Abstracts
The nymphal instars I and III - V of Sigara (Tropocorixa) denseconscripta (Breddin, 1897) are figured and described in detail, for the first time, with emphasis on morphometry and chaetotaxy of selected structures. The useful characters to identify the nymphal instars and the nymphs of the species of Sigara are provided.
Nepomorpha; Argentina; morphometry; chaetotaxy; aquarium rearing
Se ilustran y describen en detalle, por primera vez, las ninfas I y III - V de Sigara (Tropocorixa) denseconscripta (Breddin, 1897) con énfasis en la morfometría y quetotaxia de ciertas estructuras. Se proveen los caracteres útiles para identificar los estadios ninfales y las ninfas de las especies de Sigara.
Nepomorpha; Argentina; morfometría; quetotaxia; cría en acuario
Susana A. Konopko
Museo Argentino de Ciencias Naturales "Bernardino Rivadavia", División de Entomología. Av. Angel Gallardo 470, C1405DJR, Buenos Aires, Argentina. Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina). (konopko@macn.gov.ar)
ABSTRACT
The nymphal instars I and III - V of Sigara (Tropocorixa) denseconscripta (Breddin, 1897) are figured and described in detail, for the first time, with emphasis on morphometry and chaetotaxy of selected structures. The useful characters to identify the nymphal instars and the nymphs of the species of Sigara are provided.
KEYWORDS: Nepomorpha, Argentina, morphometry, chaetotaxy, aquarium rearing.
RESUMO
Se ilustran y describen en detalle, por primera vez, las ninfas I y III - V de Sigara (Tropocorixa) denseconscripta (Breddin, 1897) con énfasis en la morfometría y quetotaxia de ciertas estructuras. Se proveen los caracteres útiles para identificar los estadios ninfales y las ninfas de las especies de Sigara.
Palabras-clave: Nepomorpha, Argentina, morfometría, quetotaxia, cría en acuario.
The genus Sigara Fabricius, 1775 is placed within the tribe Corixini, subfamily Corixinae, it is divided into various subgenera, and includes approximately 70 species in America of medium to large sized water boatmen (BACHMANN, 1981; MORRONE et al., 2004). The subgenera Aphelosigara Hungerford, 1948 and Tropocorixa Hutchinson, 1940 are present in Argentina and are represented by 18 species (BACHMANN, 1981) of which Sigara (Tropocorixa) denseconscripta (Breddin, 1897) is studied in the present contribution.
Sigara denseconscripta is a medium to large sized water boatmen which is distributed in Bolivia, Argentina (from Jujuy and Misiones to Rio Negro provinces), Paraguay and Brazil (state of Rio Grande do Sul) (BACHMANN, 1981; MORRONE et al., 2004).
The adults of this species are characterized by a postnodal pruinose area longer than the one of the claval suture; a medial pronounced notch on posterior margin of the pronotal disc; a subtriangular male pala, wider basally; and a large male genital capsule (BACHMANN, 1981). This species in Argentina is very common in lentic, usually small, temporary, muddy and sunny water bodies, with scanty or without vegetation (BACHMANN, 1981).
The systematics of the adults of Sigara are comparatively well known (HUTCHINSON, 1940; HUNGERFORD, 1948; BACHMANN, 1960, 1961, 1962a - d, 1963, 1966, 1979, 1981, 1987), but there are few descriptions of nymphs of Sigara available in the literature (European species) (COBBEN & MOLLER-PILLOT, 1960; JANSSON, 1969; NIESER, 1969; LÓPEZ et al., 1996). Regarding Argentina, the nymphal instars of Sigara (Tropocorixa) jensenhaarupi Jaczewski, 1927 were described superficially by MELO & SCHEIBLER (2011), but the nymphal instars of S. (Tropocorixa) santiagiensis (Hungerford, 1928), S. (T.) schadei (Hungerford, 1928), and S. (Aphelosigara) tucma Bachmann, 1961 were recently described in detail, with an emphasis on morphometry and chaetotaxy of selected structures, by KONOPKO (2012a,b; 2013, in press). This contribution and the works made by KONOPKO (2012a,b; 2013, in press) analize the nymphal morphology of the genus Sigara based on a comparative approach.
The main objectives of this contribution are: to describe and illustrate, in detail the instars I and III - V of S. denseconscripta with an emphasis on morphometry and chaetotaxy of selected structures; and to establish the nymphal characters useful in identifying instars and species of Sigara.
MATERIAL AND METHODS
Material. Some of the adults (20 specimens: 10♂, 10♀) and the nymphs of S. denseconscripta (III - V) have been collected in the field, fixed, and preserved in 96% ethanol. Some of the adults (30 specimens: 15♂, 15♀) were reared in the laboratory, and only the eggs and the first instar were obtained in aquarium, then fixed and preserved in 96% ethanol. The material is held in the collection of the División de Entomología, Museo Argentino de Ciencias Naturales "Bernardino Rivadavia" (MACN), Buenos Aires, Argentina.
Rearing in aquarium. The adults from Reserva Laguna El Cristal, Santa Fe province, were reared in aquarium based on KONOPKO et al. (2011).
Taxonomic descriptions. The adults that were found in association with the nymphs were identified as S. denseconscripta based on the work of BACHMANN (1981). Besides, the chaetotaxy of the three pairs of legs on all surfaces was studied, starting from the adult, comparing the chaetotaxy of this stage with the chaetotaxy of all the nymphal instars, associating the nymphs with the adults and supporting the identification of the species. The taxonomic descriptions of the nymphs were performed using a stereomicroscope (at magnifications up to 144X). For each nymph only differences with the previous nymph description are emphasized. Some specimens were cleared in lactic acid for several days, dissected, and mounted on glass slides in polyvinyl-lacto-glycerol. Observations (at magnifications up to 1000X) and drawings were made using a compound microscope equipped with a drawing tube. Drawings were scanned and digitally edited.
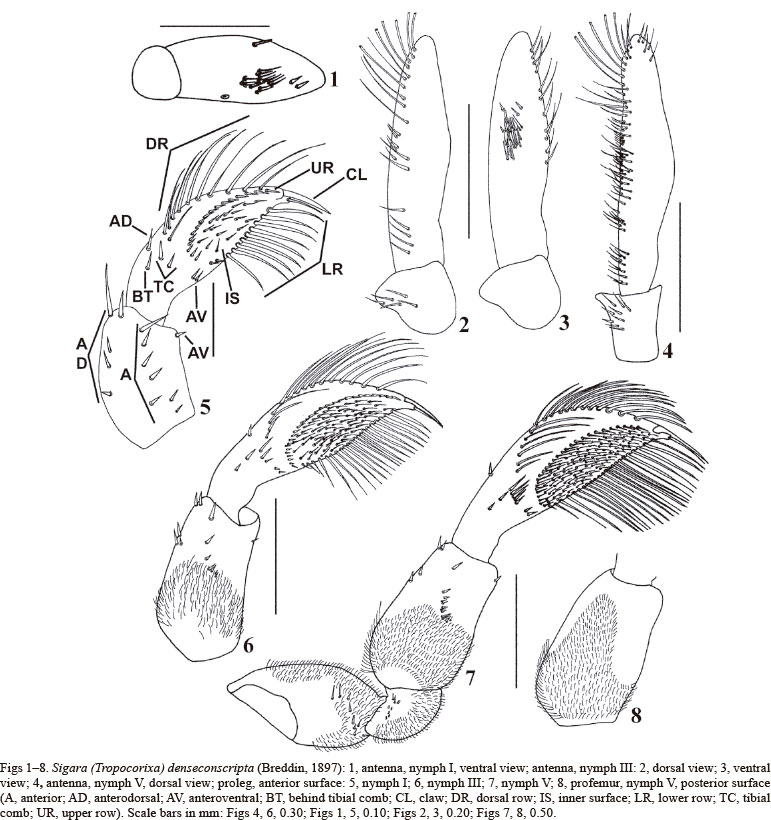
Morphometric and chaetotaxic analysis. Alcohol preserved material was observed in a Petri dish with 96% ethanol. Nymphal structures were measured utilizing a stereomicroscope equipped with a micrometric ocular. Paired structures of each individual were considered independently. The following measurements were taken: body length (BL); body width (BW); head length (HL); head width (HW); synthlipsis (S); width of an eye (eW); ocular index (OI); lengths of the antennal segments I (A1L) and II (A2L); width of the antennal segment II (A2W); length of antenna (AL); length of pterothorax (PL); length of pro-, meso- and metafemur (FE1L, FE2L, FE3L, respectively); length of protibiotarsus, meso- and metatibia (TITA1L, TI2L, TI3L, respectively); length of meso- and metatarsus (TA2L, TA3L, respectively); width of protibiotarsus, metatibia and metatarsus (TITA1W, TI3W, TA3W, respectively); length of the claw/s of the anterior legs, meso- and metalegs (CLL1, CL1L2/CL2L2, CL1L3/CL2L3, respectively); length of anterior legs, meso- and metalegs (L1L, L2L, L3L, respectively); distance between the scent gland openings on segments III, IV, and V (G3, G4, and G5, respectively); and scent gland openings diameter in segments III, IV, and V (D3, D4, and D5, respectively). The interpretation of the chaetotaxy of the legs follows KONOPKO et al. (2010b). The following material was measured: instars I and III - V, ten specimens each (Tab. I). The chaetotaxy of the following material was studied: instars I and III - V, ten specimens each. Figs 5, 9, 12 - 13 and 15 show the position of spines, setae and bristles on the legs.
RESULTS
Sigara (Tropocorixa) denseconscripta
(Breddin, 1897)
(Figs 1 - 16; Tabs I - III)
Examined material. ARGENTINA, La Rioja: Anillaco (1800 m a.s.l.), 14 specimens (instar III), 15 (IV), 12 (V), 2.IV.1998, M. Archangelsky coll.; Santa Fe: Reserva Laguna El Cristal, 9 specimens (instar I), 5.XII.2010, S. A. Mazzucconi col.; Arroyo El Rabón, 9 specimens (instar I), 2 (V), 6.XII.2010, S. A. Mazzucconi col.
First nymph (Figs 1, 5, 9, 11)
Color. Ground color testaceous with dark markings. Head testaceous with dark markings, except ecdysial line and ventral and lateral surfaces. Eyes reddish brown. Rostrum testaceous, darker apically. Antennae testaceous with pale setae. Pro-, meso- and metanotum testaceous with a dark medial marking growing towards the anterior, posterior and lateral margins of each segment. Thoracic pleurae and sterna testaceous. Legs testaceous; except metafemur and metatibia dorsoapically, and claws of the metalegs, darker. Abdominal terga I - VIII testaceous with dark markings; scent glands reddish brown on segments IV and V; abdominal sternites testaceous, except last segment with a middle rounded dark area. Connexiva testaceous with dark markings on anterior margin of each segment.
Body. Suboval, BL/BW: 1.27 - 1.35. Measurements are shown in Tab. I.
Head. Short, subrectangular, HL/HW: 0.31 - 0.34; anterior margin rounded; Y-shaped ecdysial line visible; with some long, stout and short, slender setae and trichobothria in frontal view. S/eW: 1.41 - 1.60. OI: 2.21 - 2.44. HW/BL: 0.52 - 0.54. Rostrum short, with three transverse sulcations. Antenna AL/BL: 0.14 - 0.17; two-segmented; segment II longer than segment I, A1:A2= 0.42 - 0.45:1.00; A1 subconical; A2 subcylindrical; A2 width/length: 0.36 - 0.42. A1 and A2 dorsal surface and A1 ventral surface bare (Fig. 1); A2 ventral surface with a medial campaniform sensillum, a set of short, slender spines, two short stout spines on the apical half of the segment, and a short stout spine on ventral margin (Fig. 1).
Thorax. Pronotum visible, subrectangular and short, anterior margin slightly concave, lateral margins convex, and posterior margin straight at middle. Mesonotum posterior margin convex at middle. Metanotum posterior margin straight at middle. Ecdysial line visible on pro-, meso-, and metanotum. Thoracic terga with few short, slender setae; meso- and metanotum with one lateral spine on both sides each. PL/BL: 0.26 - 0.29. Metaxyphus wider than long, rounded apically. Thoracic sterna with few short, slender setae. Meso- and metathoracic spiracles small and rounded, located ventrally, near posterior margin of each segment. Meso- and metathoracic wing pads absent. Prolegs short, L1/BL: 0.42 - 0.44; femur shorter than tibiotarsus, FE1/TITA: 0.69 - 0.74 (Fig. 5). Procoxa short, subconical; anterior surface with some short, apical spines; anterodorsal and anteroventral surfaces with chloride cells; posterior surface bare. Protrochanter short; anterior surface with two short setae, four basal campaniform sensilla, and two short spines; posterior surface with two short spines and two basal campaniform sensilla. Profemur short, stout and subrectangular (Fig. 5); anterior surface with seven spines [two short slender, four short stout (two single and two apically serrate), and one long stout]; anterodorsal surface with four or five spines (two apical ones longest), and chloride cells; anteroventral surface with one short apical spine; posterodorsal surface with one long slender seta. Protibiotarsus TITA width/length: 0.31 - 0.37 (Fig. 5); anterior surface with 10 - 12 long setae in dorsal, 18 - 19 setae in upper and 10 - 13 bristles in lower rows, tibial comb represented by two spines (one short and one long), and one or two spines behind the tibial comb; anterodorsal surface with one short spine; anteroventral surface with two short spines; inner surface with 16 - 20 slender setae; posterior surface with some long, slender setae; posterodorsal surface with four spines. One short, slender and falcate claw. Mesolegs long and slender; L2/BL: 0.96 - 1.05; femur longest, tarsus longer than tibia; FE2:TI2:TA2=1.00:0.47 - 0.51:0.50 - 0.57. Mesocoxa short, subconical; anterior surface with two short apical spines; anterodorsal surface with chloride cells; posterior surface bare. Mesotrochanter short; anterior surface with three basal campaniform sensilla, two short basal setae, and three short slender spines; posterior surface with three basal campaniform sensilla; posteroventral surface with three long slender spines. Mesofemur straight, subcylindrical (Fig. 9); anterodorsal surface with 10 - 11 short and long spines and chloride cells; anteroventral surface with seven or eight spines (three or four short and four long), and chloride cells (not illustrated); posterodorsal surface with chloride cells; posteroventral surface with five or six spines (two or three short and three long) and chloride cells. Mesotibia straight, subcylindrical (Figs 9, 11); anterior surface with four or five spines; anterodorsal surface with two long slender spines (one prebasal and one preapical); anteroventral surface with six spines; posterior surface with six spines (five short and one long), and six or seven long slender setae; posterodorsal surface with three spines; posteroventral surface with two spines and tibial comb represented by one long spine. Mesotarsus straight, subcylindrical (Figs 9, 11); anterodorsal surface with six or seven spines (one short and five or six long); anteroventral surface with seven or eight spines; posterior surface with five to seven spines; posterodorsal surface with seven long slender setae and four spines (three short and one long); posteroventral surface with one apical spine. Two long, slender, falcate claws of different length. TA2/CL1: 0.79 - 0.83; TA2/CL2: 0.75 - 0.77. Metalegs long, slender; L3/BL: 1.18 - 1.23; tarsus longest, tibia shorter than femur; FE3:TI3:TA3= 0.78 - 0.86:0.60 - 0.62:1.00. Metacoxa large, subconical; anterior surface with two spines (one short medial and one long apical); anterodorsal surface with chloride cells; posterior surface bare. Metatrochanter short; anterior surface with two short spines, two short, slender, basal setae, and three basal campaniform sensilla; posterior surface with two basal campaniform sensilla. Metafemur wide basally, narrow apically, slightly curved and flattened anteroposteriorly; anterior surface with four short spines in two areas (upper, one; lower, three); anterodorsal surface with six spines, and chloride cells; anteroventral surface with chloride cells; posterodorsal surface with two short apical spines; posteroventral surface with two short spines, and chloride cells. Metatibia wide apically, narrow basally, straight and subcylindrical; TI3 width/length: 0.26 - 0.36; anterodorsal surface with four to six spines; anteroventral surface with nine spines; posterior surface with six or seven spines (apical one longest), and seven long, slender setae; posterodorsal surface with eight to ten spines; posteroventral surface with five or six spines, and apical comb of one long spine. Metatarsus wide basally, narrow apically, margins straight to slightly curved apically and flattened anteroposteriorly; TA3 width/length: 0.13 - 0.16; anterior surface with nine or ten spines; anteroventral surface with 17 - 18 swimming hairs; posterodorsal surface with 18 - 19 spines, 23 - 26 swimming hairs and six long, slender setae; posteroventral surface with 15 - 17 spines. Two long, slender, straight claws of different length. TA3/CL1: 2.39 - 2.62; TA3/CL2: 2.36 - 2.50.
Abdomen. Posterior margins of the segments: I - VII concave medially (dorsal and ventrally), and VIII straight to slightly concave medially (dorsal and ventrally). G3/D3: 6 - 7, G4/D4: 9 - 11, and G5/D5: 9 - 10. Spiracles visible on segments I - VIII, small and rounded; located ventro-laterally near posterior margin (I) or ventrally near anterior margin (II - VIII) of each segment; the one on segment I is bigger than the others abdominal spiracles. Terga with few short, slender setae, and abundant chloride cells. Lateral spines of the abdominal segments (right side): I, zero; II - III, one short; IV, zero or one short, one long; V - VI, three short, one long; VII, four short, one long; VIII, three short, five or six long. Sterna with few short, slender setae, and chloride cells. Central spines on urosternites: I - IV: zero; V: two or three (zero or one short, two long); VI: three (zero or one short, two or three long); VII - VIII: two long.
Second nymph. No specimens were available for study.
Third nymph (Figs 2 - 3, 6, 10, 12, 13, 15)
Similar to first instar except for the following features:
Color. Posterior margin of head, pro- and mesonotum darker. Dark medial marking on metanotum with a pale eye shaped marking on each side of the notum, which join to a medial pale inverted triangle shaped marking near the posterior margin of the segment, forming between the three a single pale marking. Dark markings on abdominal terga expanding towards the anterior and posterior margins of each segment.
Body. Elongate, BL/BW: 1.72 - 1.75. Measurements are shown in Tab. I.
Head. HL/HW: 0.42 - 0.45; with chloride cells in frontal view; posterior margin with long, slender setae laterally, and short, apically serrate setae medially. S/eW: 1.24 - 1.75. OI: 1.45 - 2.53. HW/BL: 0.48 - 0.49. Rostrum with five transverse sulcations. Antenna AL/BL: 0.16 - 0.18; A1:A2=0.28 - 0.30:1.00; A1 with a lateral protuberance (Figs 2 - 3); A2 with a notch on the basal fifth of the segment (Figs 2, 3); A2 width/length: 0.24 - 0.26; A1 dorsal surface with short, slender setae (Fig. 2); A2 dorsal surface with short and long, slender setae, and long apically serrate setae (Fig. 2); A2 ventral surface with four short, stout spines on the apical half of the segment, and ventral margin with short, slender setae, and without the short stout spine (Fig. 3).
Thorax. Pronotum totally hidden by head in dorsal view; with a medial area of short slender setae, on each side of the notum, and posterior half with short, slender setae; posterior margin with long, slender setae (not exposed). Meso- and metathoracic wing pads present. Meso-, metathoracic wing pads and the anterior half of the mesonotum with abundant short, slender setae; metanotum with abundant short, slender setae, more scattered than on mesonotum, and chloride cells in two areas, on each side of the notum; posterior margin of the setose area of the mesonotum and internal margins of the wing pads with short and long lanceolate setae, respectively; posterior and external margins of the wing pads with long, slender setae. PL/BL: 0.27 - 0.29. Pro-, meso- and metapleura with short, slender setae. Meso- and metathoracic spiracles elongate; the metathoracic ones smaller than the ones of the mesothorax, and longer than the ones of the abdomen. Mesothoracic wing pads covering less than the anterior half of the pterothorax, along midline; metathoracic wing pads reaching the posterior margin of urotergite I. Prolegs L1/BL: 0.39 - 0.40; FE1/TITA: 0.71 - 0.79. Procoxa anterior surface with abundant short, slender setae, and some short apical spines; anterodorsal and anteroventral surfaces without chloride cells. Protrochanter anterior surface with abundant short, slender setae; posterior surface with three basal campaniform sensilla and abundant short, slender setae. Profemur anterior surface with an hydrophobic setose area covering the basal half of the segment (Fig. 6); anterodorsal surface with five spines (two apical); posterior surface with an hydrophobic setose area covering the basal 2/3 of the segment. Protibiotarsus TITA width/length: 0.33; anterior surface with 15 - 16 long setae in dorsal, 28 - 29 setae in upper and 21 - 22 bristles in lower rows, tibial comb represented by three spines, and two spines behind the tibial comb; inner surface with 62 - 64 slender setae (Fig. 6). Mesolegs L2/BL: 0.92 - 0.95 (Figs 10, 12); tibia longest than tarsus, FE2:TI2:TA2=1.00:0.47 - 0.49:0.41 - 0.44. Mesocoxa anterior and posterior surfaces with abundant short, slender setae; anterodorsal surface without chloride cells. Mesotrochanter anterior and posterior surfaces with four and five basal campaniform sensilla, respectively, and abundant short, slender setae. Mesofemur anterior surface with basal hydrophobic setose area of short slender setae; anterodorsal surface with 23 - 24 spines; anteroventral surface with 38 - 50 spines (eight to ten long, single; 17 - 25 short, single; 10 - 19 short, apically serrate); posterior surface with a transverse basal row of short slender setae; posterodorsal surface with two short spines; posteroventral surface with seven or eight long slender setae, five or six long spines and 30 - 35 short spines. Mesotibia anterior surface with 10 - 11spines; anterodorsal surface with two short spines; anteroventral surface with 12 - 14 spines; posterior surface with five or six spines, and 26 - 27 long, slender setae; posteroventral surface with seven to ten spines (two or three single and four to seven apically serrate), and apical comb of three spines. Mesotarsus anterodorsal and posterior surfaces with six spines each; posterodorsal surface with 22 - 25 long, slender setae and four or five spines; posteroventral surface with eight to 11 spines. TA2/CL1: 0.89 - 1.05; TA2/CL2: 0.82 - 1.00. Metalegs L3/BL: 1.07 - 1.09 (Figs 13, 15); FE3:TI3:TA3=0.73 - 0.77:0.63 - 0.66:1.00. Metacoxa anterior surface with abundant short slender setae, and a prebasal set of short and stout spines; posterior surface with abundant short slender setae, and long apical spines; anterodorsal surface without chloride cells. Metatrochanter anterior and posterior surfaces with four and five basal campaniform sensilla, respectively, and abundant short slender setae; anteroventral surface with three long slender spines. Metafemur anterior surface with an hydrophobic setose area reaching the basal half of the segment on dorsal margin, and five short spines in three areas (upper, one; middle, three; lower, one); anterodorsal surface with four or five spines and without chloride cells; anteroventral surface without chloride cells; posterior surface with transverse basal row of long slender setae; posteroventral surface with three short spines and without chloride cells; ventral surface with four to six long slender preapical setae. Metatibia TI3 width/length: 0.20 - 0.24; anterodorsal surface with seven to nine spines; anteroventral surface with 12 - 14 spines and 26 - 27 long, slender setae; posterior surface with six spines, four or five short, slender setae, and 15 - 16 long, slender setae; posterodorsal surface with 12 spines and seven short, slender setae; posteroventral surface with 16 - 19 spines (apical one longest and apically serrate), and tibial comb of four spines. Metatarsus TA3 width/length: 0.15 - 0.18; anteroventral surface with 21 - 22 spines and 218 - 223 swimming hairs; posterodorsal surface with 29 - 31 spines, nine to 12 long slender setae and 285 - 290 swimming hairs; posteroventral surface with 30 - 36 spines. TA3/CL1: 6.01 - 7.36; TA3/CL2: 5.73 - 6.20.
Abdomen. G3/D3 and G4/D4: 7, G5/D5: 6. Terga with abundant short, slender setae, and more chloride cells than on previous instar. Lateral spines of the abdominal segments (right side): I, zero; II - III, one short; IV, four short, one long; V, six or seven short, three or four long; VI, five or six short, three or four long; VII, nine or ten short, three to five long; VIII, eight short, seven long. Sterna with abundant short and slender setae, some long, and without chloride cells. Urosternites: I - VIII without central spines.
Fourth nymph
Similar to third instar except for the following features:
Color. Abdominal terga I - II and VIII with one pale marking and III - VII with a pale marking on each side of the notum.
Body. BL/BW: 1.91 - 1.99. Measurements are shown in Table I.
Head. HL/HW: 0.39 - 0.47. S/eW: 1.17 - 1.50. OI: 1.35 - 1.94. HW/BL: 0.42 - 0.44. Rostrum with six transverse sulcations. Antenna AL/BL: 0.16 - 0.17; A1:A2= 0.27 - 0.32:1.00; A2 width/length: 0.21 - 0.25; A2 ventral surface with eight short, stout spines on the apical half of the segment.
Thorax. Mesonotum short, lanceolate setae on posterior margin of the setose area, reaching the anterior margin of the metanotum medially. PL/BL: 0.28 - 0.29. Mesothoracic wing pads reaching the anterior half of the urotergite I; metathoracic wing pads reaching the anterior margin of the urotergite II. Prolegs L1/BL: 0.34 - 0.35; FE1/TITA: 0.70 - 0.77. Procoxa posterior surface with abundant short and slender setae. Protrochanter anterior and posterior surfaces with five and seven basal campaniform sensilla, respectively. Profemur anterior surface with 10 - 12 spines [three short, slender, four to five short, stout, and three long, stout (one single and two apically serrate)]; anterodorsal surface with four spines (two apical); posterior surface with an hydrophobic setose area covering the basal 3/4 of the segment. Protibiotarsus TITA width/length: 0.32 - 0.33; anterior surface with 18 - 20 long setae in dorsal, 33 - 35 setae in upper and 23 - 26 bristles in lower rows, tibial comb represented by four spines (two short and two long); inner surface with 98 - 104 long, slender setae. Mesolegs L2/BL: 0.89 - 0.92; FE2:TI2:TA2=1.00:0.45 - 0.49:0.36 - 0.40. Mesotrochanter anterior and posterior surfaces with five and seven basal campaniform sensilla, respectively. Mesofemur anterodorsal surface with 31 - 34 spines, and without chloride cells; anteroventral surface with 72 - 73 spines (six long single, 32 - 34 short single, and 32 - 35 short apically serrate) and without chloride cells; posteroventral surface with 23 - 27 long, slender setae, and 50 - 52 short spines. Mesotibia anterior surface with 13 - 14 spines; anteroventral surface with 13 - 15 spines; posterior surface with six spines, and 31 - 40 long, slender setae; posteroventral surface with 14 - 18 spines (five single and nine to 13 apically serrate), and apical comb of five spines. Mesotarsus anterodorsal surface with six or seven spines; anteroventral surface with 10 - 12 spines; posterior surface with seven or eight spines; posterodorsal surface with 36 - 40 long, slender setae. TA2/CL1: 0.84 - 0.90; TA2/CL2: 0.83 - 0.89. Metalegs L3/BL: 0.95 - 0.99; FE3:TI3:TA3=0.73 - 0.74:0.65 - 0.68:1.00. Metatrochanter anteroventral surface with two or three long, slender spines; posterior surface with seven basal campaniform sensilla. Metafemur anterior surface with 11 - 13 short spines in three areas (upper, one or two; middle, seven; lower, three or four); anterodorsal surface with four spines; ventral surface with six or seven long slender preapical setae. Metatibia TI3 width/length: 0.22 - 0.25; anterodorsal surface with nine or ten spines; anteroventral surface with 17 spines and 39 - 42 long, slender setae; posterior surface with five spines, four short, slender setae, and 35 long, slender setae; posterodorsal surface with 16 spines and 8 short slender setae; posteroventral surface with 26 spines, and tibial comb of six spines. Metatarsus TA3 width/length: 0.17 - 0.19; anteroventral surface with 290 - 295 swimming hairs and 26 - 27 spines; posterodorsal surface with 41 - 45 spines, 11 - 12 long slender setae and 310 - 314 swimming hairs; posteroventral surface with 41 - 42 spines. TA3/CL1: 7.54 - 9.70; TA3/CL2: 7.36 - 8.63.
Abdomen. Scent gland openings of segment III not distinct. G4/D4: 6 - 7 and G5/D5: 5 - 7. Lateral spines of the abdominal segments (right side): I, zero; II - III, one short; IV, six short, two long; V, nine short, four or five long; VI, seven or eight short, four or five long; VII, 21 - 24 short, four or five long; VIII, 10 - 13 short, seven long.
Fifth nymph (Figs 4, 7 - 8, 14, 16)
Similar to fourth instar except for the following features:
Color. Posterior margin of metathoracic wing pads darker. Mesotibia basally, mesotarsus and metafemur apically, and metatibia basal and apically, darker.
Body. BL/BW: 2.09 - 2.13. Measurements are shown in Tab. I.
Head. HL/HW: 0.39 - 0.40; with more chloride cells in frontal view than on previous instar. S/eW: 0.83 - 0.97. OI: 0.97 - 1.19. HW/BL: 0.40 - 0.45. Rostrum with seven transverse sulcations. Antenna AL/BL: 0.14 - 0.15; A1:A2=0.25 - 0.26:1.00; A2 width/length: 0.19 - 0.21; A2 dorsal surface with abundant short and long slender setae, and some long apically serrate setae (Fig. 4); A2 ventral surface with 13 - 16 short stout spines, on the apical half of the segment, and ventral margin with abundant long, slender setae.
Thorax. Mesonotum short lanceolate setae reaching and covering the anterior margin of the metanotum medially in a T shape. Posterior margin of the mesothoracic wing pads with long slender setae, long lanceolate setae, and long apically serrate setae. PL/BL: 0.27 - 0.28. Mesothoracic wing pads covering the anterior 2/3 of the urotergite III; metathoracic wing pads covering the anterior half of the urotergite III. Prolegs L1/BL: 0.30 - 0.32; FE1/TITA: 0.69 - 0.74 (Figs 7 - 8). Protrochanter anterior and posterior surfaces with six and nine basal campaniform sensilla, respectively. Profemur anterior surface with 14 - 16 spines [four short slender, seven to nine short stout, three long stout (one single and two apically serrate)]; posterior surface with an hydrophobic setose area covering the basal 5/6 of the segment (Fig. 8). Protibiotarsus TITA width/length: 0.32 - 0.39; anterior surface with 16 - 18 long setae in dorsal, 34 - 37 setae in upper and 23 - 24 bristles in lower rows, tibial comb represented by five spines (two short and three long); inner surface with 150 - 154 long slender setae. Mesolegs L2/BL: 0.84 - 0.87; FE2:TI2:TA2=1.00:0.47 - 0.48:0.35 - 0.36. Mesotrochanter anterior and posterior surfaces with six and nine basal campaniform sensilla, respectively. Mesofemur anterodorsal surface with 48 - 50 spines; anteroventral surface with 89 - 91 spines (six to seven long single, 38 short single, and 45 - 46 short apically serrate); posteroventral surface with 72 - 78 short spines, five long spines and 35 - 39 long slender setae. Mesotibia anteroventral surface with 15 - 16 spines; posterior surface with five to seven spines, and 59 - 62 long slender setae; posteroventral surface with 34 - 36 spines (five single, 29 - 31 apically serrate), and apical comb of five or six spines. Mesotarsus anterodorsal surface with six spines; anteroventral surface with ten spines; posterior surface with six to seven spines; posterodorsal surface with four spines, and 57 - 60 long slender setae. TA2/CL1: 0.79 - 0.85; TA2/CL2: 0.77 - 0.83. Metalegs L3/BL: 0.94 - 0.98; FE3:TI3:TA3= 0.67 - 0.71:0.63 - 0.66:1.00 (Figs 14, 16). Metatrochanter anterior and posterior surfaces with seven and nine basal campaniform sensilla, respectively; anteroventral surface with two to four long slender spines. Metafemur anterior surface with 18 - 21 short spines in three areas (upper, one to three; middle, ten; lower, seven or eight); anterodorsal surface with four to six spines; posteroventral surface with three to five spines. Metatibia TI3 width/length: 0.18 - 0.20; anterodorsal surface with 10 - 12 spines; anteroventral surface with 27 - 29 spines, and 49 - 50 long, slender setae; posterior surface with six spines, and 52 - 54 long slender setae; posterodorsal surface with nine or ten short slender setae; posteroventral surface with 29 - 30 spines, and tibial comb of eight spines. Metatarsus TA3 width/length: 0.15 - 0.18; anterior surface with 10 - 12 spines; anteroventral surface with 34 - 36 spines, and 385 - 390 swimming hairs; posterodorsal surface with 480 - 486 swimming hairs, 14 - 15 long slender setae, and 46 - 48 spines; posteroventral surface with 40 - 43 spines. TA3/CL1: 11.41 - 11.71; TA3/CL2: 11.12 - 11.41.
Abdomen. G4/D4: 5 and G5/D5: 5 - 6. Lateral spines of the abdominal segments (right side): I, zero; II - III, one short; IV, five short, two long; V, seven short, five long; VI, six or seven short, five or six long; VII, 17 - 18 short, nine or ten long; VIII, 10 - 12 short, seven or eight long.
DISCUSSION
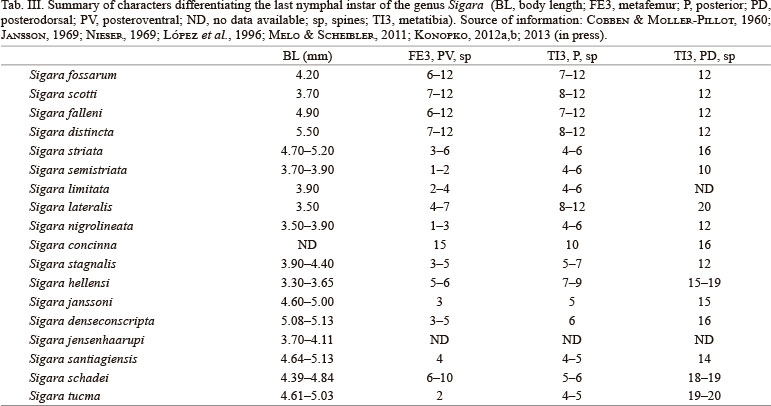
The main characters separating the nymphal instars I and III - V of Sigara denseconscripta are (Tabs I, II): the body, head, antenna, pterothorax and legs lengths; the body, head, and eye widths; the number of transverse sulcations of the rostrum; the number of campaniform sensilla on posterior surface of the protrochanter; the number of setae on the inner surface of the protibiotarsus; number of spines on the tibial comb of the protibiotarsus and metatibia; the number of campaniform sensilla on anterior and posterior surfaces of the mesotrochanter; the number of spines on anterodorsal and anterior surfaces of the meso- and metafemora, respectively; the number of long, slender setae on posterior and posterodorsal surfaces of the mesotibia and mesotarsus, respectively; the number of spines on the posteroventral surface of the mesotibia (single and apically serrate); the number of campaniform sensilla on posterior surface of the metatrochanter; the number of spines and long, slender setae on the anteroventral surface, and the number of long setae on the posterior surface of the metatibia; the number of swimming hairs on anteroventral and posterodorsal surfaces of the metatarsus; and the grade of development of the wing pads. In addition, the nymphs of Sigara denseconscripta can be separated on the basis of the presence (instars III - V) or the absence (instar I) of: a basal hydrophobic setose area on the anterior and posterior surfaces of the profemur, and on the anterior surfaces of the meso- and metafemora; a transversal basal row of long slender setae on posterior surfaces of the meso- and metafemora; and a prebasal set of short and stout spines on the anterior surface of the metacoxa (Tab. II). Besides, the last three nymphal instars of S. denseconscripta can be distinguished by the coverage of the basal hydrophobic setose area on posterior surface of the profemur (2/3, instar III; 3/4, IV; 5/6, V).
The last three nymphal instars of Sigara denseconscripta, S. santiagiensis, S. schadei, and S. tucma can be separated from each other based on: the hydrophobic setose area on anterior surface of the profemur covering the basal half of the segment (S. denseconscripta, S. santiagiensis and S. tucma) or more than the basal half (S. schadei); the hydrophobic setose area on posterior surface of the profemur covering the basal half of the segment (S. santiagiensis) or more than the basal half (S. denseconscripta, S. schadei and S. tucma); the number of long setae on anterior surface in dorsal row of the protibiotarsus (III: S. tucma and S. santiagiensis, nine, S. schadei, 11 - 12, S. denseconscripta, 15 - 16; IV: S. tucma eight to nine, S. santiagiensis, nine, S. schadei, 13, S. denseconscripta, 18 - 20; V: S. tucma, eight to nine, S. santiagiensis, 10, S. schadei, 12 - 13, S. denseconscripta, 16 - 18); the hydrophobic setose area on anterior surface of the metafemur covering the basal half of the segment (S. denseconscripta, S. schadei and S. tucma) or more than the basal half (S. santiagiensis); the number of spines on posteroventral surface of the metafemur (III: S. tucma, two, S. denseconscripta, three, S. santiagiensis, four to five, S. schadei, seven to eight; IV: S. tucma, two, S. denseconscripta, three, S. santiagiensis, five, S. schadei, four; V: S. tucma, two, S. denseconscripta, three to five, S. santiagiensis, four, S. schadei, six to 10); the number of swimming hairs on anteroventral surface of the metatarsus (III: S. tucma, 173 - 177, S. santiagiensis, 106 - 108, S. schadei, 198 - 200, S. denseconscripta, 218 - 223; IV: S. tucma, 321 - 328, S. santiagiensis, 260 - 265, S. schadei, 210 - 214, S. denseconscripta, 290 - 295; V: S. tucma, 540 - 550, S. santiagiensis, 344 - 348, S. schadei, 260 - 265, S. denseconscripta, 385 - 390); the shape of the metaxyphus (longer than wide and apically truncate in S. tucma, and wider than long and apically rounded in S. denseconscripta, S. santiagiensis and S. schadei); and the presence/absence of central spines on the urosternite V in nymphs III (two long spines in S. santiagiensis; and without spines in S. denseconscripta, S. schadei and S. tucma) (KONOPKO, 2012a,b; 2013, in press).
The chaetotaxy of the mesonotum, metafemur, and metatibia distinguish group of species belonging to the last nymphal instar of the genus Sigara (Tab. III): the body length [bigger in Sigara (Subsigara) distincta (Fieber, 1848) and smaller in S. (Sigara) hellensi (Sahlberg, 1819]; short, lanceolate setae on posterior margin of the setose area of the mesonotum reaching the anterior margin of the metanotum medially, covering the segment in a narrow T shape [S. (Subsigara) fossarum (Leach, 1817), S. (Subsigara) scotti (Douglas & Scott, 1868), S. (Subsigara) falleni (Fieber, 1848), S. distincta, S. (Sigara) striata (Linnaeus, 1758), S. (Sigara) semistriata (Fieber, 1848), S. hellensi, S. (Sigara) janssoni Lucas, 1983, S. (Tropocorixa) denseconscripta, S. (T.) santiagiensis, S. (T.) schadei and S. (Aphelosigara) tucma] or in a V shape [S. (Sigara) limitata (Fieber, 1848), S. (Sigara) lateralis (Leach, 1817), S. (Sigara) nigrolineata (Fieber, 1848), S. (Sigara) concinna (Fieber, 1848) and S. (Sigara) stagnalis (Leach, 1817)]; number of spines on posteroventral surface of the metafemur (one to two in S. limitata, S. semistriata and S. tucma; 15 in S. concinna); number of spines on posterior surface of the metatibia (four to five in S. striata, S. semistriata, S. limitata, S. nigrolineata, S. stagnalis, S. janssoni, S. santiagiensis, S. schadei and S. tucma; 8-12 in S. scotti, S. distincta and S. lateralis); and number of spines on posterodorsal surface of the metatibia (10 - 12 in S. fossarum, S. scotti, S. falleni, S. distincta, S. semistriata, S. nigrolineata and S. stagnalis; 14 - 20 in S. striata, S. lateralis, S. concinna, S. hellensi, S. janssoni, S. denseconscripta, S. santiagiensis, S. schadei and S. tucma) (COBBEN & MOLLER-PILLOT, 1960; JANSSON, 1969; NIESER, 1969; LÓPEZ et al., 1996; KONOPKO, 2012a,b; 2013, in press).
Nymphs of species of Sigara are still unknown, and therefore future studies should focus on providing detailed descriptions and re-descriptions (including chaetotaxy) of the nymphs of this genus. Besides, future works should focus on providing useful morphological data, which with molecular data, will improve the resolution of the analyses of the phylogeny of the Corixidae.
Acknowledgements
I am deeply grateful to Miguel Archangelsky and Silvia Mazzucconi for the donation of the material. Field and laboratory work were supported by a postgraduate scholarship from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina.
REFERENCES
BACHMANN, A. O. 1960. Notas sobre Corixidae (Hemiptera). Revista de la Sociedad Entomológica Argentina 22(1959):34-40.
_____. 1961. Notas sobre Corixidae (Hemiptera) (2ª serie). Neotropica 7:19-24.
_____. 1962a. Notas sobre Corixidae (Tercera serie). Acta Zoologica Lilloana 18:139-145.
_____. 1962b. Catálogo de las Corixidae de la República Argentina (Insecta, Hemiptera). Neotropica 8(25):15-25.
_____. 1962c. Clave para la determinación de las subfamilias, géneros y especies de las Corixidae de la República Argentina (Insecta, Hemipt.). Physis 23(64):21-25.
_____. 1962d. Apuntes para una hidrobiología argentina. V. Los hemípteros acuáticos de los parques nacionales Lanín, Nahuel Huapi y Los Alerces y zonas vecinas (Insecta - Hemipt.). Physis 23(64):103-107.
_____.1963. Apuntes para una hidrobiología argentina. VI. Los Hemiptera Cryptocerata de la Patagonia extracordillerana. Physis 24(67):35-37.
_____.1966. Presencia de Sigara (Tropocorixa) hungerfordi Jaczewski en la República Argentina (Hemiptera, Corixidae). Revista de la Sociedad Entomológica Argentina 28:44.
_____.1979. Notas para una monografía de las Corixidae argentinas (Insecta, Heteroptera). Acta Zoologica Lilloana 35(1):305-350.
_____. 1981. Insecta Hemiptera Corixidae. Fauna de Agua Dulce de la República Argentina 35(2):1-270.
_____.1987. Notas sobre Corixidae (Heteroptera) (Quinta serie). Revista de la Sociedad Entomológica Argentina 44(1):33-36.
COBBEN, R. H. & MOLLER-PILLOT, H. 1960. The larvae of Corixidae and an attempt to key the last larval instar of the Dutch species (Hemip., Heteroptera). Hydrobiologia 16(4):323-356.
HUNGERFORD, H. B. 1948. The Corixidae of the Western Hemisphere (Hemiptera). The University of Kansas Science Bulletin 32:1-827.
HUTCHINSON, G. E. 1940. A revision of the Corixidae of India and adjacent regions. Transactions of the Connecticut Academy of Arts and Sciences 33:339-476.
JANSSON, A. 1969. Identification of larval Corixidae (Heteroptera) of Northern Europe. Annales Zoologici Fennici 6:289-312.
KONOPKO, S. A. 2012a. Description of the immature stages of Sigara (Tropocorixa) schadei (Hungerford) (Hemiptera: Heteroptera: Corixidae). Zootaxa 3487:41-57.
_____. 2012b. Description of the immature stages of Sigara (Tropocorixa) santiagiensis (Hungerford, 1928) (Insecta: Heteroptera: Corixidae). Journal of Natural History 47(29-30):1959-1982. Available at: <http://dx.doi.org/10.1080/00222933.2012.763066>. Accessed on: 7 July 2013.
_____. 2013. The immature stages of Sigara (Aphelosigara)tucma Bachmann, 1961 (Hemiptera: Heteroptera: Corixidae). Journal of Insect Science (in press).
KONOPKO, S. A.; MAZZUCCONI, S. A. & BACHMANN, A. O. 2010a. Description of the nymphs of Tenagobia (Incertagobia) incerta Lundblad 1929 and Tenagobia (Schadeogobia) schadei Lundblad 1929 (Hemiptera: Heteroptera: Micronectidae), with emphasis on morphometry and chaetotaxy. Zootaxa 2511:39-58.
_____. 2010b. Description of the nymphs of Ectemnostega (Ectemnostegella) stridulata (Hungerford 1948) (Hemiptera: Heteroptera: Corixidae). Zootaxa 2639:19-34.
_____. 2011. Description of the immature stages of Trichocorixa mendozana Jaczewski (Hemiptera: Heteroptera: Corixidae). Zootaxa 3060:47-61.
LÓPEZ, T.; COSTAS, M. & VÁZQUEZ, M. A. 1996. Phenology and immature stages of Sigara (Sigara) janssoni Lucas, 1983 (Heteroptera: Corixidae). Boletín de la Asociación Española de Entomología 20(3-4):19-29.
MELO, M. C. & SCHEIBLER, E. E. 2011. Description of the immature stages of Sigara (Tropocrixa) jensenhaarupi (Hemiptera: Heteroptera: Corixidae: Corixini), with ecological notes. Revista Mexicana de Biodiversidad 82:117-130.
MORRONE, J. J.; MAZZUCCONI, S. A. & BACHMANN, A. O. 2004. Distributional patterns of Chacoan water bugs (Heteroptera: Belostomatidae, Corixidae, Micronectidae and Gerridae). Hydrobiologia 523:159-173.
NIESER, N. 1969. The Heteroptera of the Netherlands Antilles - VII Corixidae. Studies on the Fauna of Curacao and other Caribbean Islands 28(107):135-164.
Received 8 July 2013
Accepted 5 September 2013
- BACHMANN, A. O. 1960. Notas sobre Corixidae (Hemiptera). Revista de la Sociedad Entomológica Argentina 22(1959):34-40.
- _____. 1961. Notas sobre Corixidae (Hemiptera) (2ª serie). Neotropica 7:19-24.
- _____. 1962a. Notas sobre Corixidae (Tercera serie). Acta Zoologica Lilloana 18:139-145.
- _____. 1962b. Catálogo de las Corixidae de la República Argentina (Insecta, Hemiptera). Neotropica 8(25):15-25.
- _____. 1962c. Clave para la determinación de las subfamilias, géneros y especies de las Corixidae de la República Argentina (Insecta, Hemipt.). Physis 23(64):21-25.
- _____. 1962d. Apuntes para una hidrobiología argentina. V. Los hemípteros acuáticos de los parques nacionales Lanín, Nahuel Huapi y Los Alerces y zonas vecinas (Insecta - Hemipt.). Physis 23(64):103-107.
- _____.1963. Apuntes para una hidrobiología argentina. VI. Los Hemiptera Cryptocerata de la Patagonia extracordillerana. Physis 24(67):35-37.
- _____.1966. Presencia de Sigara (Tropocorixa) hungerfordi Jaczewski en la República Argentina (Hemiptera, Corixidae). Revista de la Sociedad Entomológica Argentina 28:44.
- _____.1979. Notas para una monografía de las Corixidae argentinas (Insecta, Heteroptera). Acta Zoologica Lilloana 35(1):305-350.
- _____. 1981. Insecta Hemiptera Corixidae. Fauna de Agua Dulce de la República Argentina 35(2):1-270.
- _____.1987. Notas sobre Corixidae (Heteroptera) (Quinta serie). Revista de la Sociedad Entomológica Argentina 44(1):33-36.
- COBBEN, R. H. & MOLLER-PILLOT, H. 1960. The larvae of Corixidae and an attempt to key the last larval instar of the Dutch species (Hemip., Heteroptera). Hydrobiologia 16(4):323-356.
- HUNGERFORD, H. B. 1948. The Corixidae of the Western Hemisphere (Hemiptera). The University of Kansas Science Bulletin 32:1-827.
- HUTCHINSON, G. E. 1940. A revision of the Corixidae of India and adjacent regions. Transactions of the Connecticut Academy of Arts and Sciences 33:339-476.
- JANSSON, A. 1969. Identification of larval Corixidae (Heteroptera) of Northern Europe. Annales Zoologici Fennici 6:289-312.
- KONOPKO, S. A. 2012a. Description of the immature stages of Sigara (Tropocorixa) schadei (Hungerford) (Hemiptera: Heteroptera: Corixidae). Zootaxa 3487:41-57.
- _____. 2012b. Description of the immature stages of Sigara (Tropocorixa) santiagiensis (Hungerford, 1928) (Insecta: Heteroptera: Corixidae). Journal of Natural History 47(29-30):1959-1982. Available at: <http://dx.doi.org/10.1080/00222933.2012.763066>. Accessed on: 7 July 2013.
- _____. 2013. The immature stages of Sigara (Aphelosigara)tucma Bachmann, 1961 (Hemiptera: Heteroptera: Corixidae). Journal of Insect Science (in press).
- KONOPKO, S. A.; MAZZUCCONI, S. A. & BACHMANN, A. O. 2010a. Description of the nymphs of Tenagobia (Incertagobia) incerta Lundblad 1929 and Tenagobia (Schadeogobia) schadei Lundblad 1929 (Hemiptera: Heteroptera: Micronectidae), with emphasis on morphometry and chaetotaxy. Zootaxa 2511:39-58.
- _____. 2010b. Description of the nymphs of Ectemnostega (Ectemnostegella) stridulata (Hungerford 1948) (Hemiptera: Heteroptera: Corixidae). Zootaxa 2639:19-34.
- _____. 2011. Description of the immature stages of Trichocorixa mendozana Jaczewski (Hemiptera: Heteroptera: Corixidae). Zootaxa 3060:47-61.
- LÓPEZ, T.; COSTAS, M. & VÁZQUEZ, M. A. 1996. Phenology and immature stages of Sigara (Sigara) janssoni Lucas, 1983 (Heteroptera: Corixidae). Boletín de la Asociación Española de Entomología 20(3-4):19-29.
- MELO, M. C. & SCHEIBLER, E. E. 2011. Description of the immature stages of Sigara (Tropocrixa) jensenhaarupi (Hemiptera: Heteroptera: Corixidae: Corixini), with ecological notes. Revista Mexicana de Biodiversidad 82:117-130.
- MORRONE, J. J.; MAZZUCCONI, S. A. & BACHMANN, A. O. 2004. Distributional patterns of Chacoan water bugs (Heteroptera: Belostomatidae, Corixidae, Micronectidae and Gerridae). Hydrobiologia 523:159-173.
- NIESER, N. 1969. The Heteroptera of the Netherlands Antilles - VII Corixidae. Studies on the Fauna of Curacao and other Caribbean Islands 28(107):135-164.
The nymphs of Sigara (Tropocorixa) denseconscripta (Hemiptera, Heteroptera, Corixidae)
Publication Dates
-
Publication in this collection
18 Nov 2013 -
Date of issue
Sept 2013
History
-
Received
08 July 2013 -
Accepted
05 Sept 2013