Abstracts
Synchronization in the events of the reproductive cycle in female Neohelice granulata Dana, 1851 were studied from samples taken weekly and biweekly from September to December 2006 in the Laguna Mar Chiquita. The timing and larval hatching and synchronicity were inferred from numbers of ovigerous females and observing the stages of embryonic development. Synchronization in larval hatching also was observed in females in experiments in dark for a period of 48 hours, at three different salinities (10, 23 and 33 ppm). In addition plankton sampling were performed in order to study larval exportation at the field and its link to the tidal and light/dark cycles. We found that ovigerous females of N. granulata have a marked synchronization in embryonic development which results in that most of berried females are close to hatching within a period of maximum tidal range (days). Within this period, there is a synchronization of hatching at a time scale of hours, governed by environmental conditions. The salinity range used in this study (10-32‰) did not affect hatching synchronicity neither time to hatch. Hatching was synchronized according to endogenous rhythms governed mainly by the tidal cycle and secondarily by the breadth of it. It is also conditioned by the light-dark cycle through an exogenous cycle, so that the hatchings would occur mostly at night high tides.
Hatching synchronicity; endogenous rhythm; larval exportation strategy; estuaries; southwestern Atlantic
La sincronización en los eventos del ciclo reproductivo en las hembras de Neohelice granulata Dana, 1851 fue estudiada a partir de muestras semanales y quincenales realizadas entre septiembre y diciembre del 2006 en la Laguna Mar Chiquita. La sincronización y eclosión larval fue inferida a partir de la variación en el porcentaje de las hembras ovígeras y la observación de los estadios del desarrollo embrionario. La sincronización en la eclosión larval, además, fue observada en hembras puestas a oscuridad en el laboratorio por un periodo de 48 hs, a tres salinidades distintas (10, 23 y 33 ppm). También se realizaron muestreos de plancton en el campo, donde se estudió el proceso de exportación larval relacionándolo con el ciclo de mareas y el de luz/oscuridad. Se muestra que las hembras ovígeras de N. granulata tienen una marcada sincronización en el desarrollo embrionario lo que se traduce en que la mayoría de las hembras ovígeras estén próximas a la eclosión dentro de un periodo de máximas amplitudes de marea (días). Dentro de este periodo, se produce una sincronización de la eclosión a una escala temporal más chica (horas) regida por condiciones ambientales. Dentro del rango de salinidad empleado en este trabajo (10-32‰) la eclosión no resultó afectada. Las eclosiones se sincronizan de acuerdo a ritmos endógenos regidos principalmente por el ciclo de mareas y secundariamente por la amplitud de la misma. A su vez, también está condicionado por el ciclo de luz-oscuridad a través de un ciclo exógeno, de forma que las eclosiones se producirían mayoritariamente en mareas altas nocturnas.
Sincronía en la eclosión larval; ritmos endógenos; estrategia de exportación larval; estuarios; Atlántico sudoeste
Hatching and larval export of the intertidal crab Neohelice granulata in Mar Chiquita coastal lagoon, Argentina
Eclosión y exportación de larvas en el cangrejo intermareal Neohelice granulata en la laguna costera Mar Chiquita, Argentina
Guillermina Sánchez VuichardI; Nahuel FaríasII; Tomás LuppiII
IDepartamento de Biología, Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Mar del Plata (UNMdP), Funes 3250, 7600 Mar del Plata, Argentina
IIInstituto de Investigaciones Marinas y Costeras (IIMyC), Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET) - UNMdP, Funes 3350, 7600 Mar del Plata, Argentina
ABSTRACT
Synchronization in the events of the reproductive cycle in female Neohelice granulata Dana, 1851 were studied from samples taken weekly and biweekly from September to December 2006 in the Laguna Mar Chiquita. The timing and larval hatching and synchronicity were inferred from numbers of ovigerous females and observing the stages of embryonic development. Synchronization in larval hatching also was observed in females in experiments in dark for a period of 48 hours, at three different salinities (10, 23 and 33 ppm). In addition plankton sampling were performed in order to study larval exportation at the field and its link to the tidal and light/dark cycles. We found that ovigerous females of N. granulata have a marked synchronization in embryonic development which results in that most of berried females are close to hatching within a period of maximum tidal range (days). Within this period, there is a synchronization of hatching at a time scale of hours, governed by environmental conditions. The salinity range used in this study (10-32) did not affect hatching synchronicity neither time to hatch. Hatching was synchronized according to endogenous rhythms governed mainly by the tidal cycle and secondarily by the breadth of it. It is also conditioned by the light-dark cycle through an exogenous cycle, so that the hatchings would occur mostly at night high tides.
Keywords: Hatching synchronicity, endogenous rhythm, larval exportation strategy, estuaries, southwestern Atlantic.
RESUMEN
La sincronización en los eventos del ciclo reproductivo en las hembras de Neohelice granulata Dana, 1851 fue estudiada a partir de muestras semanales y quincenales realizadas entre septiembre y diciembre del 2006 en la Laguna Mar Chiquita. La sincronización y eclosión larval fue inferida a partir de la variación en el porcentaje de las hembras ovígeras y la observación de los estadios del desarrollo embrionario. La sincronización en la eclosión larval, además, fue observada en hembras puestas a oscuridad en el laboratorio por un periodo de 48 hs, a tres salinidades distintas (10, 23 y 33 ppm). También se realizaron muestreos de plancton en el campo, donde se estudió el proceso de exportación larval relacionándolo con el ciclo de mareas y el de luz/oscuridad. Se muestra que las hembras ovígeras de N. granulata tienen una marcada sincronización en el desarrollo embrionario lo que se traduce en que la mayoría de las hembras ovígeras estén próximas a la eclosión dentro de un periodo de máximas amplitudes de marea (días). Dentro de este periodo, se produce una sincronización de la eclosión a una escala temporal más chica (horas) regida por condiciones ambientales. Dentro del rango de salinidad empleado en este trabajo (10-32) la eclosión no resultó afectada. Las eclosiones se sincronizan de acuerdo a ritmos endógenos regidos principalmente por el ciclo de mareas y secundariamente por la amplitud de la misma. A su vez, también está condicionado por el ciclo de luz-oscuridad a través de un ciclo exógeno, de forma que las eclosiones se producirían mayoritariamente en mareas altas nocturnas.
Palabras-clave: Sincronía en la eclosión larval, ritmos endógenos, estrategia de exportación larval, estuarios, Atlántico sudoeste.
Species inhabiting estuarine habitats must deal with daily ample oscillations of temperature and salinity and fast changes in physical-chemical environmental factors that require well developed physiological and behavioral adaptations. Species with complex life cycles can either spend their whole life within the estuary (to which they had to develop adaptations to variable conditions in all the phases of their life cycle) or avoid the physiologically stressful conditions for the sensitive larval phase (Anger et al., 2008) by exporting their larvae out of the estuary. Exportation also has the additional advantage of permitting the escape from the predatory risk posed by planktivorous fishes, often present at high densities in estuaries (Morgan, 1990, 1995; Anger, 2001; Queiroga & Blanton, 2005).
Exporting larvae requires a suit of adaptations (i.e. a strategy) of adult and larval phases to perceive the specific environmental conditions that favor the migration of larvae out of the estuary and react in consequence. A synchronized hatching rhythm, maternal migration and developmental changes in physiological tolerance and swimming behavior of larvae (Sandifer, 1975; Anger, 2001; Bilton et al., 2002; Queiroga & Blanton, 2005; Christy, 2011) are expected adaptations to an exportation strategy. Egg bearing females often synchronize hatching with the tides, lunar cycle and/or the light/dark cycle (Forward, 1987; Morgan & Christy, 1994; Anger, 2001). In this scenario larval release usually occur around the high tide time (Forward, 1987) and mostly during the night (Saigusa, 1981; Christy, 1986; Paula, 1989). Larval release can also show a lunar or semi-lunar rhythm (Wolcott & Wolcott, 1982), with hatching taking place more often around spring tides, during new or full moon when tides are stronger (e.g. Wheeler, 1978; Christy, 1982). In newly hatched larvae, horizontal displacements are almost insignificant compared to the distances they have to be transported (Queiroga & Blanton, 2005) and therefore behavioral mechanisms to regulate their depth become more important (Forward & Tankersley, 2001; Cronin & Forward, 1986; Epifanio, 1988; Anger, 2001; Queiroga & Blanton, 2005) to take advantage of seaward currents during ebb or flow tides. Recently it has been pointed out about the lack of attention that has had this type of studies in temperate and polar environments (Christy, 2011).
The varunid crab Neohelice (formerly Chasmagnatus) granulata Dana, 1851 is a predominant species in salt marshes, estuaries and mangroves of South America, ranging from northern Patagonia, Argentina, to Rio de Janeiro, Brazil (Spivak, 2010). As most brachyurans this species has a complex life cycle with benthic adult and juvenile stages, and a planktonic phase with at least four zoeae and one megalopa. Females may hatch several times during a single reproductive season (from September to March) and an individual clutch may produce up to 100,000 larvae (Luppi et al., 1997) after an embryonic development of 20 days at 20ºC (Bas & Spivak, 2000; Ituarte et al., 2004). The species has a significant role in the intertidal and supratidal coastal fringe by shaping the habitat they inhabit and affecting its community structure by several ways (e.g. Iribarne et al., 2005; Alberti et al., 2007; Martinetto et al., 2007; Méndez Casariego et al., 2011; Luppi et al., 2013 and earlier papers cited therein). Such ecological importance along with adaptations to an estuarine and semiterrestrial life has made of it an animal model for many biochemicals, physiological and ecological studies (Spivak, 2010).
This crab has been particularly well studied in Mar Chiquita, a brackish coastal lagoon of Argentina. In this population the reproduction is seasonal extending from mid-spring to early autumn (October - March) (Ituarte et al., 2004). At the beginning of the reproductive season the ovaries and the embryos are highly synchronized, but as the reproductive season progress, this synchronization disappears (Ituarte et al., 2004). Although late larval stages and juveniles are well adapted to settle and recruit in brackish and even limnetic habitats (Charmantier et al., 2002; Anger et al., 2008), intermediate stages do not resist the extreme hypoosmotic conditions found into the estuary (Anger et al., 2008). Accordingly, field observations (Anger et al., 1994) have demonstrated that zoeae hatch within adult habitats, usually at night, and freshly hatched larvae are exported from the estuary towards coastal marine waters on ebb tides, and subsequent larval stages until megalopae remain very close to the coast (Bas et al., 2010).
Although it has been postulated that larval export strategy is mainly based upon exogenously coordinated egg hatching (Anger et al., 1994) the role of endogenous rhythms could not been discarded, neither the environmental cues involved in hatch synchronicity to achieve a successful exportation out from the estuary, have been appropriately studied yet. Actually, the first larvae of N. granulata can tolerate retention in the mesohaline reaches of estuaries (Charmantier et al., 2002), suggesting that polyhaline conditions may be optimal for successful larval development of this species (Anger et al., 2008). Thus, a hatching finely tuned to conditions highly dependent on stochastic environmental variables in the brackish lagoon as wind direction and strength and rainfall intensity (which in turn affect salinity, temperature and the flow of tides toward the estuary) would not be necessary for a successful exportation. Instead, the ability of the first zoea to survive for some days in the unfavorable estuarine environment, would allow hatching coordination based on endogenous cues, much more predictable at short term.
By means of field sampling and experimental manipulations we test whether the proximate causes for hatching synchronicity are exogenous factors as salinity, tides and/or the light/dark cycle or it is mainly due to endogenous rhythms.
METHODS
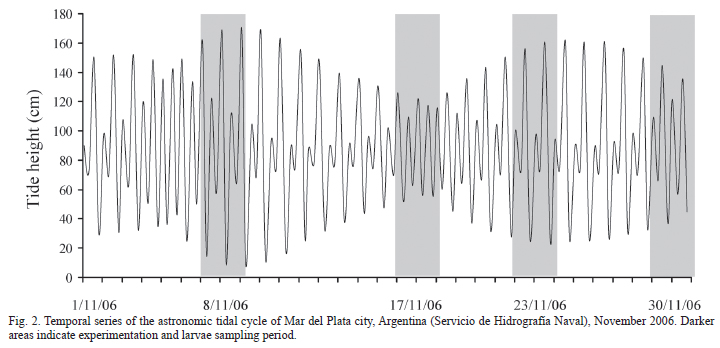
Study Area. The experiments were conducted in the Mar Chiquita Lagoon (37º40'S; 57º20'W), situated 25 km northeast from Mar del Plata city, Argentina (Fig. 1). This brackish coastal lagoon is communicated with the Atlantic Ocean through a narrow channel. The lagoon constitutes an estuary characterized by a salinity gradient that varies with the influence of tides (Olivier et al., 1972). The tidal regimen is semidiurnal, with two high tides and two low tides per day, both differing in amplitude. The distance from the lagoon's mouth and the speed and direction of the winds, influence the tidal amplitude and in the delay of the high tides and the low tides (Reta et al., 2001; see Fig. 2).
Hatching synchronicity at the field and the effect of salinity on hatching. To study the synchrony of embryonic development at the field we sampled female crabs weekly or biweekly in a tidal flat in the brackish lagoon of Mar Chiquita (Fig. 1), from September 13 to December 1 of 2006. Animals were caught by digging them from their burrows. The number of ovigerous and non ovigerous females was recorded and egg bearing females were carried to a nearby field laboratory in buckets containing water from the estuary; their embryo observed under stereo microscope and classified by developmental stage according to Bas & Spivak (2000) as follows: early (S1 and S2), intermediate (S3 and S4, S5, S6 and S7) and late (S8 and S9).
To assess whether salinity affects hatching rhythms we randomly took 75 females bearing late embryos (stages 8 and 9) from the above sampling and divided them in three groups each one corresponding to a 10, 23 and 33 salinity treatments. Each crab was placed separately in a 1 liter recipient containing water at the respective assigned salinity and left for two hours to acclimate. After this period females were held in a dark room for 48 hours and hatching monitored at one-hour intervals. To avoid any interference of common light on hatching, observations were made under infrared light to which adult crabs and larvae are not sensitive (Forward & Cronin, 1979). Females were not fed during the experiments. Experiments started on November 8, 17, 23 and 30. The amplitude of maximum tides predicted for those days was 1.63, 0.74, 1.37 and 1.28 meters respectively (Fig. 2).
We used χ2 test to evaluate differences among the frequency distributions of developmental stages of the embryos and in the proportion of hatching females at different salinities (Zar, 2009). One way ANOVA was applied to test overall differences in percentages of hatching females among experimental dates and further pair differences were tested by the Holm-Sidak method. One way ANOVA was also used to test differences in time to hatching among salinity treatments and experimental dates. In turn, to test whether females hatched differentially during the day or the night and high tide or low tides, we calculated the proportion of hatchings in each category and then applied a χ2 test (Zar, 2009) to determine differences between them. Lastly, circular descriptive statistics (Zar, 2009) were used to evaluate the existence of hatching peaks related to tide in each experiment and Rayleigh test (Zar, 2009) used to evaluate differences between two particular mean angles.
Effect of tide and light cycle on larval abundance in the field. Field surveys of plankton collection were taken in parallel to the above experiments to study fluctuations on the larvae density that may be related to the tides, and light/dark cycle. Each sampling device consisted of two pairs of traps installed at two vertical levels each. Each level had two traps, oriented in a manner that one sample inward currents and the other one the coastward currents (Fig. 3). Traps were constructed with a 1 liter plastic recipient with a funnel introduced in one side, the mouth of the funnel oriented to the exterior, and the other side with a 300 µm mesh to retain the larvae (Fig. 4). The two levels of horizontal traps were located at 10 cm, 60 cm above the bottom of the lagoon respectively. Five sampling devices were buried in the lagoon bed, to approximate 10 meters from the ebb tide line, with a distance of 10 meters between devices. These collections also lasted 48 hours, and samples were obtained by filtering the content of each trap every six hours in the middle of flooding (3 h before high tide) and ebbing (3 h after high tide). The collected material was fixed with 4% formaldehyde, and carried to the laboratory for further identification and counting the Zoea I under microscope based on Bas et al. (2010).
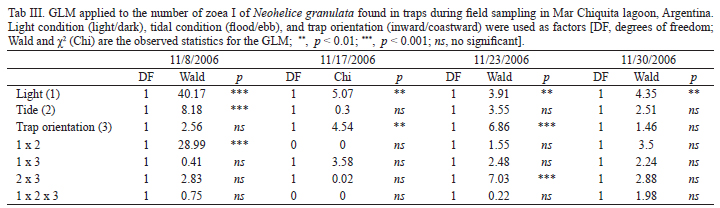
To detect per trap differences in the mean number of Zoea I at different conditions, we used a generalized lineal model (GLM), with Wald and χ2as the observed statistics. It was used per trap number of zoea I as a variable and tidal condition (flood/ebb), light condition (light/dark) and trap orientation (inward/coastward) as factors. To perform this test we used a Poisson distribution for the variable Zoea I, with a logarithmic link function (Statistica v6.0, Statsoft, 2001).
For all the parametric statistical analysis it was verified the compliance of the assumptions of normal distribution and homoscedasticity.
RESULTS
Hatching synchronicity at the field, effect of salinity on hatching. Field sampling of mature females were performed each 6 to 9 days, the minimum sample having 62 and the maximum 108 individuals. The percentage of ovigerous females increased progressively from a minimum of 4% at the beginning of the sampling period on September 13, to a maximum between 80% to 90% in October 12 and November 6, decreasing afterwards to a minimum of 40% on November 17, and rose again by the end of the sampling period on November 30, reaching 73% (Fig. 5).
The embryonic development also showed a progressive growth. The proportion of early embryos (S1-S2) rose to a maximum of 39% on October 20, then fall to 7% on November 17, rising again to 30% on December 1. Embryos in mid stage (S3 to S7) and late stages (S8-S9) raised and decreased in relation with the duration of the embryonic development and the incorporation of new ovigerous females to the population, the late stages reached a peak of 25% on November 6th (Fig. 5).
Although the proportion of hatched females differed among experimental dates (F = 16.3, p < 0.001), the manipulated variations in salinity did not affect that proportion, independently of the date of experiment (p > 0.6) (Fig. 6). The percentage of hatching was maximum for the experiment started on November 17 (81.3 ± 4.6%) and it was significantly different to those started on November 8 (40 ± 10.5%, t = 5.8, p < 0.01) and November 30 (44 ± 9.8%, t = 5.2, p < 0.01), meanwhile it showed no differences with the one started on November 23 (70.7 ± 8.3%, t = 1.5, p > 0.1).
Mean time to hatching neither vary among salinity treatments (F = 0.95, p = 0.38). Nevertheless we found significant differences between the mean time to hatching among sampling dates (F = 35.2, p < 0.001), with maximum values for the experiment started on November 8 (43.7 ± 2 hours) and minimum values for the started on November 23 (23.4 ± 0.9 hours) (Fig. 7).
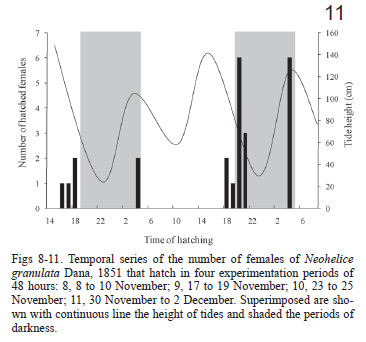
Since salinities did not affect the time to hatching we pooled the data from the three salinity treatments in order to analyze the relationship of hatching and tidal cycles. In the four experimentation dates, the hatching peaks occurred few hours after the predicted time of the high tide independently of the day or night conditions (Figs 8-11).
Comparing the number of hatchings occurring at high-high tides (127) with that occurring at high-low tides (39) differences related to light/dark cycle becomes noticeable. In the first experimental period (on November 8) no differences were evident in the number of hatchings between both tides types, although the biggest hatching pulse took place during a daily high-high tide (Fig. 8). On the experiment that was conducted on November 17 and November, 23, the number of hatchings during the higher tides were significantly bigger (Figs 9, 10). No significant differences were shown between the hatchings occurred during both tides types, in the last experiment date, although there was a tendency towards the ones occurred during the high-high tide (Tab. I). By grouping the four experimentation periods, it was evident that the tidal cycle was significant (χ2= 25.1, p < 0.001), and that the light/dark cycle was not significant (χ2 = 1.2, p = 0.27).
The time of hatching in the four experiment events showed a significant grouping according to Rayleigh test (Tab. II). The r vector, which indicates the dispersion of the results (to a bigger value, lower dispersion) was larger during the first three dates and showed a moderate value during the last experiment (Tab. II, Figs 12-15). The mean angles indicate that during the experiments of November, 8, November, 23 and November, 30, the hatching took place 1 or 2 hours after the high tide, whereas in the one started on November, 17, the hatchings took place 3 and 4 hours after the high tide.
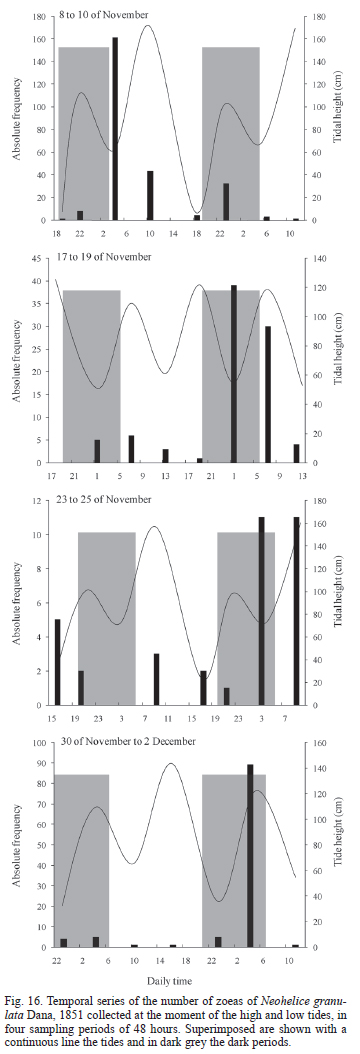
Effect of tide and light cycle on larval abundance in the field. During the first sampling event, on November, 8, the largest number of Zoeas I of N. granulata was collected (194). During the two following sampling events the number of collected larvae decreased to minimum of 25, and increased again in the last collecting sampling to 103. The majority of the N. granulata larvae collecting took place during night hours and descending tides (Fig. 16).
For the four samplings events the number of zoeas I of N. granulata collected during the night was significantly bigger (Tab. III). In the sampling started on November, 8, the number of collected larvae was significantly larger during ebb tide than during the flood tide, and it showed only a positive tendency in the larvae collected in the direction towards the ocean. On the samplings started on November, 17 and November, 23 significant differences were detected in the direction of the zoeas collected, towards the ocean for the most part in the first part of the period, and towards the lagoon by the end of the sampling (Tab. III). Only for the sampling started on November, 17 differences in the number of zoeas collected at different heights of the water column were found for N. granulata (Wald = 7.91, p < 0.01).
Overall light and tidal conditions and their interactions explained the variations in the presence of zoeas.
DISCUSSION
Accordingly with Ituarte et al. (2004) our results show high synchronization of the embryonic development in Neohelice granulata at the beginning of the reproductive season in 2006, which is evidenced in the progression of the embryonic stages and the proportion of ovigerous females (see Fig. 5). However this synchronization is not complete, since from the moment that ovigerous females appeared, in all the samplings performed are females with embryos in the early stages of development.
Variations in salinity within the range used in this study (10 to 33) have no effect on the proportion of hatching females or on the time to hatch. When the hatching comes, the crab eggs goes through changes in the permeability of the membranes that coves them, the entrance of water is facilitated to the eggs interior to increase its volume and help in its rupture (Anger et al., 1994). It is possible that at this moment the egg is too close to the hatching, and the effect could not be detected with an observation frequency of one hour like the one used in this research.
Although we did not find differences in time to hatch among salinities, we did find differences between dates of experimentation. This can be explained by differences in the selection of ovigerous females for the experiments. It is possible that more females in stage 8 were selected for those experiment dates where the time to hatching was higher (e. g. November 8). And more females in stage 9 were selected for those experiment dates where the time to hatching was lower. The difference between the average duration of each stage and hatching was calculated to be 2.9 and 1.2 days for the stages 8 and 9 respectively (Ituarte et al., 2004). This would explain the differences in the averages of the time to hatching between dates, because it does not exceed 24 hours.
Neohelice granulata zoeas I that hatched in the laboratory under a regime of total darkness have a clear endogenous rhythm related to the tidal cycle, a minor endogenous response related to the tidal amplitude, and asynchrony according to the natural light/dark cycle. All the hatchings occurred in dark conditions took place during high tides, and most of them during high-high tides, while there were no differences between the hatchings during the day or the night. In previous studies, with hatching experiences in laboratory, differences between high tides and low tides were not found (Ituarte et al., 2004), nonetheless the higher temporal resolution of the observations used in this work might explain this disparity.
Almost all the studied estuarine crab species show specific hatching times related to tidal cycles, light cycles, lunar cycles or semilunar cycles (e.g. Morgan & Christy, 1995; Yamaguchi, 2001; Park et al., 2005). The answers to these environmental stimuli can be endogenous, meaning that the specific response remains in absence of the environmental stimuli, or conditioned by the cycle of the environmental stimuli, in which case there is no response in absence of the natural environmental cycle. It has been found the existence of endogenous cycle related to the high tides in several estuarine crab species, related to the dark conditions, or related to light conditions. Each estuarine crab species has a combination of endogenous and conditioned responses that allow them to choose the most appropriate time for hatching and ensure the best conditions for the larvae to be transported to the ocean adjacent to the estuary (e.g. Morgan & Christy, 1995; Yamaguchi, 2001; Petrone et al., 2005).
During the four experimentation dates the hatchings occurred shortly after the predicted time of the high tide. 1-2 hours after for three of the experiment events and for the other one the hatchings occurred 3-4 hours after the high tide time, thus ensuring the hatching during ebb tide. Even though the pattern is consistent between dates, the possible discordance between the time of the real high tide and the time of the predicted ones could be responsible for the differences found between dates of experiments. It has been studied that keeping females in captivity can also have an effect on the synchronization of hatching with the high tide, even for short periods of time (Bergin, 1981; Yamaguchi, 2001).
The amount of zoea captured at the field varied widely among collection dates but also among collection devices and individual collectors. There was a marked peak during the break of dawn of December, 2; the most larvae found in an individual collector (80 from 85). This suggests that the larvae drift in discrete patches or clumps rather to be evenly distributed in the water column. In such scenario, a passive method of sampling as the one we used here might present severe pitfalls for quantifying larvae dispersion since depend both to the number and location of collectors relative to the main currents, the currents speed at the sampling time and the swimming abilities of the larvae to avoid collectors; all variables we did not assessed here. To this, we recognize that the efficacy of our collectors as sampling instruments is debatable and therefore we must interpret this data carefully.
Field results coincide with those of hatching experiments in laboratory. For example, on November 8 the largest number of collected zoea occurred during the first sampling event, in coincidence with full moon and maximum tidal amplitude. Zoeas I were collected mostly during the night and with ebb tide. The nocturnal hatching pattern is reinforced by the fact that in all the sampling periods significantly more zoeas were collected during the night than during the day. Furthermore, the strong endogenous hatching cycle related to high tides shown in the laboratory also reinforces the pattern of more zoeas I with ebb tide. Anger et al. (1994) reported a pattern with most of the zoeas I during night hours and ebb tide, but also found with nocturnal flood tide and diurnal ebb tide.
Two are the most important forces that model the strategy of larval export in estuarine crab species, physiological stress and predator evasion (McConaugha, 1988; Morgan & Christy, 1995). Physiological stress is associated to the low capacity of the zoeas I to resist low salinities, and predation is related to the role of estuaries as breeding areas for fish, many of them planktivorous, but is also considered the risk of predation over the females and her embryos. These two forces are not mutually exclusive; their effects are added, depending on the estuary characteristics. Morgan & Christy (1995) proposed that synchronization in hatching is given by three cycles: light/dark, tidal and tidal amplitude. Hatching under a determined combination of conditions of these cycles will not only allow export from the estuary, but this export will be as faster as possible in order to minimize exposure to predators and physiological stress.
In the laboratory, Neohelice granulata hatchings are governed primarily by a strong endogenous high tide rhythm, secondarily by the higher tides and shows no endogenous rhythm related to the light/dark cycle. However in the natural environment, the hatching rhythm is still given by the high tides, but is also conditioned by the light/dark cycle. The combination of nocturnal high tides as the moment for hatchings to occur is the classic larval export model with maximum potential for export in the shortest possible time.
Received 29 October 2012.
Accepted 27 June 2013.
- Alberti, J.; Escapa, M.; Daleo, P.; Iribarne, O.; Silliman, B. & Bertness, M. 2007. Local and geographic variation in grazing intensity by herbivorous crabs in SW Atlantic salt marshes. Marine Ecology Progress Series 349:235-243.
- Anger, K. 2001. The Biology of Decapod Crustacean Larvae Rotterdam, Balkema Publishers. 300p.
- Anger, K.; Spivak, E.; Bas, C. C.; Ismael, D. & Luppi, T. 1994. Hatching rhythms and dispersion of decapod crustacean larvae in a brackish coastal lagoon in Argentina. Helgoländer Meeresuntersuchungen 48:445-466.
- Anger, K.; Spivak, E.; Luppi, T.; Bas, C. & Ismael, D. 2008. Larval salinity tolerance of the South American salt-marsh crab, Neohelice (Chasmagnatus) granulata: physiological constraint to estuarine retention, export and reinmigration. Helgoland Marine Research 62(2):93-102.
- Bas, C. C. & Spivak, E. D. 2000. Effect of salinity on embryos of two Southwestern Atlantic estuarine grapside crab species cultures in vitro Journal of Crustacean Biology 20:647-656.
- Bas, C.; Luppi, T.; Spivak, E. & Shejter, L. 2010. Larval dispersion of the estuarine crab Neohelice granulata in coastal marine waters of Southwest Atlantic. Estuarine Coastal and Shelf Science 83:569-576.
- Bergin, M. E. 1981. Hatching rhythms in Uca pugilator (Decapoda: Brachyura). Marine Biology 63:151-158.
- Bilton, D.; Paula, J. & Bishop, D. 2002. Dispersal, genetic differentiation and speciation in estuarine organism. Estuarine, Coastal and Shelf Science 55:937-952.
- Charmantier, G.; Gimenez, L.; Charmantier-Daures, M. & Anger, K. 2002. Ontogeny of osmoregulation, physiological plasticity, and larval export strategy in the grapsid crab Chasmagnatus granulata (Crustacea, Decapoda). Marine Ecology Progress Series 229:185-194.
- Christy, J. H. 1982. Adaptative significance of semilunar cycles of larvae release in fiddler crabs (genus Uca): test of an hypothesis. Biological Bulletin 163:251-263.
- ______. 1986. Timing of larval release by intertidal crabs on an exposed shore. Bulletin of Marine Science 39:176-191.
- ______. 2011. Timing of Hatching and Release of Larvae by Brachyuran Crabs: Patterns, Adaptive Significance and Control. Integrative and Comparative Biology 51:62-72.
- Cronin, T. W. & Forward, R. B. Jr. 1986. Vertical migration cycles of crab larvae and their role in larval dispersal. Bulletin of Marine Science 39:192-201.
- Epifanio, C. E. 1988. Dispersal strategies of two species of swimming crab on the continental shelf adjacent to Delaware Bay. Marine Ecology Progress Series 49:243-248.
- Forward, R. B. Jr. 1987. Larval release rhythms of decapod crustaceans: an overview. Bulletin of Marine Science 41:165-176.
- Forward, R. B. Jr. & Cronin, T. W. 1979. Spectral sensitivity of larvae from intertidal crustaceans. Journal of Comparative Physiology 133:311-315.
- Forward, R. B. Jr. & Tankersley, R. A. 2001. Selective tidal-stream transport of marine animals. Oceanography and Marine Biology: Annual Review 39:305-353.
- Iribarne, O.; Bruschetti, M.; Escapa, M.; Bava, J.; Botto, F.; Gutierrez, J. & Palomo, G. 2005. Small- and large-scale effect of the SW Atlantic burrowing crab Chasmagnathusgranulatus on habitat use by migratory shorebirds. Journal of Experimental Marine Biology and Ecology 315(1):87-101.
- Ituarte, R.; Spivak, E. & Luppi, T. 2004. Female reproductive cycle of the Southwestern Atlantic estuarine crab Chasmagnatus granulatus (Brachyura, Grapsoidea, Varunidae). Scientia Marina 68:127-137.
- Luppi, T. A.; Bas, C. C.; Spivak, E. D. & Anger, K. 1997. Fecundity of two grapsid crab species in the Laguna Mar Chiquita, Argentina. Archive of Fishery and Marine Research 45:149-166.
- Luppi, T.; Bas, C.; Méndez Casariego, A.; Albano, M.; Lancia, J.; Kittlein, M.; Rosenthal, A.; Farías, N.; Spivak, E. & Iribarne, O. 2013. The influence of habitat, season and tidal regime in the activity of the intertidal crab Neohelice (=Chasmagnathus) granulata Helgoland Marine Research 67:1-15.
- Martinetto, P.; Valiñas, M.; Palomo, G. & Iribarne, O. 2007. Negative interactions between two SW Atlantic intertidal crabs in soft-bottom habitats. Marine Biology 151(4):1479-1490.
- McConaugha, J. R. 1988. Export and reinvasion of larvae as regulators of estuarine decapod populations. American Fisheries Society Symposium 3:90-103.
- Méndez Casariego, A.; Luppi, T.; Iribarne, O. & Daleo, P. 2011. Increase of organic matter transport between marshes and tidal flats by the burrowing crab Neohelice (Chasmagnathus) granulata Dana in SW Atlantic saltmarshes. Journal of Experimental Marine Biology and Ecology 401:110-117.
- Morgan, S. G. 1990. Impact of planktivorous fishes on dispersal, hatching and morphology of estuarine crab larvae. Ecology 71:1639-1652.
- ______. 1995. Life and death in the plankton: Larval mortality and adaptation. In: McEdward, L. ed. Ecology of Marine Invertebrate Larvae. CRC Press, Boca Raton. p. 279-321.
- Morgan, S. G. & Christy, J. H. 1994. Plasticity, constraint and optimality in reproductive timing. Ecology 75:2185-2203.
- ______. 1995. Adaptative significance of the timing of larval release by crabs. American Naturalist 145:457-479.
- Olivier, S. R.; Escofet, A.; Penchaszadeh, P. & Orensanz, J. M. 1972. Estudios ecológicos de la región estuarial de Mar Chiquita (Buenos Aires, Argentina) I. Las comunidades bentónicas. Anales de la Sociedad Científica Argentina 193:237-262.
- Park, S.; Epifanio, C. E. & Iglay, R. B. 2005. Patterns of larval release by the Asian shore crab Hemigrapsus sanguineus (DE HANN): Periodicity at diel and tidal frequencies. Journal of Shellfish Research 24(2):591-595.
- Paula, J. 1989. Rhythms of larval realease of decapod crustaceans in the Mira estuary, Portugal. Marine Biology 100:309-312.
- Petrone, C. J.; Jancaitis, L. B.; Jones, M. B.; Natunewicz, C. C.; Tilburg, C. E. & Epifanio, C. E. 2005. Dynamics of larval patches: spatial distribution of fiddler crab larvae in Delaware Bay and adjacent waters. Marine Ecology Progress Series 293:177-190.
- Queiroga, H. & Blanton, J. 2005. Interactions between behavior and physical forcing in the control of horizontal transport of decapods crustacean larvae. Advances in Marine Biology 47:107-214.
- Reta, R.; Martos, P.; Perillo, G. M. E.; Piccolo, M. C. & Ferrante, A. 2001. Características hidrográficas del estuario de la laguna Mar Chiquita. In: Iribarne, O. ed. Reserva de Biosfera Mar Chiquita: características físicas, biológicas y ecológicas. Mar del Plata, Editorial Martin. p. 31-52.
- Saigussa, M. 1981. Adaptative significance of a semilunar rhythm in the terrestrial crab sesarma. Biological Bulletin 160:311-321.
- Sandifer, P. A. 1975. The role of pelagic larvae in recruitment to populations of adult decapod crustaceans in the York River estuary and adjacent lower Chesapeake Bay, Virginia. Estuarine and Coastal Marine Science 3:269-279.
- Spivak, E. D. 2010. The crab Neohelice (Chasmagnathus) granulata: an emergent animal model from emergent countries. Helgoland Marine Research 64:149-154.
- StatSoft. 2001. Statistica for Windows (version 6.0). Available at <http://www.statsoft.com>
- Wheeler, D. E. 1978. Semilunar hatching periodicity in the mud fiddler crab, Uca pugnax Estuaries 1:268-269.
- Wolcott, T. G. & Wolcott, D. L. 1982. Larval loss and spawning behavior in the land crab Gecarcinus laterales (Freminville). Journal of Crustacean Biology 2:477-485.
- Yamaguchi, T. 2001. Daytime larval release of the fiddler crab, Uca lacteal (Decapoda, Brachyura, Ocypodidae). Crustaceana 74:545-555.
- Zar, J. H. 2009. Biostatistical analysis 5ed. Englewood Cliffs, Prentice-Hall. 960p.
Publication Dates
-
Publication in this collection
08 Aug 2013 -
Date of issue
June 2013
History
-
Received
29 Oct 2012 -
Accepted
27 June 2013