Abstracts
The aim of the present study was to determine the size at sexual maturity in the freshwater crab Dilocarcinus pagei Stimpson, 1861, from a population located in Mendonça, state of São Paulo, Brazil. The crabs were sampled monthly (July 2005 to June 2007), at Barra Mansa reservoir. The specimens were captured manually or in sieves passed through the aquatic vegetation. The crabs were captured and separated by sex based on morphology of the pleon and on the number of pleopods. The following dimensions were measured: carapace width (CW); carapace length (CL); propodus length (PL); and abdomen width (AW). The morphological analysis of the gonads was used to identify and categorize individuals according to their stage of development. The morphological maturity was estimated based on the analysis of relative growth based on the allometric equation y = ax b. The gonadal maturity was based on the morphology of the gonads by the method CW50 which indicates the size at which 50% of the individuals in the population showed gonads morphologically mature to reproduction. The biometric relationships that best demonstrated the different patterns of growth for the juvenile and adult stages were CW vs. PL for males and CW vs. AW for females (p<0.001). Based on these relationships, the estimated value to morphological sexual maturity was 21.5 mm (CW) in males and 19.7 mm (CW) in females. The determination of the size at sexual maturity and the adjustment of the data based on the logistic curve (CW50) resulted in a size of 38.2 mm for males and 39.4 mm for females (CW). Based on the data obtained for sexual maturity for D. pagei, we can estimate a minimum size for capture of 40 mm (CW). This minimum size allows at least half of the population to reproduce and retains the juveniles and a portion of the adults in the population.
Allometric growth; gonadal maturity; morphological maturity; reproduction
Este estudo foi desenvolvido com o objetivo de determinar o tamanho da maturidade sexual de machos e fêmeas de uma população natural de Dilocarcinus pagei Stimpson, 1861 de Mendonça, São Paulo, Brasil. Os caranguejos foram coletados mensalmente (julho de 2005 a junho de 2007) na Represa Barra Mansa, capturados manualmente, ou por meio de peneiras passadas junto à vegetação aquática. Os caranguejos foram separados por sexo, de acordo com a morfologia do abdome e pleópodos. As dimensões mensuradas foram: largura da carapaça (LC); comprimento da carapaça (CC); comprimento do própodo (CP) e largura do abdome (LA). Além disso, análise morfológica das gônadas foram realizadas e caracterizadas em relação ao estágio de desenvolvimento. A maturidade morfológica foi estimada com base no estudo do crescimento relativo, esta análise é baseada na equação alométrica y = ax b e a maturidade gonadal através da morfologia das gônadas, utilizando o método LC50 indicando o tamanho em que 50% dos indivíduos encontram-se com gônadas desenvolvidas para a reprodução. As relações biométricas que melhor indicaram os diferentes padrões de crescimento das fases jovem e adulta foram LCxCP para os machos e LCxAB para fêmeas (p<0,001). Baseado nessas relações, o valor estimado para a maturidade sexual morfológica em machos foi de 21,5 mm (LC) e de 19,7 mm de (LC) para fêmeas. Na determinação da maturidade sexual gonadal, o ajuste da curva logística (LC50) resultou em um tamanho de 38,2 mm para os machos e 39,4 mm para as fêmeas (LC). Com base nos dados obtidos para a maturidade sexual é possível estimar o tamanho mínimo de captura para D. pagei de 40mm (LC), possibilitando que pelo menos metade da população consiga se reproduzir, mantendo os jovens e parte dos adultos na população.
Alometria; maturidade gonadal; maturidade morfológica; reprodução
Crescimento relativo e maturidade sexual do caranguejo dulcícola dilocarcinus pagei (brachyura, trichodactylidae) na região noroeste do estado de São Paulo
Daphine R. HerreraI; Thiago M. DavansoI; Rogerio C. CostaI; Fabiano G. TaddeiII
ILaboratório de Biologia de Camarões Marinhos e Dulcícolas (LABCAM), Departamento de Ciências Biológicas, Faculdade de Ciências, Universidade Estadual Paulista (UNESP), Av. Eng. Luiz Edmundo Corrijo Coube, 14-01, 17033-360 Bauru, SP, Brasil. (daphine_herrera@hotmail.com; tdavanso@ibb.unesp.br; corresponding author: rccosta@fc.unesp.br)
IILaboratório de Estudos dos Crustáceos Amazônicos (LECAM), Universidade do Estado do Amazonas UEA/CESP, Centro de Estudos Superiores de Parintins, Estrada Odovaldo Novo, s/n°, 69152-470, Parintins, AM, Brasil. (fgtaddei@hotmail.com)
ABSTRACT
The aim of the present study was to determine the size at sexual maturity in the freshwater crab Dilocarcinus pagei Stimpson, 1861, from a population located in Mendonça, state of São Paulo, Brazil. The crabs were sampled monthly (July 2005 to June 2007), at Barra Mansa reservoir. The specimens were captured manually or in sieves passed through the aquatic vegetation. The crabs were captured and separated by sex based on morphology of the pleon and on the number of pleopods. The following dimensions were measured: carapace width (CW); carapace length (CL); propodus length (PL); and abdomen width (AW). The morphological analysis of the gonads was used to identify and categorize individuals according to their stage of development. The morphological maturity was estimated based on the analysis of relative growth based on the allometric equation y = axb. The gonadal maturity was based on the morphology of the gonads by the method CW50 which indicates the size at which 50% of the individuals in the population showed gonads morphologically mature to reproduction. The biometric relationships that best demonstrated the different patterns of growth for the juvenile and adult stages were CW vs. PL for males and CW vs. AW for females (p<0.001). Based on these relationships, the estimated value to morphological sexual maturity was 21.5 mm (CW) in males and 19.7 mm (CW) in females. The determination of the size at sexual maturity and the adjustment of the data based on the logistic curve (CW50) resulted in a size of 38.2 mm for males and 39.4 mm for females (CW). Based on the data obtained for sexual maturity for D. pagei, we can estimate a minimum size for capture of 40 mm (CW). This minimum size allows at least half of the population to reproduce and retains the juveniles and a portion of the adults in the population.
KEYWORDS: Allometric growth, gonadal maturity, morphological maturity, reproduction.
RESUMO
Este estudo foi desenvolvido com o objetivo de determinar o tamanho da maturidade sexual de machos e fêmeas de uma população natural de Dilocarcinus pagei Stimpson, 1861 de Mendonça, São Paulo, Brasil. Os caranguejos foram coletados mensalmente (julho de 2005 a junho de 2007) na Represa Barra Mansa, capturados manualmente, ou por meio de peneiras passadas junto à vegetação aquática. Os caranguejos foram separados por sexo, de acordo com a morfologia do abdome e pleópodos. As dimensões mensuradas foram: largura da carapaça (LC); comprimento da carapaça (CC); comprimento do própodo (CP) e largura do abdome (LA). Além disso, análise morfológica das gônadas foram realizadas e caracterizadas em relação ao estágio de desenvolvimento. A maturidade morfológica foi estimada com base no estudo do crescimento relativo, esta análise é baseada na equação alométrica y = axb e a maturidade gonadal através da morfologia das gônadas, utilizando o método LC50 indicando o tamanho em que 50% dos indivíduos encontram-se com gônadas desenvolvidas para a reprodução. As relações biométricas que melhor indicaram os diferentes padrões de crescimento das fases jovem e adulta foram LCxCP para os machos e LCxAB para fêmeas (p<0,001). Baseado nessas relações, o valor estimado para a maturidade sexual morfológica em machos foi de 21,5 mm (LC) e de 19,7 mm de (LC) para fêmeas. Na determinação da maturidade sexual gonadal, o ajuste da curva logística (LC50) resultou em um tamanho de 38,2 mm para os machos e 39,4 mm para as fêmeas (LC). Com base nos dados obtidos para a maturidade sexual é possível estimar o tamanho mínimo de captura para D. pagei de 40mm (LC), possibilitando que pelo menos metade da população consiga se reproduzir, mantendo os jovens e parte dos adultos na população.
PALAVRAS-CHAVE: Alometria, maturidade gonadal, maturidade morfológica, reprodução.
Trichodactylidae consists of crabs that inhabit lowland rivers, generally at elevations greater than 300 m. Crabs belonging to this family are found in Central and South America from southern Mexico to Argentina (MAGALHÃES, 2003). According to NG et al. (2008), this family includes 50 species, 15 genera and 2 subfamilies: Trichodactylinae and Dilocarcininae. Dilocarcinus pagei Stimpson, 1861 belongs to the latter subfamily and the common name is the red crab. Dilocarcinus pagei occurs in the states of Amapá, Pará, Mato Grosso, Rondônia, Acre, Mato Grosso do Sul and São Paulo (MAGALHÃES, 2003). Individuals of this species reaches 60 mm in cephalothorax width and is used commercially as live bait for fishery (PINHEIRO & TADDEI, 2005a). It is considered an important member of the trophic chain in the streams and reservoirs of northwestern state of São Paulo (ESTEVES, 1988).
Among the studies addressed to crustacean ecology, few of them have considered freshwater species. Some these studies include analyses of reproduction (LIU & LI, 2000), fecundity (MANSUR & HEBLING, 2002), investigations about distribution (MAGALHÃES et al., 2005), physiological topics (ONKEN & MCNAMARA, 2002; AMADO et al., 2006), development (PINHEIRO & TADDEI, 2005a,b; MANSUR et al., 2005; TADDEI & HERRERA, 2010) and population dynamics (DAVANSO et al., 2013).
The process of sexual maturation in crabs is characterized by morphological and physiological variation and by changes in the social role of individuals in the population, accompanied by changes in the ethological characteristic of different life stages of the species and influencing the occurrence of new habits and behaviors (HARTNOLL, 1969; FERNÁNDEZ-VERGAZ et al., 2000; MOURA & COELHO, 2004). HARTNOLL (1974) showed that ontogenetic variation occurs in the relative growth patterns of certain body parts (e.g. the chelipeds for males and the abdomen for females). These findings indicate that growth patterns differ not only between the sexes but also between juvenile and adult individuals.
Another method used to estimate the size in the decapods reaches its reproductive status can be monitored by changes occurring in the ovary during the reproductive cycle, in which there is multiplication of gonadal cells and growth, maturation of gametes and ovulation (GRASSÈ, 1996). This gonadal maturity is performed using the CW50 method, which estimates the size at which 50% of individuals in the population exhibit gonads morphologically designed for reproduction (CORGOS & FREIRE, 2006).
The harvest of several commercially exploited crab species is regulated by a management plan that includes a minimum size of capture (KNUCKEY, 1996). This management strategy is important because it allows the individuals in a population to reach the maturity to permit the reproduction and contributing to the maintenance of the population.
It is necessary to compare the values of the size at sexual maturity obtained with different methodologies to ensure the reliability of the estimates (CASTIGLIONI & COELHO, 2011). Thus, the exact estimated size at maturity should include significant changes in the external morphology, in addition to the examination of gonadal development, as sizes obtained are often different (GONZÁLEZ-GURRIARÁN, 1985). The aim of this study was to estimate the size at sexual maturity in a natural population of D. pagei based on the study of relative growth and on gonad morphology and additionally to evaluate a minimum size for capture which may be used to species.
MATERIALS AND METHODS
Sampling procedure and laboratory analysis. The Barra Mansa reservoir, Mendonça, state of São Paulo (21°14'27"S, 49°56'28"W) is located in the Paraná Basin, in the Tietê/Batalha watershed on the Western Plateau of São Paulo. The reservoir has a drainage area of 13,394 km2 and a total flow of 98 m3/s.
The population of D. pagei at Barra Mansa reservoir was sampled monthly from July 2005 to June 2007, during the day by four individuals over a period of 2 h. The specimens were captured manually or in sieves passed vigorously through the aquatic vegetation. The vegetation consists primarily of Eichhornia azurea Kunt, E. crassipes (Mart.), Salvinia molesta D. S. Mitchell, Cabomba sp. and Egeria densa Planch.
After capture, the animals were stored in plastic bags. To prevent loss of eggs and early juveniles in the incubatory chamber ovigerous females were individually placed in plastic bags. The organisms were then transported to the laboratory and kept frozen until the time for analysis.
All individuals were thawed at room temperature before analysis and were sexed using pleon morphology and the number of pleopods (PINHEIRO & TADDEI, 2005b). A precision caliper (0.05 mm) was used to measure the following structures in each crab: carapace width (CW); carapace length (CL); propodus length (PL); and abdomen width (AW) (MANSUR et al., 2005) (Fig. 1).
After measurements, the carapace of the individuals was batted to allow the visualization of the gonads for the morphological analysis and estimative of gonadal maturity. The gonads were classified macroscopically into three stages: immature (IM), characterized by the absence of visible gonads in both males and females; maturing (Mt), characterized by an early folding of the gonads in both sexes, with white coloration in males and orange in females; mature (M) characterized by greater folding of the gonads, occupying a large space in the body cavity and with milky white coloration in males and red in females. Individuals with undeveloped gonads but with a size equal to or greater than that estimated for morphological sexual maturity were subsequently classified as rudimentary (RU) adapted from PINHEIRO & FRANSOZO (1998).
Relative growth and determination of morphological sexual maturity. To estimate the values of the measurements corresponding to morphological sexual maturity, an analysis of relative growth was performed. This analysis was based on allometric methods (HUXLEY, 1950) applied to the morphometric data obtained for the crabs. The analysis produced an empirical characterization of the relationships between CW (independent variable) and selected dependent variables (AW, PL and CL). The data were displayed on scatterplots and fitted with the allometric equation y = axb (HARTNOLL, 1974, 1978, 1982), where y = dependent variable; x = independent variable; a = source index; b = constant of allometric growth. The allometric equation was expressed in linear form (logy = loga + b logx) (HUXLEY, 1950), and the parameters were estimated from a linear regression of the log-transformed data. The value of the allometric constant b was calculated for each biometric relationship, and the null hypothesis (H0: b = 1) was tested with a Student t test (α = 0.05). The values of the allometric constant correspond to isometric growth (b = 1), positive allometry (b > 1) or negative allometry (b < 1) (ZAR, 1996). The fit of the mathematical model was verified according to the coefficient of determination (R2) for each relationship (PINHEIRO & FRANSOZO, 1993).
Based on the linear relationships for relative growth, a non-hierarchical K-means cluster analysis was performed to characterize the morphometric relationships of the data. This method classifies the data into a number of groups. The analysis divides the data into groups of interest (e.g., juveniles and adults). The results of the K-means classification were refined with a discriminant analysis (SAMPEDRO et al., 1999).
After the establishment of the correct division into demographic categories, a covariance analysis (ANCOVA) of the log-transformed data was performed to test the differences among formed groups in the angular (b) and linear (a) coefficients. Based on this analysis, it was possible to determine whether the data for each relationship should be represented by a single straight line or should be represented by different linear equations for the groups (PANTALEÃO et al., 2011).
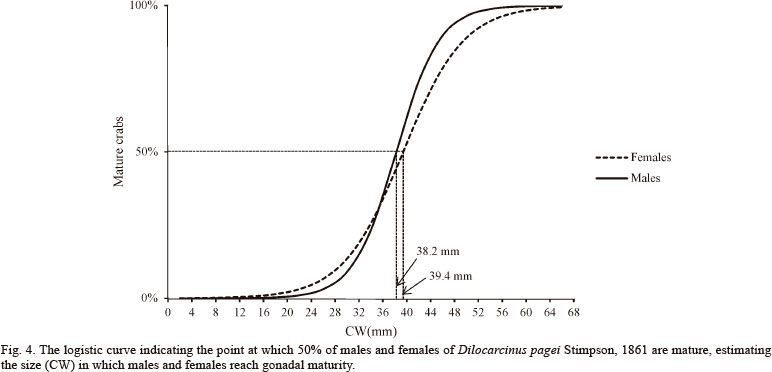
Determination of the size at gonad maturity. The CW50 method was utilized to identify the size at gonad maturity, i.e., the size at which 50% of the individuals in the population showed morphologically mature gonads (Mt + M stages). This method analyzes the distribution of individuals according to size classes based on carapace width (CW). A graph based on the logistic curve equation y = 1 /( 1+ er(CW-CW50)) was plotted to relate the size of the animal (CW), the independent variable, where r is the slope of the curve, for 50% of the population to reach the adult phase (CW50) to the relative frequency of mature individuals (Mt + M), the dependent variable. The size at gonad maturity is defined by the interpolation point corresponding to a relative frequency of 50% on the graph. The equation of the logistic curve was fitted to the data by the method of least squares (VAZZOLER, 1996).
RESULTS
During the study, 1,340 crabs were captured (804 males and 536 females). Ten of the females were ovigerous. The CW of the males ranged from 13.3 to 53.2 mm (mean 27.3 ± 13.8 mm); in the females, the CW ranged from 13.5 to 58.3 mm (mean 28.8 ± 12.9 mm). The smallest ovigerous female had a CW of 39.5 mm. The mean CW of the ovigerous females was 44.1 ± 2.56 mm.
Estimate of size at morphological and gonad maturity. The plots of the morphological data (Figs 2, 3) illustrated the growth patterns associated with the demographic categories analyzed: juvenile male (JM), adult male (AM), juvenile female (JF) and adult female (AF). The biometric relationships that best demonstrated the different growth patterns of the juvenile and adult stages were CW vs. PL for males (p<0.001) and CW vs. AW for females (p<0.001) (Tab. I). The cheliped growth patterns (CW vs. PL) were isometric for the males and females in the juvenile stage and positively allometric in the adult stage. The CW vs. CL relationship showed negative allometry for both sexes. The relationships with the growth of the abdomen (CW vs. AW) showed negative allometry for males in both phases. As for the females showed a negative allometry for the juveniles and positive allometry for the adults (Tab. I). The CW vs. CL relationship showed no statistically significant changes between the growth stages for both sexes and the CW vs. AW only for the males (Tab. II).
According to the K-means analysis, was observed by the relationship CW vs. PL for males a significant difference between the stages of growth after the size categories were separated. Based on this relationship, the estimated size of morphological sexual maturity corresponds to the CW of the smaller individual after the break point of the equations for immature and adult, in male was estimated as a carapace width of 21.5 mm (Fig. 2).
For females was found significant difference for the relationship CW vs. AW which due to overlap of the growth lines (juveniles and adults) to estimate size of morphological sexual maturity was applied the CW50 analysis to this individuals, resulting in an estimated as a carapace width of 19.7 mm (Fig. 3).
From the total number of specimens collected during the study, 441 crabs were with gonads developed to reproduction (Mt+M). These crabs included 320 males and 121 females, which were distributed in 15 size classes defined by intervals of 4 mm.
For gonad sexual maturity, the adjustment of the data based on the logistic curve (CW50) resulted in a CW at gonad maturity of 38.2 mm for males and 39.4 mm for females (Fig. 4).
DISCUSSION
This study identified marked differences between juveniles and adults in the growth pattern of the cheliped propodus and of the abdomen for males and females, respectively, and yielded the best indicators of morphological sexual maturity. These results indicate that the differential growth of these structures is related with secondary sexual character.
This type of differential growth was also observed for the specie investigated in the present study by MANSUR et al. (2005) in state of Mato Grosso do Sul, midwestern Brazil. Similar meaningful results were obtained by other authors: FERNÁNDEZ-VERGAZ et al. (2000) for Chaceon affinis (A. Milne-Edwards & Bouvier, 1894); NEGREIROS-FRANSOZO & FRANSOZO (2003) for Panopeus austrobesus Williams, 1983; GREGATI & NEGREIROS-FRANSOZO (2007) for Neohelice granulata (Dana, 1851) (as Chasmagnathus granulatus); COBO & ALVES (2009) for Mithrax tortugae Rathbun, 1920; SAL MOYANO et al. (2011) for Libinia spinosa (Milne-Edwards, 1834) and LIMA et al. (2013) for Trichodactylus fluviatilis Latreille, 1828.
The positive allometry found for the male cheliped propod in the adult stage indicates the possibility of a greater energy investment in the development of this structure after the pre-pubertal stage. This implies that the cheliped propod is associated with reproductive functions, as observed for the same species collected from state of Mato Grosso do Sul by MANSUR et al. (2005) and also observed in Uca burgersi Holthuis, 1967 by BENETTI & NEGREIROS-FRANSOZO (2004) and in Austinixa patagoniensis (Rathbun, 1918) by ALVES et al. (2005).
GHERARDI & MICHELI (1989) noted that copulation in freshwater crabs occurs when both sexes are in intermolt. The males use the chelipeds to hold the female. This observation emphasizes the importance of this structure in the reproductive process and in several behavioral interactions, including courtship and signaling to attract females, as in species of the genus Uca (BENETTI & NEGREIROS-FRANSOZO, 2004; MASUNARI & DISSENHA, 2005); defense and courtship in Ocypode quadrata (FRANSOZO et al., 2002); and other agonistic behaviors.
The CW vs. PL relationship also showed positive allometry in adult females, a characteristic also found by DANIELS (2001) for the freshwater species Potamonautes warreni (Calman, 1918) and attributed to the use of the chelipeds to defend the eggs and early juveniles against predators.
The negative allometric growth of the abdomen in the females during the juvenile stage and the positive allometric growth during the adult stage correspond to the increase in the reproductive potential of the females. This characteristic of the adult stage has also been verified in other brachyuran species (PINHEIRO & FRANSOZO, 1993; MANTELATTO & FRANSOZO, 1994, 1999; SANTOS et al, 1995; FLORES & NEGREIROS-FRANSOZO, 1999; NEGREIROS-FRANSOZO et al., 2002, 2003). In brachyuran, the abdomen of females and their pleopods present an important reproductive function, especially for most freshwater crabs because they form a "chamber incubatory" with the function of retaining the eggs and early juveniles newly hatched. According to KAESTNER (1970) this fact features a parental care typical of freshwater crabs. In freshwater crabs, the enhanced development of the abdomen increases the volume of the incubatory chamber so that the chamber can contain a greater number of eggs (HINES, 1982).
The difference in the size at morphological sexual maturity between females and males is expected and is consistent with the pattern proposed by SHINE (1988) for brachyurans. According to this author, this pattern is explained by a difference between the sexes in the requirements for reproduction. When females allocate their energy for reproductive purposes, such as spawning and egg incubation, they tend to mature at smaller sizes than males, who invest their resources in somatic growth and reach maturity at greater sizes.
The present study found that the females reached morphological sexual maturity at smaller sizes than the males. The difference in the size at morphological sexual maturity between males and females (21.5 and 19.7 mm for males and females respectively) is expected and is consistent with the pattern proposed by SHINE (1988) for brachyurans. According to this author, this pattern is explained by a difference between the sexes in the requirements for reproduction. When females allocate their energy for reproductive purpose, such as spawning and egg incubation, they tend to mature at smaller sizes than males, who invest their resources in somatic growth and reach maturity at greater sizes.
According to TADDEI & HERRERA (2010), the females of D. pagei can reproduce during the first year of life. However, an estimate sizes similar to the gonadal maturity in both sexes, in this study, probably, favors the formation of pairs in a population and the copulation (HARTNOLL, 1969; PINHEIRO & TADDEI, 2005a; LIMA et al., 2013).
Most likely, copulation in D. pagei occurs when both sexes are in intermolt, as observed for the freshwater species Candidiopotamon rathbunae (De Man, 1914), whose females lack male protection during the intermolt stage (LIU & LI, 2000; TADDEI & HERRERA, 2010). Males exhibit the behavior of forcing copulation, which occurs in hours, as opposed to days of marine species, which discards the protection of the male (PINHEIRO & FRANSOZO, 1998; TADDEI & HERRERA, 2010).
The emergence of the observed secondary sexual characters at the transition from the juvenile to the adult stage (HARTNOLL, 1978), is not necessarily synchronous with the maturity of the gonads. In a study of the crab Chaceon affinis, by FERNÁNDEZ-VERGAZ et al. (2000), it was suggested that although the functional capacity for mating to be related with the morphological maturity, this is attained before the individual becomes physiologically mature and is, therefore, able to reproduce. The first evidence of the morphometric changes required for reproduction occurs before the females attain a size sufficient for successful copulation, which occurs at the end of this process. This fact was verified for D. pagei in the present study where there has been a positive allometry of the abdomen in relation to carapace width in adult females showing that at this stage they are morphologically able for reproduction.
Because the studied species is used in the region as bait for sport fishing, it is important to establish a minimum size for capture to avoid both overexploitation and the resulting harm to the environment, as these organisms serve as the basis of many food chains (MAGALHÃES, 2003). Thus, it can be conclude that to estimate a size of sexual maturity should include studies of secondary sexual characters and examination of the gonads, as these values may not occur synchronously.
Based on the data obtained for sexual maturity (males and females) and to facilitate a practicable inspection due to a possible implementation of a minimum size to capture of D. pagei, the value was rounded to 40 mm to carapace width. This minimum potentially allows at least one-half of the population to reproduce and maintains juveniles and adults in the population. In addition, the present study will contribute to the implementation of future projects involving this species and, consequently, to the maintenance of natural stocks.
Received 21 November 2012
Accepted 22 July 2013
- ALVES, E. S.; RODRIGUES, S. A. & PEZZUTO, P. R. 2005. Estudo do crescimento relativo de Austinixa patagoniensis (Rathbun) (Decapoda, Pinnnotheridae) simbionte de Callichirus major (Say) (Decapoda, Callianassidae) no Mesolitoral da praia de Balneário Camboriú, Santa Catarina, Brasil. Revista Brasileira de Zoologia 22(3):784-792.
- AMADO, E. M.; FREIRE, C. A. & SOUZA, M. M. 2006. Osmoregulation and tissue water regulation in the freshwater red crab Dilocarcinus pagei (Crustacea, Decapoda), and the effect of waterborne inorganic lead. Aquatic Toxicology 79:1-8.
- BENETTI, A. S. & NEGREIROS-FRANSOZO, M. L. 2004. Relative growth of Uca burgersi (Crustacea, Ocypodidae) from two mangroves in the southeastern Brazilian coast. Iheringia, Série Zoologia 94(1):67-72.
- CASTIGLIONI, D. S. & COELHO, P. A. 2011. Determinação da maturidade sexual de Ucides cordatus (Crustacea, Brachyura, Ucididae) em duas áreas de manguezal do litoral sul de Pernambuco, Brasil. Iheringia, Série Zoologia 101(1-2):138-144.
- COBO, V. J. & ALVES, D. F. R. 2009. Relative growth and sexual maturity of the spider crab, Mithrax tortugae Rathbun, 1920 (Brachyura, Mithracidae) on a continental island off the southeastern Brazilian Coast. Crustaceana 82(10):1265-1273.
- CORGOS, A. & FREIRE, J. 2006. Morphometric and gonad maturity in the spider crab Maja brachydactyla: a comparison of methods for estimating size at maturity in species with determinate growth. Journal of Marine Science 63:851-859.
- DANIELS, S. R. 2001. Allometric growth, handedness, and morphological variation in Potamonautes warren (Calman, 1918) (Decapoda, Brachyura, Potamonautidae) with a redescription of the species. Crustaceana 74:237-253.
- DAVANSO, T. M.; TADDEI, F. G.; SIMÕES, S. M.; FRANSOZO, A. & COSTA, R. C. 2013. Population dynamics of the freshwater crab Dilocarcinus pagei in tropical waters in southeastern Brazil. Journal of Crustacean Biology 33(2):235-243.
- ESTEVES, F. A. 1988. Fundamentos de Limnologia São Paulo, Editora Interciências/FINEP. 288p.
- FERNÁNDEZ-VEGAZ, V.; LÓPEZ ABELLÁN, L. J. & BALGUERÍAS, E. 2000. Morphometric, functional and sexual maturity of the deep-sea red crab Chaceon affinis inhabiting Canary Island Water: chronology of maturation. Marine Ecology Progress Series 204:169-178.
- FLORES, A. & NEGREIROS-FRANSOZO, M. L. 1999. Allometry of the secondary sexual characters of the shore crab Pachygrapsus transversus (Gibbes, 1850) (Brachyura, Grapsidae). Crustaceana 72(9):1051-1066(16).
- FRANSOZO, A.; NEGREIROS-FRANSOZO, M. L. & BERTINI, G. 2002. Morphometric studies of the ghost crab Ocypode quadrata (Fabricius, 1787) (Decapoda, Ocypodidae) from Ubatuba, São Paulo, Brazil. In: ESCOBAR-BRIONES, E. & ÁLVAREZ, F. eds. Modern Approaches to the study of Crustacea New York, Kluwer/Plenum. p. 189-195.
- GHERARDI, F. & MICHELI, F. 1989. Relative growth and population structure of the freshwater crabs, Potamon potamios palestinensis, in the Dead Sea area. Israel Journal of Zoology 36:133-145.
- GONZÁLEZ-GURRIARÁN, E. 1985. Reproducción de la nécora Macropipus puber (L.) (Decapoda, Brachyura), y ciclo reproductivo en la Ría de Arousa (Galicia, NW España). Boletín Instituto Español de Oceanografía 2(1):10-32.
- GRASSÉ, P. P. 1996. Traité de Zoologie: Anotomie, Systématique, Biologie Tome VII. Crustacés; fascicule I: Morphologie, Physiologie, Reproduction, Systématique. Paris, Masson. 917p.
- GREGATI, R. A. & NEGREIROS-FRANSOZO, M. L. 2007. Relative growth and morphological sexual maturity of Chasmagnathus granulatus (Crustacea, Varunidae) from a mangrove area in southeastern Brazilian coast. Iheringia, Série Zoologia 97(3):268-272.
- HARTNOLL, R. G. 1969. Mating in Brachyura. Crustaceana 16:161-181.
- _____. 1974. Variation in growth pattern between some secondary sexual characters in crabs (Decapoda, Brachyura). Crustaceana 27(2):131-136.
- _____. 1978. The determination of relative growth in Crustacea. Crustaceana 34(3):282-292.
- _____. 1982. Growth. In: BLISS, D. E. ed. The Biology of Crustacea Embriology, Morphology and Genetics. New York, Academic Press. v.2, p. 111-185.
- HINES, A. H. 1982. Allometric contraints and variables of reproductive effort in Brachyura crabs. Marine Biology 69:309-320.
- HUXLEY, J. S. 1950. Relative growth and form transformation. Proceedings of Royal Society of London 137:465-469.
- KAESTNER, A. 1970. Invertebrate Zoology New York, Crustacea Interscience Publishers. 523p.
- KNUCKEY, I. A. 1996. Maturity in male mud crabs, Scylla serrata, and the use of mating scars as a functional indicator. Journal of Crustacean Biology 16:487-495.
- LIMA, D. J. M.; COBO, V. J.; ALVES, D. F. R.; BARROS-ALVES, S. P. & FRANSOZO, V. 2013. Onset of sexual maturity and relative growth of the freshwater crab Trichodactylus fluviatilis (Trichodactyloidea) in south-eastern Brazil. Invertebrate Reproduction & Development 57(2):1-8.
- LIU, H. C. & LI, C. W. 2000. Reproduction in the freshwater crab Candidiopotamon athbunae (Brachyura: Potamidae) in Taiwan. Journal of Crustacean Biology 20(1):89-99.
- MAGALHÃES, C. 2003. Famílias Pseudothelphusidae e Trichodactylidae. In: MELO, G. A. S. ed. Manual de Identificação dos Crustacea Decapoda de Água Doce do Brasil São Paulo, Edições Loyola. p. 143-287.
- MAGALHÃES, C.; BUENO, S. L.; BOND-BUCKUP, G.; VALENTI, W. C.; SILVA, H. M.; KIYOHARA, F.; MOSSOLIN, E. C. & ROCHA, S. 2005. Exotic species of freshwater decapod crustaceans in the state of São Paulo, Brazil: records and possible causes of their introduction. Biodiversity and Conservation 14:1929-1945.
- MANSUR, C. B. & HEBLING, N. J. 2002. Análise comparativa entre a fecundidade de Dilocarcinus pagei Stimpson e Sylviocarcinus australis Magalhães & Turkay (Crustacea, Decapoda, Trichodactylidae) no Pantanal do Rio Paraguai, Porto Murtinho, Mato Grosso do Sul. Revista Brasileira de Zoologia 19(3):797-805.
- MANSUR, C. B.; HEBLING, N. J. & SOUZA, J. A. 2005. Crescimento relativo de Dilocarcinus pagei Stimpson, 1861 e Sylviocarcinus australis Magalhães & Turkay (Crustacea, Decapoda, Trichodactylidae) no Pantanal do Rio Paraguai, Porto Murtinho, Mato Grasso do Sul. Boletim do Instituto de Pesca 31(2):103-107.
- MANTELATTO, F. L. M. & FRANSOZO, A. 1994. Crescimento relativo e dimorfismo sexual de Hepatus pudibundus (Herbst, 1785) (Decapoda, Brachyura) no litoral paulista. Papéis Avulsos de Zoologia 39(4):33-48.
- _____. 1999. Relative growth of the crab Sesarma rectum Randall, 1840 (Decapoda, Brachyura, Grapsidae) from Bertioga, São Paulo, Brazil. Pakistan Journal of Marine Biology 5(1):11-21.
- MASUNARI, S. & DISSENHA, N. 2005. Alometria no crescimento de Uca mordax (Smith) (Crustacea, Decapoda, Ocypodidae) na Baía de Guaratuba, Paraná, Brasil. Revista Brasileira de Zoologia 22(4):984-990.
- MOURA, N. F. O. & COELHO, P. A. 2004. Maturidade sexual fisiológica em Goniopsis cruentata (Latreille) (Crustacea, Brachyura, Grapsidae) no estuário do Paripe, Pernambuco, Brasil. Revista Brasileira de Zoologia 21(4):1011-1015.
- NEGREIROS-FRANSOZO, M. L. & FRANSOZO, V. 2003. Morphometric study of the mud crab, Panopeus austrobesus Willians, 1983 (Decapoda, Brachyura) from a subtropical mangrove in south America. Crustaceana 76(3):281-294.
- NEGREIROS-FRANSOZO, M. L.; COLPO, K. D. & COSTA, T. M. 2003. Allometric growth in the fiddler crab Uca thayeri (Brachyura, Ocypodidae) from a subtropical mangrove. Journal of Crustacean Biology 23(2): 1-7.
- NEGREIROS-FRANSOZO, M. L.; FRANSOZO, A. & BERTINI, G. 2002. Reproductive cycle and recruitment period of Ocypode quadrata (Decapoda, Ocypodidae) at a sandy beach in southeastern Brazil. Journal of Crustacean Biology 22(1):157-161.
- NG, P. K. L; GUINOT, D. & DAVIE, P. J. F. 2008. Systema Brachyurorum, Part I. An Annotated Checklist of Extant Brachyuran Crabs of the World. The Raffles Bulletin of Zoology 17:1-286.
- ONKEN, H. & MCNAMARA, J. C. 2002. Hyperosmoregulation in the red freshwater crab Dilocarcinus pagei (Brachyura, Trichodactylidae): structural and functional asymmetries of the posterior gills. The Journal of Experimental Biology 205:167-175.
- PANTALEÃO, J. A. F.; HIROSE, G. L. & COSTA, R. C. 2011. Relative growth, morphological sexual maturity, and size of Macrobrachium amazonicum (Heller, 1862) (Crustacea, Decapoda, Palaemonidae) in a population with an entirely freshwater life cycle. Invertebrate Reproduction & Development 56(3):180-190.
- PINHEIRO, M. A. A. & FRANSOZO, A. 1993. Relative growth of speckled swimming crab Arenaeus cribrarius (Lamarck, 1818) (Bachyura, Portunidae), near Ubatuba, State of São Paulo, Brasil. Crustaceana 65(3):377-389.
- _____. 1998. Sexual maturity of the speckled swimming crab Arenaues cribrarius (Lamarck, 1818) (Decapoda, Brachyura, Portunidae) in the Ubatuba littoral, São Paulo State, Brasil. Crustaceana 71(4):434-452.
- PINHEIRO, M. A. A. & TADDEI, F.G. 2005a. Relação peso/largura da carapaça e fator de condição em Dilocarcinus pagei Stimpson (Crustacea, Brachyura, Trichodactylidae). Revista Brasileira de Zoologia 22(3):522-528.
- _____. 2005b. Crescimento do caranguejo de água doce Dilocarcinus pagei Stimpson (Crustacea, Brachyura, Trichodactylidae). Revista Brasileira de Zoologia 22(3):522-528.
- SAL MOYANO, M. P.; GAVIO, M. A. & MAGGI, M. D. 2011. Morphometric and gonad maturity of the spider crab Libinia spinosa (Crustacea: Brachyura: Majoidea: Epialtidae) in Argentina. Journal of the Marine Biological Association of the United Kingdom 91(4):837-844.
- SAMPEDRO, M. P.; GONZÁLEZ-GURRIARÁN, E.; FREIRE, J. & MUIÑO, R. 1999. Morphometry and sexual maturity in the spider crab Maja squinado (Decapoda: Majidae) in Galicia, Spain. Journal of Crustacean Biology 19(3):578-592.
- SANTOS, S.; NEGREIROS-FRANSOZO, M. L. & FRANSOZO, A. 1995. Morphometric relationships and maturation in Portunus spinimanus Latreille, 1819 (Crustacea, Brachyura, Portunidae). Revista Brasileira de Biologia 55(4):545-553.
- SHINE, R. 1988. The Evolution of Large Body Size in Females: A Critique of Darwin's "Fecundity Advantage" Model. The American Naturalist 131(1):124-131.
- TADDEI, F. G. & HERRERA, D. R. 2010. Crescimento do caranguejo Dilocarcinus pagei Stimpson, 1861 (Crustacea, Brachyura, Trichodactylidae) na represa Barra Mansa, Mendonça, SP. Boletim do Instituto de Pesca 36(2):99-110.
- VAZZOLER, A. E. A. M. 1996. Biologia da Reprodução de Peixes Teleósteos: teorias e práticas Maringá, Eduem. 169p.
- ZAR, J. H. 1996. Biostatistical analysis. Upper Saddle River, Prentice-Hall. 662p.
The relative growth and sexual maturity of the freshwater crab
Publication Dates
-
Publication in this collection
18 Nov 2013 -
Date of issue
Sept 2013
History
-
Received
21 Nov 2012 -
Accepted
22 July 2013