Abstracts
We investigated the following aspects of the biology of a population of Cnemidophorus vacariensis Feltrim & Lema, 2000 during the four seasons: thermal biology, relationship with the thermal environment, daily and seasonal activity, population structure and growth rate. Cnemidophorus vacariensis is restricted to rocky outcrops of the "campos de cima da serra" grasslands on the Araucaria Plateau, southern Brazil, and is currently listed as regionally and nationally threatened with extinction. Data were collected from October 2004 through September 2007 in the state of Rio Grande do Sul. Sampling was conducted randomly from 08:00 a.m. to 6:00 p.m. The capture-mark-recapture method was employed. The lizards were captured by hand, and their cloacal temperature, sex, snout-ventral length (SVL), mass, and the temperature of their microhabitat (substrate temperature and air temperature) were recorded. Individuals were then marked by toe-clipping and released at the site of capture. Body temperatures were obtained for 175 individuals, activity data for 96 individuals, and data on population structure and growth for 59 individuals. All data were obtained monthly, at different times of the day. Cnemidophorus vacariensis average body temperature was 23.84ºC, ranging between 9.6 and 38.2ºC. Temperatures ranged between 21 and 29ºC. The correlation between external heat sources, substrate and air were positive and significant and there was a greater correlation between lizard's temperature and the temperature of the substrate (tigmothermic species). The relatively low body temperatures of individuals are associated with the climate of their environment (altitude up to 1,400 m), with large variations in temperature throughout the day and the year, and low temperatures in winter. The average body temperature observed for C. vacariensis was low when compared with that of phylogenetically related species, suggesting that the thermal biology of this species reflects adaptations to the temperate region where it lives. The monthly rates of activity of lizards were related to monthly variations in the ambient temperatures. Our data suggest that the daily and seasonal activity of C. vacariensis result from the interaction between two factors: changes in the environment temperature and the relationship between individuals and their thermal environment. The population structure of C. vacariensis varied throughout the study period, with maximum biomass in January and maximum density in February (recruitment period). The sex ratio diverged from the expected 1:1. The growth analysis showed a negative relationship between the growth rate of individuals and the SVL, revealing that young individuals grow faster than adults, a typical pattern for short-lived species. The population studied showed a seasonal and cyclical variation associated with the reproductive cycle. The life strategy of C. vacariensis seems to include adaptations to the seasonal variations in temperature, typical of its environment.
Body temperature; growth; density-biomass; temperate region; tigmothermy
Este estudo teve por objetivo investigar a biologia termal, as relações térmicas com o ambiente, atividade diária e sazonal, estrutura da população e crescimento de C. vacariensis Feltrim & Lema, 2000, durante as quatro estações do ano. A espécie é restrita aos afloramentos rochosos dos "campos de cima da serra", no Planalto das Araucárias, sul do Brasil, e é considerada ameaçada de extinção regional e nacionalmente. Os dados foram coletados de outubro de 2004 até setembro de 2007 percorrendo-se as áreas de amostragem aleatoriamente entre 08:00 h e 18:00 h. Utilizou-se o método de captura-marcação-recaptura. Os lagartos eram capturados manualmente, sendo registrada sua temperatura cloacal, o sexo, comprimento rostro-cloacal, a massa, e as temperaturas do micro-hábitat (substrato e ar). Após, eram marcados por amputação da última falange dos dígitos e liberados no local da captura. Foram obtidas as temperaturas cloacais de 175 indivíduos, dados de atividade de 96 indivíduos e dados de estrutura da população e crescimento de 59 indivíduos. Todos os dados foram obtidos com periodicidade mensal, em diferentes horários. A temperatura média corporal foi de 23,84ºC, variando entre 9,6 e 38,2ºC. A faixa de temperaturas mais frequentes variou entre 21 e 29ºC. A correlação entre as fontes de calor externas, substrato e ar foram positivas e significativas, verificando-se uma correlação maior entre a temperatura do lagarto e a temperatura do substrato (espécie tigmotérmica). As temperaturas corpóreas relativamente baixas nesta espécie estão associadas à região que habita, a qual pode atingir até 1.400 m de altitude, apresentando grandes variações de temperatura ao longo do ano e do dia, com baixas temperaturas no inverno. A temperatura corpórea observada para C. vacariensis é baixa quando comparada às espécies filogeneticamente relacionadas, o que permite inferir que a biologia termal da espécie reflete sua adaptação à região de clima temperado onde habita. As taxas mensais de atividade dos lagartos estiveram relacionadas às variações mensais das temperaturas ambientais. Os dados sugerem que a atividade diária e sazonal da espécie resulta da interação de dois fatores: variações das temperaturas ambientais e relações térmicas com o ambiente. Cnemidophorus vacariensis apresentou variação na estrutura populacional ao longo do estudo, com a máxima biomassa ocorrendo em janeiro e a máxima densidade em fevereiro (período de recrutamento). A proporção sexual encontrada para a população foi diferente de 1:1. As análises de crescimento demonstram uma relação negativa entra a taxa de crescimento e o tamanho dos indivíduos, revelando que os jovens crescem mais rapidamente que os adultos, o que é típico em espécies de vida curta. A população estudada apresentou uma variação cíclica e sazonal associado ao ciclo reprodutivo. Cnemidophorus vacariensis parece ter sua estratégia de vida adaptada às variações sazonais da temperatura de seu ambiente.
Temperatura corpórea; crescimento; densidade-biomassa; região temperada; tigmotermia
Thermal biology, activity, and population parameters of Cnemidophorus vacariensis (Squamata, Teiidae), a lizard endemic to southern Brazil
Biologia termal, atividade e parâmetros populacionais de Cnemidophorus vacariensis (Squamata, Teiidae), lagarto endêmico do sul do Brasil
Rodrigo Caruccio; Renata Cardoso Vieira; Laura Verrastro; Denise Mello Machado* * denise-machado@sema.rs.gov.br
Departamento de Zoologia, Instituto de Biociências, Universidade Federal do Rio Grande do Sul, Av. Bento Gonçalves, 9500, prédio 43.435, 91501-970, Porto Alegre, RS, Brazil (rodrigocaruccio@gmail.com, renatacva@yahoo.com.br, lauraver@ufrgs.br)
ABSTRACT
We investigated the following aspects of the biology of a population of Cnemidophorus vacariensis Feltrim & Lema, 2000 during the four seasons: thermal biology, relationship with the thermal environment, daily and seasonal activity, population structure and growth rate. Cnemidophorus vacariensis is restricted to rocky outcrops of the "campos de cima da serra" grasslands on the Araucaria Plateau, southern Brazil, and is currently listed as regionally and nationally threatened with extinction. Data were collected from October 2004 through September 2007 in the state of Rio Grande do Sul. Sampling was conducted randomly from 08:00 a.m. to 6:00 p.m. The capture-mark-recapture method was employed. The lizards were captured by hand, and their cloacal temperature, sex, snout-ventral length (SVL), mass, and the temperature of their microhabitat (substrate temperature and air temperature) were recorded. Individuals were then marked by toe-clipping and released at the site of capture. Body temperatures were obtained for 175 individuals, activity data for 96 individuals, and data on population structure and growth for 59 individuals. All data were obtained monthly, at different times of the day. Cnemidophorus vacariensis average body temperature was 23.84ºC, ranging between 9.6 and 38.2ºC. Temperatures ranged between 21 and 29ºC. The correlation between external heat sources, substrate and air were positive and significant and there was a greater correlation between lizard's temperature and the temperature of the substrate (tigmothermic species). The relatively low body temperatures of individuals are associated with the climate of their environment (altitude up to 1,400 m), with large variations in temperature throughout the day and the year, and low temperatures in winter. The average body temperature observed for C. vacariensis was low when compared with that of phylogenetically related species, suggesting that the thermal biology of this species reflects adaptations to the temperate region where it lives. The monthly rates of activity of lizards were related to monthly variations in the ambient temperatures. Our data suggest that the daily and seasonal activity of C. vacariensis result from the interaction between two factors: changes in the environment temperature and the relationship between individuals and their thermal environment. The population structure of C. vacariensis varied throughout the study period, with maximum biomass in January and maximum density in February (recruitment period). The sex ratio diverged from the expected 1:1. The growth analysis showed a negative relationship between the growth rate of individuals and the SVL, revealing that young individuals grow faster than adults, a typical pattern for short-lived species. The population studied showed a seasonal and cyclical variation associated with the reproductive cycle. The life strategy of C. vacariensis seems to include adaptations to the seasonal variations in temperature, typical of its environment.
Keywords: Body temperature, growth, density-biomass, temperate region, tigmothermy.
RESUMO
Este estudo teve por objetivo investigar a biologia termal, as relações térmicas com o ambiente, atividade diária e sazonal, estrutura da população e crescimento de C. vacariensis Feltrim & Lema, 2000, durante as quatro estações do ano. A espécie é restrita aos afloramentos rochosos dos "campos de cima da serra", no Planalto das Araucárias, sul do Brasil, e é considerada ameaçada de extinção regional e nacionalmente. Os dados foram coletados de outubro de 2004 até setembro de 2007 percorrendo-se as áreas de amostragem aleatoriamente entre 08:00 h e 18:00 h. Utilizou-se o método de captura-marcação-recaptura. Os lagartos eram capturados manualmente, sendo registrada sua temperatura cloacal, o sexo, comprimento rostro-cloacal, a massa, e as temperaturas do micro-hábitat (substrato e ar). Após, eram marcados por amputação da última falange dos dígitos e liberados no local da captura. Foram obtidas as temperaturas cloacais de 175 indivíduos, dados de atividade de 96 indivíduos e dados de estrutura da população e crescimento de 59 indivíduos. Todos os dados foram obtidos com periodicidade mensal, em diferentes horários. A temperatura média corporal foi de 23,84ºC, variando entre 9,6 e 38,2ºC. A faixa de temperaturas mais frequentes variou entre 21 e 29ºC. A correlação entre as fontes de calor externas, substrato e ar foram positivas e significativas, verificando-se uma correlação maior entre a temperatura do lagarto e a temperatura do substrato (espécie tigmotérmica). As temperaturas corpóreas relativamente baixas nesta espécie estão associadas à região que habita, a qual pode atingir até 1.400 m de altitude, apresentando grandes variações de temperatura ao longo do ano e do dia, com baixas temperaturas no inverno. A temperatura corpórea observada para C. vacariensis é baixa quando comparada às espécies filogeneticamente relacionadas, o que permite inferir que a biologia termal da espécie reflete sua adaptação à região de clima temperado onde habita. As taxas mensais de atividade dos lagartos estiveram relacionadas às variações mensais das temperaturas ambientais. Os dados sugerem que a atividade diária e sazonal da espécie resulta da interação de dois fatores: variações das temperaturas ambientais e relações térmicas com o ambiente. Cnemidophorus vacariensis apresentou variação na estrutura populacional ao longo do estudo, com a máxima biomassa ocorrendo em janeiro e a máxima densidade em fevereiro (período de recrutamento). A proporção sexual encontrada para a população foi diferente de 1:1. As análises de crescimento demonstram uma relação negativa entra a taxa de crescimento e o tamanho dos indivíduos, revelando que os jovens crescem mais rapidamente que os adultos, o que é típico em espécies de vida curta. A população estudada apresentou uma variação cíclica e sazonal associado ao ciclo reprodutivo. Cnemidophorus vacariensis parece ter sua estratégia de vida adaptada às variações sazonais da temperatura de seu ambiente.
Palavras-chave: Temperatura corpórea, crescimento, densidade-biomassa, região temperada, tigmotermia.
Nearly all reptiles are capable of regulating and maintaining relatively stable body temperatures by using heat sources from the environment (Andrade & Abe, 2007). In order to take advantage of the thermal variability in their habitats, lizards assume positions or behaviors that facilitate heat gain or loss. They also increase or decrease activity rates in their microhabitat in order to maintain optimal body temperatures while active (Heath, 1970; Grant & Dunham, 1988; Grant, 1990; Pianka & Vitt; 2003; Bujes & Verrastro, 2006). However, thermal relationships between lizards and the environment vary according to the species, habitat type, foraging mode, time of day or year, and differences in the relative importance of heat sources available for thermoregulation (Menezes et al., 2000).
There seems to be an important phylogenetic component in the expression of body temperatures in lizards: temperatures during activity are often consistent among species of the same genus (and genera of the same family) (Bogert, 1949). Despite this apparent phylogenetic trend, several studies have demonstrated that the environment can be a determining factor in the local expression of lizard body temperatures (Huey & Slatkin, 1976; Jaksic & Schwenk, 1983; Hertz et al., 1993; Andrews, 1998; Kiefer et al., 2005).
Because thermoregulation is critical to attain body temperatures necessary to facilitate physiological processes in ectotherms (Nicholson et al., 2005), temperature becomes a limiting factor in the ecology of populations, as a considerable portion of the activity cycle is used to respond to the thermal environment (Heatwole, 1970). Moreover, ectotherms living at high altitudes where environmental temperatures are relatively low often have their activity periods limited (Grant & Dunham, 1990). Thus, the temporal activity of lizards is strictly connected with their thermoregulatory needs (Pianka, 1969), resulting in different patterns of daily and seasonal activities (Huey et al., 1977; Pianka & Vitt, 2003).
Some authors (Tinkle, 1969; Tinkle et al., 1970; Rocha, 1998) have argued that many aspects of the population and life history traits of tropical and temperate lizards - especially those living in wet and dry (arid) environments - differ considerably. For example, attributes such as body size, growth, density, mortality, age class distribution and spawning frequency tend to show variations in accordance with precipitation in populations inhabiting regions characterized by seasonal rainfall. By contrast, population parameters of temperate species vary according to the species' reproductive cycle, which is closely associated with the annual changes in temperature (James & Shine, 1985; Magnusson, 1987; Rocha, 1992; Clerke & Alford, 1993; Vitt & Zani, 1996; Verrastro & Krause, 1999; Wiederhecker et al., 2002; Bujes & Verrastro, 2006).
Growth rate is another important factor determining the life history of species (Tinkle & Ballinger, 1972; Sinervo & Adolph, 1989). In lizards, growth rate is mainly affected by temperature, time of exposure to solar heat, social behavior, population density and availability of food and water (Andrews, 1982). Two factors seem to severely affect the growth rate of lizard populations, particularly in the temperate zone. First, low environment temperatures reduce the metabolic rates of individuals, resulting in lower rates of growth or dormancy in winter (Van Devender, 1978; Dunham, 1981; Rose, 1981; Quintana, 1991). Second, rainfall directly influences the primary productivity and abundance of insects, impacting the growth and survival of their lizard predators (Nussbaum & Diller, 1976).
Differences in life history strategies among phylogenetically related species inhabiting different environments suggest that ecological resources influence life history traits, and hypotheses to explain the evolution of distinct strategies within these groups are needed (Niewiarowski, 1994).
Lizards of the genus Cnemidophorus Wagler, 1830 generally have high body temperatures which are associated with high levels of activity and an active foraging strategy (Huey & Pianka, 1981; Magnusson et al., 1985; Anderson & Karasov, 1988). In order to maintain high body temperatures throughout the activity period, these lizards thermoregulate in the warmest hours of the day and are generally heliothermal (Bergallo & Rocha, 1994). In open environments, they alternate between micro-habitats in the shade and in the sun, to avoid excessive heat from the substrate (Vitt et al., 1993; Mesquita & Colli, 2003). Etheridge & Wit (1993) concluded that lizard activity is influenced by physical and ecological factors, and differences in predation pressure, social behavior, and energetic expenditure related to foraging can give insights into the costs and benefits of activity and inactivity.
Cnemidophorus vacariensis Feltrim & Lema, 2000, a lizard endemic to southern Brazil, lives on rocky outcrops in high elevation grasslands of the Araucaria Plateau (Feltrim & Lema, 2000; Di-Bernardo et al., 2003). Its seasonal reproduction occurs between October and February (Rezende-Pinto et al., 2009; Caruccio et al., 2010). Individuals of this species are found in small, shallow holes they build in the substrate underneath outcrops of loose stones (Caruccio et al., 2010). The species has been categorized as "Vulnerable" in the state of Rio Grande do Sul (Marques et al., 2002; Di-Bernardo et al., 2003) based on limited information regarding its sensitivity to habitat alteration. Cnemidophorus vacariensis is also regarded as nationally threatened with extinction (MMA, 2008) and included in the Red Data Book of endangered fauna in the state of Paraná (Mikich & Bérnils, 2009).
The main goal of the present study was to investigate the following aspects of the biology of a population of C. vacariensis: 1) body temperature range during activity; 2) seasonal, sex and age related variations in body temperature; 3) thermoregulation strategy; 4) daily activity patterns; 5) seasonal variations in activity; 6) age and sex related variations in activity levels; 7) population structure; 8) relationship between activity levels and thermal biology and population parameters.
MATERIAL AND METHODS
We conducted our research in the highland grasslands known as the "campos de cima da serra", in the state of Rio Grande do Sul, Brazil. Thermal biology data were obtained in the municipality of Vacaria (28º27'26"S, 50º93'91"W), approximately 1,020 m above sea level, and data on activity and population parameters were gathered primarily at an area in the municipality of Bom Jesus (28º18'29"S, 50º42'56"W). The latter is characterized by the presence of two rocky formations with a total area of 1.3 ha and vegetation composed predominantly of shrubs and grasses, at 950 m altitude.
Both localities are included in the Atlantic Forest domain, specifically in the Araucaria Forest ecoregion, which is characterized by a mosaic of Araucaria-dominated woodlands (Araucaria angustifolia, Araucariaceae) and grasslands (Rambo, 1994). The grassland, though apparently uniform, is characterized by a mixture of several species (see Boldrini, 1997 for a detailed description). The climate is temperate humid, with mild summers and no dry season, corresponding to the "Cfb" climate in Köppen's classification (Moreno, 1961). The average temperature in the warmest months - January and February - is below 19.5ºC, and that of the coldest months - June and July - is lower than 10.5ºC. The mean annual rainfall is 1,545 mm and the maximum altitude is 1,398 m (Maluf, 2000).
Data on thermal biology were collected on two days per month, from October 2004 to August 2006, between 8:00 a.m. and 6:00 p.m. Lizards were located on the rocky outcrops and captured manually. Immediately after capture, cloacal, microenvironment, substrate and air temperatures were recorded with a Schultheis cloacal thermometer (0.2ºC accuracy). Air temperature was taken consistently one centimeter above the exact place where the specimen was captured. Additionally, the following parameters were recorded: snout-ventral length (SVL), with a caliper (Mitutoyo; precision 0.02 mm), and body mass, with a spring scale (Pesola®; capacity of 30 g; 0.25 g accuracy).
In order to record population parameters, we used stakes to delimit a known area of 1.3 ha, according to site characteristics (vegetation, rocky outcrops and occurrence of lizards). Data were collected on one day every month, always under sunny conditions, from September 2006 to September 2007. During field work, all activities performed by each individual lizard were recorded when it was first spotted. Subsequently, the lizard was captured manually to allow the collection of data on SVL, weight and sex. Each individual was marked by amputation of its last phalanx and released at the site of capture (adapted from Bujes & Verrastro, 1998). The area was searched randomly from 08:00 a.m. to 6:00 p.m and the following information was recorded for each lizard observed: 1) activity level, as either (A) active, when the lizard was moving, or (B) inactive, when the lizard was static or hiding in a burrow (Di-Bernardo et al., 2007); 2) time of observation; 3) temperature of the substrate (TS) where the specimen was first located, measured with an infrared surface thermometer (precision of 0.1ºC); 4) air temperature (TA), measured with a digital thermometer (precision of 0.1ºC) at 1 cm from the ground, at the same place of TS. Every hour the following environmental temperatures were systematically recorded: air temperature (TA) (10 cm from ground), temperature of the substrate exposed to the sun (TSs), and temperature of the substrate in the shade (TSsh). In order to measure the temperature of the substrate in the sun, we positioned the thermometer under the surface of the stone exposed to the sun. When measuring the substrate temperature in the shade, the thermometer was slightly buried in the earth beneath the bushes of grass surrounding the site. Hourly temperatures were always taken at the same place, throughout the study period, in order to avoid variations due to changes in substrate and/or location relative to the sun.
Individuals were separated into two age classes. Adults were identified based on the minimum reproductive size for the species (males: SVL> 48.8 mm, females: SVL> 57.4 mm) (Rezende-Pinto et al., 2009). Sex was determined only for adult individuals, based on the following characteristics outlined by Rezende-Pinto et al. (2009) for Cnemidophorus species: males with black spots on the abdomen and throat, and a yellowish coloration on the first rows of ventral scales; females lacking spots on the abdomen and larger than males.
Seasons were defined as: spring (October-December 2006), summer (January-March 2007), autumn (April-June 2007), and winter (July-September 2007).
The relationship between body temperatures and environment temperatures (substrate and air) was tested by regression analysis, and the relationship between cloacal temperature and body size was tested using correlation analysis. The differences in temperature and body size between males and females were tested using the t-test. The differences in body and environmental temperatures (substrate and air) among the seasons were tested using nonparametric analysis of variance, the Kruskal-Wallis test (Dunn's method) (Zar, 1996).
Because the number of researchers collecting data was not constant throughout the study period, we standardized the sampling effort a posteriori according to a record rate (adapted from Maciel et al., 2003). This record rate was calculated by dividing the number of lizards recorded by the total search effort (in person-hours). Thus, the record rate of active lizards was calculated considering only individuals that were found in activity. Temporal variation of record rates of active lizards was categorized as hourly (daily), monthly, and seasonal intervals. The total search effort throughout the study period was 330 hours. The relationship between monthly record rates and mean monthly environmental temperatures (TA, TSs and TSsh) was tested using a simple linear regression (Zar, 1996). Differences in daily activity among seasons, age classes and sexes (in each season) were tested using the Kolmogorov-Smirnov two-by-two test (Siegel, 1975). The t-test was used to investigate differences between age classes and sex of the lizards with respect to the environmental temperatures (TA, TSs and TSsh) in which they were active (Zar, 1996).
The Jolly-Seber (Jolly, 1965; Seber, 1965; Rocha, 1998) method was used in population estimates. Density (number of individuals/ha) was estimated for each month by dividing the size of the population in that month by the number of hectares in the study area. Biomass (g/ha) was estimated by multiplying lizard density in each month by the average weight (grams) of lizards captured in that month.
Population age structure was estimated using the frequency of monthly distribution of age classes; the recruitment period was ascertained based on the monthly distribution of the SVL (mm) of the lizards captured. The sex ratio was estimated only for adults. We used the χ2 (chi-square) statistics to test sex and age ratios (Rocha, 1998).
The average, maximum and minimum SVL (mm) and mass (g) were measured using all individuals captured in the study area. The growth rates of C. vacariensis were calculated based on measurements (SVL and mass) of individuals previously captured and marked. This procedure, previously tested on snakes, is considered to produce the most realistic growth estimates (Parker & Plummer, 1987). It has also been widely used for lizards (Van Devender, 1978; Verrastro & Krause, 1994; Van-Sluys, 1998).
Additionally, seven eggs of C. vacariensis, collected in January 2009, were incubated in laboratory for assessment of the SVL (mm) and mass (g) at hatching.
RESULTS
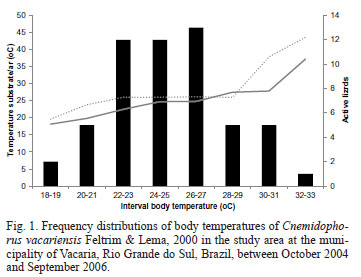
The body temperature of C. vacariensis ranged between 9.60-38.20ºC (mean 23.84 ± 6.50ºC; n = 175) (Fig. 1). Body temperatures correlated significantly with microhabitat and substrate (R2 = 0.8320, p < 0.0001, n = 173) and air (R2 = 0.5808, p < 0.0001, n = 163) temperatures. Multiple regression analysis revealed a significant correlation between body temperature (Tb) and the temperature of the environment (Ts and Tar) (R2 = 0.46; p < 0.0001; n = 163). The average temperature of the substrate was 22.32 ± 5.72ºC (range 10.00-37.30ºC), while the average air temperature was 22.68 ± 6.76ºC (range 9.00-44.85ºC).
Body size was not significantly correlated with body temperature (r = -0.003; p = 0.9724; n = 137). The body temperatures of males and females were not significantly different (t = 0.3157, p = 0.7529).
Body temperature differed significantly between seasons (H = 21.81; p < 0.0001). These differences were verified when the two seasons with the highest temperatures (spring 2004 and summer 2005-2006) and the three seasons with the lowest temperatures (autumn 2005, spring 2005 and winter 2006) were compared.
Microhabitat temperatures were also significantly different between the four seasons. Substrate temperatures (H = 38.70, p < 0.0001) differed between the seasons with higher average temperatures (spring 2004, summer 2004-2005 and summer 2005-2006) and those with lower average temperatures (fall 2005, spring 2005 and winter 2006). The average air temperature (H = 49.31, p < 0.0001) was significantly higher in the summer of both years than in the other seasons.
A total of 94 individuals of C. vacariensis were recorded throughout the activity survey: 39 adults (25 males and 14 females), 36 juveniles, and 19 non-captured specimens (it was not possible to determine their sex or age). Of these, 59 individuals were considered for the study of population parameters (35 adults: 22 males and 13 females; and 24 juveniles). The mean SVL of females was 58.93 ± 6.54 mm and the largest specimen was 72.9 mm long. The mean SVL of males was 58.93 ± 6.54 mm and the largest specimen was 68.6 mm. The mean SVL of juveniles was 44.17 ± 7.02 mm. The average weights, discriminated by sex, were: females: 7.45 ± 1.84 g; males: 5.75 ± 1.95 g; juveniles: 2.48 ± 0.98 g. The lightest weight recorded among juveniles was 0.75 g. Considering the many variables that may affect the development of individuals before they reach adulthood, the maximum weight was discarded because it was believed not to contribute with significant information.
Means of environmental temperatures (TA, TSs and TSsh) varied greatly throughout the year, reflecting the seasonality of the regional climate. The temperatures in the shade showed the greatest variances (H = 53.35, p < 0.0001), followed by air temperatures (H = 48.06, p < 0.0001) and temperatures of the substrate exposed to the sun (H = 32.2; p < 0.0001) (Tab. I).
The monthly mean record rate throughout the study period was 0.288 lizards per hour/person of search, which corresponds to approximately one lizard recorded every 3 hours and 28 minutes. The monthly mean record rate of active lizards was 0.106 lizards per hour/person of search, which corresponds to one active lizard every 9 hours and 26 minutes (Tab. II).
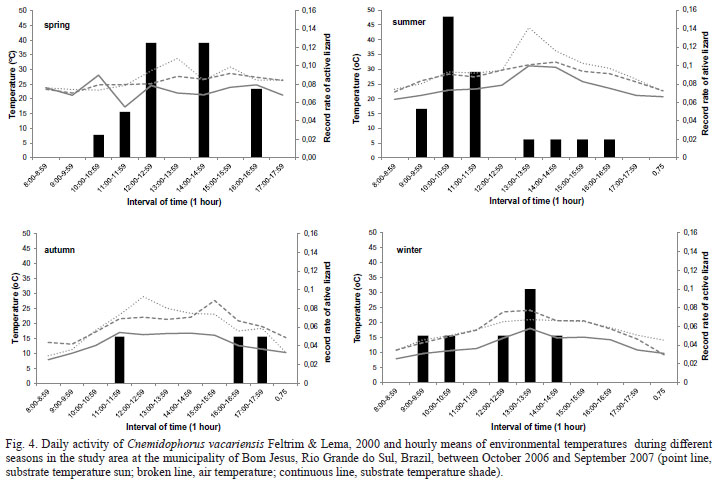
Active lizards were found during the entire year, except in November and April (Fig. 3). The mean record rates were related to the mean variation of environmental temperature (p < 0.05; R2active x tssh = 0.33, p = 0.049; n = 12) (Fig. 4).
The activity pattern of C. vacariensis showed a unimodal pattern in all seasons except autumn. Specimens were active between 9:00 a.m. and 5:35 p.m. In previous studies on C. vacariensis (unpublished data), night sampling indicated that the species is active only during daylight.
Periods of daily activity of C. vacariensis varied significantly between spring and summer (Dmax = 0.55, p < 0.05). During spring, the first individual was observed at 10:50 a.m. The number of active lizards peaked at 12:00 p.m. and 2:00 p.m., when environmental temperatures were highest. After 4:25 p.m., no lizard was observed. During summer, the first active lizard was observed at 9:20 a.m. and activity peaked between 10:00 and 11:00 a.m. After 12:00 p.m., when environmental temperatures were highest, lizard activity decreased considerably. During the autumn we recorded only three active specimens: the first at 11:30 a.m. and the others between 4:00 and 5:30 p.m. In the winter, active lizards were observed between 9:00 a.m. and 3:00 p.m. with a maximum of two records between 1:00 p.m. and 2:00 p.m. (Fig. 4). However, four of these records were in September, when environmental temperatures were highest (Fig. 4).
No statistically significant differences were found in daily activity between sexes and age classes in any season (p > 0.05). However, when the records for the entire year are clustered, males and females differed in their activity patterns (Dmax = 0.83; p < 0.05). Furthermore, females were less active than males: 14.29% of the observed females were active, compared with 24% of the observed males.
The majority of the active lizards (70%) were found when air temperatures varied between 24 and 28.9ºC (Fig. 5). Most of the active lizards (~80%) were observed on the substrate exposed to the sun (temperature between 22 and 30.9ºC), opposed to the ones in the shade (62%) (temperature between 21 and 25.9ºC) (Fig. 5).
No statistically significant difference was observed between age classes in relation to the air (TA) (t = 1.145; df = 20; p = 0.266) and substrate temperatures (TSsh) (t = 1.763; df = 20; p = 0.093) when lizards were active. The same was observed when sexes were compared: air temperature (TA) (t = 0.734; df = 6; p = 0.491) and substrate temperature (TSsh) (t = - 1.041; df = 6; p = 0.338).
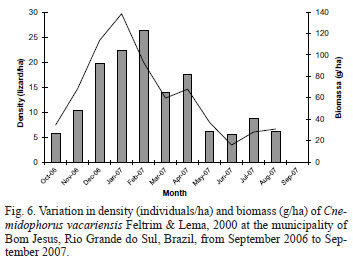
The density and biomass (Fig. 6) of the C. vacariensis population varied markedly throughout the year. The density was higher in February, and biomass in January, both decreasing after this period. Conversely, the lowest density and biomass occurred in June, coinciding with the lowest environmental temperature recorded during the study period.
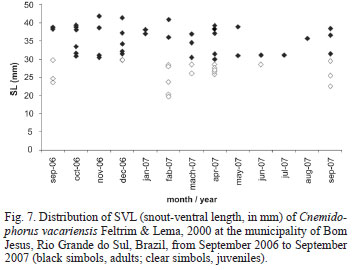
The distribution of age classes in the population varied significantly in the study months (c2 = 752.76; df = 12; p < 0.0001) (Fig. 7). Individuals with the lowest SVL were found in February. Juvenile individuals predominated over adults in February and March. The proportion of males and females also differed significantly throughout the year (c2 = 308.9; df = 10; p < 0.0001).
The growth rates presented in tables III and IV (considering SVL and mass, respectively) were calculated based on recaptures (one to five) of twelve individuals of C. vacariensis.

The analysis of the relationship between mass and SVL revealed that both are strongly correlated in males (r = 0.89, p < 0.05, n = 22), females (r = 0.71, p < 0.05, n = 13) and juveniles (r = 0.79, p < 0.05, n = 24), indicating that mass in these lizards increases in direct proportion to their length. However, we found a negative association between the growth rates of individuals and their SVL (r = - 0.126, p < 0.05, n = 12).
The seven collected eggs hatched in the laboratory between January and February 2009. The average SVL of these hatchlings was 29.71 ± 0.55 mm and the mass was 0.63 ± 0.05 g. These values indicate that the smallest individuals captured in the field were newborns (Fig. 7).
DISCUSSION
Cnemidophorus lizards are generally heliothermal and strongly rely on sun exposure for thermoregulation, especially in open environments (Mesquita & Colli, 2003). Cnemidophorus vacariensis, however, was not observed thermoregulating through direct exposure to the sun, a tigmothermic behavior. The correlation between the TB and the TA, and between the TB and the TS indicates that both (TA and TS) are important heat sources (R2 = 0.46, p < 0.0001, n = 163). As verified by Caruccio et al. (2010) for this species, only 1.06% of the lizards were observed walking through the grass, and 80.85% were recorded underneath rocky outcrops. Also, in warmer seasons, during the hottest hours of the day, these authors found only 18.6% of the individuals at the edge of the outcrop in the herbaceous vegetation. According to the same study, lizards select stones with different thickness in different seasons: during the cold seasons they are found under rocks which are thinner than those selected during the warm seasons. Thinner rocks provide more heat. This reinforces the idea that the thermoregulatory behavior of this species occurs by conduction, in burrows under rocks, characterizing a tigmothermic thermoregulatory behavior, which differentiates C. vacariensis from most congeneric species.
Coloration is part of an animal's thermoregulatory mechanism. Some colors absorb heat from the environment more quickly than others. Generally, animals with the darkest tegument inhabit environments where temperatures are, on average, lower (Smith, 1979). The color pattern of C. vacariensis is dark, which can be advantageous in a region with temperate climate, because it optimizes heat absorption.
The significant seasonal variation in the body temperature of C. vacariensis was expected, because the region it inhabits has a temperate climate with four seasons defined by temperature changes. This variation in body temperature was strongly correlated with variations in substrate temperature throughout the seasons, except in the summer of 2004-2005, when substrate temperatures were significantly warmer than in the autumn of 2005, spring 2005, and winter 2006, but lizard body temperatures were not. This is in line with what had already been observed in studies of lizard thermal biology (Pianka & Vitt, 2003). Animals avoid very warm microhabitats during extremely hot periods of the day or the season. By doing so, they maintain a temperature range suitable for their metabolic needs (Borgert, 1959; Heatwole & Taylor, 1987). The substrate temperature in the summer of 2004-2005 was very high; however, the body temperatures of the lizards remained relatively low, indicating that individuals of C. vacariensis avoided extreme temperatures by occupying thermally cooler microhabitats such as the vegetation surrounding the rocky outcrops, or crevices underneath thicker rocks (Caruccio et al., 2010).
Differences in body size correspond to differences in surface-volume ratio. Therefore, differences in the temperature of lizards of different sizes were expected. However, this trend has not been verified by us. Caruccio et al. (2010) found that adults and juveniles use rocks that are significantly different in thickness. This may indicate a differentiation in the spatial thermoregulatory behavior between individuals of different sizes. Smaller lizards should heat up faster due to their larger surface area with respect to their volume (Begon et al., 2007).
No sexual differences were found in body temperature, indicating that sex probably is not an important factor in the thermal biology of the species. Still, it is noteworthy to mention that Caruccio et al. (2010) found that, during the reproductive season, females select the thinnest rocks to build their dens.
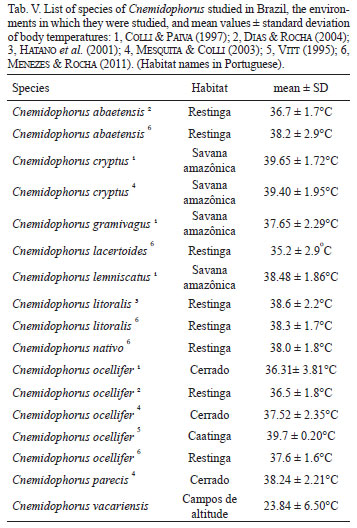
According to some studies, phylogenetically close lizard species generally have similar average body temperatures, even when they inhabit different environments (Brattstrom, 1965; Licht et al., 1966; Crowley, 1985; Van Damme et al., 1990; Colli & Paiva, 1997; Andrews, 1998; Menezes & Rocha, 2011). However, body temperature regulation in lizards is a complex process, influenced by several factors such as ecology, biology and abiotic aspects (weather, heat sources) (Pianka, 1986). With respect to geographical variation in the ecology of species of Cnemidophorus, Mesquita & Colli (2003) suggested that the patterns of life history of these lizards are strongly influenced by differences in environmental conditions among regions. The average temperature of C. vacariensis, when compared with that of other Cnemidophorus species, is lower and more variable (Tab. V). Most previous studies on the genus in Brazil have been carried out in areas with tropical climate, which imposes environmental constraints very different from those encountered by C. vacariensis. Thus, the more variable body temperatures throughout the year and the periods of more intense activity concentrated during the day, together with a lower average body temperature in this species, are characteristics that reflect the temperate climate to which it is adapted.
Additionally, the temperature - or more precisely, heat availability - is the main limiting factor for these animals (Pianka & Vitt, 2003). Accordingly, the results of the present study revealed a relationship between the level of activity of C. vacariensis and the marked seasonal temperature variations, which determine the seasonality in the region.
Cnemidophorus vacariensis had two distinct periods of seasonal activity during the year: a long period of activity in the seasons with high temperatures (spring and summer) and a period of reduced activity in seasons with low temperatures (autumn and winter). Studies have demonstrated that the intensity of environmental temperatures could influence lizard activity (Pianka, 1970a; Magnusson et. al., 1985; Haigen & Fengxiang, 1995; Mesquita & Colli, 2003). Furthermore, the period of most intense activity recorded for C. vacariensis coincided with the species' reproductive (September to December) and recruitment periods (January and February), as suggested by Rezende-Pinto et al. (2009). According to those authors, the reproductive activity of C. vacariensis is associated with the temperature: the reproductive period occurs when temperatures are most favorable for egg incubation, which in turn is dependent on the environmental temperature. During the reproductive season, males combine foraging activity with mate search (Vitt & Breitenbach, 1993). According to Etheridge & Wit (1993), in addition to food acquisition, reproduction is the most important benefit of activity. Favorable temperatures in this period also benefit the dispersion of juveniles. Thus, the temperature has a direct effect on the daily and seasonal activity of individuals of the species, which in turn influences reproduction and recruitment.
The region where this study was conducted has a temperate and humid climate. There, temperatures are on average lower and more variable when compared with other environments in Brazil. During colder months, the climate in the study area is similar to the climate of temperate zones in the Northern Hemisphere, where species of Aspidoscelis Fitzinger, 1843 (a closely related genus from North America; Reeder et al., 2002) are inactive during cold periods (Etheridge & Wit, 1993). Experimental data indicate that the increase in environmental temperatures probably triggers egg hatching in these lizards (Etheridge et al., 1983). In the Southern Hemisphere, other species of the family Teiidae also hibernate during unfavorable periods (e.g. Bujes, 1998; Cruz et al., 1999).
Studies focusing on the activity patterns of Brazilian species of Cnemidophorus, carried out in habitats with a tropical climate, either failed to mention inactivity periods or were performed during only part of the year (C. nativo Rocha, Bergallo & Peccinini-Seale, 1997, from Linhares Restinga - Bergallo & Rocha, 1993; C. lemniscatus (Linnaeus, 1758) in open habitats of the Amazon biome - Vitt & Carvalho, 1995; Vitt et al., 1997, C. ocellifer (Spix, 1825) from Caatinga - Vitt, 1995; C. litorallis Rocha, Araújo, Vrcibradic & Costa, 2000, from Macaé restinga - Hatano et al., 2001; C. abaetensis Dias, Rocha & Vrcibradic, 2002, and C. ocellifer from dunes in Salvador - Dias & Rocha, 2004). According to Vitt (1983), in species from temperate zones, activity is generally seasonal, whereas in tropical habitats activity may be continuous.
Periods of absolute inactivity were not recorded for C. vacariensis. However, low environmental temperatures during autumn and winter may have contributed to the marked increase of inactive periods in these seasons (Caruccio et al., 2010). According to Andrade & Abe (2007), lizard performance should decrease in colder environments due to a decrease in the temperature of their bodies. Thus, keeping inactive may be an important strategy to avoid extreme environmental temperatures, to decrease the risk of predation, and to conserve energy by decreasing body temperature and activity (Etheridge & Wit, 1993). According to Rose (1981), inactivity levels are part of the adaptive strategy of lizards, and are as vital as activity levels for the survival and reproductive success of these animals.
The high number of active juveniles during the summer may reflect the demographic effect of the incorporation of new individuals, resulting from recruitment during this time of the year. The pattern found in the population parameters of C. vacariensis seems to result from the reproductive strategy of the species. Rezende-Pinto et al. (2009) comment that a seasonal pattern occurs in other species of lizards that inhabit temperate regions (e.g., C. lemniscatus - Magnusson, 1987; C. ocellifer - Mesquita & Colli, 2003), and suggest that temperature and photoperiod, or the interaction between both, are associated with the seasonality of the reproduction of C. vacariensis. Thus, the variation in age classes recorded during the study period was directly influenced by the seasonal pattern of reproduction of the species, reflected in two distinct periods: i) breeding season (October-December), with great predominance of adults; ii) recruitment period (February-March), with a predominance of juveniles. The predominance of juveniles, at least during part of the year, is typical of species with a short life span (Pianka, 1970b, 1976; Howland, 1992), resulting in a significant annual replacement of the population (Wiederhecker et al., 2003). This assertion is supported by the studies of Rezende-Pinto et al. (2009) which found that individuals of C. vacariensis are able to reproduce in the first breeding season after birth. Moreover, this seasonal pattern in the proportion of age classes may reflect a strategy of producing juveniles which grow rapidly during the summer, a period with temperatures favorable for activity and dispersion (Rocha, 1996).
The negative relationship between growth rates of individuals and the SVL seems to corroborate what has been said above. Young individuals have higher growth rates than adults. Andrews (1982) mentioned two reasons that could explain the rapid growth of lizards before they reach adult size: (1) when the reproduction has a seasonal pattern, the slow growth of juveniles could harm the species' reproduction, (2) because smaller individuals are more prone to predation, a rapid growth can increase the juveniles' chance of survival. Additionally, it should be noted that there are seasonal variations in lizard growth. For instance, Andrews (1982) mentioned that the annual growth rate of reptiles in temperate zones is determined by inherent and external factors. Growth rates are highest in summer and lowest in winter. The fact that the recruitment of the population of C. vacariensis occurs in the warmer months of the year (Rezende-Pinto et al., 2009) corroborates the hypothesis that the juveniles grow rapidly in summer, when there is greater abundance of food for lizards that consume arthropods. Another indication that maximum growth rates occur in summer is the fact that individuals of C. vacariensis find themselves reproductively active in the year following their recruitment (Rezende-Pinto et al., 2009).
The daily activity period of C. vacariensis differed among seasons. In spring, lizard activity was concentrated on days with high environmental temperatures, similar to other Brazilian species of Cnemidophorus from tropical environments (Bergallo & Rocha, 1993; Hatano et al., 2001; Mesquita & Colli, 2003; Dias & Rocha, 2004). During summer, the decrease in lizard activity was related to periods of high environmental temperatures. This resulted in an anticipation of both the beginning and end of the activity peak. According to Huey & Pianka (1977), the activity varies seasonally according to ambient temperatures. These changes in activity probably result in lower thermoregulatory costs, less variation in body temperature during activity, or both. Still, the number of hours of activity during the winter is always reduced. In this sense, a reduction in activity in the warmest periods of the day could be a way to escape extreme environmental temperatures (Hatano et al., 2001). During the present study, the mean monthly temperature of the substrate, recorded at 1:00 p.m. in the summer, was 44.1ºC. According to Bogert (1968), the maximum lethal temperature for reptiles is around 45ºC. Beyond that, very brief exposure will result in death. Pianka & Vitt (2003) also reported the existence of a critical physiological and metabolic maximum temperature for lizards, close to 44.5ºC. Thus, knowing the parameters of the thermal relationship of species and their environment may contribute to understand the behavior of organisms when regulating their body temperatures along the day and the seasons (Huey et al., 1977; Caruccio et al., 2010).
The activity pattern recorded for C. vacariensis may be considered similar to the pattern observed by Belver & Ávila (2001) for C. longicaudus (Bell, 1843) in a region with temperate climate in Argentina. Both species share a unimodal activity related to the substrate temperature, and greater levels of activity in the months when high environmental temperatures prevail. However, the study of Belver & Ávila (2001) did not include the cooler seasons. In this study, the majority of active lizards were found in a narrow interval of air temperature (26 to 28.9ºC), substrate temperature in the shade (21 to 24.9ºC) and substrate temperature in the sun (24 to 29.9ºC). This indicates the existence of a thermal interval appropriate for the activity of C. vacariensis. According to Pianka & Vitt (2003), the thermal environment is particularly important for ectotherms, being more complex than the physical environment in several aspects.
Wide variations in body temperature during periods of activity and relatively low body temperatures are associated with a strong interaction between the thermal patterns of the local environment and long periods of activity. The relatively low temperature of activity recorded for C. vacariensis may be adaptive because it provides longer periods of daily and seasonal activity in cold environments (Jaksic & Schwenk, 1983). Bergallo & Rocha (1993) reported that lower temperature requirements may allow lizards to extend their period of foraging. Because C. vacariensis has a restricted distribution in the high altitude grasslands of southern Brazil, the decrease in the range of temperature of activity when compared to other species of the genus seems to suggest an adaptation to the milder climate of the region, which has seasons with well-marked differences in temperature and includes harsh winters.
Acknowledgements. We would like to thank Dr. Márcio Borges-Martins and Dr. Clóvis Bujes for their suggestions, revisions, and constant discussions throughout the development of the present study. We also thank Dr. Carlos Frederico Duarte da Rocha and Dr. Roberto Baptista de Oliveira for their valuable contributions to the study. Thanks to UFRGS and its Post-Graduate Program in Animal Biology (Programa de Pós-Graduação em Biologia Animal). Thanks to everyone who helped in the field work, especially Gabriele Volkmer. BAESA funded the study through the project "Projeto de Monitoramento da fauna pós-enchimento do Reservatório da Área de Influência do UHE Barra Grande" in collaboration with Instituto de Biociências, UFRGS.
Recebido em 29 de setembro de 2010.
Aceito em 23 de dezembro de 2011.
- Anderson, R. A. & Karasov, W. H. 1988. Energetics of the lizard Cnemidophorus tigris and life history consequences of food-acquisition mode. Ecological Monographs 58:79-110.
- Andrade, D. V. & Abe, A. S. 2007. Herpetologia no Brasil, II. In: Nascimento, L. B. & Oliveira, M. E. eds. Fisiologia de répteis Belo Horizonte, Sociedade Brasileira de Herpetologia. p.171-182.
- Andrews, R. M. 1982. Patterns of growth in reptiles. In: Gans, C. & Pough, F. H. eds. Biology of the Reptilia Vol. 13. New York, Academic Press. p.273-320.
- ______. 1998. Geographic variation in field body temperature of Sceloporus lizards. Journal Thermal of Biology 23(6):329-334.
- Begon, M.; Townsend, C. R. & Harper, J. L. 2007. Ecologia: de indivíduos a ecossistemas 4 ed. Porto Alegre, Artmed. 752p.
- Belver, L. C. & Avila, L. J. 2001. Ritmo de actividad diario y estacional de Cnemidophorus longicaudus (Squamata, Teiidae, Teiinae) en el norte de La Rioja, Argentina. Boletín Sociedad de Biologia de Concepción 72:37-42.
- Bergallo, H. G. & Rocha, C. F. D. 1993. Activity patterns and body temperatures of two sympatric lizards with different foraging tactics in southeastern Brazil. Amphibia-Reptilia 14:312-315.
- ______. 1994. Spatial and trofic niche diffentiation in two sympatric lizards (Tropidurus torquatus and Cnemidophorus ocellifer) with different foraging tatics. Australiam Journal Ecology 19:72-75.
- Bogert, C. M. 1949. Thermoregulation in reptiles. American Midland Naturalist 73(2):376-422.
- ______. 1968. How reptiles regulate their body temperature. Scientific American 22:213-221.
- Boldrini, I. I. 1997. Campos do Rio Grande do Sul: caracterização fisionômica e problemática ocupacional. Boletim Instituto de Biociências da Universidade Federal do Rio Grande do Sul 56:1-39.
- Brattstrom, B. H. 1965. Body temperature of reptiles. American Midland Naturalist 73:376-422.
- Bujes, C. S. 1998. Padrões de atividade de Teius oculatus (Sauria, Teiidae) na Reserva Biológica do Lami, Estado do Rio Grande do Sul - Brasil. Cuadernos de Herpetologia 12(2):13-21.
- Bujes, C. S. & Verrastro, L. 1998. Observações sobre o comportamento de Liolaemus occipitalis em cativeiro (Sauria: Tropiduridae). Revista Brasileira de Zoologia 15(4):907-912.
- ______. 2006. Termal biology of Liolaemus occipitalis (Squamata, Tropiduridae) in the coastal sand dunes of Rio Grande do Sul, Brazil. Brazilian Journal of Biology 66:945-954.
- Caruccio, R.; Vieira, R. C. & Verrastro, L. 2010. Microhabitat use by Cnemidophorus vacariensis (Squamata: Teiidae) in the grasslands of the Araucaria Plateau, Rio Grande do Sul, Brazil. Zoologia 27(6):902-908.
- Clerke, R. B. & Alford, R. A. 1993. Reproductive biology of four species of tropical Australian lizards and coments on the factor regulating lizard reproductive cycles. Journal of Herpetology 27:400-406.
- Colli, G. R. & Paiva, M. S. 1997. Estratégias de forrageamento e termorregulação em lagartos do cerrado e savanas amazônicas In: Leite, L. L. & Saito, C. H. orgs. Contribuição do conhecimento ecológico do Cerrado - Trabalhos selecionados do 3ş Congresso de Ecologia do Brasil (Brasília, 6-11.10.1996). Brasília, Depto. Ecologia - Universidade de Brasília. p. 224-231.
- Crowley, S. R. 1985. Thermal sensitivity of sprint-running in the lizard Sceloporus undulatus: support for conservative view of thermal physiology. Oecologia 66:219-225.
- Cruz, F. B.; Teisaire, E.; Nieto, L. & Roldán, A. 1999. Reproductive biology of Teius teyou in the Semiarid Chaco of Salta, Argentina. Journal of Herpetolology 33(3):420-429.
- Dias, E. J. R. & Rocha, C. F. D. 2004. Thermal ecology, activity patterns, and microhabitat use by two sympatric whiptail lizards (Cnemidophorus abaetensis and Cnemidophorus ocellifer) from Northeastern Brazil. Journal of Herpetology 38(4):586-588.
- Di-Bernardo, M.; Borges-Martins, M. & Oliveira, R. B. 2003. Répteis. In: Fontana C. S.; Bencke, G. A. & Reis R. E. orgs. Livro vermelho da fauna ameaçada de extinção no Rio Grande do Sul Porto Alegre, EDIPUCRS. p.165-188.
- Di-Bernardo, M.; Borges-Martins, M.; Oliveira, R. B. de & Pontes, G. M. F. 2007. Taxocenoses de serpentes de regiões temperadas do Brasil. In: Nascimento, L. B. & Oliveira, E. orgs. Herpetologia no Brasil II Belo Horizonte, Sociedade Brasileira de Herpetologia. p.222-263.
- Dunham, A. E. 1981. Population in fluctuating environments. The comparative population ecology of the iguanid lizard Sceloporus meriami and Urosaurus ornatus Miscellaneous Publications Museum of Zoology 158:1-62.
- Etheridge, K. & Wit, L. C. 1993. Factors affecting activity in Cnemidophorus (Sauria: Teiidae). In: Wright, J. W. &. Vitt, L. J. eds. Biology of whiptail lizards (genus Cnemidophorus) Norman, Oklahoma Museum of Natural History. p.151-162.
- Etheridge, K.; Wit, L. C. & Sellers, J. C. 1983. Hibernation in the lizard Cnemidophorus sexlineatus (Lacertilia: Teiidae). Copeia 1983(1):206-214.
- Feltim, A. C & Lema, T. 2000. Uma nova espécie de Cnemidophorus Wagler, 1830 do Estado do Rio Grande do Sul, Brasil (Sauria, Teiidae). Biociências 8(1):103-114.
- Grant, B. W. 1990. Trade-offs in activity time and physiological performance for thermoregulating desert lizards, Sceloporus merriami Ecology 71:2323-2333.
- Grant, B. W. & Dunham, A. E. 1988. Thermally imposed time constraints on the activity of the desert lizard Sceloporus merriami Ecology 69:167-176.
- ______. 1990. Elevational covariation in environmental constraints and life histories of the desert lizard Sceloporus merriami Ecology 71:1765-1776.
- Haigen, X. & Fengwiang, Y. 1995. Simulation model of activity of Phrynocephalus przewalskii Ecological Modelling 77:197-204.
- Hatano, F. H.; Vrcibradic, D.; Galdino, C. A. B.; Cunha-Barros, M.; Rocha, C. D. F. & Van Sluys, M. 2001. Thermal ecology and activity patterns of the lizards community of the restinga of Jurubatiba, Macaé, RJ. Revista Brasileira de Biologia 61(2):287-294.
- Heath, J. E. 1970. Behavioral regulation of body temperature in poikilotherms. Physiologist 13:399-410.
- Hertz, P. E.; Huey, R. B. & Stevenson, R. D. 1993. Evaluating temperature regulation by field-active ectotherms: The fallacy of the inappropriate question. The American Naturalist 142(5):796-818.
- Heatwole, H. 1970. Thermal ecology of the desert dragon, Amphibolurus inermis Ecological Monographs 40:425-457.
- Heatwole, H. F. & Taylor, J. 1987. Ecology of reptiles Sydney, Surrey Beatty & Sons. 325p.
- Howland, J. M. 1992. Life history of Cophosaurus texanus (Sauria: Iguanidae): environmental correlates and interpopulational variation. Copeia 1992:82-93.
- Huey, R. B. & Slatkin, M. 1976. Costs and benefits of lizard thermoregulation. Quarterly Review of Biology 51:363-384.
- Huey, R. B. & Pianka, E. R. 1981. Ecological consequences of foraging mode. Ecology 62:991-999.
- Huey, R. B.; Pianka, E. R. & Hoffman, J. A. 1977. Seasonal variation in thermoregulatory behavior and body temperature of diurnal Kalahari lizards. Ecology 58(5):1066-1075.
- Jaksic, F. M. & Schwenk, K. 1983. Natural history observations on Liolaemus magellanicus, the southernmost lizard in the world. Herpetologica 39:457-46.
- James, C. D. & Shine, R. 1985. The seasonal timing of reproduction: a tropical-temperate comparison in Australian lizards. Oecologia 67:464-474.
- Jolly, G. M. 1965. Explicit estimates form capture-recapture data with both death and immigration stochastic model. Biometrika 52:225-247.
- Kiefer, M. C.; Van-Sluys, M. & Rocha, C. F. D. 2005. Body temperatures of Tropidurus torquatus (Squamata, Tropiduridae) from coastal populations: Do body temperatures vary along their geographic range? Journal of Thermal Biology 30(6):449-456.
- Licht, P. W.; Dawson, R.; Shoemaker, V. H. & Main, A. R. 1966. Observations on the thermal relation of western Australian lizards. Copeia 1966:97-110.
- Maciel, A. P.; Di-Bernardo, M.; Hartz, S. M.; Oliveira, R. B. & Pontes, G. M. F. 2003. Seasonal and daily activity patterns of Liophis poecilogyrus (Serpentes: Colubridae) on the north coast of Rio Grande do Sul, Brazil. Amphibia-Reptilia 24(2):189-200.
- Magnusson, W. E. 1987. Reproductive cycles of teiid lizards in Amazonian savannah. Journal of Herpetology 21:307-316.
- Magnusson, W. E.; Paiva, L. J. D.; Rocha, R. M. D.; Franke, C. R.; Kasper, L. A. & Lima, A. P. 1985. The correlates of foraging mode in a community of Brazilian lizards. Herpetologica 41(3):324-332.
- Maluf, J. R. T. 2000. Nova classificação climática do Estado do Rio Grande do Sul. Revista Brasileira de Agrometeorologia 8(1):141-150.
- Marques, A. A. B.; Fontana, C. S.; Vélez, E.; Bencke, G. A.; Schneider, M. & Reis, R. E. 2002. Lista das espécies de fauna ameaçadas de extinção no Rio Grande do Sul Decreto nş 41.672, de 11 de junho de 2002. Porto Alegre, FZB/MCT-PUCRS/PANGEA (Publicações Avulsas FZB, 11). 52p.
- Menezes, V. A. & Rocha, C. F. D. 2011. Thermal ecology of five Cnemidophorus species (Squamata: Teiidae) in east coast of Brazil. Journal of Thermal Biology 36:232-238.
- Menezes, V. A.; Rocha, C. F. D. & Dutra, G. F. 2000. Termorregulação no lagarto partenogenético Cnemidophorus nativo (Teiidae) em uma área de restinga do Nordeste do Brasil. Revista de Etologia 2(2):103-109.
- Mesquita, D. O. & Colli, G. R. 2003a. Geografical variation in the ecology of populations of some Brazilian species of Cnemidophorus (Squamata, Teiidae). Copeia 2003(2):285-298.
- ______. 2003b. The ecology of Cnemidophorus ocellifer (Squamata, Teiidae) in a Neotropical Savanna. Journal of Herpetology 37(3):498-509.
- Mikich, S. B. & Bérnils, R. S. eds. 2004. Livro vermelho da fauna ameaçada do Estado do Paraná Curitiba, Instituto Ambiental do Paraná. 272p.
- MMA - Ministério do Meio Ambiente. 2008. Livro vermelho da fauna brasileira ameaçada de extinção 1 ed. Brasília, Belo Horizonte, MMA, Fundação Biodiversitas.
- Moreno, J. A. 1961. Clima do Rio Grande do Sul Porto Alegre, Secretaria da Agricultura.
- Nicholson, K. L.; Torrence, S. M.; Ghioca, D. M.; Bhattacharjee, J.; Andrei, A. E.; Owen, J.; Radke, N. J. A. & Perry, G. 2005. The influence of temperature and humidity on activity patterns of the lizards Anolis stratulus and Ameiva exsul in the British Virgin Islands. Caribbean Journal of Science 41:870-873.
- Niewiarowski, P. H. 1994. Understanding geographic life-history variation in lizard. In: Vitt, L. J. & Pianka, E. R. Lizard ecology: historical and experimental perspectives Princeton, American Society of Ichthyologists and Herpetologists, Princeton University Press. 430p.
- Nussbaum, R. A. & Diller, L. V. 1976. The life history of the Side-blotched lizard Uta stansburiana Baird and Girard in North Central Oregon. Northwest Science 4:243-260.
- Parker, W. S. & Plummer, M. V. 1987. Population ecology. In: Seigel, R. A.; Collins, J. T. & Novak, S. S. eds. Snakes: ecology and evolutionary biology New York, McGraw-Hill. p.253-301.
- Pianka, E. R. 1969. Sympatry of desert lizard (Ctenotus) in western Australia. Ecology 50:1012-1030.
- ______. 1970a. Comparative autoecology of the lizard Cnemidophorus tigris in different part of its geographic range. Ecology 51:703-720.
- ______. 1970b. On r and K selection. American Naturalist 104:592-597.
- ______. 1976. Natural selection of optimal reproductive tactics. American Zoologist 16:775-784.
- ______. 1986. Ecology and natural history of desert lizards: analyses of ecological niche and community structure Princeton, Princeton University Press.
- Pianka, E. R. & Vitt, L. J. 2003. Lizards: windows to the evolution of diversity Barkeley, University of California Press. 333p.
- Quintana, M. G. 1991. Estimaciones sobre morfometría y crescimiento en la Iguana colorada, Tubinambis rufescens (Sauria:Teiidae) en la Argentina. Revista del Museo Argentino Bernardino Rivadavia 3:193-217.
- Rambo, B. 2004. A fisionomia do Rio Grande do Sul: ensaio de monografia natural 3 ed. São Leopoldo, UNISINOS. 473p.
- Reeder, T. W.; Cole, C. J. & Dessauer, H. C. 2002. Phylogenetic relationships of whiptail lizards of the genus Cnemidophorus (Squamata: Teiidae): a test of monophyly, reevaluation of karyotypic evolution, and review of hybrid origins. American Museum Novitates 3365:1-61.
- Rezende-Pinto, F. M.; Verrastro, L.; Zanotelli, J. C. & Barata, P. C. R. 2009. Reproductive biology and sexual dimorphism in Cnemidophorus vacariensis (Sauria, Teiidae) in the grasslands of the Araucaria Plateau, southern Brazil. Iheringia, Série Zoologia 99(1):82-91.
- Rocha, C. F. D. 1992. Reproductive and fat body cycles of the tropical sand lizard Liolaemus lutzae of southeastern Brazil. Journal of Herpetology 26(1):17-23.26.
- ______. 1996. Seasonal shift in lizard diet: the seasonality in food resources affecting the diet of Liolaemus lutzae (Tropiduridae). Ciência & Cultura 48(4):264-270.
- ______. 1998. Population dynamics of the endemic tropi durid lizard Liolaemus lutzae in a tropical seasonal resting habitat. Ciência & Cultura 50(6):446-451.
- Rose, B. 1981. Factors affecting activity in Sceloporus virgatus Ecology 62:706-716.
- Seber, G. A. 1965. A note on the multiple-recapture census. Biometrika 52:249-259.
- Siegel, S. 1975. Estatística não paramétrica para as ciências do comportamento Rio de Janeiro, McGraw-Hill. 350p.
- Sinervo, B. & Adolph, S. C. 1989. Thermal sensitivy of growth rate in hatchlings of Sceloporus lizards: environmental, behavioral and genetic aspects. Oecologia 78:411-419.
- Smith H. M. 1979. Handbook of lizards: Lizards of the United States and of Canada Binghamton, Comstock Publishing Company. 557p.
- Tinkle, D. W. 1969. The concept of reprodutive effort and its relation to the evolution of life histories of lizard. American Naturalist 103:501-516.
- Tinkle, D. W. & Ballinger, R. F. 1972. Scloporus undulates, a study of the intraspecific comparative demography of a lizard. Ecology 53:570-584.
- Tinkle, D. W.; Wilbur, H. M. & Tilley, S. G. 1970. Evolutionary strategies in lizard reproduction. Evolution 24:55-74.
- Van Damme, R.; Bauwen, D.; Castilla, A. M. & Verheyen, R. F. 1990. Altitudinal variation of the thermal biology and running performance in the lizard Podarcis tiliguerta Oecologia 80:516-524.
- Van Devender, R. W. 1978. Growth ecology of a tropical lizard: Basiliscus basiliscus Ecology 59:1031-1038.
- Van-Sluys, M. 1998. Growth and body condition of the saxicolous lizard Tropidurus itambere in southeastern Brazil. Journal of Herpetology 32(3):359-365.
- Verrastro, L. & Krause, L. 1994. Analysis of growth in a population of Liolaemus occipitalis Boul. 1885, from the coastal sand-dunes of Tramandaí, RS, Brazil (Reptilia, Tropiduridae). Studies on Neotropical Fauna and Environment 29(2):99-111.
- ______. 1999. Ciclo reprodutivo de machos de Liolaemus occipitalis Boulenger (Sauria-Tropiduridae). Revista Brasileira de Zoologia 16(1):227-231.
- Vitt, L. J. 1983. Reproduction and sexual dimorphism in the tropical teiid lizard Cnemidophorus ocellifer Copeia 1983:359-366.
- ______. 1995. The ecology of tropical lizards in the caatinga of northeast Brazil. Occasional Papers of the Oklahoma Museum Natural History 1:1-29.
- Vitt, L. J. & Breitenbach, G. L. 1993. Biology of whiptail lizards (genus Cnemidophorus). In: Wright, J. W. & Vitt, L. J. Life histories and reproductive tactics among lizards in the genus Cnemidophorus (Sauria: Teiidae) Oklahoma, Museum of Natural History. p.211-244.
- Vitt, L. J. & Carvalho, C. M. 1995. Niche partitioning in a tropical wet season: lizards in the lavrado area of northern Brazil. Copeia 1995:305-329.
- Vitt, L. J. & Zani, P. 1996. Ecology of the elusive tropical lizard Tropidurus flaviceps (Tropiduridae) in lowland rain forest in Ecuador. Herpetologica 52:121-132.
- Vitt, L. J.; Ávila-Pires, T. C. S.; Araújo, M. C. D. & Magnusson, W. E. 1997. Ecology of whiptail lizard (Cnemidophorus) in the Amazon region of Brazil. Copeia 1997:745-757.
- Vitt, L. J.; Zani, P. A.; Caldwell, J. P. & Durtsche, R. D. 1993. Ecology of the whiptail lizard Cnemidophorus deppii on a tropical beach. Canadian Journal of Zoology 71:2391-2400.
- Wiederhecker, H. C.; Pinto, A. C. S.; Paiva, M. S. & Colli, G. R. 2002. Reproductive ecology of Tropidurus torquatus (Squamata: Tropiduridae) in the Highly Seasonal Cerrado Biome of Central Brazil. Journal of Herpetology 36(1):82-91.
- _______. 2003. The demography of the lizard Tropidurus torquatus (Squamata, Tropiduridae) in a highly seasonal Neotropical savanna. Phyllomedusa 2(1):9-19.
- Zar, J. H. 1996. Biostatistical analysis 3 ed. Prentice Hall. 662p.
Publication Dates
-
Publication in this collection
08 Mar 2012 -
Date of issue
Dec 2011
History
-
Received
29 Sept 2010 -
Accepted
23 Dec 2011