessment of Myocardial Perfusion - Summary of Recommendations and Evidence
11.3. Nuclear Cardiology Methods
MPS is fundamental when an ET cannot be performed and when there are difficulties interpreting the exercise ECG. One advantage of MPS is that it is among the most widely used non-invasive techniques for low-risk UA patients. It allows assessment of patients who cannot undergo physical exertion through pharmacological stress and has a better correlation with anatomical data than ET.
MPS and radionuclide ventriculography have great diagnostic and prognostic value in acute and chronic coronary diseases.316316. Brown KA. Evaluation of the unstable angina patient in 2005: is there still a role for non invasive risk stratification? J Nucl Cardiol. 2005;12(1):9-11.
317. Amanullah AM. Noninvasive testing in the diagnosis and management of unstable angina. Int J Cardiol. 1994;47(2):95-103.-318318. Miller DD. Risk stratification in unstable angina pectoris. In: Zaret BL, Beller GA. (eds) Nuclear cardiology: stateoftheartand future directions. Saint Louis: Mosby Inc; 1999. p. 490-9. One of the main advantages of MPS is that it can be performed early in ACS and has a wide safety margin, using vasodilating agents such as dipyridamole and adenosine. For this application, MPS was found to be superior to other tests. It should also be pointed out that tomographic scintigraphy can be synchronized with ECG (gated SPECT) to assess regional systolic function and measure ventricular ejection fraction with a single exam.319319. Stratmann HG, Younis LT, Wittry MD, Amato M, Miller DD. Exercise technetium-99m myocardial tomography for the risk stratification of men with medically treated unstable angina pectoris. Am J Cardiol. 1995;76(4):236-40. Studies have consistently demonstrated that patients diagnosed with UA who have normal findings belong to a subgroup with a markedly reduced risk of serious events (about 1% in 1 year), while reversible defects indicate an unfavorable prognosis, with an event rate of approximately 20% in 1 year.320320. Freeman MR, Chisholm RJ, Armstrong PW. Usefulness of exercise electrocardiography and thallium scintigraphy in unstable angina pectoris in predicting the extent and severity of coronary artery disease. Am J Cardiol. 1988;62(17):1164-70. Erratum in: Am J Cardiol 1989;63(5):392.
321. Zhu YY, Chung WS, Botvinick EH, Dae MW, Lim AD, Ports TA, et al. Dipyridamole perfusion scintigraphy: the experience with its application in one hundred seventy patients with known or suspected unstable angina. Am Heart J. 1991;121(1 Pt 1):33-43.
322. Brown KA. Prognosticvalueof thallium-201 myocardial perfusion imaging in patients with unstable angina who respond to medical treatment. J Am Coll Cardiol. 1991;17(5):1053-7.-323323. Madsen JK, Stubgaard M, Utne HE, Hansen JF, van Duijvendijk K, Reiber JH, et al. Prognosis and thallium-201 scintigraphy in patients admitted with chest pain without confirmed acute myocardial infarction. Br Heart J. 1988;59(2):184-9.
Positron emission tomography, a non-invasive technique for assessing obstructive coronary disease, has a very high accuracy for detecting myocardial ischemia and myocardial viability. It has been found useful for diagnostic and prognostic assessment of CAD. It also provides a very accurate assessment of coronary flow reserve by absolute quantification. This information can be useful for assessing infarction in patients without significant coronary obstructions (MINOCA).324324. Beanlands RS, Nichol G, Huszti E, Humen D, Racine N, Freeman M, et al. F-18-fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: A randomized, controlled trial (PARR-2). J Am Coll Cardiol. 2007;50(2):2002–12.,325325. Ziadi MC, Dekemp RA, Williams KA, Guo A, Chow BJ, Renaud JM, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol.2011;58(7):740-8. It is difficult to obtain radiotracers (rubidium and ammonia) in Brazil, although new gamma chamber detectors of cadmium-zinc-tellurium can also quantify flow reserve using technetium tracers.326326. de Souza ACDAH, Gonçalves BKD, Tedeschi AL, Lima RSL. Quantification of Myocardial Flow Reserve Using aGamma Camera With Solid-State Cadmium-Zinc-Telluride Detectors: Relation to Angiographic Coronary Artery Disease. J Nucl Cardiol. 2019; Jun 20. Doi 10.1007/s12350-019-01775-z. ahead of print
https://doi.org/10.1007/s12350-019-01775...
11.4. Cardiovascular Magnetic Resonance
Cardiovascular magnetic resonance (CMR) is a very useful method of morphological and functional cardiac assessment, providing important diagnostic and prognostic information about the ischemic disease and nonischemic cardiomyopathies. The test can provide accurate information about morphological aspects of the heart, quantify volumes, mass, and global and regional ventricular function, assess myocardial ischemia (by analyzing the segmental contractility under dobutamine stress without using contrast medium or by stress myocardial perfusion with vasodilators, such as dipyridamole and adenosine, and gadolinium-based contrast), as well as assess myocardial fibrosis/necrosis with the myocardial enhancement technique.327327. Hundley WG, Bluemke DA, Finn JP, Flamm SD, Fogel MA, Friedrich MG, et al; American College of Cardiology Foundation Task Force on Expert Consensus Documents. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus documenton cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;121(22):2462-508. All of this information is provided in an integrated manner through a single exam. The method also allows, by combining these and other forms of image acquisition, visualization of the pericardium and the great vessels, as well as flow and valve function analysis.
Several clinical studies and meta-analyses have shown that CMR is a highly accurate method for detecting myocardial ischemia, even compared to stress echocardiography,328328. Nagel E, Lehmkuhl HB, Bocksch W, Klein C, Vogel U, Frantz E, et al. Non invasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI: comparison with dobutamine stress echocardiography. Circulation. 1999;99(6):763-70. myocardial scintigraphy,329329. Jaarsma C, Leiner T, Bekkers SC, Crijns HJ, Wildberger JE, Nagel E, et al. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and pósitron emission tomography imaging for the detection of obstructive coronary artery disease: a meta-analysis. J Am Coll Cardiol. 2012;59(19):1719-28.,330330. Schwitter J, Wacker CM, Wilke N, Al-Saadi N, Sauer E, Huettle K, et al; MR-IMPACT Investigators. MR-IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery diseaseTrial: perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial. Eur Heart J. 2013;34(10):775-81. and FFR.331331. Watkins S, McGeoch R, Lyne J, Steedman T, Good R, McLaughlin MJ, et al. Validation of magnetic resonance myocardial perfusion imaging with fractional flow reserve for the detection of significant coronary heart disease. Circulation. 2009;120(22):2207-13.
The use of delayed enhancement imaging to assess myocardial viability has also been studied and validated in ACS. This method provides important diagnostic and prognostic information and is considered the gold standard for assessing myocardial viability and detecting infarctions.332332. Steel K, Broderick R, Gandla V, Larose E, Resnic F, Jerosch-Herold M, et al. Complementary prognostic values of stress myocardial perfusion and late gadolinium enhancement imaging by cardiac magnetic resonance in patients with known or suspected coronary artery disease. Circulation. 2009;120(14):1390-400.
333. Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343(20):1445-53.
334. Choi KM, Kim RJ, Gubernikoff G, Vargas JD, Parker M, Judd RM. Transmural extent of acute myocardial infarction predicts long-term improvement in contractile function. Circulation. 2001;104(10):1101-7.
335. Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, et al. Contrast-enhanced MRI androutine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial Myocardial infarcts: an imaging study. Lancet. 2003;361(9355):374-9.-336336. Cheong BY, Muthupillai R, Wilson JM, Sung A, Huber S, Amin S, et al. Prognostic significance of delayed-enhancement magnetic resonance imaging: survival of 857 patients with and without left ventricular dysfunction. Circulation. 2009;120(21):2069-76. Uni- and multicenter studies have shown that CMR is an extremely sensitive technique for detecting and localizing infarction, even small subendocardial infarctions,337337. Wu E, Judd RM, Vargas JD, Klocke FJ, Bonow RO, Kim RJ. Visualisation of presence, location, and transmural extent of healed Q-wave and non-Q wave myocardial infarction. Lancet. 2001;357(9249):21-8.
338. Kim RJ, Albert TS, Wible JH, Elliott MD, Allen JC, Lee JC, et al. Performance of delayed-enhancement magnetic resonance imaging with gadoversetamide contrast for the detection and assessment of myocardial infarction: na international, multicenter, double-blinded, randomized trial. Circulation.2008;117(5):629-37.-339339. Kim HW, Farzaneh-Far A, Kim RJ. Cardiovascular magneticresonance in patients with myocardial infarction: current and emerging applications. J Am Coll Cardiol. 2009;55(1):1-16. which have prognostic importance, perhaps due to their frequent association with critical coronary stenosis and the fact that they may go unnoticed both in clinical evaluation and other diagnostic tests.340340. Eitel I, de Waha S, Wöhrle J, Fuernau G, Lurz P, Pauschinger M, et al. Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2014 Sep 23;64(12):1217-26.
341. Schelbert EB, Cao JJ, Sigurdsson S, Aspelund T, Kellman P, Aletras AH, et al. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA. 2012;308(9):890-6.-342342. Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S, et al. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or Symptoms of coronary artery disease. Circulation. 2006;113(23):2733-43. Erratum in Circulation. 2006;114(8):e365. In addition, the infarcted area, which is measured by CMR, has a great prognostic impact independent of other clinical factors; some studies have found it more important than LVEF itself.343343. Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation.1998;97(8):765-72.,344344. Kelle S, Roes SD, Klein C, Kokocinski T, de Roos A, Fleck E, et al. Prognostic value of myocardial infarct size and contractile reserve using magnetic resonance imaging. J Am Coll Cardiol. 2009;54(19):1770-7. When techniques for identifying areas of edema are associated with the measurement of areas of delayed enhancement, the rate of myocardial salvage can also be obtained, which has prognostic value, especially in patients undergoing thrombolytic treatment.345345. Kendziora B, Dewey M. Prognostic value of the myocardial salvage index measured by T2-weighted and T1-weighted late gadolinium enhancement magnetic resonance imaging after ST-segment elevation myocardial infarction: A systematic review and meta-regression analysis. PLoS One. 2020 Feb 13;15(2):e0228736.
Delayed enhancement imaging also allows detection of hypoenhanced (dark) areas in the midst of the hyperenhanced area (infarction), which are related to microvascular obstruction (the no-reflow phenomenon), adding prognostic information about this population.343343. Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation.1998;97(8):765-72.
344. Kelle S, Roes SD, Klein C, Kokocinski T, de Roos A, Fleck E, et al. Prognostic value of myocardial infarct size and contractile reserve using magnetic resonance imaging. J Am Coll Cardiol. 2009;54(19):1770-7.
345. Kendziora B, Dewey M. Prognostic value of the myocardial salvage index measured by T2-weighted and T1-weighted late gadolinium enhancement magnetic resonance imaging after ST-segment elevation myocardial infarction: A systematic review and meta-regression analysis. PLoS One. 2020 Feb 13;15(2):e0228736.
346. Rochitte CE. Microvascular obstruction the final frontier for a complete myocardial reperfusion. J Am Coll Cardiol. 2008;51(23):2239-40.-347347. Klug G, Mayr A, Schenk S, Esterhammer R, Schocke M, Nocker M, et al. Prognostic value at 5 years of microvascular obstruction after acute myocardial infarction assessed by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14(1):46.
In addition to these applications, CMR is very useful for differentiating ischemic from nonischemic cardiomyopathies;327327. Hundley WG, Bluemke DA, Finn JP, Flamm SD, Fogel MA, Friedrich MG, et al; American College of Cardiology Foundation Task Force on Expert Consensus Documents. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus documenton cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;121(22):2462-508.,348348. Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magneticresonance assessment of non ischaemic cardiomyopathies. Eur Heart J. 2005;26(15):1461-74. it is used to diagnose myocarditis349349. Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, et al; International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53(17):1475-87.,350350. Gannon MP, Schaub E, Grines CL, Saba SG. State of the Art: Evaluation and Prognostication of Myocarditis Using Cardiac MRI. J Magn Reson Imaging, 2019;49 (7), e122-e131 and Takotsubo syndrome.351351. Athanasiadis A, Schneider B, Sechtem U. Role of cardiovascular magnetic resonance in takotsubo cardiomyopathy. Heart Fail Clin. 2013;9(2):167-76.,352352. Scally C, Abbas H, Ahearn T, Srinivasan J, Mezincescu A, Rudd A, et al. Myocardial and Systemic Inflammation in Acute Stress-Induced (Takotsubo) Cardiomyopathy. Circulation. 2019;139 (13):1581-92. Furthermore, when there are elevated myocardial necrosis biomarkers and “normal” coronariography, CMR can confirm the presence of infarction, which could be related to spasm, thrombophilic syndromes, etc. Other differential diagnoses in which CMR can help include LV hypertrophy (primary of hypertrophic cardiomyopathy or secondary) and isolated acute pericarditis without associated myocarditis.
Due to its sensitivity for finding areas of infarction and diagnosing other cardiac abnormalities, CMR has been recommended to assess patients with infarction and those without significant coronary obstructions (MINOCA). The examination allows the correct differentiation between inflammatory processes and actual ischemic necrosis, even without significant atheroma plaque, and can provide fundamental data for managing these cases. Such an approach may be particularly relevant for women, who have a higher incidence of MINOCA.353353. Vidal-Perez R, Abou Jokh Casas C, Agra-Bermejo RM, Alvarez-Alvarez B, Grapsa J, Fontes-Carvalho R, et al. Myocardial infarction with non-obstructive coronary arteries: A comprehensive review and future research directions. World J Cardiol. 2019 Dec 26;11(12):305-15.
In cases of confirmed CAD, CMR can also help define the infarction-related coronary anatomy when it is not clear in catheterization, such as in patients with severe multivessel diseaseand non-specific ECG. In these patients, CMR can also differentiate between old and recent infarctions that are related to the current condition. Finally, CMR can provide high-resolution images for assessing post-infarction mechanical complications, such as LV rupture, mitral regurgitation, an extension of the infarction to the right ventricle, and interventricular communication.340340. Eitel I, de Waha S, Wöhrle J, Fuernau G, Lurz P, Pauschinger M, et al. Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2014 Sep 23;64(12):1217-26.,354354. Perazzolo Marra M, Lima JA, Iliceto S. MRI in acute myocardial infarction. Eur Heart J. 2011;32(3):284-93.
12. Cardiac Catheterization and Intravascular Coronary Imaging (fractional flow reserve, intravascular ultrasound, optical coherence tomography)
Coronary angiography, which is commonly used to diagnose and determine treatment for patients with NSTEMI, may not show the culprit lesion in UA and NSTEMI. This is due to the fact that the test is not 100% sensitive to plaque ruptures.355355. Sandoval Y, Jaffe AS. Type 2 Myocardial Infarction: JACC Review Topic of the Week. J Am Coll Cardiol. 2019 Apr 16;73(14):1846-60. The triggering factors for ACS can range from obstructive atherosclerotic disease (which may be incipient or with or without ruptured plaque) to spasm, embolism, intramural coronary hematoma, spontaneous coronary dissection, and aortic dissection.
In a recent meta-analysis, Barcarawi et al. demonstrated that for patients with NSTE-ACS, an early invasive strategy resulted in a lower incidence of major cardiovascular events (defined by each included study) than late invasive strategy (RR 0, 65, 95% CI 0.49-0.87; p = 0.003), although the benefits persisted only in patients with GRACE scores > 140.356356. Barbarawi, M, Kheiri, B, Zayed, Y, Barbarawi O, Chahine A, Haykal T, et al. Meta‐analysis of optimal timing of coronary intervention in nonST‐elevation acute coronary syndrome. Catheter Cardiovasc Interv. 2020; 95(2): 185– 93. However, there were no significant differences in all-cause mortality, cardiovascular mortality, myocardial infarction or hemorrhagic events between the groups. Early invasive treatment was also associated with a lower risk of ischemic outcomes in the SWEDEHEART study.357357. Lindholm D, Alfredsson J, Angerås O, Bohm F, Calais F, Koul S, et al. Timing of percutaneous coronary intervention in patients with non-ST-elevation myocardial infarction: a SWEDEHEART study. Eur Heart J Qual Care Clin Outcomes. 2017;3(1):53‐60.
The therapeutic strategy, especially with respect to invasive evaluation, depends not only on the anatomical and functional diagnosis but on the comorbidities, clinical presentation, frailty, cognitive status, life expectancy, and, especially, risk stratification, because “very high risk” patients must be evaluated in less than 2 hours and “intermediate risk” patients must be evaluated within 72 hours, as is presented in Table 2.1.358358. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization Eur Heart J. 2019;40(2): 87-165.
The invasive strategy allows diagnosis of underlying CAD, identifies the lesion, guides treatment for antithrombotic drugs, and determines the coronary anatomy for PCI or CABG surgery, since up to 40% of NSTE-ACS patients have plaques of complex morphology and up to 25% have acute coronary occlusion.
The European Society of Cardiology and the European Association of Cardiothoracic Surgery consider FFR to be the diagnostic standard for functional assessment of injury severity in patients with intermediate-grade stenosis (typically around 40% to 50%) without evidence of ischemia in non-invasive tests or in patients with multivessel disease. This guideline includes the instantaneous wave-free ratio (iFR), a new measure that does not require adenosine-induced hyperemia and has a class IA recommendation like FFR.
For left main coronary disease, functional assessment by FFR or iFR can be technically complex, and evidence supporting their use in this condition is scarce. Consequently, intravascular ultrasound is a class IIaB recommendation, and revascularization should be excluded when the minimum luminal area is > 6 mm2. For all lesions outside this area, functional assessment is preferable to intracoronary imaging. Intravascular ultrasound and optical coherence tomography (OCT) are recommended to optimize stenting (class IIaB).
FFR assessment requires the administration of adenosine to obtain maximum and stable hyperemia, unlike iFR, which assesses rest indexes derived only from resting gradients, i.e., the ratio of distal coronary pressure to aortic pressure (Pd/Pa). In the iFR-SWEDEHEART trial, which evaluated iFR-guided revascularization, 17.5% of the patients had ACS at the time of the assessment and, this strategy was not inferior to FFR-guided revascularization regarding the rate of major cardiac events and events up to 12 months after the procedure.359359. Götberg M, Christiansen EH, Gudmundsdottir I J, Sandhall L, Danielewicz M, Jakobsen L, et al. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. N Engl J Med. 2017;376(19):1813-23.
The DEFINE–FLAIR trial,360360. Davies JE, Sen S, Dehbi HM, Al-Lamee R, Petraco R, Nijjer S S, et al. Use of the Instantaneous Wave-free Ratio or Fractional Flow Reserve in PCI. N Engl J Med. 2017;376(19), 1824-34., which evaluated 2,492 patients with CAD, found that iFR-guided coronary revascularization was no less efficacious than FFR-guided revascularization regarding the risk of major adverse cardiac events in 1 year, with the added benefit that iFR was more economical than FFR. The cut-off points for defining a lesion as hemodynamically significant were: iFR ≤ 0.89 and FFR ≤ 0.8.
OCT uses infrared light to provide high-resolution in vivo cross-sectional images of the coronary artery. This imaging technique allows a detailed assessment of plaque morphology in patients with ACS and can clarify the underlying mechanisms, including plaque rupture or erosion and calcification. Therefore, OCT is useful for optimizing PCI and evaluating vascular response to coronary intervention and pharmacological therapy.361361. Matsuo Y, Kubo T, Akasaka T. The use of optical coherence tomography in acute coronary syndrome. Expert Rev Cardiovasc Ther. 2016;14(5):649-57.
By identifying the thrombus and plaque rupture or erosion, OCT can help clarify the nature of lesions and determine the underlying mechanisms of ACS, especially when the lesions are ambiguous on angiography.362362. Ali ZA, Galougahi, KK, Maehara A, Shlofmitz RA, Ben-Yehuda O, Mintz GS, Stone GW. Intracoronary Optical Coherence Tomography 2018. JACC: Cardiovasc Interv. 2017;10(24), 2473–87.
The DOCTORS trial assessed ACS patients referred for angioplasty and stenting in whom a single localized lesion in the culprit artery was considered responsible for ACS.363363. Meneveau N, Souteyrand G, Motreff P, Caussin C, Amabile N, Ohlmann P, et al. Optical Coherence Tomography to Optimize Results of Percutaneous Coronary Intervention in Patients with Non-ST-Elevation Acute Coronary Syndrome: Results of the Multicenter, Randomized DOCTORS Study (Does Optical Coherence Tomography Optimize Results of Stenting). Circulation. 2016;134(13):906‐17. This trial was designed to evaluate the effectiveness of OCT in optimizing percutaneous coronary intervention among patients with NSTE-ACS, demonstrating that stent underexpansion was common (approximately 42%) and an independent predictor of adverse outcomes. The authors concluded that an intervention with OCT results in greater functional benefits according to post-intervention FFR assessment than an intervention guided by routine angiography.
Although long-term data demonstrate that physiological assessment has better results in CAD treatment, FFR and iFR are underused in current practice.364364. Chowdhury M, Osborn EA. Physiological assessment of coronary lesions in 2020. Curr Treat Options Cardiovasc Med. 2020; 22(1):2. Nevertheless, advances in alternative approaches to FFR, including non-invasive coronary computed tomography, invasive angiography, and OCT, are already being guided by artificial intelligence algorithms and robust tools that allow detailed pre-procedure intervention.
13. Myocardial Revascularization (Myocardial Revascularization Surgery and Percutaneous Coronary Intervention)
The difference between UA and NSTEMI treatment in chronic CAD is a quick decision to indicate revascularization, thus avoiding cardiovascular complications.365365. Choudhry NK, Singh JM, Barolet A, Tomlinson GA, Detsky AS. How should patients with unstable angina and non-ST-segment elevation myocardial infarction be managed? A meta-analysis of randomized trials. Am J Med. 2005;118(5):465-74.
366. Damman P, van Geloven N, Wallentin L, Lagerqvist B, Fox KA, Clayton T, et al. Timing of angiography with a routine invasive strategy and long-term outcomes in non-ST-segment elevation acute coronary syndrome: a collaborative analysis of individual patient data from the FRISC II (Fragmin and Fast Revascularization During Instability in Coronary Artery Disease), ICTUS (Invasive Versus Conservative Treatment in Unstable Coronary Syndromes), and RITA-3 (Intervention Versus Conservative Treatment Strategy in Patients With Unstable Angina or Non-ST Elevation Myocardial Infarction) Trials. JACC Cardiovasc Interv. 2012;5(2):191-9.-367367. Zimarino M, Curzen N, Cicchitti V, De Caterina R. The adequacy of myocardial revascularization in patients with multivessel coronary artery disease. Int J Cardiol. 2013;168(3):1748-57. Risk stratification is essential for the proper choice of conservative or invasive treatment. The most common risk scores are TIMI and GRACE.2828. Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000 Aug 16;284(7):835-42.,368368. Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, et al. Predictors of hospital mortality in the Global Registry of Acute Coronary Events. Arch Intern Med 2003;163:2345–53138.
Coronary angiography determines the treatment strategy; revascularization should be immediate in cases of refractory angina and electrical or hemodynamic instability. Earlier approaches also have favorable outcomes and are indicated in high-risk patients.2828. Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000 Aug 16;284(7):835-42.,368368. Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, et al. Predictors of hospital mortality in the Global Registry of Acute Coronary Events. Arch Intern Med 2003;163:2345–53138.
369. Judd E, Hollander JE, Than M, Mueller C. State-of-the-Art Evaluation of Emergency Department Patients Presenting With Potential Acute Coronary Syndromes. Circulation 2016;134:547–564.-370370. Damman P, Hirsch A, Windhausen F, Tijssen JGP, Winter RJ, ICTUS Investigators. 5-year clinical outcomes in the ICTUS (Invasive versus Conservative Treatment in Unstable coronary Syndromes) trial a randomized comparison of an early invasive versus selective invasive management in patients with non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol. 2010;55:858–64. In a cohort of 363,500 diabetic patients with NSTEMI, an early invasive assessment strategy was used in 164,740 patients (45.3%). In an analysis adjusted for propensity score, 21,681 diabetics were paired in each arm. Lower in-hospital mortality was associated with the invasive strategy in NSTEMI but not in UA (2.2% vs. 3.8%; RR 0.57, 95% CI; 0.50-0.63, p < 0.01).371371. Mahmoud AN, Elgendy IY, Mansoor H, Xuerong W, Mojadid MK, Bavry AA, et al. Early invasive strategy and in-hospital survival of diabetics with non-st-elevation Myocardial infarction: a contemporary national insight. JACC. 2017;(69):182.
Invasive strategies include angioplasty or myocardial revascularization surgery.372372. Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1(2):219-27. The SYNTAX Score is a fundamental tool for making this decision based on coronary anatomy.373373. Mohr FW, Morice MC, Kappetein AP, Feldman TE, Ståhle E, Colombo A, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381(9867):629-38. Patients with SYNTAX scores > 22 (intermediate or high) have the greatest long-term benefits from surgical revascularization.374374. Jones EL, Craver JM, Guyton RA, Bone DK, Hatcher CR Jr, Riechwald N. Importance of complete revascularization in performance of the coronary bypass operation. Am J Cardiol. 1983;51(1):7-12.,375375. Bundhun PK, Sookharee Y, Bholee A, Huang F. Application of the SYNTAX score in interventional cardiology: A systematic review and meta-analysis. Medicine (Baltimore). 2017;96(28):e7410.
Approximately 5% to 10% of patients with NSTE-ACS require CABG.376376. Ranasinghe I, Alprandi-Costa B, Chow V, Elliott JM, Waites J, Counsell JT, et al. Risk stratification in the setting of non-ST elevation acute coronary syndromes 1999-2007. Am J Cardiol 2011;108:617–624. This is a challenging subgroup due to its high-risk characteristics compared to patients undergoing elective myocardial revascularization.377377. Fukui T, Tabata M, Morita S, Takanashi S. Early and long-term outcomes of coronary artery bypass grafting in patients with acute coronary syndrome versus stable angina pectoris. J Thorac Cardiovasc Surg 2013;145:1577–1583.
In patients with ongoing ischemia or hemodynamic instability who are indicated for CABG, surgery should be performed as soon as possible and should not be delayed as a consequence of antiplatelet treatment.
Since data from randomized trials are lacking, the ideal time for myocardial revascularization should be determined individually. The risk of ischemic events related to suboptimal antiplatelet therapy while awaiting surgery is < 0.1%, while that of perioperative bleeding complications associated with platelet inhibitors is > 10%.378378. Malm CJ, Hansson EC, Akesson J, Andersson M, Hesse C, Shams Hakimi C, et al. Preoperative platelet function predicts perioperative bleeding complications in ticagrelor-treated cardiac surgery patients: A prospective observational study. Br J Anaesth 2016;117:309–315.
If the patient's clinical condition allows for delayed surgery, antiplatelet therapy should be stopped in a timely manner. Depending on the type and dosage of the agent, the effects should be reduced, e.g., 5, 5, and 7 days before the procedure for clopidogrel, ticagrelor, and prasugrel, respectively. The waiting time for surgery could be shortened by daily platelet aggregation testing to determine whether the patient has recovered from aggregation inhibition. An ongoing Brazilian study (NCT 02516267) is testing this hypothesis.
Current surgical management of NSTE-ACS is similar to that of stable disease. Therefore, after discussion with the heart team and appropriate surgical indication, the most important issue is determining the ideal time to perform the procedure. The best results are achieved by considering the systemic repercussions of the acute event while optimizing the patient's clinical condition. This means that patients could undergo surgery in the same hospital immediately, wait for a reduction of antiplatelet action, or be discharged with an upcoming surgery scheduled.
In an emergency, venous grafts are preferential to arterial grafts. Surgery may be performed with or without cardiopulmonary bypass, according to the technical conditions relevant to each case. In patients with cardiogenic shock, complete revascularization through an angioplasty procedure is an initial option; however, in view of the procedure's limitations, surgery may be indicated according to multi-professional assessment.358358. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization Eur Heart J. 2019;40(2): 87-165.
Patients who undergo surgery 1-2 days and 3-7 days after AMI have similar mortality rates, which suggests that, for some patients, it would be possible to reduce the AMI-CABG interval without compromising the results. A higher mortality rate was observed in patients who underwent surgery on the first day after AMI.379379. Nichols EL, McCullough JN, Ross CS, Kramer RS, Westbrook BM, Klemperer JD, et al. Optimal Timing From Myocardial Infarction to Coronary Artery Bypass Grafting on Hospital Mortality. Ann of Thorac Surg. 2017;103:(1)162–171.
The After Eighty study, conducted in 16 Norwegian centers with NSTEMI and UA patients > 80 years of age, randomized patients to an invasive strategy (ATC or CABG + optimized clinical treatment) vs. conservative strategy with optimized clinical treatment alone. The best outcomes occurred in 93 (40.6%) out of 229 patients in the invasive strategy group and in 140 (61.4%) out of 228 patients in the non-invasive strategy group (RR 0.53 [95% CI 0.41-0.69], p = 0.0001). There was no difference in bleeding complications between the two groups.380380. Tegn N, Abdelnoor M, Aaberge L, Endresen K, Smith P, Aakhus S, et al. After Eighty study investigators. Invasive versus conservative strategy in patients aged 80 years or older with non-ST-elevation myocardial infarction or unstable angina pectoris (After Eighty study): an open-label randomised controlled trial. Lancet. 2016;387(10023):1057–1065.
A multicenter study of 1810 AMI patients without ST elevation or UA within 48 hours of pain onset also compared invasive and conservative strategies. Although there was no difference in infarction or death rates in the first year of follow up, these rates were significantly lower in the invasive strategy group in 5 years.381381. Fox KA, Poole-Wilson P, Clayton TC, Henderson RA, Shaw TRD, Wheatley DJ, et al. 5-year outcome of an interventional strategy in non-ST-elevation acute coronary syndrome: the British Heart Foundation RITA 3 randomised trial. Lancet. 2005;366(9489):914–20.
A systematic review that included 8915 patients, 4545 of whom were assigned to invasive strategy and 4370 to conservative strategy, also observed lower mortality between 6 and 12 months with the invasive strategy.382382. Fanning JP, Nyong J, Scott IA, Aroney CN, Walters DL. Routine Invasive Strategies Versus Selective Invasive Strategies for Unstable Angina and non-ST Elevation Myocardial Infarction in the Stent Era. Cochrane Database Syst Rev. 2016;(5):CD004815. More advanced techniques, such as FFR and intravascular ultrasound, can help determine risk stratification and the therapeutic strategy.383383. Smits PC, Abdel-Wahab M, Neumann FJ, Boxma-de Klerk BM, Lunde K, Schotborgh CE, et al. Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N Engl J Med, 2017;376(13):1234–44.
384. Choi KH, Song YB, Lee JM, Lee SY, Park TK, Yang JH, et al. Impact of Intravascular Ultrasound-Guided Percutaneous Coronary Intervention on Long-Term Clinical Outcomes in Patients Undergoing Complex Procedures. JACC Cardiovasc Interv, 2019;12(7):607–20.-385385. Gao XF, Wang ZM, Wang F, Gu Y, Ge Y, et al. Intravascular Ultrasound Guidance Reduces Cardiac Death and Coronary Revascularization in Patients Undergoing Drug-Eluting Stent Implantation: Results From a Meta-Analysis of 9 Randomized Trials and 4724 Patients. Int J Cardiovasc Imaging. 2019;35(2):239–47.
Part 3 – Discharge Recommendations and Post-discharge Care
1. Lifestyle Change
Secondary prevention is a fundamental part of post-ACS patient care. This stage includes lifestyle changes, cardiovascular rehabilitation, education about risk factors, and promoting better treatment adherence. Despite its proven effectiveness in preventing negative outcomes, secondary prevention is still suboptimal.386386. Nicolau JC, Franken M, Lotufo PA, Carvalho AC, Marin-Neto JA, Gallego F, et al. Use of demonstrably effective therapies in the treatment of acute coronary syndromes:comparison between different Brazilian regions. Analysis of the Brazilian Registry on Acute Coronary Syndromes (BRACE). Arq Bras Cardiol. 2012;98(4):282–9.,387387. Coleman EA. Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc. 2003;51(4):549–55. Patients and family members must be guided in a clear and understandable way regarding evidence-based benefits.388388. Coleman EA, Boult C, American Geriatrics Society Health Care Systems Committee. Improving the quality of transitional care for persons with complex care needs. J Am Geriatr Soc. 2003;51(4):556–7.
389. Coleman EA, Mahoney E, Parry C. Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure. Med Care. 2005;43(3):246–55.-390390. Smith SC Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124(22):2458–73. Increasing the scope of secondary prevention is an important goal in this patient population.
Adequate guidance should be given about returning to work, sexual activities, and driving vehicles, working to gradually reintegrate patients into their routine. In addition, special attention should be paid to psychosocial and socioeconomic issues, including attention to the risk of depression and social isolation.391391. Bernheim SM, Spertus JA, Reid KJ, Bradley E, Desai RA, Peterson ED, et al. Socioeconomic disparities in outcomes after acute myocardial infarction. Am Heart J. 2007;153(2):313–9.
392. Rahimi AR, Spertus JA, Reid KJ,Bernheim SM, Krumholz HM. Financial barriers to health care and outcomes after acute myocardial infarction. JAMA. 2007;297(10):1063–72.
393. Smolderen KG, Spertus JA, Reid KJ, Buchanan DM, Krumholz HM, Denollet J, et al. The association of cognitive and somatic depressive symptoms with depression recognition and outcomes after myocardial infarction. Circ Cardiovasc Qual Outcomes. 2009;2(4):328–37.
394. Taylor RS, Brown A, Ebrahim S, Jollife J, Noorani H, Becky KR, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116(10):682–92.
395. Leon AS, Franklin BA, Costa F, Balady GB, Berra KA, Stewart KJ, et al. Cardiac rehabilitation and secondary prevention of coronary heart disease: an American Heart Association scientific statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity), in collaboration with the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2005;111(3):369–76.
396. Suaya JA, Stason WB, Ades PA, Normand ST, Shepard DS, et al. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol. 2009;54(1):25–33.
397. Iestra JA, Kromhout D, van der Schouw YT, Grobbee DE, Boshuizen HC, van Staveren WA. Effect size estimates of lifestyle and dietary changes on all-cause mortality in coronary artery disease patients: a systematic review. Circulation. 2005;112(6)924–34.-398398. Anderson L1, Thompson DR, Oldridge N, Zwisler AD, Rees K, Martin N, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2016 Jan 5;67(1):1-12.
1.1. Smoking Cessation
Smoking cessation, a particularly important factor in lifestyle change, is a very effective measure for reducing mortality in post-NSTE-ACS patients.399399. Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA. 2003;290(1):86–97. Cigarettes contain approximately 7,000 chemical components, many of which directly interfere with cardiovascular disease, 69 of which are carcinogenic.400400. National Center for Chronic Disease Prevention and Health Promotion(US) Office on Smoking and Health. The health consequences of smoking— 50 years of progress: a report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention,(US); 2014. Cigarettes interfere with the cardiovascular system in various ways, increasing the heart rate and myocardial oxygen demand while decreasing supply.401401. Kalkhoran S, Benowitz N, Rigotti N. Prevention and Treatment of Tobacco Use.JACC Health Promotion Series. J Am Coll Cardiol. 2018;72(9):1030-45. Smoking increases the incidence of sudden death,402402. Goldenberg I, Jonas M, Tenenbaum A, Boyoko V, Matetzky S, Shotan A, et al. Current smoking, smoking cessation, and the risk of sudden cardiac death in patients with coronary artery disease. Arch Intern Med. 2003;163(19):2301–5. the risk of thrombosis, inflammation, vasoconstriction, and increased oxidation of LDL cholesterol.403403. Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol. 2014;34(3):509–15. It is estimated that the risk of atrial fibrillation increases 1.5 to 2 times among smokers.404404. Chamberlain AM, Agarwal SK, Folsom AR, et al. (2011) Smoking and incidence of atrial fibrillation: results from the Atherosclerosis Risk in Communities (ARIC) study. Heart Rhythm.2011;8(8):1160-6.
In patients after NSTE-ACS, referral to a smoking cessation program and pharmacological agents, such as nicotine patches or gum, have proven useful.405405. Daly LE, Mulcahy R, Graham IM, Hickey N. Long term effect on mortality of stopping smoking after unstable angina and myocardial infarction. Br Med J (Clin Res Ed). 1983;287(6388):324-6. In an observational study of 12,656 patients, Parasuraman et al. found that, compared to ex-smokers and never smokers, smoking patients underwent coronary intervention at an earlier age, and the intervention was more associated with ACS. Former smokers had similar results to never smokers.406406. Parasuraman S, Zaman AG, Egred M, Bagnall A, Broadhurst PA, Ahmed J, et al. Smoking status and mortality outcomes following percutaneous coronary intervention. Eur J Prev Cardiol. 2020 Feb 3; 2047487320902325.
The smoking cessation strategy should play a central role in the patient's overall rehabilitation and begin as soon as possible, even during the hospital phase. Family members who live in the patient's house should also be encouraged to participate in smoking cessation programs, thus reinforcing the patient's commitment, in addition to reducing the risk of secondhand smoke.407407. Ranney L, Melvin C, Lux L, McClain E, Lohr KN. Systematic review: smoking cessation intervention strategies for adults and adults in special populations. Ann Intern Med. 2006;145(11):845-56.
Four steps are involved in treating nicotine addiction. The Fagerström test (Table 3.1) is commonly used for the first step, determining the addiction level. The next steps are counseling, medication/behavioral treatment, and clinical follow-up.
To prevent relapse, it is important to identify high-risk situations and act to address them. The strategies basically consist of avoiding, escaping, distracting, and postponing. Parties, alcohol or caffeine intake, and meeting with smokers should be avoided, at least during the first weeks of cessation.
Medications such as bupropion, varenicline, and nicotine replacement therapy may increase the risk of cessation, especially in association with other types of therapy, such as support and counseling groups, psychotherapy, and alcohol withdrawal.
Bupropion is an antidepressant with anxiolytic action that several studies have found to decrease withdrawal symptoms. One study that compared smoking cessation among patients treated with bupropion 300 mg/day vs. placebo found abstinence rates of 44.2% and 9.2% in the bupropion and placebo groups, respectively.408408. Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, et al. Explaining the decrease in U.S. deaths from coronary disease,1980–2000. N Engl J Med. 2007;356(23):2388-98. A literature survey revealed that bupropion appears to be more effective in patients who have abandoned and/or failed nicotine replacement therapy.409409. Lindson N, Klemperer E, Hong B, Ordóñez-Mena JM, Aveyard P. Smoking reduction interventions for smoking cassation. Cochrane Database Syst Rev. 2019 Sep 30;9(9):CD013183. On the other hand, two studies on smokers hospitalized for acute coronary events found no difference in smoking cessation between bupropion and placebo groups.410410. Eisenberg MJ, Grandi SM, Gervais A, O’Loughlin J, Paradis G, Rinfret S, et al. Bupropion for smoking cessation in patients hospitalized with acute myocardial infarction: a randomized, placebo-controlled trial. J Am Coll Cardiol 2013; 61(5):524–32.,411411. Planer D, Lev I, Elitzur Y, Sharon N, Ouzan E, Pugatsch T, et al. Bupropion for smoking cessation in patients with acute coronary syndrome. Arch Intern Med. 2011;171(12):1055-60.
Nicotine replacement therapy is effective and increases smoking cessation rates. In one study, any form of nicotine replacement resulted in up to a 60% increase in abstinence (RR: 1.60; 95% CI: 1.53 to 1.68) compared to placebo. The increase in efficacy only occurred when comparing an association of nicotine replacement modes to a single mode(RR: 1.34; 95% CI: 1.18 to 1.51).412412. Stead LF, Perera R,Bullen C,Mant D, Hartman-Boyce J, Cahill K, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev.2018;5(5):CD000146. The most common long-term nicotine replacement mode is transdermal patches, while the most common short-term modes are chewing gum, nasal sprays, and inhalers, some of which are still not available in Brazil.
Varenicline is a nicotine receptor partial agonist that, by replacing the action of nicotine, reduces the intensity of withdrawal symptoms. Its bond with the receptor also reduces the reward and pleasure effects of smoking, which can reduce some of the effects of deprivation. Its effectiveness has been demonstrated in six clinical trials with a total of 3,659 chronic smokers.413413. Wilson K, Gibson N, Willan A, Cook D. Effect of smoking cessationon mortality after myocardial infarction: meta-analysis of cohortstudies. Arch Intern Med. 2000;160(7):939-44.
414. Agency for Health Care Research and Quality. (AHRQ). Clinical Practice Guidelines: Smoking Cessation. No 18. AHCPR Publication No.96-0692; 1996.
415. Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al; Varenicline Phase 3 Study Group. Efficacy of varenicline, analpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placeboor sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56-63. Erratum in JAMA. 2006;296(11):1355.-416416. Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47-55. Varenicline doubled abstinence rates compared to placebo (RR: 2.24; 95% CI: 2.06 to 2.43).417417. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Sao Paulo Med J.2012;130(5):346-7. (Cochrane Highlights) A study involving more than 8,000 smokers compared the use of varenicline, bupropion, nicotine patches,and placebo, randomized in a 1:1:1:1 ratio, and found a higher abstinence rate in the varenicline group at 6 months than the other groups.418418. Anthenelli RM, Benowitz NL, West R, Russ C, McRae T, Lawrence D, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric isorders EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet.2016; 387(10037):P2507-20. In a randomized study in patients with chronic coronary disease, the probability of continuous abstinence after 1 year was higher in the varenicline group than the placebo group (OR: 3.14; 95% CI: 1.94 to 5.11).419419. Rigotti NA, Pipe AL, Benowitz NL, Arteaga C, Garza D, Tonstad S. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation. 2010; 121(2):221–9. Two randomized studies in smokers with ACS found higher rates of continuous abstinence at 24 weeks and a higher rate of abstinence at 52 weeks in the varenicline group than the placebo group.420420. Eisenberg MJ, Windle SB, Roy N, Old W, Grondin FR, Bata I, et al. Varenicline for smoking cessation in hospitalized patients with acute coronary syndrome. Circulation. 2016; 133(1):21–30.,421421. Windle SB, Dehghani P, Roy N, Old W, Grondin FR, Bhata I, et al. Smoking abstinence 1 year after acute coronary syndrome: follow-up from a randomized controlled trial of varenicline in patients admitted to hospital. CMAJ.2018;190(12):E347-E354. One of varenicline's main limitations is its high cost and the occurrence of nausea as a limiting side effect in some patients.
An association of methods seems to be more effective than treatment with a single method. In a recent meta-analysis of four studies, an association of varenicline + bupropion resulted in higher abstinence rates at 6 months than varenicline alone. The effects were more pronounced in more nicotine-dependent and heavier smokers. The benefits, however, did not last for 12 months, and the association significantly increased anxiety symptoms.422422. Zhong Z, Zhao S, Zhao Y, Xia S. Combination therapy of varenicline and bupropion in smoking cessation: A meta-analysis of the randomized controlled trials. Compr Psychiatry. 2019; 95:152125. Therefore, an association between methods appears to be safer and more effective when varenicline or bupropion is combined with nicotine replacement therapy.
Bupropion and varenicline also appear to be safe for patients with cardiovascular disease. Some studies have found an increased occurrence of neuropsychiatric events with these medications, which led the U.S. Food and Drug Administration to require a label warning. After further study, this risk was reassessed, and the warning was withdrawn in 2016.423423. Kalkhoran S, Benowitz NL, Rigotti N. Prevention and Treatment of Tobacco Use. J Am Coll Cardiol. 2018;72(23):2964-979.
Replacing traditional cigarettes with low-nicotine cigarettes or vaporizers (electronic cigarettes) is a controversial issue.424424. Benowitz NL,Donny EC, Hatsukami DK. Reduced nicotine content cigarettes, e-cigarettes and the cigarette end game. Addiction.2017; 112(1):6–7. Both strategies are only classified as potential damage control.
Studies on low-nicotine cigarettes indicate that they have little effect, since smokers generally compensate by smoking more cigarettes or adding other forms of nicotine.425425. Hatsukami DK, Donny EC, Koopmeiners JS, Benowitz NL. Compensatory smoking from gradual and immediate reduction in cigarette nicotine content. Cancer Epidemiol Biomarkers Prev.2015; 24(2):472–6. Vaporizers apparently expose the smoker to fewer carcinogens than traditional cigarettes, although there is great variability in the toxic substances they contain. In addition, there has been an explosive increase in their use among young people, especially in the USA.426426. MacDonald A, Middlekauff HR. Electronic cigarettes and cardiovascular health: what do we know so far? Vasc Health Risk Manag. 2019 jun 21;15:159-74. Sale of these devices is prohibited in Brazil. They have been associated with several recent deaths in the USA due to acute lung problems from inhaled substances. A total of 2,807 vaping-related hospitalizations and 68 deaths have been confirmed by the U.S. Centers for Disease Control. To date, there is no evidence that these strategies facilitate smoking cessation, and electronic cigarettes, in particular, are potentially dangerous.
1.2. Dietary Recommendations
Since patients with NSTE-ACS are classified as very high cardiovascular risk, a considerable reduction in cholesterol values is absolutely indispensable. In addition to statin therapy, which is supported by the results of several meta-analyses427427. Baigent C, Blackwell L, Emberson J, Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376: 1670–81.,428428. Collins R, Reith C, Emberson J,Bhala N, Peto R, Barnes EH, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388(9753):2532–61. and randomized trials,429429. Amarenco P, Bogousslavsky J, Callahan A 3rd,Goldstein LB, Hennerici M, Rudolph AE, et al. High-dose atorvastatin after stroke or transiente ischemic attack. N Engl J Med. 2006;355(6):549–59.
430. Athyros VG, Papageorgiou AA, Mercouris BR, Athyros VV, Symeonidis AN, Basayannis EO, et al. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus ’usual’ care in secondary coronary heart disease prevention. The GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study. Curr Med Res Opin. 2002;18(4):220–8.
431. Kjekshus J, Apetrei E, Barrios V, Bohm M, Cleland JG, Cornel JH, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357(22):2248–61.-432432. Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008; 372(9645):1231–9. dietary therapy is of central importance and is supported by significant evidence.433433. Eckel RH, Jakicic JM, Ard JD,de Jesus JM, Houston Miller N, Hubbard VS, et al. et al. 2013 AHA / ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S76–99.,434434. Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985–3023.
A standard diet rich in fruits, vegetables, whole grains, healthy protein sources (dairy products, low-fat [skinless] poultry, fish/seafood, and nuts, non-tropical vegetable oils is recommended. Sweets, sugary drinks, and sugar itself shuld be avoided, as well as excess red meat. The PREDIMED trial tested a Mediterranean-type diet, finding a reduction in cardiovascular events, especially stroke.435435. Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med. 2018 Jun 21;378(25):e34.
This diet must be adjusted for appropriate caloric requirements, cultural patterns, food preferences,,a nd nutritional therapy for other conditions, including DM. Caloric intake should be adjusted to avoid weight gain and, for overweight/obese patients, it must promote weight loss. Vitamins or antioxidant food supplements should not be routinely prescribed for secondary prevention.436436. Bjelakovic G, Nikolova D, Gluud LL,Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007; 297(8):842–57.
2. Cardiovascular Rehabilitation
Physical exercise reduces atherogenesis, promotes anti-inflammatory action, improves endothelial function, decreases sympathetic tone, increases HDL, and reduces blood pressure and insulin resistance, among other beneficial effects, and should not be denied to patients after ACS.437437. Carvalho T, Milani M, Ferraz AS, Silveira AD, Herdy AH, Hossri CAC, et al. Diretriz Brasileira de Reabilitação Cardiovascular – 2020. Arq Bras Cardiol. 2020; 114(5):943-87. Exercise is included in the therapeutic context of cardiovascular rehabilitation programs.. Sedentary lifestyle, a worldwide problem, is the main target of primary prevention and is even more important for secondary prevention of coronary events.
The cardiovascular rehabilitation process of ACS patients should begin during hospitalization withphysical therapy, including early walkingand passive and active movement of the main muscle groups. Even while hospitalized, the patient should be familiarized with scales of perceived effort (e.g., the BORG scale), which will be useful after discharge.
At discharge, every patient should receive adequate guidance about physical activity, which should initially be mild intensity, a continuation of the exercises performed during the hospital phase (phase 1), including self-monitoring techniques. Early assessment in a formal rehabilitation program should be indicated, or, when unavailable, the patient should be advised about an exercise program that can be administered by a physical therapist or physical educator according to doctor-imposed limits (phase 2).
Studies have shown that patients referred to exercise programs after an acute coronary event have a better quality of life, less symptom recurrence, and fewer new cardiovascular events.438438. Kureshi F, Kennedy KF, Jones PG, Thomas RJ, Arnold S V., Sharma P, et al. Association between cardiac rehabilitation participation and health status outcomes after acute myocardial infarction. JAMA Cardiol. 2016;1(9):980–8.
439. Suaya JA, Shepard DS, Normand S-LT, Ades PA, Prottas J, Stason WB. Use of Cardiac Rehabilitation by Medicare Beneficiaries After Myocardial Infarction or Coronary Bypass Surgery. Circulation. 2007 Oct 9;116(15):1653 LP – 1662.-440440. Sunamura M, Ter Hoeve N, van den Berg-Emons RJG, Boersma E, van Domburg RT, Geleijnse ML. Cardiac rehabilitation in patients with acute coronary syndrome with primary percutaneous coronary intervention is associated with improved 10-year survival. Eur Heart J Qual Care Clin Outcomes. 2018 Jul;4(3):168–72.
Despite the positive effects of rehabilitation programs, systematic referral and compliance are still major challenges.441441. Ades PA, Keteyian SJ, Wright JS, Hamm LF, Lui K, Newlin K, et al. Increasing Cardiac Rehabilitation Participation From 20% to 70%: A Road Map From the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin Proc. 2017;92(2):234-42. A lack of continuing medical education in cardiac rehabilitation, a lack of knowledge about program safety (event rate of 1 for every 112,000 patients/hour), and difficulty making these programs cost-effective are important parts of the problem.
2.1. Instructions about Physical Activity at Discharge
The first step in assessing patients for a cardiovascular rehabilitation program after clinical evaluation and complementary exams is to determine whether there is any absolute contraindication to physical exertion.442442. Correia LC, Garcia G, Kalil F, Ferreira F, Carvalhal M, Oliveira R,et al. Prognostic value of TIMI score versus GRACE score in ST-segment elevation myocardial infarction. Arq Bras Cardiol. 2014 Aug;103(2):98-106.,443443. Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, et al. Exercise standards for testing and training: A scientific statement from the American heart association. Circulation. 2013;128(8):873–934. Table 3.2 lists contraindications to regular physical activity.
The risk classification for physical activity in cardiac patients is based on clinical data regarding angina, functional classification, ventricular function, residual coronary lesions, and arrhythmias. This classification is useful for determining the level of support required during the program and the need for monitoring during exercise sessions.442442. Correia LC, Garcia G, Kalil F, Ferreira F, Carvalhal M, Oliveira R,et al. Prognostic value of TIMI score versus GRACE score in ST-segment elevation myocardial infarction. Arq Bras Cardiol. 2014 Aug;103(2):98-106.,443443. Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, et al. Exercise standards for testing and training: A scientific statement from the American heart association. Circulation. 2013;128(8):873–934.
Low-risk patients may begin exercising with a physical therapist or physical educator, without necessarily being supervised by a physician. Moderate risk patients, however, should preferably exercise in an environment with medical supervision and advanced life support resources; they can progress to a semi-supervised external program if no complications occur after 12 weeks. When a medically supervised cardiovascular rehabilitation program is not available, these patients can exercise under the supervision of a physical educator or physical therapist, conforming to safety limits predetermined by the cardiologist. The exercise team must have training in at least basic life support and an automatic external defibrillator must be available on site. High-risk patients should exercise under medical supervision, with a physical therapy and/or physical education team trained in basic life support and with local resources for advanced life support.
In all situations, heart rate monitoring should guide exertion intensity. The need for electrocardiographic monitoring during the rehabilitation program can be defined on a case-by-case basis by the medical team.
Safe exercise zones can be ideally defined through a functional test (cardiopulmonary test or ET) or, in specific cases, they can be guided by perceived effort. When a cardiopulmonary test is available, aerobic exercise should generally involve a heart rate between what is indicated by the anaerobic threshold and the respiratory compensation point, ie, a zone at which, despite the production of lactic acid, there is no decompensated metabolic acidosis, which is associated with a lower risk of arrhythmic events or cardiovascular overload. When a cardiopulmonary test is not available, exercise at between 70% and 85% of the maximum heart rate achieved in an ET or 50% to 80% of the heart rate reserve added to the resting heart rate, where: heart rate reserve = peak heart rate - resting heart rate. In patients with ECG changes or symptoms suggestive of ischemia on exertion, the limit should, in general, be 10 beats less than symptom onset or ST change. In specific cases and under medical supervision, the patient can approach the point of ischemia onset.
The doctor should ideally determine the following exercise parameters:
-
Weekly frequency.
-
Intensity (using a Borg scale and the heart rate or load determined in a cardiopulmonary or ET).
-
Mode (eg, walking, cycling, rowing).
-
Session duration.
-
Resistance exercises should also be encouraged. The recommended intensity is 40% to 60% of the maximum voluntary contraction (low to moderate intensity), 8 to 15 repetitions, 1 to 3 sets.443443. Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, et al. Exercise standards for testing and training: A scientific statement from the American heart association. Circulation. 2013;128(8):873–934.,444444. Costa RVC, Braga AMW, Carlos A. Arquivos Brasileiros de Cardiologia I CONSENSO NACIONAL DE REABILITAÇÃO CARDIOVASCULAR. Arq Bras Cardiol. 1997;69(4):267–91.
3. Medications to be Prescribed at Discharge
3.1. Antithrombotic drugs
A. Switching Antiplatelet Treatment
Although a second antiplatelet may have been previously selected for use with ASA, at the time of hospital discharge it may be necessary to switch to a different antiplatelet for some reason, such as:
-
Cost.
-
Limiting adverse effects (eg, dyspnea with ticagrelor, bleeding while using the most potent drugs, such as ticagrelor and prasugrel).
-
Patient preference for a single daily dose (eg, prasugrel, if treated with PCI and without prior stroke/TIA) rather than twice a day (ticagrelor).
The transition from clopidogrel to ticagrelor has been the only such change studied with sufficient power to assess clinical outcomes, despite the fact that the study was not developed for this purpose. In the PLATO trial, 46% of the patients randomized to receive ticagrelor had been pretreated with clopidogrel (300 to 600 mg dose).152152. Wallentin L, Becker RC, Budaj A, Cannon CP, Emmanuelson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(1):1045-57. The safety and efficacy of ticagrelor were not affected by previous use of clopidogrel.248248. Cannon CP, Harrington RA, James S, Ardissino D, Becker RC, Emanuelsson H, et al; PLATelet inhibition and patient Outcomes Investigators. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet. 2010;375(9711):283-93. In the TRITON-TIMI-38 trial, which evaluated the use of prasugrel, previous use of another P2Y12 inhibitor was considered an exclusion criterion.154154. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007; 357(20): 2001-15. Other changes in P2Y12 inhibitors (e.g., between ticagrelor and prasugrel or from ticagrelor/prasugrel to clopidogrel) have been evaluated by pharmacodynamic and observational studies, which serve more as hypothesis generating, since they were not designed to assess clinical outcomes.445445. Loh JP, Pendyala LK, Kitabata H, Torguson R, Chen F, Kent KM, et al. Safety of reloading prasugrel in addition to clopidogrel loading in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Am J Cardiol.2013;111(6):841-5.
446. Zettler ME, Peterson ED, McCoy LA, Effron MB, Anstrom KJ, Henry TD, et al. Switching of adenosine diphosphate receptor inhibitor after hospital discharge among myocardial infarction patients: Insights from the Treatment with Adenosine Diphosphate Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events after. Am Heart J. 2017J Jan; 183:62-8.
447. Angiolillo DJ, Curzen N, Gurbel P, Vaitkus P, Lipkin F, Li W, et al. Pharmacodynamic evaluation of switching from ticagrelor to prasugrel in patients with stable coronary artery disease: Results of the swap-2 study (switching anti platelet-2). J Am Coll Cardiol. 2014;63(15):1500–9.
448. Pourdjabbar A, Hibbert B, Chong AY, Le May MR, Labinaz M, Simard T, et al. A randomised study for optimising crossover from ticagrelor to clopidogrel in patients with acute coronary syndrome. Thromb Haemost. 2017;117(2):303–10.-449449. Franchi F, Rollini F, Rios JR, Rivas A, Agarwal M, Kureti M, et al. Pharmacodynamic effects of switching from ticagrelor to clopidogrel in patients with coronary artery disease results of the SWAP-4 study. Circulation. 2018;137(23):2450–62. Thus, there is less evidence to support such transitions. Figure 3.1 shows the recommendations for switching P2Y12 agents in patients with ACS for less than 30 days.450450. Angiolillo DJ, Rollini F, Storey RF, Bhatt DL, James S, Schneider DJ, et al. International expert consensus on switching platelet P2Y12 receptor-inhibiting therapies. Circulation. 2017;136(20):1955–75.,451451. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur Heart J. 2017;53(1):213–54.
Recommendations for switching P2Y12 inhibitors in the first 30 days after acute coronary syndrome. A loading dose of the new agent is routinely recommended. *If prasugrel or ticagrelor therapy is being de-escalated due to bleeding or an increased risk of bleeding, only a maintenance dose of clopidogrel can be considered. Adapted from Valgimigli et al.451451. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur Heart J. 2017;53(1):213–54.
Thus, as a general rule, when switching P2Y12 antiplatelet therapy during the acute phase of coronary disease (< 30 days), a loading dose of the new medication should be used, followed by a maintenance dose. One exception to this rule is when prasugrel or ticagrelor is replaced with clopidogrel due to bleeding or increased risk of bleeding. The new drug should be administered 24 hours after the final administration of the first drug, with the exception of when clopidogrel is being replaced.
When the patient has had ACS for more than 30 days, and it becomes necessary to switch agents, see Figure 3.2.
Recommendations for changing P2Y12 inhibitors in patients after 30 days of acute coronary syndrome. * If ticagrelor to clopidogrel therapy is being de-escalated due to bleeding or an increased risk of bleeding, only a maintenance dose of clopidogrel can be considered. Adapted from Valgimigli et al.451451. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur Heart J. 2017;53(1):213–54.
Some differences should be considered:
-
In all situations, a 24-hour wait is recommended between the final dose of the previous medication and the first dose of the new medication.
-
A loading dose of the new agent is recommended only when ticagrelor is being replaced. Clinical studies have shown greater platelet reactivity in the first 48 hours when changing from ticagrelor to clopidogrel448448. Pourdjabbar A, Hibbert B, Chong AY, Le May MR, Labinaz M, Simard T, et al. A randomised study for optimising crossover from ticagrelor to clopidogrel in patients with acute coronary syndrome. Thromb Haemost. 2017;117(2):303–10.,449449. Franchi F, Rollini F, Rios JR, Rivas A, Agarwal M, Kureti M, et al. Pharmacodynamic effects of switching from ticagrelor to clopidogrel in patients with coronary artery disease results of the SWAP-4 study. Circulation. 2018;137(23):2450–62. or prasugrel.447447. Angiolillo DJ, Curzen N, Gurbel P, Vaitkus P, Lipkin F, Li W, et al. Pharmacodynamic evaluation of switching from ticagrelor to prasugrel in patients with stable coronary artery disease: Results of the swap-2 study (switching anti platelet-2). J Am Coll Cardiol. 2014;63(15):1500–9. This difference is due to the fact that ticagrelor is a reversible P2Y12 inhibitor, while thienopyridines (clopidogrel and prasugrel) are non-reversible.
B. Duration of Dual Antithrombotic Therapy
B.1. More than 12 Months
B.1.1. Antiplatelets
Studies have assessed the use of clopidogrel, prasugrel, and ticagrelor in ACS patients on DAPT for an average of 12 months. This is the recommended time, regardless of therapeutic strategy (clinical treatment, angioplasty, or myocardial revascularization surgery). For patients who receive clinical treatment, the only options for antiplatelet drugs are ticagrelor and clopidogre, since it has been demonstrated that prasugrel is not superior to clopidogrel in such cases.452452. Roe MT, Armstrong PW, Fox KAA, White HD, Prabhakaran D, Goodman SG, et al. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med. 2012;367(14):1297–309.
DAPT is a very effective way to prevent stent thrombosis. The risk of late (between 1 month and one year after stent implantation) and very late stent thrombosis (> 1 year after angioplasty) has been decreasing considerably with the use of more modern drug-eluting stents. Thus, continuing DAPT more than one year after an ACS event and angioplasty seems to carry an increased risk of bleeding that is more harmful than the potential benefits of preventing very late stent thrombosis. However, the PEGASUS trial demonstrated that in patients with a history of infarction (> 1 year) and additional high risk criteria (DM, age > 65 years, chronic kidney disease, multivessel coronary diseas or two or more previous infarctions), prolonged DAPT with ASA + ticagrelor reduced the incidence of ischemic outcomes, including new cases of infarction unrelated to stent thrombosis or stroke, compared to ASA monotherapy (HR 0.84; 95% CI 0.74 to 0.95; p = 0.004 and HR 0.85; 95% CI 0.75 to 0.96; p = 0.008, for doses of 60 mg and 90 mg, respectively).453453. Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, et al. Long-Term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372(19):1791–800. Continuing DAPT beyond 12 months would thus be more associated with systemic protection against thrombotic events than with preventing late stent thrombosis. This finding was reinforced by a subanalysis of the same trial, which found a similar reduction of ischemic cardiovascular events in patients with no history of stent angioplasty and patients with previous angioplasty.454454. Furtado RHM, Nicolau JC, Magnani G, Im K, Bhatt DL, Storey RF, et al. Long-term ticagrelor for secondary prevention in patients with prior myocardial infarction and no history of coronary stenting: insights from PEGASUS-TIMI 54. Eur Heart J.2020;41(17):1625-32. However, the decrease in cardiovascular events was accompanied by an increase in major bleeding episodes (HR 2.32; 95% CI 1.68 to 3.21; p < 0.001 and HR 2.69, 95% CI 1.96 to 3, 70; p < 0.001, for doses of 60 and 90 mg, respectively). There was no increase in fatal bleeding or intracranial hemorrhage with ticagrelor. The highest dose of ticagrelor (90 mg, twice a day) did not reduce ischemic risk and led to higher bleeding rates. Thus, the 60 mg dose is approved for selected patients 1 year after NSTEMI who tolerated DAPT during this period.
The DAPT trial also assessed the use of DAPT for > 12 months with clopidogrel and prasugrel (65.2% and 34.8% of patients, respectively).455455. Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014;371(23):2155–66. All included patients had undergone angioplasty with a drug-eluting st,ent and angioplasty in the context of NSTE-ACS was performed in approximately one third of the patients. In this study, patients received DAPT for a standard 12-month period and, if they adhered to treatment and did not experience relevant bleeding, they were then randomized to continue DAPT or ASA + placebo for another 18 months. Like the PEGASUS trial, a reduction in ischemic events occurred, but this benefit was offset by the increased risk of bleeding. Although stent thrombosis was reduced by one case in every 100 patients treated with prolonged DAPT, major or moderate bleeding occurred in one out of every 111 patients.
A meta-analysis of > 32,000 patients before the publication of the PEGASUS trial found a significant increase in infarction incidence in the group that received DAPT for 12 months (compared with > 12 months; OR = 1.57, 95% CI 1.30 to 1.90), as well as a lower incidence of bleeding (OR = 0.65, 95% CI 0.52 to 0.81).456456. Giustino G, Baber U, Sartori S, Mehran R, Mastoris I, Kini AS, et al. Duration of dual antiplatelet therapy after drug-eluting stent implantation: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2015 Apr 7;65(13):1298-310. Subsequent meta-analysis, including the PEGASUS trial, found a significant decrease in all-cause mortality in the DAPT > 12 months group (HR = 0.89, 95% CI 0.79 to 0.99).457457. Bonaca MP, Sabatine MS. Antiplatelet Therapy for Long-term Secondary Prevention After Myocardial Infarction. JAMA Cardiol. 2016 Sep 1;1(6):627-8. It was concluded that any of the three P2Y12 inhibitors available in Brazil (clopidogrel, ticagrelor, and prasugrel) can reduce ischemic events when used in association with ASA for more than 12 months, although at an increased risk of bleeding. Thus, patient risk for ischemic events and bleeding must be individually assessed. Clinical scores allow ischemic and hemorrhagic risk to be estimated with more objective parameters according to the patient's clinical characteristics, assisting with decision making regarding the duration of DAPT.
The PRECISE-DAPT trial assessed 14,963 patients who had undergone angioplasty, using a score that consisted of five variables (age, creatinine clearance, hemoglobin, leukocyte count, and a history of spontaneous bleeding) to predict the risk of bleeding.458458. Costa F, van Klaveren D, James S, Heg D, Räber L, Feres F, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet.2017;389(10073):1025-34. The score was also validated in a group of more than 14,000 patients. Patients with low scores (< 25) benefitted from prolonged DAPT, including a reduction in the composite ischemic outcome (AMI, definitive stent thrombosis, stroke, and need for revascularization); the number needed to treat was 65, and there was no significant increasing in risk of bleeding. On the other hand, in patients with high scores (≥ 25), prolonged DAPT was associated with an increased risk of bleeding (number needed to harm = 38) and provided no benefits regarding ischemic outcome prevention. It should be pointed out, however, that this score was derived from randomized studies with PCI (with or without ACS) and not exclusively from a population with ACS, whose risk of ischemic events tends to be higher than that of patients with stable CAD.
The DAPT score (Table 3.3) was derived from a cohort in the DAPT trial.459459. Yeh RW, Secemsky EA, Kereiakes DJ, Normand SLT, Gershlick AH, Cohen DJ, et al. Development and validation of a prediction rule for benefit and harm of Dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA - J Am Med Assoc 2016;315(16):1735–49. In this analysis, patients with a high risk score (≥ 2) who received DAPT for 30 months had a significant reduction in ischemic outcomes (number needed to treat = 34) and a small increase in the risk of bleeding (number needed to harm = 272). On the other hand, in patients with a low score (< 2), prolonged DAPT led to no reduction in ischemic risk and a considerable increase in the risk of bleeding (number needed to harm = 64).
B.1.2. Anticoagulants
The COMPASS study randomized > 27,000 patients with chronic coronary disease to rivaroxaban 2.5 mg twice a day + ASA 100 mg once a day (n = 9152) or rivaroxaban 5 mg twice a day (n = 9117) or ASA 100 mg (n = 9126). In an average follow-up of 23 months, rivaroxaban + ASA was superior to ASA alone (HR = 0.76, 95% CI 0.66 to 0.86 for the primary outcome (cardiovascular death, AMI or stroke), although it increased the incidence of bleeding (HR = 1.70, 95% CI 1.40 to 2.05). Importantly, there was a significant decrease in all-cause mortality (HR = 0.82, 95% CI 0.71 to 0.96) but no significant difference in the incidence of fatal or intracerebral bleeding.460460. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, et al; COMPASS Investigators. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N Engl J Med. 2017 Oct 5;377(14):1319-30. Considering only the rivaroxaban + ASA and ASA groups, 2423 patients had a history of AMI < 2 years, 3279 between 2 and 5 years, and 5673 > 5 years. The comparison between groups showed hazard ratios of 0.70 for patients with AMI < 2 years, 0.81 for those with AMI between 2 and 5 years, and 0.72 for those with AMI > 5 years (P-interaction = 0.93).461461. Connolly SJ, Eikelboom JW, Bosch J, Dagenais G, Dyal L, Lanas F, et al; COMPASS investigators. Rivaroxaban with or without aspirin in patients with stable coronary artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018 Jan 20;391(10117):205-18.
B.2. Less than 12 Months
Ticagrelor monotherapy was evaluated in the GLOBAL LEADERS trial 462462. Vranckx P, Valgimigli M, Jüni P, Hamm C, Steg PG, Heg D, et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-la. Lancet. 2018;392(10151):940–9. in 15,968 patients undergoing PCI (34% with NSTE-ACS). The groups received ASA + ticagrelor for 1 month, followed by ticagrelor monotherapy for 23 months or by standard DAPT with ASA + clopidogrel (stable CAD) or ASA + ticagrelor (acute CAD) for 12 months, followed by ASA monotherapy for 12 more months. At the end of 2 years of follow-up, the primary outcome (all-cause mortality or infarction) was similar in both groups (RR 0.87, 95% CI 0.75 to 1.01). The secondary outcome, bleeding (BARC 3 or 5), was also similar in both groups (RR = 0.97, 95% CI 0.78 to 1.20). Secondary analysis in a later publication found a significant decrease in the primary outcome in patients with acute CAD and PCI in more than one coronary artery.463463. Takahashi K, Serruys PW, Chichareon P, Chang CC, Tomaniak M, Modolo R, et al. Efficacy and Safety of Ticagrelor Monotherapy in Patients Undergoing Multivessel PCI. J Am Coll Cardiol. 2019 Oct 22;74(16):2015-27.
The TWILIGHT trial evaluated whether early discontinuation of ASA would be safe in patients treated with PCI who had a high risk of thrombosis or bleeding according to clinical and angiographic characteristics.464464. Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, et al. Ticagrelor with or without Aspirin in High-Risk Patients after PCI. N Engl J Med. 2019;381(21):2032-42. A total of 7119 patients (4614 with NSTE-ACS) were included, all treated for 3 months after PCI with ASA + ticagrelor. After this period, they were randomized to continue with DAPT or ticagrelor monotherapy for another 9 months. The main outcome was bleeding (BARC 2, 3 or 5). At the end of follow-up, the ticagrelor monotherapy group there was a 44% reduction (HR 0.56; 95% CI: 0.45-0.68; p < 0.001) in the main outcome, with no increase in ischemic risk (secondary outcome, insufficient sampling power for this conclusion).
In a recently published meta-analysis, O'Donoghue et al. assessed monotherapy with P2Y12 inhibitors vs. DAPT in approximately 17,000 patients with acute coronary disease, finding a hazard ratio of 0.50 (95% CI 0.41 to 0.61) for bleeding, and a hazard ratio of 0.85 (95% CI 0.70 to 1.03) for major adverse cardiac events.465465. O’Donoghue ML, Murphy SA, Sabatine MS. The Safety and Efficacy of Aspirin Discontinuation on a Background of a P2Y12 Inhibitor in Patients After Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. Circulation. 2020 Aug 11;142(6):538-45.
Care should be taken when prescribing antiplatelet drugs to patients with ACS who, during hospitalization, undergo CABG. Subanalyses of the CURE,466466. Fox KAA, Mehta SR, Peters R, Zhao F, Lakkis N, Gersh BJ, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non- ST-elevation acute coronary syndrome: The clopidogrel in unstable angina to prevent recurrent ischemic events (CURE) trial. Circulation 2004;110(10):1202–8. PLATO,249249. Held C, Asenblad N, Bassand JP, Becker RC, Cannon CP, Claeys MJ, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes undergoing coronary artery bypass surgery: results from the PLATO (Platelet Inhibition and Patient Outcomes) trial. J Am Coll Cardiol. 2011;57(6):672-84. and TRITON237237. Smith PK, Goodnough LT, Levy JH, Poston RS, Short MA, Weerakkody GJ, et al. Mortality benefit with prasugrel in the TRITON-TIMI 38 coronary artery bypass grafting cohort: risk-adjusted retrospective data analysis. J Am Coll Cardiol. 2012,31;60(5):388-96. trials showed that patients undergoing surgical revascularization seemed to benefit like the other patients included in these trials. Thus, thienopyridine should be reintroduced as soon as it is considered safe after surgical revascularization.
C. Antiplatelet Management in Patients Who Need Chronic Anticoagulation
Evidence about antiplatelet management in patients who need chronic anticoagulation is, for the most part, restricted to atrial fibrillation (AF) patients. The incidence of AF in patients with NSTE-ACS ranges from 5% to 23%.467467. Lopes RD,Pieper KS, Horton JR, Al-Khatib SM, Newby LK,Mehta RH, et al. Short- and long-term outcomes following atrial fibrillation in patients with acute coronary syndromes with or without ST-segment elevation. Heart. 2008,94(7):867-73. The standard therapy for ACS is DAPT for at least 12 months to prevent ischemic events. In patients with AF and a CHA2DS2VASC score ≥ 2, chronic anticoagulation is recommended to prevent thromboembolic events. When both of these conditions are present, it becomes important to determine the best combination of antithrombotic agents, including the appropriate dose and the ideal duration of each treatment. These questions were evaluated in a series of phase 3 clinical trials.
The first of these studies was the WOEST trial,468468. Dewilde WJM, Oirbans T, Verheugt FWA, Kelder JC, De Smet BJGL, Herrman JP, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet 2013;381(9872):1107–15. which recruited 573 patients with AF who were on anticoagulants and underwent angioplasty. The patients were then randomized into two groups: one that used clopidogrel associated with anticoagulant and another that used triple therapy (anticoagulant + clopidogrel + ASA). Dual therapy was associated with a lower risk of bleeding at 1 year (19.4% vs. 44.4%, HR 0.36, 95% CI 0.26-0.50, p <0.0001) without a greater risk of ischemic events.
The first randomized trial to assess DOAC in the context of double and triple therapy in patients with AF who underwent angioplasty was PIONEER AF-PCI.268268. Gibson CM, Mehran R, Bode C, Halpecin J, Verheugt FW, Goose P, Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375(25):2423–34. This trial compared three antithrombotic strategies: rivaroxaban 15 mg/day associated with a P2Y12 inhibitor; triple therapy with DAPT and low-dose rivaroxaban (2.5 mg every 12 h); triple therapy with DAPT and a vitamin K antagonist. Strategies involving rivaroxaban were associated with a lower risk of bleeding than triple therapy with vitamin K antagonists. There was no difference in ischemic outcomes, but the sample size (2124 patients) had insufficient power for this outcome. One criticism of this study was the fact that the low doses of rivaroxaban had not been approved for antithrombotic treatment in AF.
The following year, the RE-DUAL PCI trial was published, which randomized 2725 patients with AF who underwent angioplasty.269269. Cannon CP, Bhatt DL, Oldgren J, Thomas L, Ansell J, Fonarow GC, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377(25):1513–24. Dual therapy with dabigatran at different doses (150 mg twice a day and 110 mg twice a day) associated with a P2Y12 inhibitor was compared to triple therapy with warfarin. In the triple therapy group, ASA was continued for 1 month in patients who received a non-drug-eluting stent, and for 3 months in patients who received a drug-eluting stent. The two dabigatran regimens were associated with a lower risk of bleeding than the triple therapy warfarin regimen. No interaction was found between proton pump inhibitors and the effect of the drug, since this class of drugs did not influence the main results of the study.469469. Nicolau JC, Bhatt DL, Hohnloser SH, Kimura T, Lip GYH, Miede C, et al. RE-DUAL PCI Steering Committee and Investigators. Dabigatran Dual Therapy vs Warfarin Triple Therapy Post-Percutaneous Coronary Intervention in Patients with Atrial Fibrillation With/Without a Proton Pump Inhibitor: A Pre-Specified Analysis of the RE-DUAL PCI Trial. Drugs. 2020 Jul;80(10):995–1005. The design of RE-DUAL PCI and PIONEER AF-PCI did not allow assessment of whether the reduced risk of bleeding in dual therapy was secondary to replacing vitamin K antagonists with DOAC or to excluding ASA from the treatment.
The AUGUSTUS trial, published in 2019,270270. Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, et al Antithrombotic Therapy After Acute Coronary Syndrome or PCI in Atrial Fibrillation. N Engl J Med. 2019;380(16):1509-24. set out to answer this question by randomizing 4,614 patients with AF who had undergone PCI or had ACS in the last 14 days. The study used a 2x2 factorial design; patients were randomized into groups that compared apixaban 5 mg twice a day to warfarin, and groups that compared ASA to placebo. All patients received a P2Y12 inhibitor, which was clopidogrel in more than 90% of cases. Thus, four groups were studied: (1) P2Y12 inhibitor + warfarin + ASA; (2) P2Y12 inhibitor + warfarin + placebo; (3) P2Y12 inhibitor + apixaban + ASA; (4) P2Y12 inhibitor + apixaban + placebo. In the comparison between apixaban and warfarin, there was a lower risk of bleeding with apixaban. One major or clinically relevant bleeding episode was avoided during the study period for every 24 patients treated with apixaban when compared with warfarin. In the comparison of ASA vs. placebo, it was demonstrated that ASA increased the risk of bleeding. There was one additional significant bleeding episode in 6 months for every 14 patients treatedwith ASA when compared with placebo. Compared to triple therapy, there was no increase in ischemic outcomes with double therapy; however, the study was not powered to assess efficacy outcomes. It is important to point out that in the AUGUSTUS trial, patients were randomized an average of 6 days after PCI or ACS. Thus, patients received at least a short course of ASA and triple therapy prior to randomization. Another relevant fact about this study is that it also included patients with AF and medically managed ACS (approximately 25% of the sample).
The ENTRUST trial randomized 1506 patients with AF who underwent coronary angioplasty, following them for 12 months.271271. Vranckx P, Valgimigli M, Eckardt L, Tijsssen J, Lewatter T, Gargiuo G, et al. Edoxaban-based Versus Vitamin K Antagonist-Based Antithrombotic Regimen After Successful Coronary Stenting in Patients With Atrial Fibrillation (ENTRUST-AF PCI): A Randomised, Open-Label, Phase 3b Trial. Lancet, 2019;394 (10206), 1335-43. The study design compared double therapy with edoxaban 60 mg/day associated with clopidogrel 75 mg/day to traditional triple therapy (warfarin + double antiplatelet therapy with ASA + clopidogrel). Dual therapy with edoxaban was non-inferior to triple therapy with warfarin regarding the primary outcome of bleeding. However,this was the only randomized clinical trial that did not demonstrate a superiority for bleeding outcomes of a dual therapy with a DOAC compared to a triple therapy with warfarin.
One possible concern with dual therapy in AF patients who undergo angioplasty would be an increased ischemic risk, especially for stent thrombosis. The five previously mentioned trials (WOEST, PIONEER AF-PCI, RE-DUAL PCI, AUGUSTUS and ENTRUST) were designed to determine whether less aggressive antithrombotic therapy resulted in reduced bleeding. None of them, however, had sufficient power to adequately assess increased ischemic risk. To assess this issue, two meta-analyses were developed, both of which demonstrated that, compared to warfarin + DAPT, dual antithrombotic therapy decreases hemorrhagic events.272272. Lopes RD, Hong H, Harskamp RE, Bhatt DL, Mehran R, Cannon CP, et al. Optimal Antithrombotic Regimens for Patients with Atrial Fibrillation Undergoing Percutaneous Coronary Intervention: an Updated Network Meta-analysis. JAMA Cardiol. 2020;5(5):582-589.,470470. Golwala HB, Cannon CP, Steg PG, Doros G, Qamar A, Ellis SG, Oldgren J, Ten Berg JM, Kimura T, Hohnloser SH, Lip GYH, Bhatt DL. Safety and efficacy of dual vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of randomized clinical trials. Eur Heart J. 2018; 39(19):1726-1735.,471471. Lopes RD, Hong H, Alexander JH. Antithrombotic therapy after acute coronary syndrome and/or percutaneous coronary intervention in atrial fibrillation: finding the sweet spot. Eur Heart J. 2019 Dec 7;40(46):3768–70. However, when comparing double and triple antithrombotic therapy, Gargiulo et al.472472. Gargiulo G, Goette A, Tijssen J, Eckardt L, Lewalter T, Vranckx P, et al. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur Heart J. 2019 Dec 7;40(46):3757-67. found that dual antithrombotic therapy led to a significant decrease in bleeding at the expense of a significantly higher incidence of stent thrombosis (RR = 1.59, 95% CI 1.01 to 2.50) and a trend toward increased AMI, suggesting that some patients with a very high risk of ischemic events and (especially) a low risk of bleeding events may benefit from triple antithrombotic therapy for up to 30 days after the index event (Figure 3.3).
Antithrombotic regimen proposed for patients with non-ST-elevation acute coronary syndrome who have AF and are indicated for anticoagulation.
3.2. Renin-Angiotensin-Aldosterone System Inhibitors
ACE inhibitors are recommended for post-ACS patients with systolic LV dysfunction/HF, systemic arterial hypertension or DM. Angiotensin receptor blockers can be used in patients intolerant to ACE inhibitors.
Mineralocorticoid antagonists are indicated in post-ACS patients with LVEF ≤ 40% associated with evidence of HF or DM.283283. Hoedemaker NPG, Damman P, Ottervanger JP, Dambrink JHE, Gosselink ATM, Kedhi E, et.al. Trends in Optimal Medical Therapy Prescription and Mortality After Admission for Acute Coronary Syndrome: A 9-year Experience in a Real-World Setting. Eur Heart J Cardiovasc Pharmacother,2018; 4(2):102-10.
When managing arterial hypertension after hospitalization for ACS and in the long term, special care must be taken to maintain adequate blood pressure control, as recommended by the Brazilian Hypertension Guidelines.473473. Malachias MVB, Souza WKSB, Plavnik FL, Rodrigues CIS, Brandão AA, Neves MFT, et al,Sociedade Brasileira de Cardiologia.. 7ª Diretriz Brasileira de Hipertensão Arterial. Arq Bras Cardiol 2016; 107(3Supl.3):1-83. However, the J-curve should also be considered, despite the controversy about its existence and role. The ONTARGET study474474. Sleight P, Redon J, Verdecchia P, Mancia G, Gao P, Fagard R, et al. ONTARGET investigators. Prognostic value of blood pressure in patients with high vascular risk in the Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial study. J Hypertens. 2009;27(7):1360–9.,475475. Banach M, Michalska M, Kjeldsen SE, Małyszko J, Mikhailidis DP, Rysz J. What should be the optimal levels of blood pressure: Does the J-curve phenomenon really exist? Expert Opin Pharmacother. 2011;12(12):1835–44. demonstrated that excessive reductions in SBP were associated with an increase in ischemic events and that a SBP of 126 mmHg was associated with the lowest occurrence of AMI. The TNT study476476. Bangalore S, Messerli FH, Wun C, Zuckerman AL, DeMicco D, Kostis JB,et al. J-curve revisited: an analysis of the Treating to New Targets (TNT) Trial. Eur Heart J. 2010; 31(23):2897–908. assessed the impact of blood pressure levels on more than 10,000 coronary disease patients. More ischemic events occurred when SBP was below 110-120 mmHg, and diastolic blood pressure was below 60-70 mmHg. However, the SPRINT study, which randomized 9,361 patients without DM and high cardiovascular risk to an intensive control goal (SBP < 120 mmHg) or a conventional goal (SBP < 140 mmHg), found that intensive treatment reduced the occurrence of the composite outcome (cardiovascular death, infarction, stroke, ACS, or HF)(HR 0.75; 95% CI 0.64 to 0.89; p < 0.001), as well as all-cause mortality (HR 0.73; 95% CI 0, 60 to 0.90; p = 0.003). There was an increased incidence of adverse events from falls, syncope, hypotension, and acute renal failure in the intensive group. No increase in infarction or ACS was found in the intensive treatment group. However, the study included less than 20% of patients with overt cardiovascular disease.477477. SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015 Nov 26;373(22):2103-16. Erratum in: N Engl J Med. 2017 Dec 21;377(25):2506. No other studies have been conducted specifically on patients with a history of ACS. Thus, due to the potential for worsening ischemia, lowering the patient's blood pressure should be viewed with caution. To avoid reducing coronary perfusion pressure, diastolic blood pressure should not fall below 60 mmHg.
3.3. Beta-blockers
Long-term use of beta-blockers is recommended in patients with HF and an EF ≤ 40%. In patients with preserved LV systolic function, the data are less robust. Evidence favorable to beta-blockers emerged in the early 1980s, when ACS treatment differed substantially from current protocols.478478. A randomized trial of propranolol in patients with acute myocardial infarction. I. Mortality results. JAMA 1982;247(12):1707–14.,479479. Hjalmarson Å, Herlitz J, Málek I, Rydén L, Vedin A, Waldenström A, et al. Effect On Mortality of Metoprolol In Acute Myocardial Infarction. A Double-blind Randomised Trial. Lancet. 1981;318(8251):823–7. Their use in NSTE-ACS patients without HF has not been evaluated in randomized clinical trials. The data available for this population are derived from observational studies suggesting that long-term beta-blocker use after AMI is useful.480480. Goldberger JJ, Bonow RO, Cuffe M, Liu L, Rosenberg Y, Shah PK, et al. Effect of Beta-Blocker Dose on Survival after Acute Myocardial Infarction. J Am Coll Cardiol. 2015;66(13):1431–41.,481481. Bangalore S, Steg G, Deedwania P, Crowley K. B-Blocker Use and Clinical Outcomes in Stable Outpatients With and Without Coronary Artery Disease. J Am Med Assoc. JAMA. 2012;308(13):1340–9. A specifically designed Brazilian study that followed NSTE-ACS patients for approximately 17 years suggested that the long-term use of beta-blockers benefits populations with LV dysfunction, but not those with normal EF.137137. Nicolau JC, Furtado RHM, Baracioli LM, Lara LM, Dalçóquio TF, Scanavini Junior MA, et al. The Use of Oral Beta-Blockers and Clinical Outcomes in Patients with Non-ST-Segment Elevation Acute Coronary Syndromes: a Long-Term Follow-Up Study. Cardiovasc Drugs Ther. 2018 Oct;32(5):435-42. The ongoing DANBLOCK study (NCT 03778554) is randomizing patients with recent myocardial infarction and EF > 40% to assess whether beta-blockers reduce ischemic outcomes in the context of current ACS therapy. The results are expected in 2023.
3.4. Antidiabetic drugs
Over 30% of NSTE-ACS patients have DM.482482. Piva e Mattos LA, Berwanger O, Santos ES, Reis HJ, Romano ER, Petriz JL,et al. Clinical outcomes at 30 days in the Brazilian Registry of Acute Coronary Syndromes (ACCEPT). Arq Bras Cardiol. 2013 Jan;100(1):6-13. The treatment of this comorbidity has undergone major changes in recent years with the arrival of drugs that not only lower blood glucose levels, but also reduce the incidence of cardiovascular events. There is evidence that more intensive control of glycated hemoglobin (HbA1c) in diabetic patients reduces the risk of microvascular complications.483483. Lachin JM, Genuth S, Cleary P. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive insulin therapy. N Engl J Med. 2000;342(6):381–9.,484484. Holman RR, Paul SK, Bethel MA. Follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89. Their impact on macrovascular events has been more heterogeneous, although there seem to be benefits in long-term follow-up (> 10 years), as long as treatment begins soon after diagnosis.485485. Laiteerapong N, Ham SA, Gao Y, Moffet HH, Liu JY, Huang ES, et al. The legacy effect in type 2 diabetes:impactofearlyglycemic control on future complications (the Diabetes & Aging study). Diabetes Care. 2019;42(3):416–26. Based on microvascular event findings, an HbA1c target of less than 7% is recommended in most diabetic patients. It is important to point out, however, that this number is more of a general principle and it should be individualized for each patient. In the ACCORD trial, for example, more aggressive glycemic control, in addition to not reducing major cardiovascular outcomes, was associated with higher overall mortality, which led to early interruption of the study.486486. Gerstein C, Miller M, Byington R. Effects of Intensive Glucose Lowering in Type 2 Diabetes. N Engl J Med. 2008;358(24):2545–59. The included patients had long-standing DM (10 years on average), in addition to a high prevalence of cardiovascular disease (approximately 35%). Therefore, in patients with low life expectancy, long-standing DM, a history of severe hypoglycemia, advanced microvascular or macrovascular disease, less stringent HbA1c targets (eg, < 8%) can be considered. Conversely, in patients with recent-onset DM, no relevant comorbidities, and a good understanding of the disease, more intensive targets (eg, HbA1c < 7%) can be targeted.
Once the glycemic targets have been determined, the drugs must be chosen. Every patient diagnosed with DM should receive metformin unless it is formally contraindicated.487487. American Diabetes Association. Standards of Medical Care in Diabetes — 2020. Diabetes Care. 2020 Jan;43((Suppl 1):S14-S31). This low-cost medication is widely available in the public health system and has favorable effects on weight reduction and glycemic control, in addition to potentially reducing the incidence of cardiovascular events.484484. Holman RR, Paul SK, Bethel MA. Follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89. Recent randomized trials have shown that treatment with new antidiabetics in association with metformin has reduced cardiovascular outcomes in more than 70% to 80% of patients.
The main side effects of metformin are gastrointestinal (feeling of bloating, abdominal pain, diarrhea) and can be avoided with a slower dose progression. Patients with creatinine clearance below 30 mL/min are formally contraindicated to the drug.488488. U.S. Food and Drug Administration. FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function.[Internet]. [Cited in 2019 Apr 12] Available from: Available from: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM494140.pdf
http://www.fda.gov/downloads/Drugs/DrugS...
Chronic metformin treatment is associated with a higher incidence of low vitamin B12 levels, and monitoring should be considered in this population, especially if there is associated anemia or neuropathy.489489. Aroda VR, Edelstein SL, Goldberg RB, Knowler WC, Marcovina SM, Orchard TJ, et al. Long-term metformin use and vitamin B12 deficiency in the diabetes prevention program outcomes study. J Clin Endocrinol Metab. 2016;101(4):1754–61.
In patients with a history of coronary disease, therapy with SGLT2 inhibitors or GLP1 receptor agonists should be considered, regardless of the HbA1c level. Medications that have already shown benefits in reducing cardiovascular events in phase 3 studies should be selected.
Choosing between SGLT2 inhibitors and GLP1 receptor agonists depends on several factors, some of which are shown in Table 3.4. Both drug classes have similar reductions in major cardiovascular outcomes in secondary prevention patients.490490. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus: Systematic review and meta-analysis of cardio. Circulation. 2019;139(17):2022–31. Ideally, the decision should be shared with the patient, weighing practical aspects such as route of administration (oral vs. subcutaneous) and price, as well as the clinical benefits found in randomized studies. Due to their benefits beyond glycemic control, these drugs should be prescribed as soon as possible after ACS, since diabetic patients with ACS are a very high risk group and therapeutic inertia should be avoided. In a subanalysis of patients with previous AMI from the DECLARE trial, dapagliflozin reduced the composite outcome of cardiovascular death, infarction and ischemic stroke (mainly driven by a reduction in reinfarction) compared to placebo, and this benefit appeared to be greater closer to the acute phase of a coronary event.491491. Furtado RHM, Bonaca MP, Raz I, Zelniker TA, Mosenzon O, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. Circulation. 2019;139222516-27.,Circulation 2019; 139(22): 2516-27.
Factors to be considered when deciding on the best new antidiabetic treatment in coronary patients
Importantly, in the DAPA-HF study, dapagliflozin showed cardiovascular benefits in patients with HF and a LVEF <40%, regardless of DM.492492. McMurray JJ V, Solomon SD, Inzucchi SE, Kǿber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019,381:1995-2008. In this trial, coronary disease was the cause of HF in 55% of the patients and a there was a 23% reduction in the composite outcome (worsening HF and cardiovascular death), regardless of the patient's HbA1c levels. Thus, in patients with LV dysfunction due to coronary disease and associated DM, there is more evidence for the benefit of SGLT2 inhibitors than GLP1 receptor agonists.
Another situation in which SGLT2 inhibitors showed benefits was in diabetic patients with chronic kidney disease. In the CREDENCE trial, canagliflozin at a dose of 100 mg per day reduced the progression of nephropathy in diabetic patients with creatinine clearance between 30 and 90 mL/min associated with proteinuria (albumin to urinary creatine ratio ≥ 300 mg/g).493493. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380(24):2295–306. In end-stage renal disease, the primary endpoint (a 2-fold increase in creatinine levels or death from renal or cardiovascular causes) was reduced by 30% with the use of SGLT2 inhibitors (HR 0.70; 95% CI 0.59 to 0.82; p = 0.00001).
Most of the other antidiabetics had no effect on cardiovascular events. However, in some cases, the outcomes worsened. In the SAVOR-TIMI 53 trial, the DPP-4 inhibitor saxagliptin was tested against placebo in a population of 16,492 diabetic patients.494494. Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369(14):1317–26. In addition to not reducing cardiovascular outcomes, saxagliptin increased the hospitalization rate for HF (from 2.8% to 3.5%, HR 1.27; 95%CI 1.07 to 1, 51; p = 0.007). Thus, this medication should be avoided in patients with HF.
3.5. Lipid lowering drugs
Treatment with statins should begin early, preferably with high-intensity therapy (ie, rosuvastatin 20-40 mg or atorvastatin 40-80 mg). In patients who are already using this medication, treatment should not be interrupted. If the previously used statin is of low or moderateintensity, changing to a high potency statin should be considered.
In outpatient follow-up, consider therapeutic LDL-c and HDL-c goals of < 50 mg/dL and < 80 mg/dL, respectively. If the goals are not achieved, and the patient is not yet using high-potency statins, prescbing a medication from this group is recommended. However, if LDL targets are still not achieved, an association with ezetimibe 10 mg/day is recommended. After these measures, if the LDL remains above the target, PCSK9 inhibitors should be considered.
The FOURIER trial (2017) was the first study to evaluate clinical outcomes associated with PCSK9 inhibitors495495. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med 2017;376(18):1713–22.. It included 27,564 patients with a history of cardiovascular disease with LDL ≥70 mg/dL despite statin use. The patients were randomized to evolocumab (140 mg subcutaneously every 2 weeks or 420 mg subcutaneously once a month, according to the patient's preference) or placebo. A significant reduction in the composite outcome (cardiovascular death, AMI, stroke, coronary revascularization, or hospitalization due to UA) occurred in the evolocumab group. This outcome occurred in 11.3% of the control group and in 9.8% of the evolocumab group over a mean follow-up of 26 months. There was no difference in mortality.
The ODYSSEY-OUTCOMES trial (2018) evaluated alirocumab in patients with ACS in the year prior to inclusion.496496. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med. 2018;379(22):2097-107. The patients, who had LDL ≥ 70 mg/dL despite using high-potency statins, were randomized to receive alirocumab 75 mg subcutaneously every 2 weeks or placebo. In an average follow-up of 2.8 years, there was a 14.4% reduction in the composite outcome (death from coronary heart disease, AMI, stroke, or hospitalization for UA). In this trial there was an apparent reduction in overall mortality (from 4.1% to 3.5%), although there was no reduction in cardiovascular death. The benefits of the new medication were more prominent in patients with baseline LDL above 100 mg/dL. Although the absolute risk of mortality was reduced by 0.6% in the general group, the reduction was 1.7% in the group with LDL > 100 mg/dL.
One relevant fact about the ODYSSEY-OUTCOMES trial is that the PCSK9 inhibitor was initiated an average of 2.6 months after the ACS episode. In the FOURIER study, however, in patients with a history of AMI (about 80% of the total population), evolocumab was initiated an average 3.4 years after the last AMI. In other words, based on the evidence from these studies, PCSK9 inhibitors, as a rule, should be considered in outpatient follow-up for infarcted patients and in the acute in-hospital phase.
Another lipid lowering drug that reduced cardiovascular events in secondary prevention was omega-3 supplementation (eicosapentaenoic acid: icosapent-ethyl 4 g/day). This strategy was evaluated in the REDUCE-IT study, which randomized high-risk patients (secondary prevention or DM with another associated risk factor) who had hypertriglyceridemia despite statin use.497497. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med. 2019;380(1):11-22. Most patients (70.7%) were onsecondary prevention. There was a reduction in the primary endpoint (cardiovascular death, AMI, stroke, need for revascularization, or UA) from 22% to 17.2%. There was also a reduction in cardiovascular death from 5.2% to 4.3% (CI 0.66 to 0.98; p = 0.03). For every 21 patients treated during the mean 4.9 years of follow-up, one cardiovascular event was prevented. One criticism about the REDUCE-IT trial was that the placebo, mineral oil, led to an LDL increase of 5 mg/dL in the control group compared to the EPA group, which could have increased the risk of events in the placebo group.
It should be pointed out that in the REDUCE-IT trial, a specific formulation of omega 3 based on eicosapentaenoic acid (icosapent-ethyl) was used, which had no relevant impact on LDL during the trial (an increase of 2 mg/dL). There is evidence that omega 3 formulations containing docosahexaenoic acid raise LDL levels.498498. Chang CH, Tseng PT, Chen NY, Lin PC, Lin PY, Chang JPC, et al. Safety and tolerability of prescription omega-3 fatty acids: A systematic review and meta-analysis of randomized controlled trials. Prostaglandins Leukot Essent Fat Acids. 2018 Feb;129:1-12. Thus, it is recommended that, for omega-3 supplementation in post-ACS patients, the REDUCE-IT formulation should be used. It will not be available in Brazil until after this Guideline has been published.
3.6. Other Medications
Post-ACS patients are at increased risk of bleeding due to antithrombotic therapy. The gastrointestinal tract is the most frequent site of clinically relevant bleeding in patients on chronic antiplatelet therapy.499499. Moukarbel G V, Signorovitch JE, Pfeffer MA, McMurray JJ V, White HD, Maggioni AP, et al. Gastrointestinal bleeding in high risk survivors of myocardial infarction: the VALIANT Trial. Eur Heart J. 2009;30(18):2226–32. Gastric protection is one way to reduce the risk of these events. PPIs have been shown to be more effective than H2 receptor antagonists in observational studies.500500. Lin KJ, Hernandez-Diaz S, Garcia Rodriguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology. 2011;141(1):71–9. However, some in vitro studies have suggested that associating PPIs with clopidogrel could reduce its antiplatelet activity.225225. Gilard M, Arnaud B, Cornily JC, Le Gal G, Lacut K, Le Calvez G, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (OmeprazoleCLopidogrel Aspirin) study. J Am Coll Cardiol. 2008;51(3):256-60.,226226. Ho PM, Maddox TM, Wang L, Fihn SD, Jesse RL, Peterson ED, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009;301(9):937-44. The COGENT trial (see Part 2) showed that combining omeprazole with clopidogrel reduced the risk of gastrointestinal complications without an apparent increase in ischemic events. However, given that the study was discontinued early, it cannot be ruled out that omeprazole would have led to an increase in ischemic events.231231. Bhatt DL, Cryer BL, Contant CF, Cohen M, Lanas A, Schnitzer TJ, et al; COGENT Investigators. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363(20):1909-17.
Therefore, PPIs are recommended for patients receiving DAPT who are at increased risk of bleeding, i.e., those with a history of gastrointestinal bleeding, a history of peptic ulcer disease, anticoagulant use, chronic NSAID use, chronic corticosteroid use, or those with two or more of the following factors: age ≥ 65 years, dyspepsia, gastroesophageal reflux disease, H. pylori infection, or chronic alcohol use.
After discharge, patients must be instructed that if they have angina at rest, they should use sublingual nitrate (dinitrate, mononitrate, or nitroglycerin), as long as there are no contraindications. If the pain does not improve in 3 to 5 minutes, the emergency department should be called.
Viral and bacterial infections can trigger an acute cardiovascular event in patients with heart disease. There is evidence that vaccinating for influenza and pneumococcus can reduce hospitalizations and even mortality in these patients.501501. Nichol KL, Nordin J, Mullooly J, Lask R, Fillbrandt K, Iwane M. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med. 2003;348(14):1322–32. Thus, annual vaccination for influenza is recommended for patients with coronary disease. Ideally, the vaccine should be administered during the normal vaccination campaign (April and May in Brazil). Regarding the pneumococcal vaccine, a dose of the 23-valent polysaccharide (Pneumo 23) vaccine with a booster after 5 years is recommended.502502. Brasil. Ministerio da Saúde. Manual de Normas e Procedimentos para Vacinação [Internet]. [Citado em 2020 Jan 12]. Disponível em: http://bvsms.saude.gov.br/bvs/publicacoes/manual_procedimentos_vacinacao.pdf
http://bvsms.saude.gov.br/bvs/publicacoe...
An ongoing Brazilian randomized trial is testing whether a double-dose of anti-influenza vaccine during hospitalization for ACS is superior to conventional vaccination 30 days after the event for reducing cardiovascular outcomes (NCT 04001504).
Therapy aimed at reducing plaque inflammation has begun to yield promising results. In the CANTOS trial, canakinumab, an anti-interleukin 1-β monoclonal antibody, reduced major cardiovascular events (cardiovascular death, reinfarction or stroke) in patients with previous infarction (at least 30 days after the index event) and elevated C-reactive protein (HR 0.88; 95% CI 0.79 to 0.97; p = 0.02).503503. Ridker PM, Everett B, Thuren T, Mac Fadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017; 377(12): 1119–31. However, there was an increase in death from infection. Moreover, due to its prohibitive cost, this medication has not been incorporated into normal clinical practice. Colchicine, a widely available and low-cost drug, significantly reduced the composite outcome (cardiovascular death, reinfarction, hospitalization for UA with a need for revascularization, stroke, or resuscitated cardiac arrest (HR 0.77; 95% CI 0.61 to 0.96; p = 0.02) in patients with recent AMI (within 30 days of the index event). However, there was an increase in the incidence of pneumonia.504504. Tardif JC, Kouz S, Waters DD, Bertrand D, Diaz R, Maggioni A, et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Eng J Med. 2019;381(26):2497-505. Figure 3.4 summarizes the recommended drugs for NSTE-ACS patients after discharge.
4. Post-discharge Clinical Screening
When early reassessment is recommended for low-risk patients and those revascularized after NSTE-ACS, it should be performed between 2 and 6 weeks after discharge. In patients with greater severity, reassessment is recommended within 14 days.505505. Nicolau, JC,Timerman A, Marin-Neto JÁ, Piegas LS, Barbosa CJ, Franci A, et al, Diretrizes da Sociedade Brasileira de Cardiologia sobre Angina Instável e Infarto Agudo do Miocárdio sem Supradesnível do Segmento ST (II Edição, 2007) - Atualização 2013/2014. Arq Bras Cardiol. 2014;102(3 supl 1):1-61. Obviously, these deadlines must consider the particularities of each situation since the procedural complications, health care access, and individual characteristics of each patient can vary considerably.
Since there is a risk of complications even in asymptomatic patients, risk assessment should be applied to both symptomatic and asymptomatic patients. Patients should be closely monitored in the first 12 months after revascularization and/or stabilized ACS because they are at higher risk for complications and are subject to changes in pharmacological treatment.506506. Daly CA, De Stavola B, Sendon JL, Tavazzi L, Boersma E, Clemens F, et al. Euro Heart Survey Investigators. Predicting prognosis in stable angina–results from the Euro heart survey of stable angina: prospective observational study. BMJ. 2006;332(7536):262-7. Therefore, at least two visits are recommended in the first year of follow-up, the first occurring ideally within 3 months.
In patients with LV systolic dysfunction prior to the revascularization procedure or after ACS, LV function should be reassessed 8 to 12 weeks after the intervention.507507. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T,et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277-314.,508508. Steeds RP, Garbi M, Cardim N, Kasprzak JD, Sade E, Nihoyannopoulos P, et al. 2014_2016 EACVI Scientific Documents Committee. EACVI appropriateness criteria for the use of transthoracic echocardiography in adults: a report of literature and current practice review. Eur Heart J Cardiovasc Imaging. 2017;18(11):1191-204.
Cardiac function may improve due to mechanisms such as recovery from myocardial dizziness or hibernating myocardium, which can be reversed through revascularization or worsened due to other comorbidities, such as heart valve disease, arrhythmia, inflammation, etc. These conditions should be identified and treated.509509. Knuuti J,Wijins W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al, 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart j. 2020;41(3):407-77.
Similarly, non-invasive ischemia testing can be performed after revascularization to identify residual ischemia and serve as a reference for subsequent comparisons.509509. Knuuti J,Wijins W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al, 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart j. 2020;41(3):407-77. This approach must be individualized since each revascularization involves anatomical peculiarities and varies greatly from patient to patient. These recommendations are mostly based on expert opinion.
In patients who have been asymptomatic for more than 1 year, at least one annual reassessment is recommended.509509. Knuuti J,Wijins W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al, 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart j. 2020;41(3):407-77. The assessment should include the patient's general clinical status, treatment adherence, and risk profile based on risk scores. Laboratory tests, including a lipid profile, kidney function, a complete blood count, and biomarkers, should be performed at least every 2 years.507507. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T,et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277-314.,508508. Steeds RP, Garbi M, Cardim N, Kasprzak JD, Sade E, Nihoyannopoulos P, et al. 2014_2016 EACVI Scientific Documents Committee. EACVI appropriateness criteria for the use of transthoracic echocardiography in adults: a report of literature and current practice review. Eur Heart J Cardiovasc Imaging. 2017;18(11):1191-204.
The lipid profile and glycemic status should be evaluated periodically and, although there is no evidence to support a specific periodicity, an annual evaluation is generally recommended.509509. Knuuti J,Wijins W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al, 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart j. 2020;41(3):407-77.
Certain inflammatory biomarkers appear to be event markers. The highsensitive C-reactive protein has been tested in multiple studies, even in patients in primary prevention. NT-proBNP, von Willebrand factor, and interleukin-6 also seem to predict events.510510. van Holten TC, Waanders LF, de Groot PG, Vissers J, Hoefer IE, Pasterkamp G,et al. Circulating biomarkers for redicting cardiovascular disease risk; a systematic review and comprehensive overview of meta-analyses. PLoS One. 2013;8(4):e62080. Scores based on an association of biomarkers (LDL, NT-proBNP, troponin T, fibrin degradation products) have been found to have better reclassification power and higher C-statistics than scores based on clinical models, and they are promising tools for predicting events in patients with coronary disease.511511. Lindholm D, Lindback J, Armstrong PW, Budaj A, Cannon CP, Granger CB, et al. Biomarker-based risk model to predict cardiovascular mortality in patients with stable coronary disease. J Am Coll Cardiol. 2017;70(7):813-26.,512512. Eapen DJ, Manocha P, Patel RS, Hammadah M, Veledar E, Wassel C, et al. Aggregate risk score based on markers of inflammation, cell stress, and coagulation is an independent predictor of adverse cardiovascular outcomes. J Am Coll Cardiol. 2013;62(4):329-37. However, the lack of more robust evidence limits the routine application of these biomarkers, which should be reserved for selected cases.
An ECG should be requested at each visit to determine heart rate and rhythm. Echocardiograms can help assess LV function (diastolic and systolic), valve status, and cardiac dimensions in apparently asymptomatic patients every 3 to 5 years. Likewise, it may be beneficial to non-invasively assess the presence of silent ischemia in apparently asymptomatic patients every 3 to 5 years, preferably with stress imaging tests.513513. Harb SC, Marwick TH. Prognostic value of stress imaging after revascularization: a systematic review of stress echocardiography and stress nuclear imaging Am Heart J. 2014;167(1):7-85.
Due to the lack of functional information, coronary CCTA should not be used for evaluation. In specific cases, it can help assess the patency of coronaries and grafts.512512. Eapen DJ, Manocha P, Patel RS, Hammadah M, Veledar E, Wassel C, et al. Aggregate risk score based on markers of inflammation, cell stress, and coagulation is an independent predictor of adverse cardiovascular outcomes. J Am Coll Cardiol. 2013;62(4):329-37.
For patients with a history of NSTE-ACS and/or revascularization and unequivocal symptoms of angina, the vast majority of evidence indicates that invasive stratification is the best option. For patients with unclear symptoms, stress imaging is recommended.513513. Harb SC, Marwick TH. Prognostic value of stress imaging after revascularization: a systematic review of stress echocardiography and stress nuclear imaging Am Heart J. 2014;167(1):7-85.
-
Development: The Brazilian Society of Cardiology's Department of Clinical Cardiology
-
Norms and Guidelines Council (2020-2021): Brivaldo Markman Filho, Antonio Carlos Sobral Sousa, Aurora Felice Castro Issa, Bruno Ramos Nascimento, Harry Correa Filho, Marcelo Luiz Campos Vieira
-
Norms and Guidelines Coordinator (2020-2021): Brivaldo Markman Filho
-
Associate Editors: Gilson Feitosa Filho, João Luiz Petriz, Dalton Bertolim Précoma, Walmor Lemke, Ari Timerman, José A. Marin-Netto, Luiz Bezerra Neto, Bruno Ferraz, Eduardo Lapa, Leopoldo S. Piegas
-
Writing Committee: José Carlos Nicolau, Gilson Feitosa Filho, João Luiz Petriz, Remo Holanda de Mendonça Furtado, Dalton Bertolim Précoma, Walmor Lemke, Renato D. Lopes, Ari Timerman, José A. Marin-Netto, Luiz Bezerra Neto, Bruno Ferraz, Eduardo Lapa, Leopoldo S. Piegas.
-
Note: These guidelines are for information purposes and should not replace the clinical judgment of a physician, who must ultimately determine the appropriate treatment for each patient.
Referências
-
1Goodacre S, Cross E, Arnold J, Angelini K, Capewell S, Nicholl J. The health care burden of acute chest pain. Heart. 2005;91(2):229-30.
-
2Barstow C, Rice M, McDivitt JD. Acute Coronary Syndrome: Diagnostic Evaluation. Am Fam Physician. 2017;95(3):170-7.
-
3Gibler WB, Racadio JM, Hirsch AL, Roat TW. Continuum of Care for Acute Coronary Syndrome: Optimizing Treatment for ST-Elevation Myocardial Infarction and Non-St-Elevation Acute Coronary Syndrome. Crit Pathw Cardiol. 2018;17(3):114-38.
-
4Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40(3):237-69.
-
5Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131(10):861-70. Erratum in: Circulation. 2015;131(19):e475.
-
6Scalone G, Niccoli G, Crea F. Editor's Choice-Pathophysiology, diagnosis and management of MINOCA: an update. Eur Heart J. Acute Cardiovasc Care. 2019;8(1):54-62.
-
7Niccoli G, Camici PG. Myocardial infarction with non-obstructive coronary arteries: what is the prognosis?. Eur Heart J Suppl. 2020;22(Suppl E):E40-5.
-
8Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, et al. Contemporary Diagnosis and Management of Patients With Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease: A Scientific Statement From the American Heart Association. Circulation. 2019;139(18):e891-e908.
-
9Planer D, Mehran R, Ohman EM, White HD, Newman JD, Xu K, et al. Prognosis of patients with non-ST- segment-elevation myocardial infarction and nonobstructive coronary artery disease: propensity-matched analysis from the Acute Catheterization and Urgent Intervention Triage Strategy trial. Circ Cardiovasc Interv. 2014;7(3):285-93.
-
10Pasupathy S, Tavella R, Beltrame JF. Myocardial Infarction With Nonobstructive Coronary Arteries (MINOCA): The Past, Present, and Future Management. Circulation. 2017 Apr 18;135(16):1490-3.
-
11Effects of tissue plasminogen activator and a comparison of early invasive and conservative strategies in unstable angina and non-Q-wave myocardial infarction. Results of the TIMI IIIB Trial. Thrombolysis in Myocardial Ischemia. Circulation. 1994 Apr;89(4):1545-56.
-
12Theroux P, Fuster V. Acute coronary syndromes: unstable angina and non-Q-wave myocardial infarction. Circulation. 1998 Mar 31;97(12):1195-206.
-
13Zaacks SM, Liebson PR, Calvin JE, Parrillo JE, Klein LW. Unstable angina and non-Q wave myocardial infarction: does the clinical diagnosis have therapeutic implications? J Am Coll Cardiol. 1999 Jan;33(1):107-18.
-
14Calvin JE, Klein LW, VandenBerg BJ, Meyer P, Ramirez-Morgen LM, Parrillo JE. Clinical predictors easily obtained at presentation predict resource utilization in unstable angina. Am Heart J. 1998 Sep;136(3):373-81.
-
15Kong DF, Blazing MA, O’Connor CM. The health care burden of unstable angina. Cardiol Clin. 1999 May;17(2):247-61.
-
16Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(3):267-315.
-
17Naghavi M, Wang H, Lozano R, Davis A, Liang X, Zhou M, et al; GBD 2013Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117-71.
-
18Brasil. Ministério da Saúde. Informação em saúde: Estatísticas vitais. [Citado em 2020 fev 26]. Disponível em: http://datasus.saude.gov.br/).
» http://datasus.saude.gov.br/ -
19Franken M, Giugliano RP, Goodman SG, Baracioloi L, Godoy LC, Furtado RHM, et al. Performance of acute coronary syndrome approaches in Brazil. A report from the BRACE (Brazilian Registry in Acute Coronary syndromEs) [published online ahead of print, 2019 Aug 10]. Eur Heart J Qual Care Clin Outcomes. 2019;qcz045.
-
20Libby P. Mechanisms of Acute Coronary Syndromes and Their Implications for Therapy. N Engl J Med. 2013;368(21):2004-13.
-
21Ibáñez B, Heusch G, Ovize M, Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol.2015; 65(14):1454-71.
-
22The Principal Investigators of CASS and Their Associates. The National Heart, Lung and Blood Institute Coronary Artery Surgery Study: historical background, design, methods, the registry, the randomized trial, clinical database. Circulation. 1981;63(suppl I):I-1-I-81.
-
23Stone PH, Thompson B, Anderson HV, Kronenberg MW, Gibson RS, Rogers WJ, et al. Influence of race, sex, and age on management of unstable angina and non-Q-wave myocardial infarction: The TIMI III registry. JAMA. 1996 Apr 10;275(14):1104-12.
-
24Nicolau JC, Franci A, Barbosa CJ, Baracioli LM, Franken M, Furtado RH, et al. Influence of proven oral therapies in the very old with acute coronary syndromes: A 15 year experience. Int J Cardiol. 2015 Nov 1;198:213-5.
-
25Kleiman NS, Anderson HV, Rogers WJ, Theroux P, Thompson B, Stone PH. Comparison of outcome of patients with unstable angina and non-Q-wave acute myocardial infarction with and without prior coronary artery bypass grafting (Thrombolysis in Myocardial Ischemia III Registry). Am J Cardiol. 1996 Feb 1;77(4):227-31.
-
26Barbash GI, Reiner J, White HD, Wilcox RG, Armstrong PW, Sadowski Z, et al. Evaluation of paradoxic beneficial effects of smoking in patients receiving thrombolytic therapy for acute myocardial infarction: mechanism of the “smoker's paradox” from the GUSTO-I trial, with angiographic insights. Global Utilization of Streptokinase and Tissue-Plasminogen Activator for Occluded Coronary Arteries. J Am Coll Cardiol. 1995 Nov 1;26(5):1222-9.
-
27Barbash GI, White HD, Modan M, Diaz R, Hampton JR, Heikkila J, et al. Significance of smoking in patients receiving thrombolytic therapy for acute myocardial infarction. Experience gleaned from the International Tissue Plasminogen Activator/Streptokinase Mortality Trial. Circulation. 1993 Jan;87(1):53-8.
-
28Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000 Aug 16;284(7):835-42.
-
29Braunwald E, Jones RH, Mark DB, Brown J, Brown L, Cheitlin MD, et al. Diagnosing and managing unstable angina. Agency for Health Care Policy and Research. Circulation. 1994 Jul;90(1):613-22.
-
30Yeghiazarians Y, Braunstein JB, Askari A, Stone PH. Unstable angina pectoris. N Engl J Med. 2000 Jan 13;342(2):101-14.
-
31Braunwald E, Califf RM, Cannon CP, Fox KA, Fuster V, Gibler WB, et al. Redefining medical treatment in the management of unstable angina. Am J Med. 2000 Jan;108(1):41-53.
-
32Bosch X, Theroux P, Pelletier GB, Sanz G, Roy D, Waters D. Clinical and angiographic features and prognostic significance of early postinfarction angina with and without electrocardiographic signs of transient ischemia. Am J Med. 1991 Nov;91(5):493-501.
-
33Gottlieb SO, Weisfeldt ML, Ouyang P, Mellits ED, Gerstenblith G. Silent ischemia predicts infarction and death during 2 year follow-up of unstable angina. J Am Coll Cardiol. 1987 Oct;10(4):756-60.
-
34Armstrong PW, Fu Y, Chang WC, Topol EJ, Granger CB, Betriu A, et al. Acute coronary syndromes in the GUSTO-IIb trial: prognostic insights and impact of recurrent ischemia. The GUSTO-IIb Investigators. Circulation. 1998 Nov 3;98(18):1860-8.
-
35Cannon CP, McCabe CH, Stone PH, Rogers WJ, Schactman M, Thompson BW, et al. The electrocardiogram predicts one-year outcome of patients with unstable angina and non-Q wave myocardial infarction: results of the TIMI III Registry ECG Ancillary Study. Thrombolysis in Myocardial Ischemia. J Am Coll Cardiol. 1997 Jul;30(1):133-40.
-
36Lee TH, Goldman L. Serum enzyme assays in the diagnosis of acute myocardial infarction. Recommendations based on a quantitative analysis. Ann Intern Med. 1986 Aug;105(2):221-33.
-
37Newby LK, Christenson RH, Ohman EM, Armstrong PW, Thompson TD, Lee KL, et al. Value of serial troponin T measures for early and late risk stratification in patients with acute coronary syndromes. The GUSTO-IIa Investigators. Circulation. 1998 Nov 3;98(18):1853-9.
-
38Amsterdam EA, Kirk JD, Bluemke DA, Diercks DE, Farkouh ME, Garvey JE, et al. Testing of low-risk patients presenting to the emergency department with chest pain. A Scientific Statement From the American Heart Association. Circulation. 2010;122(17):1756-776.
-
39Ohman EM, Armstrong PW, Christenson RH, Granger CB, Katus HA, Hamm CW, et al. Cardiac troponin T levels for risk stratification in acute myocardial ischemia. GUSTO IIA Investigators. N Engl J Med. 1996 Oct 31;335(18):1333-41.
-
40Jaffe AS, Ravkilde J, Roberts R, Naslund U, Apple FS, Galvani M, et al. It's time for a change to a troponin standard. Circulation. 2000 Sep 12;102(11):1216-20.
-
41Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361(9):858-67.
-
42Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56(2):254-61.
-
43Mueller C. Biomarkers and acute coronary syndromes: an update. Eur Heart J. 2014;35(9):552–6.
-
44Westermann D, Neumann J, Sörensen N,Blankenberg S. High-sensitivity assays for troponin in patients with cardiac disease. Nat Rev Cardiol.2017;14(8):472-83.
-
45Stoyanov KM, Hund H, Biener M,Gandowitz J, Riedle C, Lohr J, et al. RAPID-CPU: a prospective study on implementation of the ESC 0/1-hour algorithm and safety of discharge after rule-out of myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2020;9(1):39-51.
-
46Boeddinghaus J, Nestelberger T, Twerenbold R, Wildi K, Badertscher P, Cupa J, et al. Direct Comparison of 4 Very Early Rule-Out Strategies for Acute Myocardial Infarction Using High-Sensitivity Cardiac Troponin I. Circulation. 2017 Apr 25;135(17):1597-11.
-
47Mair J, Morandell D, Genser N, Lechleitner P, Dienstl F, Puschendorf B. Equivalent early sensitivities of myoglobin, creatine kinase MB mass, creatine kinase isoform ratios, and cardiac troponins I and T for acute myocardial infarction. Clin Chem. 1995 Sep;41(9):1266-72.
-
48Costa TN, Strunz CM, Nicolau JC, Gutierrez PS. Comparison of MB fraction of creatine kinase mass and troponin I serum levels with necropsy findings in acute myocardial infarction. Am J Cardiol. 2008 Feb 1;101(3):311-4.
-
49Lin JC, Apple FS, Murakami M,Luepker RV. Rates of positive cardiac troponin I and creatine kinase MB mass among Patients Hospitalized for Suspected Acute Coronary Syndromes. Clin Chem. 2004;50(2):333–8.
-
50Amsterdam EA, Kirk JD, Diercks DB, Lewis WR, Turnipseed SD. Immediate exercise testing to evaluate low-risk patients presenting to the emergency department with chest pain. J Am Coll Cardiol. 2002;40(2):251-6. 165.
-
51Meneghelo RS, Araújo CG, Stein R, Mastrocolla LE, Albuquerque PF, Serra SM, et al; Sociedade Brasileira de Cardiologia. III Diretrizes da Sociedade Brasileira de Cardiologia sobre teste ergométrico. Arq Bras Cardiol. 2010;95(5 Suppl 1):1-26.
-
52Peels CH, Visser CA, Kupper AJ, Visser FC, Roos JP. Usefulness of two-dimensional echocardiography for immediate detection of myocardial ischemia in the emergency room. Am J Cardiol. 1990 Mar 15;65(11):687-91.
-
53Sabia P, Abbott RD, Afrookteh A, Keller MW, Touchstone DA, Kaul S. Importance of two-dimensional echocardiographic assessment of left ventricular systolic function in patients presenting to the emergency room with cardiac-related symptoms. Circulation. 1991 Oct;84(4):1615-24.
-
54Feigenbaum H. Role of echocardiography in acute myocardial infarction. Am J Cardiol. 1990 Nov 20;66(18):17H-22H.
-
55Parisi AF. The case for echocardiography in acute myocardial infarction. J Am Soc Echocardiogr. 1988 May;1(3):173-8.
-
56Reeder GS, Seward JB, Tajik AJ. The role of two-dimensional echocardiography in coronary artery disease: a critical appraisal. Mayo Clin Proc. 1982 Apr;57(4):247-58.
-
57Gibson RS, Bishop HL, Stamm RB, Crampton RS, Beller GA, Martin RP. Value of early two dimensional echocardiography in patients with acute myocardial infarction. Am J Cardiol. 1982 Apr 1;49(5):1110-9.
-
58Visser CA, Lie KI, Kan G, Meltzer R, Durrer D. Detection and quantification of acute, isolated myocardial infarction by two dimensional echocardiography. Am J Cardiol. 1981 May;47(5):1020-5.
-
59Heger JJ, Weyman AE, Wann LS, Rogers EW, Dillon JC, Feigenbaum H. Cross-sectional echocardiographic analysis of the extent of left ventricular asynergy in acute myocardial infarction. Circulation. 1980 Jun;61(6):1113-8.
-
60Ryan T, Vasey CG, Presti CF, O’Donnell JA, Feigenbaum H, Armstrong WF. Exercise echocardiography: detection of coronary artery disease in patients with normal left ventricular wall motion at rest. J Am Coll Cardiol. 1988 May;11(5):993-9.
-
61Sawada SG, Ryan T, Conley MJ, Corya BC, Feigenbaum H, Armstrong WF. Prognostic value of a normal exercise echocardiogram. Am Heart J. 1990 Jul;120(1):49-55.
-
62Cheitlin MD, Alpert JS, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, et al. ACC/AHA Guidelines for the Clinical Application of Echocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Clinical Application of Echocardiography). Developed in collaboration with the American Society of Echocardiography. Circulation. 1997 Mar 18;95(6):1686-744.
-
63Ersbøll M, Valeur N, Mogensen UM, Andersen MJ, Møller JE, Velazquez EJ, Hassager C,et al. Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol. 2013 Jun 11;61(23):2365-73.
-
64Lin SS, Lauer MS, Marwick TH. Risk stratification of patients with medically treated unstable angina using exercise echocardiography. Am J Cardiol. 1998 Sep 15;82(6):720-4.
-
65Colon PJ, III, Guarisco JS, Murgo J, Cheirif J. Utility of stress echocardiography in the triage of patients with atypical chest pain from the emergency department. Am J Cardiol. 1998 Nov 15;82(10):1282-4, A10.
-
66Markman Filho B, Almeida MC, Markman M, Chaves A, Moretti M,Ramires JA, Cesar LA. Stratifying the risk in unstable angina with dobutamine stress echocardiography. Arq Bras Cardiol. 2006; 87(3):294-9.
-
67Jain D, Thompson B, Wackers FJ, Zaret BL. Relevance of increased lung thallium uptake on stress imaging in patients with unstable angina and non-Q wave myocardial infarction: results of the Thrombolysis in Myocardial Infarction (TIMI)-IIIB Study. J Am Coll Cardiol. 1997 Aug;30(2):421-9.
-
68Geleijnse ML, Elhendy A, van Domburg RT, Cornel JH, Rambaldi R, Salustri A, et al. Cardiac imaging for risk stratification with dobutamine-atropine stress testing in patients with chest pain. Echocardiography, perfusion scintigraphy, or both? Circulation. 1997 Jul 1;96(1):137-47.
-
69Kontos MC, Jesse RL, Schmidt KL, Ornato JP, Tatum JL. Value of acute rest sestamibi perfusion imaging for evaluation of patients admitted to the emergency department with chest pain. J Am Coll Cardiol. 1997 Oct;30(4):976-82.
-
70Kapetanopoulos A, Heller GV, Selker HP, Ruthazer R, Beshansky JR, Feldman JA, et al. Acute resting myocardial perfusion imaging in patients with diabetes mellitus: results from the Emergency Room Assessment of Sestamibi for Evaluation of Chest Pain (ERASE Chest Pain) trial. J Nucl Cardiol. 2004 Sep;11(5):570-7.
-
71Hendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA, Pohost GM,et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging. Circulation. 2009 Jun 9;119(22):e561-87;
-
72Klocke FJ, Baird MG, Lorell BH, Bateman TM, Messer JV, Berman DS, et al; American College of Cardiology; American Heart Association; American Society for Nuclear Cardiology. American College of Cardiology; American Heart Association Task Force on Practice Guidelines; American Society for Nuclear Cardiology. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the clinical use of cardiac radionuclide imaging). J Am Coll Cardiol. 2003;42(7):1318-33.
-
73Notghi A, Low CS. Myocardial perfusion scintigraphy: past, present and future. Br J Radiol.2011;84(Spec 3):S229-36.
-
74Wackers FJ, Brown KA, Heller GV, Kontos MC, Tatum JL, Udelson JE, et al. American Society of Nuclear Cardiology position statement on radionuclide imaging in patients with suspected acute ischemic syndromes in the emergency department or chest pain center. J Nucl Cardiol. 2002;9(2):246-50.
-
75Budoff MJ, Dowe D, Jollis JG,Gitter M, Sutherland J, Halamert E, et al. iagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52(21):1724-32.
-
76Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Gottlieb NH, Clouse ME, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 2008;359(22):2324-36.
-
77Meijboom WB, Meijs MF, Schuijf JD, Cramer MJ, Mollet NR, van Mieghem CA, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52(25):2135-44.
-
78Hoffmann U, Ferencik M, Udelson JE, Picard MH, Truong QA, Patel MR, et al.; PROMISE Investigators. Prognostic Value of Noninvasive Cardiovascular Testing in Patients With Stable Chest Pain: Insights From the PROMISE Trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation. 2017;135(24):2320-32.
-
79Collet C, Onuma Y, Andreini D, Sonck J, Pompilio G, Mushtaq S,et al. Coronary computed tomography angiography for heart team decision-making in multivessel coronary artery disease. Eur Heart J. 2018 Nov 1;39(41):3689-98.
-
80Goldstein JA, Chinnaiyan KM, Abidov A, Achenbach S, Berman DS, Hayes SW,et al. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol. 2011;58(14):1414-22.
-
81Litt HI, Gatsonis C, Snyder B, et al. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med. 2012;366(15):1393-403.
-
82Hoffmann U, Truong QA, Schoenfeld DA, Chou ET, Woodard PK, Nagurney JT, et al. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med 2012;367(4):299-308.
-
83Hulten E, Pickett C, Bittencourt MS,Villines Tc, Petrillo S, Di Carlo MF, et al. Outcomes After Coronary Computed Tomography Angiography in the Emergency Department: A Systematic Review and Meta-Analysis of Randomized, Controlled Trials. J Am Coll Cardiol. 2013;61(8):880-92.
-
84Schertler T, Frauenfelder T, Stolzmann P, Scheffel H, Desbiolles L, Marincek B, Kaplan V, Kucher N, Alkadhi H. Triple rule-out CT in patients with suspicion of acute pulmonary embolism: findings and accuracy. Acad Radiol. 2009 Jun;16(6):708-17.
-
85Takakuwa KM, Halpern EJ, Shofer FS. A time and imaging cost analysis of low-risk ED observation patients: a conservative 64-section computed tomography coronary angiography “triple rule-out” compared to nuclear stress test strategy. Am J Emerg Med. 2011 Feb;29(2):187-95.
-
86Sara L, Szarf G, Tachibana A, Shiozaki AA, Villa AV, de Oliveira AC, de Albuquerque AS, et al. II Guidelines on Cardiovascular Magnetic Resonance and Computed Tomography of the Brazilian Society of Cardiology and the Brazilian College of Radiology. Arq Bras Cardiol. 2014 Dec;103(6 Suppl 3):1-86.
-
87Goehler A, Mayrhofer T, Pursnani A, Ferencik M, Lumish HS, Barth C, et al. Long-term health outcomes and cost-effectiveness of coronary CT angiography in patients with suspicion for acute coronary syndrome. J Cardiovasc Comput Tomogr. 2020 Jan-Feb;14(1):44-54.
-
88Fox KA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, Van der Wef F, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ. 2006;333(7578):1091.
-
89Granger CB, Goldberg RJ, Dabbous O, et al. Predictors of hospital mortality in the Global Registryof Acute Coronary Events. Arch Intern Med. 2003;163(19):2345-53.
-
90Fox KAA, FitzGerald G, Puymirat E,Huang W, Carruthers K, Simon T, et al. Should patients with acute coronary disease be stratified for management according to their risk? Derivation, external validation and outcomes using the updated GRACE risk score. BMJ Open. 2014;4(2):e004425.
-
91Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, et al. ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina). J Am Coll Cardiol. 2000 Sep;36(3):970-1062.
-
92Subherwal S, Bach RG, Chen AY, Gage BF, Rao SV, Newby LK,et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation. 2009;119(14):1873–82.
-
93Nicolau JC, Moreira HG, Baracioli LM, Serrano CV Jr, Lima FG, Franken M, et al. The bleeding risk score as a mortality predictor in patients with acute coronary syndrome. Arq Bras Cardiol. 2013 Dec;101(6):511-8.
-
94Mehran R, Pocock SJ, Nikolsky E, Clayton T, Dangas GD, Kirtane AJ,et al. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol. 2010;55(23):2556–66.
-
95Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2020 Aug 29:ehaa575.
-
96Six AJ, Backaus BE, Kelder SC, Chest pain in the emergency room: value of the Heart score. Neth Heart J.2008;16(6):191-6.
-
97Than M, Cullen L, Aldous S, Goodacre S, Frampton CM, Troughton R, et al. 2-Hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: the ADAPT trial. J Am Coll Cardiol. 2012;59(23):2091-8.
-
98Than M,Flaws D, Sanders S, Doust J, Glasziou P, Kline J, et al. Development and validation of the Emergency Department Assessment of Chest pain Score and 2 h accelerated diagnostic protocol. Emerg Med Australas.2014;26(1):34-44.
-
99Greenslade JH et al. Diagnostic Accuracy of a New High-Sensitivity Troponin I Assay and Five Accelerated Diagnostic Pathways for Ruling Out Acute Myocardial Infarction and Acute Coronary Syndrome. Ann Emerg Med. 2018;71(4):439-51.e3.
-
100Santi L, Farina G, Gramenzi A, Trevisani F, Baccini M, Bernardi M, et al. The HEART score with high-sensitive troponin T at presentation: ruling out patients with chest pain in the emergency room. Intern Emerg Med. 2017;12(3):357-64.
-
101Carlton EW, Cullen L, Than M, Gamble J, Khattab A, Greaves K. A novel diagnostic protocol to identify patients suitable for discharge after a single high-sensitivity troponin. Heart. 2015;101(13):1041-6.
-
102Aarts GWA, Camaro C, van Geuns RJ, et al. Acute rule-out of non-ST-segment elevation acute coronary syndrome in the (pre) hospital setting by HEART score assessment and a single point-of-care troponin: rationale and design of the ARTICA randomised trial. BMJ Open. 2020;10(2): e034403.
-
103Backus BE, Six AJ, Kelder JC, Mast TP, van den Akker F, Mast EG, Monnink SH, van Tooren RM, Doevendans PA. Chest pain in the emergency room: a multicenter validation of the HEART Score. Crit Pathw Cardiol. 2010 Sep;9(3):164-9.
-
104Backus BE, Six AL, Kelder SC,Bosschaert EG, Mast A, Mosterd RF, et al. A prospective validation of the Heart score for chest pain patients at the emergency departament. Int J Cardiol. 2013; 168:2153-8.
-
105Six Aj, Cullen L,Backus BE, Greenslade J, Parsonage W, Aldous S, et al. The HEART score for the assessment of patients with chest pain in the emergency department: a multinational validation study. Crit Pahtw Cardiol. 2013;12(3):121-6.
-
106Van Den Berg P, Body R. The HEART score for early rule out of acute coronary syndromes in the emergency department: a systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care. 2018; 7(2): 111–9.
-
107Poldervaart JM, Langedijk M, Backus BE, Dekker IMC, Six AJ, Doevendans PA, Hoes AW, Reitsma JB. Comparison of the GRACE, HEART and TIMI score to predict major adverse cardiac events in chest pain patients at the emergency department. Int J Cardiol. 2017 Jan 15;227:656-61.
-
108Sakamoto JT, Liu N, Koh ZX, Fung NX, Heldeweg ML, Ng JC. Comparing HEART, TIMI, and GRACE scores for prediction of 30-day major adverse cardiac events in high acuity chest pain patients in the emergency department. Int J Cardiol. 2016 Oct 15;221:759-64.
-
109Mahler SA, Riley RF, Hiestand BC, Russell GB, Hoekstra JW, Lefebvre CW, et al. The HEART Pathway randomized trial: identifying emergency department patients with acute chest pain for early discharge. Circ Cardiovasc Qual Outcomes. 2015;8(2):195-203.
-
110Stopyra JP, Riley RF, Hiestand BC, Russell G, Holkstra JW, Lefebvre CW, et al. The HEART Pathway Randomized Controlled Trial One-year Outcomes. Acad Emerg Med. 2019;26(1):41-50.
-
111Reinhardt SW, Lin CJ, Novak E, Brown DL. Noninvasive Cardiac Testing vs Clinical Evaluation Alone in Acute Chest Pain: A Secondary Analysis of the ROMICAT-II Randomized Clinical Trial. JAMA Intern Med. 2018;178(2):212-9.
-
112O’Neill L, Smith K, Currie P,Elder Dhj, Wei J, Lang CC. et al. Nurse-led Early Triage (NET) study of chest pain patients: a long term evaluation study of a service development aimed at improving the management of patients with non-ST-elevation acute coronary syndromes. Eur J Cardiovasc Nurs. 2014; 13(3):253–60.
-
113Roche TE, Gardner G, Jack L. The effectiveness of emergency nurse practitioner service in the management of patients presenting to rural hospitals with chest pain: a multisite prospective longitudinal nested cohort study. BMC Health Serv Res. 2017; 17(1): 445.
-
114Stub D, Smith K, Bernard S, Nehme Z, Stephenson M, Bray JE, eet al. Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation 2015;131(24):2143—50.
-
115Hofmann R, James SK, Jernberg T, Lindahl B, Erlinge D, Witt N,et al. Oxygen Therapy in Suspected Acute Myocardial Infarction. N Engl J Med. 2017 Sep 28;377(13):1240-9.
-
116Hobl EL, Stimpfl T, Ebner J, Schoergenjofer C, Derhaschnig U, Plassman RS, et al. Morphine decreases clopidogrel concentrations and effects: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol. 2014;63(7):630–5.
-
117Thomas MR, Morton AC, Hossain R, Chen b, Luo L, Shahari NN, et al. Morphine delays the onset of action of prasugrel in patients with prior history of ST-elevation myocardial infarction. Thromb Haemost. 2016; 116(7):96–102.
-
118Kubica J, Adamski P, Ostrowska M, Sikora J, Kubica JM, Srooka WD, et al. Morphine delays and attenuates ticagrelor exposure and action in patients with myocardial infarction: the randomized, double-blind, placebo-controlled IMPRESSION trial. Eur Heart J. 2016;37(3):245-52.
-
119Meine TJ, Roe MT, Chen AY, Patel MR, Washam JB, Ohman EM, et al; CRUSADE Investigators. Association of intravenous morphine use and outcomes in acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative. Am Heart J. 2005;149(6):1043-9.
-
120Duarte GS, Nunes-Ferreira A, Rodrigues FB, Pinto FJ, Ferreira JJ, Costa J, et al. Morphine in acute coronary syndrome: systematic review and meta-analysis. BMJ Open. 2019;9(3):e025232.
-
121Furtado RHM, Nicolau JC, Guo J, Im K, White JA, Sabatine MS, et al Morphine and Cardiovascular Outcomes Among Patients With Non-ST-Segment Elevation Acute Coronary Syndromes Undergoing Coronary Angiography. J Am Coll Cardiol. 2020 Jan 28;75(3):289-300.
-
122Dixon RA, Edwards IR, Pilcher J. Diazepam in immediate post-myocardial infarct period. A double blind trial. Br Heart J.1980 May;43(5):535-40.
-
123Gislason GH, Jacobsen S, Rasmussen JN,Rasmussen S, Bucch P, Fribens J, et al. Risk of death or reinfarction associated with the use of selective cyclooxygenase-2 inhibitors and nonselective nonsteroidal antiinflammatory drugs after acute myocardial infarction. Circulation. 2006;113(25):2906–13.
-
124Conaway DG, O’Keefe JH, Reid KJ, Spertus J. Frequency of undiagnosed diabetes mellitus in patients with acute coronary syndrome. Am J Cardiol. 2005;96(3):363–5.
-
125Goyal A, Mahaffey KW, Garg J, Nicolau JC, Hochman JS, Weaver WD, Theroux P, Oliveira GB, Todaro TG, Mojcik CF, Armstrong PW, Granger CB. Prognostic significance of the change in glucose level in the first 24 h after acute myocardial infarction: results from the CARDINAL study. Eur Heart J. 2006 Jun;27(11):1289-97.
-
126Nicolau JC, Serrano CV Jr, Giraldez RR, Baracioli LM, Moreira HG, Lima F, et al. In patients with acute myocardial infarction, the impact of hyperglycemia as a risk factor for mortality is not homogeneous across age-groups. Diabetes Care. 2012 Jan;35(1):150-2.
-
127Finfer S, Chittock DR, Su SY, Bleir D, Foster D, Dhingra V, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–97.
-
128DePace NL, Herling IM, Kotler MN, Hakki AH, Spielman SR, Segal BL. Intravenous nitroglycerin for rest angina. Potential pathophysiologic mechanisms of action. Arch Intern Med. 1982 Oct;142(10):1806-9.
-
129Kaplan K, Davison R, Parker M, Przybylek J, Teagarden JR, Lesch M. Intravenous nitroglycerin for the treatment of angina at rest unresponsive to standard nitrate therapy. Am J Cardiol.1983 Mar 1;51(5):694-8.
-
130Roubin GS, Harris PJ, Eckhardt I, Hensley W, Kelly DT. Intravenous nitroglycerine in refractory unstable angina pectoris. Aust N Z J Med. 1982 Dec;12(6):598-602.
-
131Curfman GD, Heinsimer JA, Lozner EC, Fung HL. Intravenous nitroglycerin in the treatment of spontaneous angina pectoris: a prospective, randomized trial. Circulation. 1983 Feb;67(2):276-82.
-
132Dellborg M, Gustafsson G, Swedberg K. Buccal versus intravenous nitroglycerin in unstable angina pectoris. Eur J Clin Pharmacol. 1991;41(1):5-9.
-
133Gottlieb SO, Weisfeldt ML, Ouyang P, Achuff SC, Baughman KL, Traill TA, et al. Effect of the addition of propranolol to therapy with nifedipine for unstable angina pectoris: a randomized, double-blind, placebo-controlled trial. Circulation. 1986 Feb;73(2):331-7.
-
134Telford AM, Wilson C. Trial of heparin versus atenolol in prevention of myocardial infarction in intermediate coronary syndrome. Lancet. 1981 Jun 6;1(8232):1225-8.
-
135Lubsen J, Tijssen JG. Efficacy of nifedipine and metoprolol in the early treatment of unstable angina in the coronary care unit: findings from the Holland Interuniversity Nifedipine/metoprolol Trial (HINT). Am J Cardiol. 1987 Jul 15;60(2):18A-25A.
-
136Yusuf S, Wittes J, Friedman L. Overview of results of randomized clinical trials in heart disease. II. Unstable angina, heart failure, primary prevention with aspirin, and risk factor modification. JAMA. 1988 Oct 21;260(15):2259-63.
-
137Nicolau JC, Furtado RHM, Baracioli LM, Lara LM, Dalçóquio TF, Scanavini Junior MA, et al. The Use of Oral Beta-Blockers and Clinical Outcomes in Patients with Non-ST-Segment Elevation Acute Coronary Syndromes: a Long-Term Follow-Up Study. Cardiovasc Drugs Ther. 2018 Oct;32(5):435-42.
-
138Chen ZM, Pan HC, Chen YP, Peto R, Collins R, Jiang LX, et al. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005 Nov 5;366(9497):1622-32.
-
139Kontos MC, Diercks DB, Ho PM, Wang TY, Chen AY, Roe MT. Treatment and outcomes in patients with myocardial infarction treated with acute β-blocker therapy: results from the American College of Cardiology's NCDR(®). Am Heart J. 2011 May;161(5):864-70.
-
140Crea F, Libby P. Acute Coronary Syndromes: The Way Forward From Mechanisms to Precision Treatment. Circulation. 2017 Sep 19;136(12):115566.
-
141Bhatt DL, Hulot JS, Moliterno DJ, Harrington RA. Antiplatelet and anticoagulation therapy for acute coronary syndromes. Circ Res. 2014;114(12):1929-43.
-
142Lüscher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation. 2007 Feb 27;115(8):1051-8.
-
143Bellemain-Appaix A, Kerneis M, O’Connor SA, Silvain J, Cucherat M, Beygui F, et al. Reappraisal of thienopyridine pretreatment in patients with non-ST elevation acute coronary syndrome: a systematic review and meta-analysis. BMJ.2014;349:g6269.
-
144Golwala H, Bhatt DL. The Timing of P2Y12 Inhibitor Initiation in the Treatment of ACS? Does the Evidence Exist in This Era? Prog Cardiovasc Dis. 2018 Jan-Feb;60(4-5):471-7.
-
145Dworeck C, Redfors B, Angerås O, Haraldsson I, Odenstedt J, Ioanes D, et al. Association of Pretreatment With P2Y12 Receptor Antagonists Preceding Percutaneous Coronary Intervention in Non–ST-Segment Elevation Acute Coronary Syndromes With Outcomes. JAMA Netw Open. 2020;3(10):e2018735.
-
146Wiviott SD, Trenk D, Frelinger AL, O’Donoghue M, Neumann FJ, Michelson AD, et al. PRINCIPLE-TIMI 44 Investigators. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation. 2007 Dec 18;116(25):2923-32.
-
147Gurbel PA1, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009 Dec 22;120(25):2577-85.
-
148Bonello L, Laine M, Camoin-Jau L, Noirot F, Guieu R, Dignet-George F, et al. Onset of optimal P2Y12-ADP receptor blockade after ticagrelor and prasugrel intake in Non-ST elevation acute coronary syndrome. Thromb Haemost. 2015;114(4):702-7.
-
149Singh S, Singh M, Grewal N, Khosla S. Comparative Efficacy and Safety of Prasugrel, Ticagrelor, and Standard-Dose and High-Dose Clopidogrel in Patients Undergoing Percutaneous Coronary Intervention: A Network Meta-analysis. Am J Ther. 2016;23(1):e52-e62.
-
150Yusuf S, Bijsterveld N, Moons A. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation: the clopidogrel in unstable angina to prevent recurrent events trial investigators. New Engl J Med. 2001; 345(7):494–502.
-
151Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358(9281):527-33.
-
152Wallentin L, Becker RC, Budaj A, Cannon CP, Emmanuelson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(1):1045-57.
-
153Cannon CP, Harrington RA, James S, Ardissino D, Becker RC, Emanuelsson H et al. PLATelet inhibition and patient Outcomes (PLATO) investigators. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet. 2010; 375(9791): 283–93.
-
154Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007; 357(20): 2001-15.
-
155Di Sciascio MG, Patti G, Pasceri V, Gatto L. Effectiveness of in-laboratory high-dose clopidogrel loading versus routine pre-load in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol.2010;56(7):550-7.
-
156Steinhubl SR, Berger PB, Mann JT 3rd, Fry ET, Delago A, Wilmer CH, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288(19):2411-20.
-
157Montalescot G, Bolognese L, Dudek D, Goldstein P, Hamm C, Tanguay J-F, ten Berg JM, et al. Pretreatment with prasugrel in non–ST-segment elevation acute coronary syndromes. N Engl J Med. 369(11):999–1010.
-
158Tarantini G, Mojoli M, Varbella F,Caporale R, Riggatieri S, Condo G, et al. Timing of Oral P2Y12 Inhibitor Administration in Non-ST Elevation Acute Coronary Syndrome. J Am Coll Cardiol. 2020;S0735-1097(20)36444-5.
-
159Nairooz R, Valgimigli M, Rochlani Y, Pothineni NV, Raina S, Sardar P, et al. Meta-analysis of clopidogrel pretreatment in acute coronary syndrome patients undergoing invasive strategy. Int J Cardiol. 2017 Feb 15;229:82-9.
-
160Komosa A, Lesiak M, Krasiński Z, Grygier M, Siniawski A, Skorupski W, et al. Optimal Timing of P2Y12 Inhibitor Loading in Patients Undergoing PCI: A Meta-Analysis. Thromb Haemost. 2019 Jun;119(6):1000-20.
-
161Topol EJ, Moliterno DJ, Herrmann HC, Powers ER, Grines CL, Cohen DJ, et al. the TARGET Investigators. Comparison of two platelet glycoprotein IIb/IIIa inhibitors, tirofiban and abciximab, for the prevention of ischemic events with percutaneous coronary revascularization. N Engl J Med. 2001;344(25):1888–94.
-
162Valgimigli M, Biondi-Zoccai G, Tebaldi M, van't Hof AWJ, Campo G, Hamm C, et al.T irofiban as adjunctive therapy for acute coronary syndromes and percutaneous coronary intervention: a meta-analysis of randomized trials. Eur Heart J. 2010;31(1):35-49.
-
163Cannon CP, Weintraub WS, Demopoulos LA, Vicari R, Frey MJ, Lakkis N, et al. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med 2001 Jun 21;344(25):1879-87.
-
164Novel dosing regimen of eptifibatide in planned coronary stent implantation (ESPRIT): a randomised, placebo-controlled trial. Lancet 2000 Dec 16;356(9247):2037-44.
-
165Platelet Receptor Inhibition in Ischemic Syndrome Management (PRISM) Study Investigators. A comparison of aspirin plus tirofiban with aspirin plus heparin for unstable angina. N Engl J Med. 1998 May 21;338(21):1498-505.
-
166International, randomized, controlled trial of lamifiban (a platelet glycoprotein IIb/IIIa inhibitor), heparin, or both in unstable angina. The PARAGON Investigators. Platelet IIb/IIIa Antagonism for the Reduction of Acute coronary syndrome events in a Global Organization Network. Circulation. 1998 Jun 23;97(24):2386-95.
-
167Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy (PURSUIT) Trial Investigators. Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. N Engl J Med. 1998 Aug 13;339(7):436-43.
-
168Mukherjee D, Mahaffey KW, Moliterno DJ, Harrington RA, Yadav JS, Pieper KS, et al. Promise of combined low-molecular-weight heparin and platelet glycoprotein IIb/IIIa inhibition: results from Platelet IIb/IIIa Antagonist for the Reduction of Acute coronary syndrome events in a Global Organization Network B (PARAGON B). Am Heart J. 2002 Dec;144(6):995-1002.
-
169Simoons ML. Effect of glycoprotein IIb/IIIa receptor blocker abciximab on outcome in patients with acute coronary syndromes without early coronary revascularisation: the GUSTO IV-ACS randomised trial. Lancet. 2001 Jun 16;357(9272):1915-24.
-
170Boersma E, Harrington RA, Moliterno DJ, White H, Theroux P, Van de WF, et al. Platelet glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: a meta-analysis of all major randomised clinical trials. Lancet. 2002 Jan 19;359(9302):189-98.
-
171EPIC Investigators. Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. N Engl J Med. 1994 Apr 7;330(14):956-61.
-
172Randomised placebo-controlled trial of abciximab before and during coronary intervention in refractory unstable angina: the CAPTURE Study. Lancet. 1997 May 17;349(9063):1429-35. Erratum in: Lancet 1997 Sep 6;350(9079):744.
-
173Platelet glycoprotein IIb/IIIa receptor blockade and low-dose heparin during percutaneous coronary revascularization. The EPILOG Investigators. N Engl J Med. 1997 Jun 12;336(24):1689-96.
-
174EPISTENT Investigators. Randomised placebo-controlled and balloon-angioplasty-controlled trial to assess safety of coronary stenting with use of platelet glycoprotein-IIb/IIIa blockade. Lancet. 1998 Jul 11;352(9122):87-92.
-
175Randomised placebo-controlled trial of effect of eptifibatide on complications of percutaneous coronary intervention: IMPACT-II. Integrilin to Minimise Platelet Aggregation and Coronary Thrombosis-II. Lancet. 1997 May 17;349(9063):1422-8.
-
176Effects of platelet glycoprotein IIb/IIIa blockade with tirofiban on adverse cardiac events in patients with unstable angina or acute myocardial infarction undergoing coronary angioplasty. The RESTORE Investigators. Randomized Efficacy Study of Tirofiban for Outcomes and REstenosis. Circulation. 1997 Sep 2;96(5):1445-53.
-
177O'shea JC, Hafley GE, Greenberg S, Hasselblad V, Lorenz TJ, Kitt MM, et al. Platelet glycoprotein IIb/IIIa integrin blockade with eptifibatide in coronary stent intervention: the ESPRIT trial: a randomized controlled trial. JAMA. 2001 May 16;285(19):2468-73.
-
178Karvouni E, Katritsis DG, Ioannidis JP. Intravenous glycoprotein IIb/IIIa receptor antagonists reduce mortality after percutaneous coronary interventions. J Am Coll Cardiol. 2003 Jan 1;41(1):26-32.
-
179Kastrati A, Mehilli J, Neumann FJ, Dotzer F, Ten BJ, Bollwein H, et al. Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: the ISAR-REACT 2 randomized trial. JAMA. 2006 Apr 5;295(13):1531-8.
-
180Stone GW, Bertrand ME, Moses JW, Ohman EM, Lincoff AM, Ware JH, et al. Routine upstream initiation vs deferred selective use of glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: the ACUITY Timing trial. JAMA. 2007 Feb 14;297(6):591-602.
-
181Giugliano RP, White JA, Bode C, Armstrong Pw, Montalescot G, Lewis BS, et al. et al. Early versus delayed, provisional eptifibatide in acute coronary syndromes. N Engl J Med 2009;360(21):2176 –90.
-
182Eikelboom JW, Anand SS, Malmberg K, Weitz JI, Ginsberg JS, Yusuf S. Unfractionated heparin and low-molecular-weight heparin in acute coronary syndrome without ST elevation: a meta-analysis. Lancet. 2000 Jun 3;355(9219):1936-42.
-
183Spinler SA, Mahaffey KW, Gallup D, Levine GM, Ferguson JJ, Rao SV,et al. Relationship between renal function and outcomes in high-risk patients with non-ST-segment elevation acute coronary syndromes: results from SYNERGY. Int J Cardiol. 2010;144(1):36-41.
-
184Bazinet A, Almanric K, Brunet C,Martineau J, Turcotte I, Caron S, et al. Dosage of enoxaparin among obese and renal impairment patients. Thromb Res. 2005;116(1):41-50.
-
185Garcia DA, Baglin TP, Weitz JI, Samama MM. Parenteral anticoagulants: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 May;141(2 Suppl):e24S-e43S.
-
186Lalama JT, Feeney ME, Vandiver JW, Beavers KD, Walter LN, McClintic JR. Assessing an enoxaparin dosing protocol in morbidly obese patients. J Thromb Thrombolysis. 2015;39(4):516-21.
-
187Lee YR, Palmere PJ, Burton CE, Benavides TM. Stratifying Therapeutic Enoxaparin Dose in Morbidly Obese Patients by BMI Class: A Retrospective Cohort Study. Clin Drug Investig. 2020;40(1):33-40.
-
188Spinler SA, Ou FS, Roe MT, Gibler WB, Ohman EM, Pollack CV,et al. Weight-based dosing of enoxaparin in obese patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE initiative. Pharmacotherapy. 2009 Jun;29(6):631-8.
-
189Chua D, Tataru A. Enoxaparin Dosing for Acute Coronary Syndromes in Obese Patients-Should There be a Maximum Dose. J Cardio Cardiovasc Med. 2016;1:002.
-
190White HD, Kleiman NS, Mahaffey KW, Lokhnygina Y, Pieper K, Cheswell K. et al. Efficacy and safety of enoxaparin compared with unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention in the Superior Yield of the New Strategy of Enoxaparin, Revascularization and Glycoprotein IIb/IIIa Inhibitors (SYNERGY) trial [published correction appears in Am Heart J. 2007 Feb;153(2):327]. Am Heart J. 2006;152(6):1042-50.
-
191Silvain J, Beygui F, Barthelemy O, Pollack C, Jr., Cohen M, Zeymer U, et al. Efficacy and safety of enoxaparin versus unfractionated heparin during percutaneous coronary intervention: systematic review and meta-analysis. BMJ. 2012;344:e553.
-
192Petersen JL, Mahaffey KW, Hasselblad V, Antman EM, Cohen M, Goodman SG, et al. Efficacy and bleeding complications among patients randomized to enoxaparin or unfractionated heparin for antithrombin therapy in non-ST-segment elevation acute coronary syndromes: a systematic overview. JAMA. 2004; 292(1): 89–96.
-
193Califf RM, Petersen JL, Hasselblad V, Mahaffey KW, Ferguson JJ. A perspective on trials comparing enoxaparin and unfractionated heparin in the treatment of non-ST-elevation acute coronary syndromes. Am Heart J. 2005 Apr;149(4 Suppl):S91-9.
-
194Yusuf S, Mehta SR, Chrolavicius S, Afzal R, Pogue J, Granger CB, et al. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med. 2006 Apr 6;354(14):1464-76.
-
195Karthikeyan G, Mehta SR, Eikelboom JW. Fondaparinux in the treatment of acute coronary syndromes: evidence from OASIS 5 and 6. Expert Rev Cardiovasc Ther. 2009 Mar;7(3):241-9.
-
196Serrano Jr. CV, Soeiro AM, Leal TCAT, Godoy LC, Biselli B, Hata LA et al. Posicionamento sobre Antiagregantes Plaquetários e Anticoagulantes em Cardiologia – 2019. Arq Bras Cardiol. 2019; 113(1):111-34.
-
197Mason PJ, Shah B, Tamis-Holland JE,Bitt JA, Cohen MG, Safirstein J, et al. An Update on Radial Artery Access and Best Practices for Transradial Coronary Angiography and Intervention in Acute Coronary Syndrome: A Scientific Statement From the American Heart Association. Circ Cardiovasc Interv. 2018;11(9):e000035. doi:10.1161/HCV.0000000000000035
» https://doi.org/10.1161/HCV.0000000000000035 -
198Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganisats TG, Holmes DR, et al. 2014 AHA/ACC Guideline for the management of patients with non-ST-elevation acute coronary syndromes. Circulation. 2014;130(25):e344-e426.
-
199Borzak S, Cannon C P, Kraft PL, Douthat L, Becker R C, Palmeri S T et al. Effects of Prior Aspirin and Anti-Ischemic Therapy on Outcome of Patients With Unstable Angina. Am J Cardiol.1998;81(6):678-81.
-
200Theroux P, Taeymans Y, Morissette D, Bosch X, Pelletier GB, Waters DD. A randomized study comparing propranolol and diltiazem in the treatment of unstable angina. J Am Coll Cardiol. 1985;5(3):717-22.
-
201Parodi O, Simonetti I, Michelassi C, Carpeggiani C, Biagini A, L’Abbate A, et al. Comparison of verapamil and propranolol therapy for angina pectoris at rest: a randomized, multiple-crossover, controlled trial in the coronary care unit. Am J Cardiol. 1986;57(11):899-906.
-
202Held PH, Yusuf S, Furberg CD. Calcium channel blockers in acute myocardial infarction and unstable angina: an overview. BMJ. 1989;299(6709):1187-92.
-
203Smith NL Reiber GE, Psaty BM, Heckbert SR, Siscovick DS, Ritchie JL, et al. Health outcomes associated with beta-blocker and diltiazem treatment of unstable angina. J Am Coll Cardiol. 1998;32(5):1305-11.
-
204Yusuf S, Held P, Furberg C. Update of effects of calcium antagonists in myocardial infarction or angina in light of the second Danish Verapamil Infarction Trial (DAVIT-II) and other recent studies. Am J Cardiol. 1991;67(15):1295-7.
-
205Boden WE, van Gilst WH, Scheldewaert RG, Starkey IR, Carlier MF, Julian DG, et al. Diltiazem in acute myocardial infarction treated with thrombolytic agents: a randomised placebo-controlled trial. Incomplete Infarction Trial of European Research Collaborators Evaluating Prognosis post-Thrombolysis (INTERCEPT). Lancet. 2000;355(9217):1751-6.
-
206De Luca G, Verdoia M, Binda G, Schaffer A, Suryapranata H, Marino P. Aspirin desensitization in patients under-going planned or urgent coronary stent implantation: a single-center experience. Int J Cardiol. 2013;167(2):561-3.
-
207Rossini R, Angiolillo DJ, Musumeci G, Scuri P, Invernizzi P, Bass TA, et al. Aspirin desensitization in patients undergoing percutaneous coronary interventions with stent implantation. Am J Cardiol. 2008;101(6):786-9.
-
208Page NA, Schroeder WS. Rapid desensitization protocols for patients with cardiovascular disease and aspirin hypersensitivity in an era of dual antiplatelet therapy. Ann Pharmacother. 2007;41(1):61-7.
-
209McMullan KL, Wedner HJ. Safety of aspirin desensitization in patients with reported aspirin allergy and cardiovascular disease. Clin Cardiol. 2013;36(1):25-30.
-
210Risk of myocardial infarction and death during treatment with low dose aspirin and intravenous heparin in men with unstable coronary artery disease. The RISC Group. Lancet. 1990;336(8719):827-30.
-
211Theroux P, Ouimet H, McCans J, Latour JG, Joly P, Levy G, et al. Aspirin, heparin, or both to treat acute unstable angina. N Engl J Med. 1988;319(17):1105-11.
-
212Mehta SR, Tanguay JF, Eikelboom JW, JollySS, J oyner CD, Granger CB, et al; CURRENT-OASIS 7 trial investigators. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376(9748):1233-43.
-
213Yusuf S, Mehta SR, Zhao F, Gersh BJ, Commerford PJ, Blumenthal M, et al; Clopidogrel in Unstable angina to prevent Recurrent Events Trial Investigators. Early and late effects of clopidogrel in patients with acute coronary syndromes. Circulation. 2003;107(7):966-72.
-
214Peters RJ, Mehta SR, Fox KA, Zhao F, Lewis BS, Kopecky SL, et al; Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) Trial Investigators. Effects of aspirin dose when used alone or in combination with clopidogrel in patients with acute coronary syndromes: observations from the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) study. Circulation. 2003;108(14):1682-7.
-
215Leon MB, Baim DS, Popma JJ, Gordon PC, Cutlip DE, Ho KK, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med.1998;339(23):1665-71.
-
216Mehta SR, Bassand JP, Chrolavicius S, Diaz R, Eikelboom JW, Fox KA, et al; CURRENT-OASIS 7 Investigators. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med. 2010;363(10):930-42. Erratum in: N Engl J Med. 2010;363(16):1585.
-
217Breet NJ, van Werkum JW, Bouman HJ, Kelder JC, Ruven HJ, Bal ET, et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA. 2010;303(8):754-62. Erratum in: JAMA. 2010;303(13):1257. JAMA. 2011;305(21):2174. JAMA. 2011;305(21):2172-3.
-
218Buonamici P, Marcucci R, Migliorini A, Gensini GF, Santini A, Paniccia R, et al. Impact of platelet reactivity after clopidogrel administration on drugeluting stent thrombosis. J Am Coll Cardiol. 2007; 49(24): 2312-7.
-
219Price MJ, Endemann S, Gollapudi RR, Valencia R, Stinis CT, Levisay JP, et al. Prognostic significance of postclopidogrel platelet reactivity assessed by a point-of-care assay on thrombotic events after drug-eluting stent implantation. Eur Heart J. 2008;29(8):992-1000.
-
220Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a metaanalysis. JAMA. 2010;304(16):1821-30.
-
221Taubert D, von Beckerath N, Grimberg G, Lazar A, Jung N, Goeser T, et al. Impact of P-glycoprotein on clopidogrel absorption. Clin PharmacolTher. 2006;80(5):486-501.
-
222Angiolillo DJ, Gibson CM, Cheng S, Ollier C, Nicolas O, Bergougnan L, et al. Differential effects of omeprazole and pantoprazole on the pharmacodynamics and pharmacokinetics of clopidogrel in healthy subjects: randomized, placebo-controlled, crossover comparison studies. Clin PharmacolTher. 2011;89(1): 65-74.
-
223Cuisset T, Frere C, Quilici J, Poyet R, Gaborit B, Bali L, et al. Comparison of omeprazole and pantoprazole influence on a high 150-mg clopidogrel maintenance dose the PACA (Proton Pump Inhibitors And Clopidogrel Association) prospective randomized study. J Am Coll Cardiol. 2009;54(13):1149-53.
-
224Frelinger AL 3rd, Lee RD, Mulford DJ, Wu J, Nudurupati S, Nigam A, et al. A randomized, 2-period, crossover design study to assess the effects of dexlansoprazole, lansoprazole, esomeprazole, and omeprazole on the steady-state pharmacokinetics and pharmacodynamics of clopidogrel in healthy volunteers. J Am Coll Cardiol. 2012;59(14):1304-11. Erratum in J Am Coll Cardiol. 2012;60(6):566-7.
-
225Gilard M, Arnaud B, Cornily JC, Le Gal G, Lacut K, Le Calvez G, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (OmeprazoleCLopidogrel Aspirin) study. J Am Coll Cardiol. 2008;51(3):256-60.
-
226Ho PM, Maddox TM, Wang L, Fihn SD, Jesse RL, Peterson ED, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009;301(9):937-44.
-
227Juurlink DN, Gomes T, Ko DT, Szmitko PE, Austin PC, Tu JV, et al. A population-based study of the drug interaction between proton pumpinhibitors and clopidogrel. CMAJ. 2009;180(7):713-8.
-
228Nicolau JC, Bhatt DL, Roe MT, Lokhnygina Y, Neely B, Corbalán R, et al. TRILOGY ACS investigators. Concomitant proton-pump inhibitor use, platelet activity, and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel and managed without revascularization: insights from the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes trial. Am Heart J. 2015;170(4):683-694.
-
229O’Donoghue ML, Braunwald E, Antman EM, Murphy SA, Bates ER, Rozenman Y, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: na analysis of two randomised trials. Lancet. 2009;374(9694):989-97.
-
230Goodman SG, Clare R, Pieper KS, Nicolau JC, Storey RF, Cantor WJ, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation. 2012;125(8):978-86.
-
231Bhatt DL, Cryer BL, Contant CF, Cohen M, Lanas A, Schnitzer TJ, et al; COGENT Investigators. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363(20):1909-17.
-
232Price MJ, Berger PB, Teirstein PS, Tanguay JF, Angiolillo DJ, Spriggs D, et al; GRAVITAS Investigators. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305(11):1097-105. Erratum in JAMA. 2011 Jun 1;305(21);2174.
-
233Collet JP, Cuisset T, Rangé G, Cayla G, Elhadad S, Pouillot C, et al; ARCTICInvestigators. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med. 2012;367(22):2100-9.
-
234Jakubowski JA, Winters KJ, Naganuma H, Wallentin L. Prasugrel: a novel thienopyridine antiplatelet agent: a review of preclinical and clinical studies and the mechanistic basis for its distinct antiplatelet profile. Cardiovasc Drug Rev. 2007;25(4):357-74.
-
235Morrow DA, Wiviott SD, White HD, Nicolau JC, Bramucci E, Murphy SA, et al. Effect of the novel thienopyridine prasugrel compared with clopidogrel on spontaneous and procedural myocardial infarction in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction 38: an application of the classification system from the universal definition of myocardial infarction. Circulation. 2009;119(21):2758-64.
-
236Wiviott SD, Braunwald E, Angiolillo DJ, Meisel S, Dalby AJ, Verheugt FW, et al; TRITON-TIMI 38 Investigators. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-Thrombolysis in Myocardial Infarction 38. Circulation. 2008;118(16):1626-36.
-
237Smith PK, Goodnough LT, Levy JH, Poston RS, Short MA, Weerakkody GJ, et al. Mortality benefit with prasugrel in the TRITON-TIMI 38 coronary artery bypass grafting cohort: risk-adjusted retrospective data analysis. J Am Coll Cardiol. 2012,31;60(5):388-96.
-
238Gurbel PA, Erlinge D, Ohman EM, Neely B, Neely M, Goodman SG, et al. Platelet function during extended prasugrel and clopidogrel therapy for patients with ACS treated without revascularization: the TRILOGY ACS platelet function substudy. JAMA. 2012;308(17):1785-94.
-
239Ohman J, Kudira R, Albinsson S, Olde B, Erlinge D. Ticagrelor induces adenosine triphosphate release from human red blood cells. Biochem Biophys Res Commun. 2012;418(4):754-8.
-
240Bonello L, Laine M, Kipson N, Mancini J, Helal O, Fromonot J, et al. Ticagrelor increases adenosine plasma concentration in patients with an acute coronary syndrome. J Am Coll Cardiol 2014;63(9):872-7.
-
241Orme RC, Parker WAE, Thomas MR, Judge HM, Baster K, Sumaya W, et.al. Study of Two Dose Regimens of Ticagrelor Compared with Clopidogrel in Patients Undergoing Percutaneous Coronary Intervention for Stable Coronary Artery Disease (STEEL-PCI). Circulation 2018;138(13):1290-300.
-
242Ortega-Paz L, Brugaletta S, Ariotti S, Akkerhuis KM, Karagiannis A, Windecker S, et al. HI-TECH investigators. Adenosine and Ticagrelor Plasma Levels in Patients With and Without Ticagrelor-Related Dyspnea. Circulation 2018;138(6):646-48.
-
243Scirica BM, Cannon CP, Emanuelsson H, Michelson EL, Harrington RA, Husted S, et al.; PLATO Investigators. The incidence of bradyarrhythmias an clinical bradyarrhythmic events in patients with acute coronary syndromes treated with ticagrelor or clopidogrel in the PLATO (Platelet Inhibition and Patient Outcomes) trial: results of the continuous electrocardiographic assessment substudy. J Am Coll Cardiol. 2011;57(19):1908-16.
-
244Nilsen DW. Potential benefits of ticagrelor beyond platelet inhibition. Cardiology. 2013;125(1):31-3.
-
245ames S, Angiolillo DJ, Cornel JH, Erlinge D, Husted S, Kontny F, et al; PLATO Study Group. Ticagrelor vs. clopidogrel in patients with acute coronary syndromes and diabetes: a substudy from the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J. 2010;31(24):3006-16.
-
246James S, Budaj A, Aylward P, Buck KK, Cannon CP, Cornel JH, et al. Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: results from the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2010;122(11):1056-67.
-
247James SK, Storey RF, Khurmi NS, Husted S, Keltai M, Mahaffey KW, et al; PLATO Study Group. Ticagrelor versus clopidogrel in patients with acute coronary syndromes and a history of stroke or transient ischemic attack. Circulation. 2012;125(23):2914-21.
-
248Cannon CP, Harrington RA, James S, Ardissino D, Becker RC, Emanuelsson H, et al; PLATelet inhibition and patient Outcomes Investigators. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet. 2010;375(9711):283-93.
-
249Held C, Asenblad N, Bassand JP, Becker RC, Cannon CP, Claeys MJ, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes undergoing coronary artery bypass surgery: results from the PLATO (Platelet Inhibition and Patient Outcomes) trial. J Am Coll Cardiol. 2011;57(6):672-84.
-
250Kohli P, Wallentin L, Reyes E, Horrow J, Husted S, Angiolillo DJ, et al. Reduction in first and recurrent cardiovascular events with ticagrelor compared with clopidogrel in the PLATO Study. Circulation. 2013;127(6):673-80.
-
251Nikolic E, Janzon M, Hauch O, Wallentin L, Henriksson M; PLATO Health Economic Substudy Group. Cost-effectivenessof treating acute coronary syndrome patients with ticagrelor for 12 months: results from the PLATO study. Eur Heart J. 2013;34(3):220-8.
-
252Schüpke S, Neumann FJ, Menichelli M, Mayer K, Bernlochner I, Wöhrle J, et al., ISAR-REACT 5 Trial Investigators Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes N Engl J Med 2019;381(16):1524-34.
-
253Cayla G, Cuisset T, Silvain J, Leclercq F, Manzo-Silberman S, Saint-Etienne C, et al.ANTARCTIC investigators. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): an open-label, blinded-endpoint, randomised controlled superiority trial. Lancet. 2016;388(10055):2015-22.
-
254Claassens DMF, Vos GJA, Bergmeijer TO, Hermanides RS, van ‘t Hof AWJ, van der Harst P, et al. A Genotype-Guided Strategy for Oral P2Y 12 Inhibitors in Primary PCI. N Engl J Med. 2019;381(17):1621-31.
-
255Pereira NL, Farkouh ME, So D. Effect of Genotype-Guided Oral P2Y12 Inhibitor Selection vs Conventional Clopidogrel Therapy on Ischemic Outcomes After Percutaneous Coronary Intervention: The TAILOR-PCI Randomized Clinical Trial JAMA. 2020;324(8):761-77
-
256Hirsh J, Warkentin TE, Shaughnessy SG, Annand SS. Heparin and Low-Molecular-Weight Heparin, Mechanisms of Action, Pharmacokinetics, Dosing, Monitoring, Efficacy, and Safety. Chest. 2001; 119(1 Suppl):64S-94S.
-
257Hirsh J, Anand SS, Halperin JL, Fuster V. Mechanism of Action and Pharmacology of Unfractionated Heparin. Arterioscler Thromb Vasc Biol. 2001;21(7):1094-96.
-
258Lee MS, Wali AU, Menon V, Berkowitz SD, Thompson TD, Califf RM, et al. The determinants of activated partial thromboplastin time, relation of activated partial thromboplastin time to clinical outcomes, and optimal dosing regimens for heparin treated patients with acute coronary syndromes: are view of GUSTO-IIb. J Thromb Thrombolysis 2002;14(2):91–101.
-
259AntmanEM, McCabe CH, Gurfinkel EP, Turpie AG, Bernink PJ, Salein D, et al. Enoxaparin prevents death and cardiac Ischemic events in unstable angina/non-Q-wave myocardial infarction: results of the Thrombolysis in Myocardial Infarction (TIMI) 11B trial. Circulation. 1999;100(15):1593–601.
-
260Cohen M, Demers C, Gurfinkel EP, Turpie AGG, Fromell GJ, Goodman S, et al. A Comparison of Low-Molecular-Weight Heparin with Unfractionated Heparin for Unstable Coronary Artery Disease. N Engl J Med. 1997;337(7),447-52.
-
261Antman EM, Cohen M, Radley D, McCabe C, Rush J, Premmereur J, Braunwald E. Assessment of the Treatment Effect of Enoxaparin for Unstable angina/non-Q-wave Myocardial Infarction. TIMI 11B-ESSENCE Meta-Analysis. Circulation. 1999;100(15), 1602–8.
-
262Ferguson JJ, Califf RM, Antman EM, Cohen M, Grines CL, Goodman S, et al. Enoxaparin VS unfractionated heparin in high risk patients with non-ST-segment elevation acute coronary syndromes managed with na intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA. 2004;292(1):45–54.
-
263Collet JP, Montalescot G, Lison L, Choussat R, Ankri A, Drobinski G, et al. Percutaneous coronary intervention after subcutaneous enoxaparin pretreatment in patients with unstable angina pectoris. Circulation. 2001;103(5):658–63.
-
264Petitou M, Casu B, Lindahl U. 1976-1983, a critical period in the history of heparin: the discovery of the antithrombin binding site. Biochimie. 2003;85(12):83–9.
-
265Holbrook A, Schulman S, Witt DM, Vandnivick PO, Fish J, Kovacs MJ, et al. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e152S–e184S.
-
266Zhang Y, Zhang M, Tan L, Pan N, Zhang L. The clinical use of Fondaparinux: A synthetic heparin pentasaccharide. Prog Mol Biol Transl Sci. 2019;163:41–53.
-
267Mehta SR, Granger CB, Eikelboom JW, Bassand JP, Wallentin L,Faxon DP, et al. Efficacy and Safety of Fondaparinux Versus Enoxaparin in Patients With Acute Coronary Syndromes Undergoing Percutaneous Coronary Intervention: Results From the OASIS-5 Trial. J Am Coll Cardiol 2007;50(18):1742-51
-
268Gibson CM, Mehran R, Bode C, Halpecin J, Verheugt FW, Goose P, Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375(25):2423–34.
-
269Cannon CP, Bhatt DL, Oldgren J, Thomas L, Ansell J, Fonarow GC, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377(25):1513–24.
-
270Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, et al Antithrombotic Therapy After Acute Coronary Syndrome or PCI in Atrial Fibrillation. N Engl J Med. 2019;380(16):1509-24.
-
271Vranckx P, Valgimigli M, Eckardt L, Tijsssen J, Lewatter T, Gargiuo G, et al. Edoxaban-based Versus Vitamin K Antagonist-Based Antithrombotic Regimen After Successful Coronary Stenting in Patients With Atrial Fibrillation (ENTRUST-AF PCI): A Randomised, Open-Label, Phase 3b Trial. Lancet, 2019;394 (10206), 1335-43.
-
272Lopes RD, Hong H, Harskamp RE, Bhatt DL, Mehran R, Cannon CP, et al. Optimal Antithrombotic Regimens for Patients with Atrial Fibrillation Undergoing Percutaneous Coronary Intervention: an Updated Network Meta-analysis. JAMA Cardiol. 2020;5(5):582-589.
-
273Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al ESC Scientific Document Group, 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2020 Aug 20 ehaa575 [online]
-
274Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, et.al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–15042.
-
275Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001-9.
-
276Heart Protection Collaborative Group. MRC/BHF Heart Protection Study of cholsterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7-22.
-
277Dondo TB, Hall M, Timmis AD, Gilthorpe MS, Alabas AO, Batin PD, et al. Excess Mortality and Guideline-Indicated Care Following non-ST-elevation Myocardial Infarction. Eur Heart J Acute Cardiovasc Care, 2107;6 (5), 412-20.
-
278Faludi AA, Izar MCO, Saraiva JFK, Bianco HT, Chacra APM, Bertoluci MC et al. Diretriz brasileira baseada em evidências sobre prevenção de doenças cardiovasculares em pacientes com diabetes: posicionamento da Sociedade Brasileira de Diabetes (SBD), da Sociedade Brasileira de Cardiologia (SBC) e da Sociedade Brasileira de Endocrinologia e Metabologia (SBEM). Arq Bras Cardiol. 2017; 109(6 Supl.1):1-31
-
279Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et.al. IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-97.
-
280Berwanger O, Santucci EV, de Barros e Silva PGM, Jesuíno IA, Damiani LP, Barbosa LM, et al. Effect of Loading Dose of Atorvastatin Prior to Planned Percutaneous Coronary Intervention on Major Adverse Cardiovascular Events in Acute Coronary Syndrome. JAMA. 2018;319(13):1331-40.
-
281Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of na angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342(3):145-53. Erratum in N Engl J Med. 2000;342(10):748 Erratum: N Engl J Med. 2000;342(18):1376.
-
282Fox KM; EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet. 2003; 362(9386):782-8.
-
283Hoedemaker NPG, Damman P, Ottervanger JP, Dambrink JHE, Gosselink ATM, Kedhi E, et.al. Trends in Optimal Medical Therapy Prescription and Mortality After Admission for Acute Coronary Syndrome: A 9-year Experience in a Real-World Setting. Eur Heart J Cardiovasc Pharmacother,2018; 4(2):102-10.
-
284Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003 Apr 3;348(14):1309-21. Erratum in: N Engl J Med. 2003; 348(22):2271.
-
285Carillo S, Zhang Y, Fay R, Angioi M, Vincent J, Sutradhor SC, et al. Heart failure with systolic dysfunction complicating acute myocardial infarction - differential outcomes but similar eplerenone efficacy by ST-segment or non-ST-segment elevation: A post hoc substudy of the EPHESUS trial. Arch Cardiovasc Dis. 2014 Mar;107(3):149-57.
-
286Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999 Sep 2;341(10):709-17.
-
287Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart AssociationTask Force on Practice Guidelines (Committee to Update the 1997 ExerciseTesting Guidelines). Circulation. 2002;106(14):1883-92.
-
288Gibler WB, Cannon CP, Blomkalns AL, Char DM, Drew BJ, Hollander JE, et al; American Heart Association Council on Clinical Cardiology (Subcommittee on Acute Cardiac Care); Council on Cardiovascular Nursing, and Quality of Care and Outcomes Research Interdisciplinary Working Group; Society of Chest Pain Centers. Practical implementation of the guidelines for unstable angina/non-ST-segment elevation myocardial infarction in the emergency department: a scientific statement from the American Heart Association Councilon Clinical Cardiology (Subcommittee on Acute Cardiac Care), Councilon Cardiovascular Nursing, and Quality of Care and Outcomes Research Interdisciplinary Working Group,in Collaboration With the Society of Chest Pain Centers. Circulation. 2005;111(20):2699-710.
-
289Amsterdam EA, Kirk JD, Diercks DB, Lewis WR, Turnipseed SD. Early exercise testing in the management of low risk patients in chest pain centers. Prog Cardiovasc Dis. 2004;46(5):438-52.
-
290Mark DB, Hlatky MA, Harrell FE Jr, Lee KL, Califf RM, Pryor DB. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med. 1987;106(6):793-800.
-
291Stein RA, Chaitman BR, Balady GJ, Fleg JL, Limacher MC, Pina IL, et al. Safety and utility of exercise testing in emergency room chest pain centers: an advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association. Circulation. 2000;102(12):1463-7.
-
292Larsson H, Areskog M, Areskog NH, Nylander E, Nyman I, Swahn E, et al. Should the exercise test (ET) be performed at discharge or one month later after an episode of unstable angina or non-Q-wave myocardial infarction? Int J Card Imaging. 1991;7(1):7-14.
-
293Hermann LK, Weingart SD, Duvall WL, Henzlova MJ. The limited utility of routine cardiac stress testing in emergency department chest pain patients younger than 40 years. Ann Emerg Med. 2009;54(1):12-6.
-
294Natsui S, Sun BC, Shen E, Wu YL, Redberg RF, Lee MS, et al. Evaluation of Outpatient Cardiac Stress Testing After Emergency Department Encounters for Suspected Acute Coronary Syndrome. Ann Emerg Med. 2019 Aug;74(2):216-23.
-
295Sabia P, Afrookteh A, Touchstone DA, Keller MW, Esquivel L, Kaul S. Value of regional wall motion abnormality in the emergency room diagnosis of acute myocardial infarction: a prospective study using two-dimensional echocardiography. Circulation. 1991;84(3 Suppl):I85-92.
-
296Stein JH, Neumann A, Preston LM, VandenBerg BJ, Parrillo JE, Calvin JE, et al. Improved risk stratification in unstable angina: identification of patients at low risk for in hospital cardiac events by admission echocardiography. Clin Cardiol. 1998;21(10):725-30.
-
297Horowitz RS, Morganroth J, Parrotto C, Chen CC, Soffer J, Pauletto FJ. Immediate diagnosis of acute myocardial infarction by two-dimensional echocardiography. Circulation. 1982;65(2):323-9.
-
298Kontos MC, Arrowood JA, Paulsen WH, Nixon JV. Early echocardiography can predict cardiac events in emergency department patients with chest pain. Ann Emerg Med. 1998;31(5):550-7.
-
299Barberato SH, Romano MMD, Beck ALS, Rodrigues ACT, Almeida ALC, Assunção BMBL, et al. Position Statement on Indications of Echocardiography in Adults - 2019. Arq Bras Cardiol. 2019; 113(1):135-81.
-
300Campos FO, Zielinsky P, Ortiz J, Maciel BC, Andrade JL, Mathias W Jr, et al; Brazilian Society of Cardiology. [Guideline for indication and utilization of echocardiography in clinical practice]. Arq Bras Cardiol. 2004;82(Suppl 2):11-34.
-
301Atici A, Ali Barman H, Durmaz E, Demir K, Cakmak R, Tugrul S, et al. Predictive value of global and territorial longitudinal strain imaging in detecting significant coronary artery disease inpatients with myocardial infarction without persistent ST-segment elevation. Echocardiography. 2019; 36(3):425-622.
-
302Solomon SD, Glynn RJ, Greaves S, Ajani U, Rouleau JL, Menapace RF, et al. Recovery of venricular function after myocardial infarction in the reperfusion era: The healing and early after local therapy study. Ann Intern Med. 2001; 134(6): 451 - 8.
-
303Liou K, Negishi K, Ho S, Russell EA, Cranney G, Ooi SY. Detectionof Obstructive Coronary Artery Disease Using Peak Systolic Global Longitudinal Strain Derived by Two-Dimensional Speckle-Tracking: A Systematic Review and Meta-Analysis. J Am Soc Echocardiogr.2016;29(8):724-35 e4.
-
304Marques-Alves P, Espirito-Santo N, Baptista R, Teixeira R, Martins R, Gonçalvrs F, et al. Two-dimensional speckle-tracking global longitudinal strain in high-sensitivity troponin-negative low-risk patients with unstable angina: a “resting ischemia test”? Int J Cardiovasc Imaging. 2017; 34(4):561-8.
-
305Dahlslett T, Karlsen S, Grenne B, Eek C, Sjoli B, Skustad H, et al. Early assessment of strain echocardiography can accurately exclude significant coronary artery stenosis in suspected non-ST-segment elevation acute coronary syndrome. J Am Soc Echocardiogr. 2014;27(5):512–9.
-
306Porter TR, Mulvagh SL, Abdelmoneim SS, Becher H, Belcik JT, Bierig M, et.al. Clinical Applications of Ultrasonic Enhancing Agents in Echocardiography: 2018 American Society of Echocardiography Guidelines Update. J Am Soc Echocardiogr. 2018; 31(3):241-74.
-
307Pellikka PA, Olson AA, Chaudhry FA, Chen MH, Marshall JE, Porter TR, Sawada SG, Guidelines for Performance, Interpretation, and Application of Stress Echocardiography in Ischemic Heart Disease: From the American Society of Echocardiography. J Am Soc Echocardiogr.2020;33(1):1-41.
-
308Sicari R, Landi P, Picano E, Pirelli S, Chiaranda G, Previtali M, et al. Exercise-electrocardiography and/or pharmacological stress echocardiography for non-invasive risk stratification early after uncomplicated myocardial infarction: a prospective international large scale multicentre study. Eur Heart J. 2002;23(13):1030-7.
-
309Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949-3003.
-
310Picano E, Mathias W Jr, Pingitore A, Bigi R, Previtali M. Safety and tolerability of dobutamine-atropine stress echocardiography: a prospective, multicentre study. Echo Dobutamine International Cooperative Study Group. Lancet. 1994;344(8931):1190-2.
-
311Caldas MA, Tsutsui JM, Kowatsch I, Andrade JL, Nicolau JC, Ramires JF, et al. Value of myocardial contrast echocardiography for predicting left ventricular remodeling and segmental functional recovery after anterior wall acute myocardial infarction. J Am Soc Echocardiogr. 2004;17(9):923-32.
-
312Mathias W Jr, Arruda AL, Andrade JL, Filho OC, Porter TR. Endocardial borderdeline action during dobutamine infusion using contrast echocardiography. Echocardiography. 2002;19(2):109-14.
-
313Porter TR, Abdelmoneim S, Belcik JT, McCulloch ML, Mulvagh SL, Olson JJ, et al. Guidelines for the cardiac sonographer in the performance of contrast echocardiography: a focused update from the American Society of Echocardiography. J Am Soc Echocardiogr. 2014;27(8):797-810.
-
314Tsutsui JM, Elhendy A, Xie F, O’Leary EL, McGrain AC, Porter TR. Safety of dobutamine stress real-time myocardial contrast echocardiography. J Am Coll Cardiol. 2005;45(8):1235-42.
-
315Tsutsui JM, Xie F, O’Leary EL, Elhendy A, Anderson JR, McGrain AC, et al. Diagnostic accuracy and prognostic value of dobutamine stress myocardial contrast echocardiography in patients with suspected acute coronary syndromes. Echocardiography. 2005;22(6):487-95.
-
316Brown KA. Evaluation of the unstable angina patient in 2005: is there still a role for non invasive risk stratification? J Nucl Cardiol. 2005;12(1):9-11.
-
317Amanullah AM. Noninvasive testing in the diagnosis and management of unstable angina. Int J Cardiol. 1994;47(2):95-103.
-
318Miller DD. Risk stratification in unstable angina pectoris. In: Zaret BL, Beller GA. (eds) Nuclear cardiology: stateoftheartand future directions. Saint Louis: Mosby Inc; 1999. p. 490-9.
-
319Stratmann HG, Younis LT, Wittry MD, Amato M, Miller DD. Exercise technetium-99m myocardial tomography for the risk stratification of men with medically treated unstable angina pectoris. Am J Cardiol. 1995;76(4):236-40.
-
320Freeman MR, Chisholm RJ, Armstrong PW. Usefulness of exercise electrocardiography and thallium scintigraphy in unstable angina pectoris in predicting the extent and severity of coronary artery disease. Am J Cardiol. 1988;62(17):1164-70. Erratum in: Am J Cardiol 1989;63(5):392.
-
321Zhu YY, Chung WS, Botvinick EH, Dae MW, Lim AD, Ports TA, et al. Dipyridamole perfusion scintigraphy: the experience with its application in one hundred seventy patients with known or suspected unstable angina. Am Heart J. 1991;121(1 Pt 1):33-43.
-
322Brown KA. Prognosticvalueof thallium-201 myocardial perfusion imaging in patients with unstable angina who respond to medical treatment. J Am Coll Cardiol. 1991;17(5):1053-7.
-
323Madsen JK, Stubgaard M, Utne HE, Hansen JF, van Duijvendijk K, Reiber JH, et al. Prognosis and thallium-201 scintigraphy in patients admitted with chest pain without confirmed acute myocardial infarction. Br Heart J. 1988;59(2):184-9.
-
324Beanlands RS, Nichol G, Huszti E, Humen D, Racine N, Freeman M, et al. F-18-fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: A randomized, controlled trial (PARR-2). J Am Coll Cardiol. 2007;50(2):2002–12.
-
325Ziadi MC, Dekemp RA, Williams KA, Guo A, Chow BJ, Renaud JM, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol.2011;58(7):740-8.
-
326de Souza ACDAH, Gonçalves BKD, Tedeschi AL, Lima RSL. Quantification of Myocardial Flow Reserve Using aGamma Camera With Solid-State Cadmium-Zinc-Telluride Detectors: Relation to Angiographic Coronary Artery Disease. J Nucl Cardiol. 2019; Jun 20. Doi 10.1007/s12350-019-01775-z. ahead of print
» https://doi.org/10.1007/s12350-019-01775-z -
327Hundley WG, Bluemke DA, Finn JP, Flamm SD, Fogel MA, Friedrich MG, et al; American College of Cardiology Foundation Task Force on Expert Consensus Documents. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus documenton cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;121(22):2462-508.
-
328Nagel E, Lehmkuhl HB, Bocksch W, Klein C, Vogel U, Frantz E, et al. Non invasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI: comparison with dobutamine stress echocardiography. Circulation. 1999;99(6):763-70.
-
329Jaarsma C, Leiner T, Bekkers SC, Crijns HJ, Wildberger JE, Nagel E, et al. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and pósitron emission tomography imaging for the detection of obstructive coronary artery disease: a meta-analysis. J Am Coll Cardiol. 2012;59(19):1719-28.
-
330Schwitter J, Wacker CM, Wilke N, Al-Saadi N, Sauer E, Huettle K, et al; MR-IMPACT Investigators. MR-IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery diseaseTrial: perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial. Eur Heart J. 2013;34(10):775-81.
-
331Watkins S, McGeoch R, Lyne J, Steedman T, Good R, McLaughlin MJ, et al. Validation of magnetic resonance myocardial perfusion imaging with fractional flow reserve for the detection of significant coronary heart disease. Circulation. 2009;120(22):2207-13.
-
332Steel K, Broderick R, Gandla V, Larose E, Resnic F, Jerosch-Herold M, et al. Complementary prognostic values of stress myocardial perfusion and late gadolinium enhancement imaging by cardiac magnetic resonance in patients with known or suspected coronary artery disease. Circulation. 2009;120(14):1390-400.
-
333Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343(20):1445-53.
-
334Choi KM, Kim RJ, Gubernikoff G, Vargas JD, Parker M, Judd RM. Transmural extent of acute myocardial infarction predicts long-term improvement in contractile function. Circulation. 2001;104(10):1101-7.
-
335Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, et al. Contrast-enhanced MRI androutine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial Myocardial infarcts: an imaging study. Lancet. 2003;361(9355):374-9.
-
336Cheong BY, Muthupillai R, Wilson JM, Sung A, Huber S, Amin S, et al. Prognostic significance of delayed-enhancement magnetic resonance imaging: survival of 857 patients with and without left ventricular dysfunction. Circulation. 2009;120(21):2069-76.
-
337Wu E, Judd RM, Vargas JD, Klocke FJ, Bonow RO, Kim RJ. Visualisation of presence, location, and transmural extent of healed Q-wave and non-Q wave myocardial infarction. Lancet. 2001;357(9249):21-8.
-
338Kim RJ, Albert TS, Wible JH, Elliott MD, Allen JC, Lee JC, et al. Performance of delayed-enhancement magnetic resonance imaging with gadoversetamide contrast for the detection and assessment of myocardial infarction: na international, multicenter, double-blinded, randomized trial. Circulation.2008;117(5):629-37.
-
339Kim HW, Farzaneh-Far A, Kim RJ. Cardiovascular magneticresonance in patients with myocardial infarction: current and emerging applications. J Am Coll Cardiol. 2009;55(1):1-16.
-
340Eitel I, de Waha S, Wöhrle J, Fuernau G, Lurz P, Pauschinger M, et al. Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2014 Sep 23;64(12):1217-26.
-
341Schelbert EB, Cao JJ, Sigurdsson S, Aspelund T, Kellman P, Aletras AH, et al. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA. 2012;308(9):890-6.
-
342Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S, et al. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or Symptoms of coronary artery disease. Circulation. 2006;113(23):2733-43. Erratum in Circulation. 2006;114(8):e365.
-
343Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation.1998;97(8):765-72.
-
344Kelle S, Roes SD, Klein C, Kokocinski T, de Roos A, Fleck E, et al. Prognostic value of myocardial infarct size and contractile reserve using magnetic resonance imaging. J Am Coll Cardiol. 2009;54(19):1770-7.
-
345Kendziora B, Dewey M. Prognostic value of the myocardial salvage index measured by T2-weighted and T1-weighted late gadolinium enhancement magnetic resonance imaging after ST-segment elevation myocardial infarction: A systematic review and meta-regression analysis. PLoS One. 2020 Feb 13;15(2):e0228736.
-
346Rochitte CE. Microvascular obstruction the final frontier for a complete myocardial reperfusion. J Am Coll Cardiol. 2008;51(23):2239-40.
-
347Klug G, Mayr A, Schenk S, Esterhammer R, Schocke M, Nocker M, et al. Prognostic value at 5 years of microvascular obstruction after acute myocardial infarction assessed by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14(1):46.
-
348Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magneticresonance assessment of non ischaemic cardiomyopathies. Eur Heart J. 2005;26(15):1461-74.
-
349Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, et al; International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53(17):1475-87.
-
350Gannon MP, Schaub E, Grines CL, Saba SG. State of the Art: Evaluation and Prognostication of Myocarditis Using Cardiac MRI. J Magn Reson Imaging, 2019;49 (7), e122-e131
-
351Athanasiadis A, Schneider B, Sechtem U. Role of cardiovascular magnetic resonance in takotsubo cardiomyopathy. Heart Fail Clin. 2013;9(2):167-76.
-
352Scally C, Abbas H, Ahearn T, Srinivasan J, Mezincescu A, Rudd A, et al. Myocardial and Systemic Inflammation in Acute Stress-Induced (Takotsubo) Cardiomyopathy. Circulation. 2019;139 (13):1581-92.
-
353Vidal-Perez R, Abou Jokh Casas C, Agra-Bermejo RM, Alvarez-Alvarez B, Grapsa J, Fontes-Carvalho R, et al. Myocardial infarction with non-obstructive coronary arteries: A comprehensive review and future research directions. World J Cardiol. 2019 Dec 26;11(12):305-15.
-
354Perazzolo Marra M, Lima JA, Iliceto S. MRI in acute myocardial infarction. Eur Heart J. 2011;32(3):284-93.
-
355Sandoval Y, Jaffe AS. Type 2 Myocardial Infarction: JACC Review Topic of the Week. J Am Coll Cardiol. 2019 Apr 16;73(14):1846-60.
-
356Barbarawi, M, Kheiri, B, Zayed, Y, Barbarawi O, Chahine A, Haykal T, et al. Meta‐analysis of optimal timing of coronary intervention in nonST‐elevation acute coronary syndrome. Catheter Cardiovasc Interv. 2020; 95(2): 185– 93.
-
357Lindholm D, Alfredsson J, Angerås O, Bohm F, Calais F, Koul S, et al. Timing of percutaneous coronary intervention in patients with non-ST-elevation myocardial infarction: a SWEDEHEART study. Eur Heart J Qual Care Clin Outcomes. 2017;3(1):53‐60.
-
358Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization Eur Heart J. 2019;40(2): 87-165.
-
359Götberg M, Christiansen EH, Gudmundsdottir I J, Sandhall L, Danielewicz M, Jakobsen L, et al. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. N Engl J Med. 2017;376(19):1813-23.
-
360Davies JE, Sen S, Dehbi HM, Al-Lamee R, Petraco R, Nijjer S S, et al. Use of the Instantaneous Wave-free Ratio or Fractional Flow Reserve in PCI. N Engl J Med. 2017;376(19), 1824-34.
-
361Matsuo Y, Kubo T, Akasaka T. The use of optical coherence tomography in acute coronary syndrome. Expert Rev Cardiovasc Ther. 2016;14(5):649-57.
-
362Ali ZA, Galougahi, KK, Maehara A, Shlofmitz RA, Ben-Yehuda O, Mintz GS, Stone GW. Intracoronary Optical Coherence Tomography 2018. JACC: Cardiovasc Interv. 2017;10(24), 2473–87.
-
363Meneveau N, Souteyrand G, Motreff P, Caussin C, Amabile N, Ohlmann P, et al. Optical Coherence Tomography to Optimize Results of Percutaneous Coronary Intervention in Patients with Non-ST-Elevation Acute Coronary Syndrome: Results of the Multicenter, Randomized DOCTORS Study (Does Optical Coherence Tomography Optimize Results of Stenting). Circulation. 2016;134(13):906‐17.
-
364Chowdhury M, Osborn EA. Physiological assessment of coronary lesions in 2020. Curr Treat Options Cardiovasc Med. 2020; 22(1):2.
-
365Choudhry NK, Singh JM, Barolet A, Tomlinson GA, Detsky AS. How should patients with unstable angina and non-ST-segment elevation myocardial infarction be managed? A meta-analysis of randomized trials. Am J Med. 2005;118(5):465-74.
-
366Damman P, van Geloven N, Wallentin L, Lagerqvist B, Fox KA, Clayton T, et al. Timing of angiography with a routine invasive strategy and long-term outcomes in non-ST-segment elevation acute coronary syndrome: a collaborative analysis of individual patient data from the FRISC II (Fragmin and Fast Revascularization During Instability in Coronary Artery Disease), ICTUS (Invasive Versus Conservative Treatment in Unstable Coronary Syndromes), and RITA-3 (Intervention Versus Conservative Treatment Strategy in Patients With Unstable Angina or Non-ST Elevation Myocardial Infarction) Trials. JACC Cardiovasc Interv. 2012;5(2):191-9.
-
367Zimarino M, Curzen N, Cicchitti V, De Caterina R. The adequacy of myocardial revascularization in patients with multivessel coronary artery disease. Int J Cardiol. 2013;168(3):1748-57.
-
368Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, et al. Predictors of hospital mortality in the Global Registry of Acute Coronary Events. Arch Intern Med 2003;163:2345–53138.
-
369Judd E, Hollander JE, Than M, Mueller C. State-of-the-Art Evaluation of Emergency Department Patients Presenting With Potential Acute Coronary Syndromes. Circulation 2016;134:547–564.
-
370Damman P, Hirsch A, Windhausen F, Tijssen JGP, Winter RJ, ICTUS Investigators. 5-year clinical outcomes in the ICTUS (Invasive versus Conservative Treatment in Unstable coronary Syndromes) trial a randomized comparison of an early invasive versus selective invasive management in patients with non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol. 2010;55:858–64.
-
371Mahmoud AN, Elgendy IY, Mansoor H, Xuerong W, Mojadid MK, Bavry AA, et al. Early invasive strategy and in-hospital survival of diabetics with non-st-elevation Myocardial infarction: a contemporary national insight. JACC. 2017;(69):182.
-
372Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1(2):219-27.
-
373Mohr FW, Morice MC, Kappetein AP, Feldman TE, Ståhle E, Colombo A, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381(9867):629-38.
-
374Jones EL, Craver JM, Guyton RA, Bone DK, Hatcher CR Jr, Riechwald N. Importance of complete revascularization in performance of the coronary bypass operation. Am J Cardiol. 1983;51(1):7-12.
-
375Bundhun PK, Sookharee Y, Bholee A, Huang F. Application of the SYNTAX score in interventional cardiology: A systematic review and meta-analysis. Medicine (Baltimore). 2017;96(28):e7410.
-
376Ranasinghe I, Alprandi-Costa B, Chow V, Elliott JM, Waites J, Counsell JT, et al. Risk stratification in the setting of non-ST elevation acute coronary syndromes 1999-2007. Am J Cardiol 2011;108:617–624.
-
377Fukui T, Tabata M, Morita S, Takanashi S. Early and long-term outcomes of coronary artery bypass grafting in patients with acute coronary syndrome versus stable angina pectoris. J Thorac Cardiovasc Surg 2013;145:1577–1583.
-
378Malm CJ, Hansson EC, Akesson J, Andersson M, Hesse C, Shams Hakimi C, et al. Preoperative platelet function predicts perioperative bleeding complications in ticagrelor-treated cardiac surgery patients: A prospective observational study. Br J Anaesth 2016;117:309–315.
-
379Nichols EL, McCullough JN, Ross CS, Kramer RS, Westbrook BM, Klemperer JD, et al. Optimal Timing From Myocardial Infarction to Coronary Artery Bypass Grafting on Hospital Mortality. Ann of Thorac Surg. 2017;103:(1)162–171.
-
380Tegn N, Abdelnoor M, Aaberge L, Endresen K, Smith P, Aakhus S, et al. After Eighty study investigators. Invasive versus conservative strategy in patients aged 80 years or older with non-ST-elevation myocardial infarction or unstable angina pectoris (After Eighty study): an open-label randomised controlled trial. Lancet. 2016;387(10023):1057–1065.
-
381Fox KA, Poole-Wilson P, Clayton TC, Henderson RA, Shaw TRD, Wheatley DJ, et al. 5-year outcome of an interventional strategy in non-ST-elevation acute coronary syndrome: the British Heart Foundation RITA 3 randomised trial. Lancet. 2005;366(9489):914–20.
-
382Fanning JP, Nyong J, Scott IA, Aroney CN, Walters DL. Routine Invasive Strategies Versus Selective Invasive Strategies for Unstable Angina and non-ST Elevation Myocardial Infarction in the Stent Era. Cochrane Database Syst Rev. 2016;(5):CD004815.
-
383Smits PC, Abdel-Wahab M, Neumann FJ, Boxma-de Klerk BM, Lunde K, Schotborgh CE, et al. Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N Engl J Med, 2017;376(13):1234–44.
-
384Choi KH, Song YB, Lee JM, Lee SY, Park TK, Yang JH, et al. Impact of Intravascular Ultrasound-Guided Percutaneous Coronary Intervention on Long-Term Clinical Outcomes in Patients Undergoing Complex Procedures. JACC Cardiovasc Interv, 2019;12(7):607–20.
-
385Gao XF, Wang ZM, Wang F, Gu Y, Ge Y, et al. Intravascular Ultrasound Guidance Reduces Cardiac Death and Coronary Revascularization in Patients Undergoing Drug-Eluting Stent Implantation: Results From a Meta-Analysis of 9 Randomized Trials and 4724 Patients. Int J Cardiovasc Imaging. 2019;35(2):239–47.
-
386Nicolau JC, Franken M, Lotufo PA, Carvalho AC, Marin-Neto JA, Gallego F, et al. Use of demonstrably effective therapies in the treatment of acute coronary syndromes:comparison between different Brazilian regions. Analysis of the Brazilian Registry on Acute Coronary Syndromes (BRACE). Arq Bras Cardiol. 2012;98(4):282–9.
-
387Coleman EA. Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc. 2003;51(4):549–55.
-
388Coleman EA, Boult C, American Geriatrics Society Health Care Systems Committee. Improving the quality of transitional care for persons with complex care needs. J Am Geriatr Soc. 2003;51(4):556–7.
-
389Coleman EA, Mahoney E, Parry C. Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure. Med Care. 2005;43(3):246–55.
-
390Smith SC Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124(22):2458–73.
-
391Bernheim SM, Spertus JA, Reid KJ, Bradley E, Desai RA, Peterson ED, et al. Socioeconomic disparities in outcomes after acute myocardial infarction. Am Heart J. 2007;153(2):313–9.
-
392Rahimi AR, Spertus JA, Reid KJ,Bernheim SM, Krumholz HM. Financial barriers to health care and outcomes after acute myocardial infarction. JAMA. 2007;297(10):1063–72.
-
393Smolderen KG, Spertus JA, Reid KJ, Buchanan DM, Krumholz HM, Denollet J, et al. The association of cognitive and somatic depressive symptoms with depression recognition and outcomes after myocardial infarction. Circ Cardiovasc Qual Outcomes. 2009;2(4):328–37.
-
394Taylor RS, Brown A, Ebrahim S, Jollife J, Noorani H, Becky KR, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116(10):682–92.
-
395Leon AS, Franklin BA, Costa F, Balady GB, Berra KA, Stewart KJ, et al. Cardiac rehabilitation and secondary prevention of coronary heart disease: an American Heart Association scientific statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity), in collaboration with the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2005;111(3):369–76.
-
396Suaya JA, Stason WB, Ades PA, Normand ST, Shepard DS, et al. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol. 2009;54(1):25–33.
-
397Iestra JA, Kromhout D, van der Schouw YT, Grobbee DE, Boshuizen HC, van Staveren WA. Effect size estimates of lifestyle and dietary changes on all-cause mortality in coronary artery disease patients: a systematic review. Circulation. 2005;112(6)924–34.
-
398Anderson L1, Thompson DR, Oldridge N, Zwisler AD, Rees K, Martin N, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2016 Jan 5;67(1):1-12.
-
399Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA. 2003;290(1):86–97.
-
400National Center for Chronic Disease Prevention and Health Promotion(US) Office on Smoking and Health. The health consequences of smoking— 50 years of progress: a report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention,(US); 2014.
-
401Kalkhoran S, Benowitz N, Rigotti N. Prevention and Treatment of Tobacco Use.JACC Health Promotion Series. J Am Coll Cardiol. 2018;72(9):1030-45.
-
402Goldenberg I, Jonas M, Tenenbaum A, Boyoko V, Matetzky S, Shotan A, et al. Current smoking, smoking cessation, and the risk of sudden cardiac death in patients with coronary artery disease. Arch Intern Med. 2003;163(19):2301–5.
-
403Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol. 2014;34(3):509–15.
-
404Chamberlain AM, Agarwal SK, Folsom AR, et al. (2011) Smoking and incidence of atrial fibrillation: results from the Atherosclerosis Risk in Communities (ARIC) study. Heart Rhythm.2011;8(8):1160-6.
-
405Daly LE, Mulcahy R, Graham IM, Hickey N. Long term effect on mortality of stopping smoking after unstable angina and myocardial infarction. Br Med J (Clin Res Ed). 1983;287(6388):324-6.
-
406Parasuraman S, Zaman AG, Egred M, Bagnall A, Broadhurst PA, Ahmed J, et al. Smoking status and mortality outcomes following percutaneous coronary intervention. Eur J Prev Cardiol. 2020 Feb 3; 2047487320902325.
-
407Ranney L, Melvin C, Lux L, McClain E, Lohr KN. Systematic review: smoking cessation intervention strategies for adults and adults in special populations. Ann Intern Med. 2006;145(11):845-56.
-
408Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, et al. Explaining the decrease in U.S. deaths from coronary disease,1980–2000. N Engl J Med. 2007;356(23):2388-98.
-
409Lindson N, Klemperer E, Hong B, Ordóñez-Mena JM, Aveyard P. Smoking reduction interventions for smoking cassation. Cochrane Database Syst Rev. 2019 Sep 30;9(9):CD013183.
-
410Eisenberg MJ, Grandi SM, Gervais A, O’Loughlin J, Paradis G, Rinfret S, et al. Bupropion for smoking cessation in patients hospitalized with acute myocardial infarction: a randomized, placebo-controlled trial. J Am Coll Cardiol 2013; 61(5):524–32.
-
411Planer D, Lev I, Elitzur Y, Sharon N, Ouzan E, Pugatsch T, et al. Bupropion for smoking cessation in patients with acute coronary syndrome. Arch Intern Med. 2011;171(12):1055-60.
-
412Stead LF, Perera R,Bullen C,Mant D, Hartman-Boyce J, Cahill K, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev.2018;5(5):CD000146.
-
413Wilson K, Gibson N, Willan A, Cook D. Effect of smoking cessationon mortality after myocardial infarction: meta-analysis of cohortstudies. Arch Intern Med. 2000;160(7):939-44.
-
414Agency for Health Care Research and Quality. (AHRQ). Clinical Practice Guidelines: Smoking Cessation. No 18. AHCPR Publication No.96-0692; 1996.
-
415Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al; Varenicline Phase 3 Study Group. Efficacy of varenicline, analpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placeboor sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56-63. Erratum in JAMA. 2006;296(11):1355.
-
416Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47-55.
-
417Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Sao Paulo Med J.2012;130(5):346-7. (Cochrane Highlights)
-
418Anthenelli RM, Benowitz NL, West R, Russ C, McRae T, Lawrence D, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric isorders EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet.2016; 387(10037):P2507-20.
-
419Rigotti NA, Pipe AL, Benowitz NL, Arteaga C, Garza D, Tonstad S. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation. 2010; 121(2):221–9.
-
420Eisenberg MJ, Windle SB, Roy N, Old W, Grondin FR, Bata I, et al. Varenicline for smoking cessation in hospitalized patients with acute coronary syndrome. Circulation. 2016; 133(1):21–30.
-
421Windle SB, Dehghani P, Roy N, Old W, Grondin FR, Bhata I, et al. Smoking abstinence 1 year after acute coronary syndrome: follow-up from a randomized controlled trial of varenicline in patients admitted to hospital. CMAJ.2018;190(12):E347-E354.
-
422Zhong Z, Zhao S, Zhao Y, Xia S. Combination therapy of varenicline and bupropion in smoking cessation: A meta-analysis of the randomized controlled trials. Compr Psychiatry. 2019; 95:152125.
-
423Kalkhoran S, Benowitz NL, Rigotti N. Prevention and Treatment of Tobacco Use. J Am Coll Cardiol. 2018;72(23):2964-979.
-
424Benowitz NL,Donny EC, Hatsukami DK. Reduced nicotine content cigarettes, e-cigarettes and the cigarette end game. Addiction.2017; 112(1):6–7.
-
425Hatsukami DK, Donny EC, Koopmeiners JS, Benowitz NL. Compensatory smoking from gradual and immediate reduction in cigarette nicotine content. Cancer Epidemiol Biomarkers Prev.2015; 24(2):472–6.
-
426MacDonald A, Middlekauff HR. Electronic cigarettes and cardiovascular health: what do we know so far? Vasc Health Risk Manag. 2019 jun 21;15:159-74.
-
427Baigent C, Blackwell L, Emberson J, Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376: 1670–81.
-
428Collins R, Reith C, Emberson J,Bhala N, Peto R, Barnes EH, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388(9753):2532–61.
-
429Amarenco P, Bogousslavsky J, Callahan A 3rd,Goldstein LB, Hennerici M, Rudolph AE, et al. High-dose atorvastatin after stroke or transiente ischemic attack. N Engl J Med. 2006;355(6):549–59.
-
430Athyros VG, Papageorgiou AA, Mercouris BR, Athyros VV, Symeonidis AN, Basayannis EO, et al. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus ’usual’ care in secondary coronary heart disease prevention. The GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study. Curr Med Res Opin. 2002;18(4):220–8.
-
431Kjekshus J, Apetrei E, Barrios V, Bohm M, Cleland JG, Cornel JH, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357(22):2248–61.
-
432Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008; 372(9645):1231–9.
-
433Eckel RH, Jakicic JM, Ard JD,de Jesus JM, Houston Miller N, Hubbard VS, et al. et al. 2013 AHA / ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S76–99.
-
434Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985–3023.
-
435Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med. 2018 Jun 21;378(25):e34.
-
436Bjelakovic G, Nikolova D, Gluud LL,Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007; 297(8):842–57.
-
437Carvalho T, Milani M, Ferraz AS, Silveira AD, Herdy AH, Hossri CAC, et al. Diretriz Brasileira de Reabilitação Cardiovascular – 2020. Arq Bras Cardiol. 2020; 114(5):943-87.
-
438Kureshi F, Kennedy KF, Jones PG, Thomas RJ, Arnold S V., Sharma P, et al. Association between cardiac rehabilitation participation and health status outcomes after acute myocardial infarction. JAMA Cardiol. 2016;1(9):980–8.
-
439Suaya JA, Shepard DS, Normand S-LT, Ades PA, Prottas J, Stason WB. Use of Cardiac Rehabilitation by Medicare Beneficiaries After Myocardial Infarction or Coronary Bypass Surgery. Circulation. 2007 Oct 9;116(15):1653 LP – 1662.
-
440Sunamura M, Ter Hoeve N, van den Berg-Emons RJG, Boersma E, van Domburg RT, Geleijnse ML. Cardiac rehabilitation in patients with acute coronary syndrome with primary percutaneous coronary intervention is associated with improved 10-year survival. Eur Heart J Qual Care Clin Outcomes. 2018 Jul;4(3):168–72.
-
441Ades PA, Keteyian SJ, Wright JS, Hamm LF, Lui K, Newlin K, et al. Increasing Cardiac Rehabilitation Participation From 20% to 70%: A Road Map From the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin Proc. 2017;92(2):234-42.
-
442Correia LC, Garcia G, Kalil F, Ferreira F, Carvalhal M, Oliveira R,et al. Prognostic value of TIMI score versus GRACE score in ST-segment elevation myocardial infarction. Arq Bras Cardiol. 2014 Aug;103(2):98-106.
-
443Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, et al. Exercise standards for testing and training: A scientific statement from the American heart association. Circulation. 2013;128(8):873–934.
-
444Costa RVC, Braga AMW, Carlos A. Arquivos Brasileiros de Cardiologia I CONSENSO NACIONAL DE REABILITAÇÃO CARDIOVASCULAR. Arq Bras Cardiol. 1997;69(4):267–91.
-
445Loh JP, Pendyala LK, Kitabata H, Torguson R, Chen F, Kent KM, et al. Safety of reloading prasugrel in addition to clopidogrel loading in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Am J Cardiol.2013;111(6):841-5.
-
446Zettler ME, Peterson ED, McCoy LA, Effron MB, Anstrom KJ, Henry TD, et al. Switching of adenosine diphosphate receptor inhibitor after hospital discharge among myocardial infarction patients: Insights from the Treatment with Adenosine Diphosphate Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events after. Am Heart J. 2017J Jan; 183:62-8.
-
447Angiolillo DJ, Curzen N, Gurbel P, Vaitkus P, Lipkin F, Li W, et al. Pharmacodynamic evaluation of switching from ticagrelor to prasugrel in patients with stable coronary artery disease: Results of the swap-2 study (switching anti platelet-2). J Am Coll Cardiol. 2014;63(15):1500–9.
-
448Pourdjabbar A, Hibbert B, Chong AY, Le May MR, Labinaz M, Simard T, et al. A randomised study for optimising crossover from ticagrelor to clopidogrel in patients with acute coronary syndrome. Thromb Haemost. 2017;117(2):303–10.
-
449Franchi F, Rollini F, Rios JR, Rivas A, Agarwal M, Kureti M, et al. Pharmacodynamic effects of switching from ticagrelor to clopidogrel in patients with coronary artery disease results of the SWAP-4 study. Circulation. 2018;137(23):2450–62.
-
450Angiolillo DJ, Rollini F, Storey RF, Bhatt DL, James S, Schneider DJ, et al. International expert consensus on switching platelet P2Y12 receptor-inhibiting therapies. Circulation. 2017;136(20):1955–75.
-
451Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur Heart J. 2017;53(1):213–54.
-
452Roe MT, Armstrong PW, Fox KAA, White HD, Prabhakaran D, Goodman SG, et al. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med. 2012;367(14):1297–309.
-
453Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, et al. Long-Term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372(19):1791–800.
-
454Furtado RHM, Nicolau JC, Magnani G, Im K, Bhatt DL, Storey RF, et al. Long-term ticagrelor for secondary prevention in patients with prior myocardial infarction and no history of coronary stenting: insights from PEGASUS-TIMI 54. Eur Heart J.2020;41(17):1625-32.
-
455Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014;371(23):2155–66.
-
456Giustino G, Baber U, Sartori S, Mehran R, Mastoris I, Kini AS, et al. Duration of dual antiplatelet therapy after drug-eluting stent implantation: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2015 Apr 7;65(13):1298-310.
-
457Bonaca MP, Sabatine MS. Antiplatelet Therapy for Long-term Secondary Prevention After Myocardial Infarction. JAMA Cardiol. 2016 Sep 1;1(6):627-8.
-
458Costa F, van Klaveren D, James S, Heg D, Räber L, Feres F, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet.2017;389(10073):1025-34.
-
459Yeh RW, Secemsky EA, Kereiakes DJ, Normand SLT, Gershlick AH, Cohen DJ, et al. Development and validation of a prediction rule for benefit and harm of Dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA - J Am Med Assoc 2016;315(16):1735–49.
-
460Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, et al; COMPASS Investigators. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N Engl J Med. 2017 Oct 5;377(14):1319-30.
-
461Connolly SJ, Eikelboom JW, Bosch J, Dagenais G, Dyal L, Lanas F, et al; COMPASS investigators. Rivaroxaban with or without aspirin in patients with stable coronary artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018 Jan 20;391(10117):205-18.
-
462Vranckx P, Valgimigli M, Jüni P, Hamm C, Steg PG, Heg D, et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-la. Lancet. 2018;392(10151):940–9.
-
463Takahashi K, Serruys PW, Chichareon P, Chang CC, Tomaniak M, Modolo R, et al. Efficacy and Safety of Ticagrelor Monotherapy in Patients Undergoing Multivessel PCI. J Am Coll Cardiol. 2019 Oct 22;74(16):2015-27.
-
464Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, et al. Ticagrelor with or without Aspirin in High-Risk Patients after PCI. N Engl J Med. 2019;381(21):2032-42.
-
465O’Donoghue ML, Murphy SA, Sabatine MS. The Safety and Efficacy of Aspirin Discontinuation on a Background of a P2Y12 Inhibitor in Patients After Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. Circulation. 2020 Aug 11;142(6):538-45.
-
466Fox KAA, Mehta SR, Peters R, Zhao F, Lakkis N, Gersh BJ, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non- ST-elevation acute coronary syndrome: The clopidogrel in unstable angina to prevent recurrent ischemic events (CURE) trial. Circulation 2004;110(10):1202–8.
-
467Lopes RD,Pieper KS, Horton JR, Al-Khatib SM, Newby LK,Mehta RH, et al. Short- and long-term outcomes following atrial fibrillation in patients with acute coronary syndromes with or without ST-segment elevation. Heart. 2008,94(7):867-73.
-
468Dewilde WJM, Oirbans T, Verheugt FWA, Kelder JC, De Smet BJGL, Herrman JP, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet 2013;381(9872):1107–15.
-
469Nicolau JC, Bhatt DL, Hohnloser SH, Kimura T, Lip GYH, Miede C, et al. RE-DUAL PCI Steering Committee and Investigators. Dabigatran Dual Therapy vs Warfarin Triple Therapy Post-Percutaneous Coronary Intervention in Patients with Atrial Fibrillation With/Without a Proton Pump Inhibitor: A Pre-Specified Analysis of the RE-DUAL PCI Trial. Drugs. 2020 Jul;80(10):995–1005.
-
470Golwala HB, Cannon CP, Steg PG, Doros G, Qamar A, Ellis SG, Oldgren J, Ten Berg JM, Kimura T, Hohnloser SH, Lip GYH, Bhatt DL. Safety and efficacy of dual vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of randomized clinical trials. Eur Heart J. 2018; 39(19):1726-1735.
-
471Lopes RD, Hong H, Alexander JH. Antithrombotic therapy after acute coronary syndrome and/or percutaneous coronary intervention in atrial fibrillation: finding the sweet spot. Eur Heart J. 2019 Dec 7;40(46):3768–70.
-
472Gargiulo G, Goette A, Tijssen J, Eckardt L, Lewalter T, Vranckx P, et al. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur Heart J. 2019 Dec 7;40(46):3757-67.
-
473Malachias MVB, Souza WKSB, Plavnik FL, Rodrigues CIS, Brandão AA, Neves MFT, et al,Sociedade Brasileira de Cardiologia.. 7ª Diretriz Brasileira de Hipertensão Arterial. Arq Bras Cardiol 2016; 107(3Supl.3):1-83.
-
474Sleight P, Redon J, Verdecchia P, Mancia G, Gao P, Fagard R, et al. ONTARGET investigators. Prognostic value of blood pressure in patients with high vascular risk in the Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial study. J Hypertens. 2009;27(7):1360–9.
-
475Banach M, Michalska M, Kjeldsen SE, Małyszko J, Mikhailidis DP, Rysz J. What should be the optimal levels of blood pressure: Does the J-curve phenomenon really exist? Expert Opin Pharmacother. 2011;12(12):1835–44.
-
476Bangalore S, Messerli FH, Wun C, Zuckerman AL, DeMicco D, Kostis JB,et al. J-curve revisited: an analysis of the Treating to New Targets (TNT) Trial. Eur Heart J. 2010; 31(23):2897–908.
-
477SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015 Nov 26;373(22):2103-16. Erratum in: N Engl J Med. 2017 Dec 21;377(25):2506.
-
478A randomized trial of propranolol in patients with acute myocardial infarction. I. Mortality results. JAMA 1982;247(12):1707–14.
-
479Hjalmarson Å, Herlitz J, Málek I, Rydén L, Vedin A, Waldenström A, et al. Effect On Mortality of Metoprolol In Acute Myocardial Infarction. A Double-blind Randomised Trial. Lancet. 1981;318(8251):823–7.
-
480Goldberger JJ, Bonow RO, Cuffe M, Liu L, Rosenberg Y, Shah PK, et al. Effect of Beta-Blocker Dose on Survival after Acute Myocardial Infarction. J Am Coll Cardiol. 2015;66(13):1431–41.
-
481Bangalore S, Steg G, Deedwania P, Crowley K. B-Blocker Use and Clinical Outcomes in Stable Outpatients With and Without Coronary Artery Disease. J Am Med Assoc. JAMA. 2012;308(13):1340–9.
-
482Piva e Mattos LA, Berwanger O, Santos ES, Reis HJ, Romano ER, Petriz JL,et al. Clinical outcomes at 30 days in the Brazilian Registry of Acute Coronary Syndromes (ACCEPT). Arq Bras Cardiol. 2013 Jan;100(1):6-13.
-
483Lachin JM, Genuth S, Cleary P. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive insulin therapy. N Engl J Med. 2000;342(6):381–9.
-
484Holman RR, Paul SK, Bethel MA. Follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89.
-
485Laiteerapong N, Ham SA, Gao Y, Moffet HH, Liu JY, Huang ES, et al. The legacy effect in type 2 diabetes:impactofearlyglycemic control on future complications (the Diabetes & Aging study). Diabetes Care. 2019;42(3):416–26.
-
486Gerstein C, Miller M, Byington R. Effects of Intensive Glucose Lowering in Type 2 Diabetes. N Engl J Med. 2008;358(24):2545–59.
-
487American Diabetes Association. Standards of Medical Care in Diabetes — 2020. Diabetes Care. 2020 Jan;43((Suppl 1):S14-S31).
-
488U.S. Food and Drug Administration. FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function.[Internet]. [Cited in 2019 Apr 12] Available from: Available from: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM494140.pdf
» http://www.fda.gov/downloads/Drugs/DrugSafety/UCM494140.pdf -
489Aroda VR, Edelstein SL, Goldberg RB, Knowler WC, Marcovina SM, Orchard TJ, et al. Long-term metformin use and vitamin B12 deficiency in the diabetes prevention program outcomes study. J Clin Endocrinol Metab. 2016;101(4):1754–61.
-
490Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus: Systematic review and meta-analysis of cardio. Circulation. 2019;139(17):2022–31.
-
491Furtado RHM, Bonaca MP, Raz I, Zelniker TA, Mosenzon O, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. Circulation. 2019;139222516-27.,Circulation 2019; 139(22): 2516-27.
-
492McMurray JJ V, Solomon SD, Inzucchi SE, Kǿber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019,381:1995-2008.
-
493Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380(24):2295–306.
-
494Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369(14):1317–26.
-
495Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med 2017;376(18):1713–22.
-
496Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med. 2018;379(22):2097-107.
-
497Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med. 2019;380(1):11-22.
-
498Chang CH, Tseng PT, Chen NY, Lin PC, Lin PY, Chang JPC, et al. Safety and tolerability of prescription omega-3 fatty acids: A systematic review and meta-analysis of randomized controlled trials. Prostaglandins Leukot Essent Fat Acids. 2018 Feb;129:1-12.
-
499Moukarbel G V, Signorovitch JE, Pfeffer MA, McMurray JJ V, White HD, Maggioni AP, et al. Gastrointestinal bleeding in high risk survivors of myocardial infarction: the VALIANT Trial. Eur Heart J. 2009;30(18):2226–32.
-
500Lin KJ, Hernandez-Diaz S, Garcia Rodriguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology. 2011;141(1):71–9.
-
501Nichol KL, Nordin J, Mullooly J, Lask R, Fillbrandt K, Iwane M. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med. 2003;348(14):1322–32.
-
502Brasil. Ministerio da Saúde. Manual de Normas e Procedimentos para Vacinação [Internet]. [Citado em 2020 Jan 12]. Disponível em: http://bvsms.saude.gov.br/bvs/publicacoes/manual_procedimentos_vacinacao.pdf
» http://bvsms.saude.gov.br/bvs/publicacoes/manual_procedimentos_vacinacao.pdf -
503Ridker PM, Everett B, Thuren T, Mac Fadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017; 377(12): 1119–31.
-
504Tardif JC, Kouz S, Waters DD, Bertrand D, Diaz R, Maggioni A, et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Eng J Med. 2019;381(26):2497-505.
-
505Nicolau, JC,Timerman A, Marin-Neto JÁ, Piegas LS, Barbosa CJ, Franci A, et al, Diretrizes da Sociedade Brasileira de Cardiologia sobre Angina Instável e Infarto Agudo do Miocárdio sem Supradesnível do Segmento ST (II Edição, 2007) - Atualização 2013/2014. Arq Bras Cardiol. 2014;102(3 supl 1):1-61.
-
506Daly CA, De Stavola B, Sendon JL, Tavazzi L, Boersma E, Clemens F, et al. Euro Heart Survey Investigators. Predicting prognosis in stable angina–results from the Euro heart survey of stable angina: prospective observational study. BMJ. 2006;332(7536):262-7.
-
507Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T,et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277-314.
-
508Steeds RP, Garbi M, Cardim N, Kasprzak JD, Sade E, Nihoyannopoulos P, et al. 2014_2016 EACVI Scientific Documents Committee. EACVI appropriateness criteria for the use of transthoracic echocardiography in adults: a report of literature and current practice review. Eur Heart J Cardiovasc Imaging. 2017;18(11):1191-204.
-
509Knuuti J,Wijins W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al, 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart j. 2020;41(3):407-77.
-
510van Holten TC, Waanders LF, de Groot PG, Vissers J, Hoefer IE, Pasterkamp G,et al. Circulating biomarkers for redicting cardiovascular disease risk; a systematic review and comprehensive overview of meta-analyses. PLoS One. 2013;8(4):e62080.
-
511Lindholm D, Lindback J, Armstrong PW, Budaj A, Cannon CP, Granger CB, et al. Biomarker-based risk model to predict cardiovascular mortality in patients with stable coronary disease. J Am Coll Cardiol. 2017;70(7):813-26.
-
512Eapen DJ, Manocha P, Patel RS, Hammadah M, Veledar E, Wassel C, et al. Aggregate risk score based on markers of inflammation, cell stress, and coagulation is an independent predictor of adverse cardiovascular outcomes. J Am Coll Cardiol. 2013;62(4):329-37.
-
513Harb SC, Marwick TH. Prognostic value of stress imaging after revascularization: a systematic review of stress echocardiography and stress nuclear imaging Am Heart J. 2014;167(1):7-85.
Edited by
Publication Dates
-
Publication in this collection
26 July 2021 -
Date of issue
July 2021





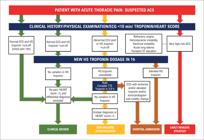









 Source: adapted from Granger et al.
Source: adapted from Granger et al.








