Abstracts
The construction of reservoirs is considered an important source of impacts on the fish fauna, severely altering the structure of the assemblage. This paper aimed to describe the structure of the fish assemblage of the Goioerê River, determining its longitudinal distribution and patterns of species dominance. The evaluation of its longitudinal variation in the diversity and abundance of the fish assemblage was conducted in July and October 2004 and January and May 2005. The collections were carried out near the headwaters (Gurucaia), middle stretch (Olaria), just above the falls (Paiquerê) and downstream (Foz). Forty-four species were captured. The Gurucaia fish assemblages differed significantly from Olaria, Paiquerê and Foz. The Olaria assemblages differed significantly from the Foz. Gurucaia showed the lowest diversity and abundance of species. Astyanax aff paranae Eigenmann,1914 (78% of the total) was found to be dominant at this site. Almost the same species richness was found at Olaria and Paiquerê, although Olaria had the greatest abundance of individuals. Astyanax aff paranae, Cyphocharax modestus (Fernández-Yépez, 1948) and Astyanax altiparanae Garutti & Britski, 2000 were the top three dominants and comprised over 71% of the total number of fish caught. At Paiquerê, Astyanax altiparanae, Hypostomus aff ancistroides (Ihering, 1911) and Loricariichthys platymetopon Isbrücker & Nijssen, 1979 composed 58% of the catches. Thirty-one species were recorded at Foz, which presented the greatest richness. The most abundant species were Apareiodon affinis (Steindachner, 1879), Galeocharax knerii (Steindachner, 1879) and A.altiparanae, which contributed to 50% of the total catches in this environment.These results record the fish biodiversity and how the community is longitudinally structured in the Goioerê River, and also demonstrate how this type of evaluation is important to understanding the fish community patterns and finding solutions to problems related to the conservation and management of the basin.
Diversity; fish community; richness; evenness; Goioerê River basin
A construção de reservatórios é considerada importante fonte de impactos para a fauna de peixes, levando a alterações significativas na estrutura da assembleia. Considerando isso, esse artigo objetiva descrever a estrutura da assembleia de peixes do rio Goioerê, determinando sua distribuição longitudinal e padrões na dominância das espécies. A avaliação da variação longitudinal na diversidade e abundância da assembleia de peixes neste rio foi conduzida em julho e outubro de 2004 e janeiro e maio de 2005. As coletas foram realizadas próximas as nascentes (Gurucaia), no segmento médio (Olaria), logo acima do salto Paiquerê (Paiquerê) e na foz do rio (Foz). Foram registradas 44 espécies. A assembleia de peixes de Gurucaia diferiu significativamente de Olaria, Paiquerê e Foz. A assembleia de Olaria diferiu significativamente de Foz. Gurucaia apresentou a menor diversidade e abundância de espécies. Astyanax aff. paranae Eigenmann,1914 (78% do total) foi dominante nesta localidade. Em Olaria e Paiquerê foi encontrada grande similaridade na riqueza de espécies, no entanto, em Olaria registrou-se a maior abundância de indivíduos. Astyanax aff. paranae, Cyphocharax modestus (Fernández-Yépez, 1948) and Astyanax altiparanae Garutti & Britski, 2000 foram as três espécies dominantes de topo e compreenderam cerca de 71% do total de peixes capturados. Na localidade Paiquerê, A. altiparanae, Hypostomus aff. ancistroides (Ihering, 1911) and Loricariichthys platymetopon Isbrücker & Nijssen, 1979 compuseram 58% das capturas. Trinta e uma espécies foram registradas na Foz, que apresentou a maior riqueza. As espécies mais abundantes foram Apareiodon affinis (Steindachner, 1879), Galeocharax knerii (Steindachner, 1879) and A. altiparanae, as quais contribuíram com 50% das capturas totais neste ambiente. Estes resultados registram a biodiversidade de peixes e como esta comunidade se estrutura longitudinalmente no rio Goioerê, além de demonstrar a importância desse tipo de avaliação para a compreensão dos padrões das comunidades de peixes e para a busca de soluções para problemas relacionados à conservação e o manejo desta bacia.
Diversidade; comunidade de peixes; riqueza; equitabilidade; bacia rio Goioerê
Claudenice Dei TosI; Luiz C. GomesI; Maria A. RodriguesII
IUniversidade Estadual de Maringá, Nupélia-DBI, Av. Colombo, 5790, Bloco H-90, Sala 7B, 87020-900, Maringá, PR, Brazil. (claudenice@nupelia.uem.br, agostinhoaa@nupelia.uem.br, lcgomes@nupelia.uem.br)
IIUniversidade Estadual de Maringá, CCB/DQI, Av. Colombo, 5790, Bloco 22, Sala 14, 87020-900, Maringá, Paraná, Brazil. (marodrigues@uem.br)
ABSTRACT
The construction of reservoirs is considered an important source of impacts on the fish fauna, severely altering the structure of the assemblage. This paper aimed to describe the structure of the fish assemblage of the Goioerê River, determining its longitudinal distribution and patterns of species dominance. The evaluation of its longitudinal variation in the diversity and abundance of the fish assemblage was conducted in July and October 2004 and January and May 2005. The collections were carried out near the headwaters (Gurucaia), middle stretch (Olaria), just above the falls (Paiquerê) and downstream (Foz). Forty-four species were captured. The Gurucaia fish assemblages differed significantly from Olaria, Paiquerê and Foz. The Olaria assemblages differed significantly from the Foz. Gurucaia showed the lowest diversity and abundance of species. Astyanax aff paranae Eigenmann,1914 (78% of the total) was found to be dominant at this site. Almost the same species richness was found at Olaria and Paiquerê, although Olaria had the greatest abundance of individuals. Astyanax aff paranae, Cyphocharax modestus (Fernández-Yépez, 1948) and Astyanax altiparanae Garutti & Britski, 2000 were the top three dominants and comprised over 71% of the total number of fish caught. At Paiquerê, Astyanax altiparanae, Hypostomus aff ancistroides (Ihering, 1911) and Loricariichthys platymetopon Isbrücker & Nijssen, 1979 composed 58% of the catches. Thirty-one species were recorded at Foz, which presented the greatest richness. The most abundant species were Apareiodon affinis (Steindachner, 1879), Galeocharax knerii (Steindachner, 1879) and A.altiparanae, which contributed to 50% of the total catches in this environment.These results record the fish biodiversity and how the community is longitudinally structured in the Goioerê River, and also demonstrate how this type of evaluation is important to understanding the fish community patterns and finding solutions to problems related to the conservation and management of the basin.
Keywords: Diversity, fish community, richness, evenness, Goioerê River basin.
RESUMO
A construção de reservatórios é considerada importante fonte de impactos para a fauna de peixes, levando a alterações significativas na estrutura da assembleia. Considerando isso, esse artigo objetiva descrever a estrutura da assembleia de peixes do rio Goioerê, determinando sua distribuição longitudinal e padrões na dominância das espécies. A avaliação da variação longitudinal na diversidade e abundância da assembleia de peixes neste rio foi conduzida em julho e outubro de 2004 e janeiro e maio de 2005. As coletas foram realizadas próximas as nascentes (Gurucaia), no segmento médio (Olaria), logo acima do salto Paiquerê (Paiquerê) e na foz do rio (Foz). Foram registradas 44 espécies. A assembleia de peixes de Gurucaia diferiu significativamente de Olaria, Paiquerê e Foz. A assembleia de Olaria diferiu significativamente de Foz. Gurucaia apresentou a menor diversidade e abundância de espécies. Astyanax aff. paranae Eigenmann,1914 (78% do total) foi dominante nesta localidade. Em Olaria e Paiquerê foi encontrada grande similaridade na riqueza de espécies, no entanto, em Olaria registrou-se a maior abundância de indivíduos. Astyanax aff. paranae, Cyphocharax modestus (Fernández-Yépez, 1948) and Astyanax altiparanae Garutti & Britski, 2000 foram as três espécies dominantes de topo e compreenderam cerca de 71% do total de peixes capturados. Na localidade Paiquerê, A. altiparanae, Hypostomus aff. ancistroides (Ihering, 1911) and Loricariichthys platymetopon Isbrücker & Nijssen, 1979 compuseram 58% das capturas. Trinta e uma espécies foram registradas na Foz, que apresentou a maior riqueza. As espécies mais abundantes foram Apareiodon affinis (Steindachner, 1879), Galeocharax knerii (Steindachner, 1879) and A. altiparanae, as quais contribuíram com 50% das capturas totais neste ambiente. Estes resultados registram a biodiversidade de peixes e como esta comunidade se estrutura longitudinalmente no rio Goioerê, além de demonstrar a importância desse tipo de avaliação para a compreensão dos padrões das comunidades de peixes e para a busca de soluções para problemas relacionados à conservação e o manejo desta bacia.
Palavras-chave: Diversidade, comunidade de peixes, riqueza, equitabilidade, bacia rio Goioerê.
Neotropical freshwater ecosystems are inhabited by rich assemblages of fish species, estimated to be about 6,000 (REIS et al., 2003). Main threats for this tremendous diversity includes several anthropogenic activities, such as the ones that cause alteration, fragmentation and losses of habitats, introduction of non-native species, overfishing, and pollution (AGOSTINHO et al., 1992, 2005a,b, 2006; ALLAN & FLECKER, 1993; OKADA et al., 1996; CUNICO et al., 2006). The construction of reservoirs is considered an important source of impacts on the ichthyofauna, altering the structure of the fish assemblage in the reservoir and below the dam (AGOSTINHO et al., 2005b; 2007; 2008). These impacts are conspicuous because lotic systems are highly variable ecosystems in space and time (MATTHEWS, 1998).
Rivers and streams vary spatially (local microhabitat), longitudinally (patterns of zonation along gradients and inter-regional faunal differences) and temporally (daily, seasonal and inter-annual scales) (WINEMILLER et al., 2008). A large number of studies reveals that species diversity in the rivers and streams of some taxonomic groups increases from the headwaters to the mouth (DARNELL, 1970; VANNOTE et al., 1980; SHELDON, 1988; ALLAN, 1995; MATTHEWS, 1998), because as the river increases in size, it offers a larger variety of ecological opportunities, more abundant resources, shelters and more stable physical conditions, favoring the addition of species. On the other hand, MATTHEWS (1998) comments that the mouth of rivers can have its species richness reduced by the smaller heterogeneity of habitats and cumulative effects of silt, pollutants, among others. Therefore, the impacts on the fish fauna will be dependent on the location in the basin where the dams will be positioned (WARD & STANFORD, 1995). Therefore, the understanding of the variation patterns of community attributes, especially richness and abundance of species, is of fundamental importance for evaluating the state of conservation of ecological systems and for planning their management (MAGURRAN, 1988). In the specific case of Brazil, these studies are of paramount importance due to the construction of small power plants. These plants are considered to generate less impact than large ones, and their constructions have easily been approved.
The Goioerê River is one of the rivers where the inventory for the construction of dams has already been approved. This river remains undammed and there is no data on its fish fauna, despite the apparent deterioration of the basin, related to soil use, the exploitation of clay in protected areas, the sugarcane crop, and potential dams. Considering these problems, this paper aims to describe the structure of the fish assemblage of the Goioerê River, determining its longitudinal distribution and patterns of species dominance. Specifically, we proposed to answer the following questions: i) What are the fish species and their dominance patterns along the main channel of the Goioerê River? ii) Are there longitudinal variations in some assemblage attributes (species richness, evenness and diversity index) and iii) Are there longitudinal patterns in the structure of the fish assemblages? The results of this investigation serve as a record of the fish biodiversity of this river basin and supply baseline information that can be used to better plan the occupation of the basin (there are plans for the construction of several dams), as well as its management and conservation.
MATERIAL AND METHODS
Study area. The Goioerê River runs through the municipalities of Araruna, Janiópolis, Moreira Sales, Tuneira do Oeste, Cruzeiro do Oeste, Umuarama, Perobal, Mariluz and Alto Piquiri (state of Paraná) and is one of the main tributaries of the Piquiri River. Its headwaters are in the municipality of Campo Mourão, in a Cerrado region. The river goes through a winding valley for about 123 km (drainage area approximately 2,424 km2). The main tributaries of its right bank are the Mouro River, Areia River, Guarani Stream, Pinhalzinho 2 River, Palmital Stream, Pinhalzinho Stream, Azul River and Água do Pinhal River. The main tributary on its left bank is the Riozinho River (Fig. 1).
Four sampling sites were established in the Goioerê River channel: near the headwaters (Gurucaia), in the middle (Olaria), just above Paiquerê Falls (Paiquerê) and below the falls in the mouth (Foz) (Tab. I).
Data collection. Samplings were carried out in July and October 2004 and January and May 2005, to coincide with the wet and dry seasons of the region, using cast nets (meshes 2.4 and 4 cm opposite knots) and 10 m long gill nets (meshes 2.4, 3, 4, 5, 6, 7, 8, 9, 10, 12 and 14 cm opposite knots) in extension around 400 m.
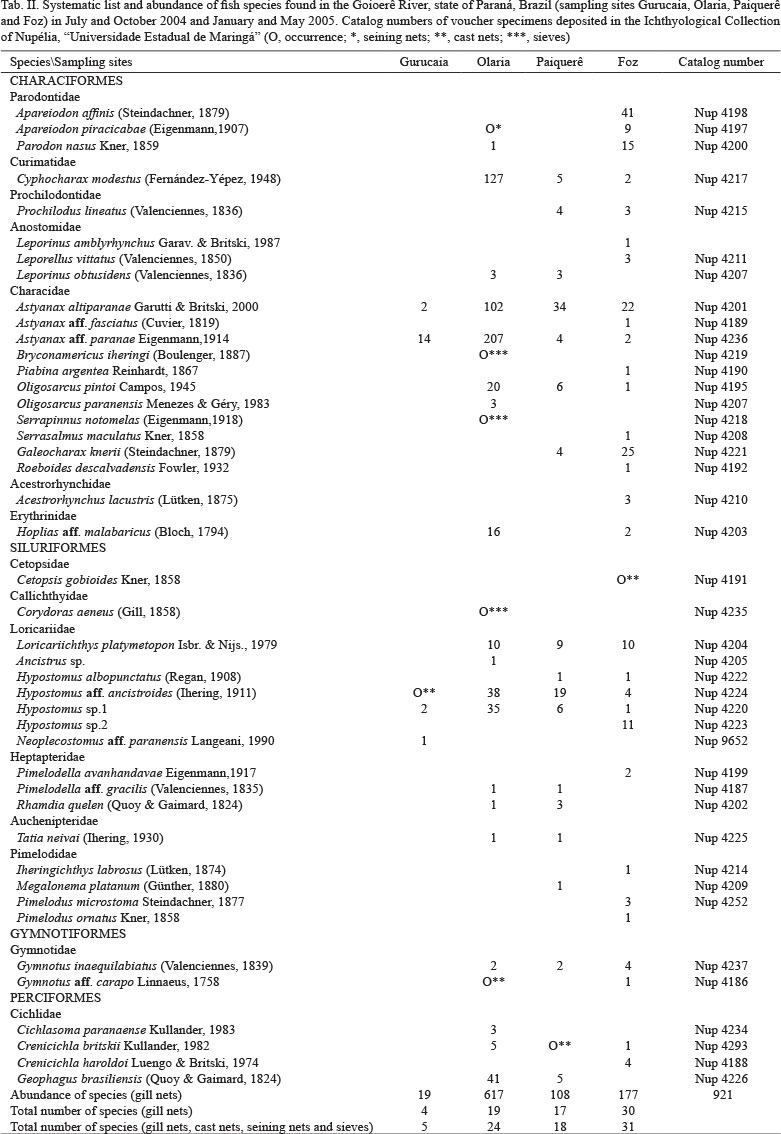
Fishes were removed in the morning (8 a.m.), in the afternoon (5 p.m.) and in the evening (10 p.m.) at each sampling site. Cast nets were thrown 10 times at each site during the day and at night. To improve the survey, we used sieves and nets to catch small-sized individuals at the Olaria site (the only one where it was possible due to the depth). The species sampled with cast nets, sieves and seining nets were used only as a record of occurrence in the Goioerê River basin. The systematic list of fish was elaborated based on REIS et al. (2003) and NELSON (2006) and specimens were deposited in the ichthyological collection of "Núcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura" (Nupélia), "Universidade Estadual de Maringá" (catalog number - Tab. II).
Data analysis. Species distribution was based on occurrence data, while species abundance data (only for gill net samplings) was analyzed using catch per unit of effort (CPUE), given in number of individuals per 1000 m2 of gill nets over 24 hours.
Species richness (S) was computed as the number of species found in each sample per site. Ichthyofaunistic diversity for each site was estimated using the Shannon Diversity Index (H') (PIELOU, 1975). Evenness (E) of the captured species estimated for each site (PIELOU, 1975). Evenness is a measure of equitability in species abundance within an assemblage. It varies between 0.0 (only one species) and 1.0 (species evenly distributed). To evaluate differences between species richness, evenness and Shannon Diversity Index, all calculated for each sampling site (4 sampling sites and four months; total of 16 samples), a one-way analysis of variance (ANOVA) (sites as factors) was used. In the cases of significant differences between the averages (ANOVA), a Tukey test was carried out to determine which sites differed. The program Statistica 7.0 (STATSOFT, 2005) was used for the analyses of variance (ANOVA) and the Tukey test.
Nonmetric multidimensional scaling (NMDS) was applied to summarize similarity patterns of the fish assemblage relative to the sampling sites (Gurucaia, Olaria, Paiquerê and Foz). NMDS was applied to the gill nets' CPUE data matrix (16 sampling sites - rows; 39 species - columns) using the Bray-Curtis coefficient as the resemblance measure. PERMANOVA was applied to test differences in fish assemblages relative to the factor sites (same as ANOVA). In the PERMANOVA design, months were considered replicates. NMDS, PERMANOVA and the Pair-Wise Test were applied and implemented using PRIMER (version 6) with the add-on PERMANOVA package (CLARKE & GORLEY, 2006; ANDERSON et al., 2008).
RESULTS
Survey of the fish assemblage and abundance. The survey of the ichthyofauna of the Goioerê River showed 44 species distributed in 4 orders and 15 families (Tab. II). Richness varied from 5 to 31 species at the different sampling sites (Tab. II). Five species were captured at the Gurucaia site (gill nets and cast nets), with Neoplecostomus aff. paranensis Langeani, 1990 restricted to this location (Tab. II). At the Olaria, twenty four species were captured but six exclusive to this location (Tab. II): Bryconamericus iheringi (Boulenger, 1887), Oligosarcus paranensis Menezes & Géry, 1983, Serrapinnus notomelas (Eigenmann,1918), Corydoras aeneus (Gill, 1858), Ancistrus sp. and Cichlasoma paranaense Kullander, 1983. Eighteen species were recorded at the Paiquerê site (Tab. II), with one exclusive to the site: Megalonema platanum (Günther, 1880) (considered rare because only one individual was caught). Thirty-one species were captured at the Foz site, and 15 were captured only in this site: Apareiodon affinis (Steindachner, 1879), Leporinus amblyrhynchus Garavello & Britski, 1987, Leporellus vittatus (Valenciennes, 1850), Astyanax aff. fasciatus (Cuvier, 1819), Piabina argentea Reinhardt, 1867, Serrasalmus maculatus Kner, 1858, Roeboides descalvadensis Fowler, 1932, Acestrorhynchus lacustris (Lütken, 1875), Cetopsis gobioides Kner, 1858, Hypostomus sp. 2, Pimelodella avanhandavae Eigenmann,1917, Iheringichthys labrosus (Lütken, 1874), Pimelodus microstoma Steindachner, 1877, Pimelodus ornatus Kner, 1858 and Crenicichla haroldoi Luengo & Britski, 1974.
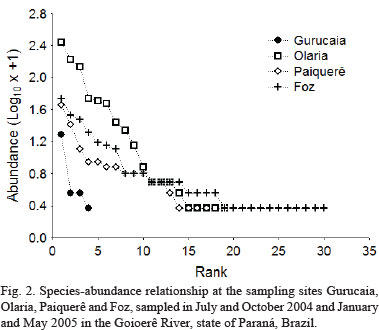
The most abundant taxa near the headwaters (Gurucaia) was Astyanax aff. paranae Eigenmann,1914, followed by Hypostomus sp. 1 and Astyanax altiparanae Garutti & Britski, 2000. Neoplecostomus aff. paranensis was considered rare (only one individual of this species was recorded) at the site (Fig. 2). The most commonly collected taxa at the Olaria were A. aff. paranae, Cyphocharax modestus (Fernández-Yépez, 1948) and A. altiparanae. Parodon nasus Kner, 1859, Ancistrus sp., Pimelodella aff. gracilis (Valenciennes, 1835), Rhamdia quelen (Quoy & Gaimard, 1824) and Tatia neivai (Ihering, 1930) were rare at this site (Fig. 2). The most abundant species at the Paiquerê site (just above Paiquerê Falls) were A. altiparanae, Hypostomus aff. ancistroides (Ihering, 1911), Hypostomus sp. 1 and Loricariichthys platymetopon Isbrücker & Nijssen, 1979. Hypostomus albopunctatus (Regan, 1908), M. platanum, P. aff. gracilis and T. neivai were rare in this environment (Fig. 2). The most abundant species at the Foz site were A. affinis, Galeocharax knerii (Steindachner, 1879), A. altiparanae and P. nasus. There were 12 rare species at this site: A. aff. fasciatus, Crenicichla britskii Kullander, 1982, Gymnotus aff. carapo Linnaeus, 1758, H. albopunctatus, Hypostomus sp.1, I. labrosus, L. amblyrhynchus, Oligosarcus pintoi Campos, 1945, P. argentea, P. ornatus, R. descalvadensis and S. maculatus (Fig. 2).
Longitudinal variations in the attributes of the assemblage. Species richness was the lowest at Gurucaia and the highest at Foz (Fig. 3). There were significant differences among the sites for richness (ANOVA, F = 7.16; p < 0.005) and Gurucaia site differed significantly from Olaria (Tukey; p < 0.01) and Foz (p < 0.005). Evenness did not differ significantly among sites (F = 1.66; p > 0.23). However, there was a conspicuously high variability at Gurucaia, which presented low mean evenness (Fig. 4).
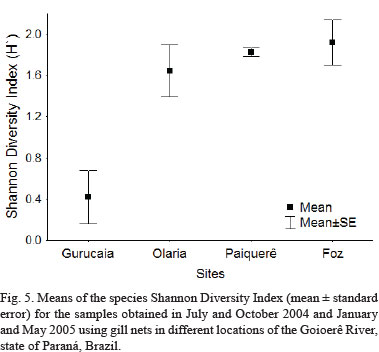
The Shannon Diversity Index, calculated for the samples obtained at the different sampling sites, varied from 0 to 2.2. The Shannon Diversity Index means showed, however, statistically significant variations (ANOVA, F = 10.75; p < 0.001). The Tukey test showed that there were differences between the means of Gurucaia in relation to Olaria (p < 0.007), Paiquerê (p < 0.003) and Foz (p < 0.002). The headwaters stretch (Gurucaia) presented the lowest value of the Shannon Diversity Index. The middle regions (Olaria and Paiquerê), together with Foz, presented the highest diversities, showing a tendency of increase from the headwaters to the mouth of the Goioerê River (Fig. 5).
Longitudinal variations in the fish assemblages (NMDS). Nonmetric multidimensional scaling (NMDS) analysis (stress of 0.11) revealed difference in the composition and abundance of the fish assemblages along the Goioerê River (Fig. 6). PERMANOVA analysis indicates significant differences in the fish assemblage for the sampling sites (Pseudo F = 3.20; p = 0.001). The Pair-Wise Test revealed significant differences between sites. Gurucaia differed significantly from Olaria (p < 0.02), Paiquerê (p < 0.02) and Foz (p < 0.03). In addition, it showed that Olaria differed significantly from Foz (p < 0.03). These differences may be related to the peculiarities of the fauna, especially at this latter site, where 15 species were exclusive.
DISCUSSION
The fauna of the Goioerê River, represented by 44 species, consists of only 11% of the 401 valid species recorded for the Paraná River basin, according to REIS et al. (2003). However, the number of species recorded in this river represents more than 75% of those in the Piquiri River (57 species; AGOSTINHO et al., 1997, 2000) and 14% of those in the upper Paraná River (310 species; LANGEANI et al., 2007). Most of the captured species are common to the fauna of the upper Paraná River, with the exception of A. aff. paranae, A. aff. fasciatus, Hoplias aff. malabaricus (Bloch, 1794), H. aff. ancistroides, Hypostomus sp. 1, Hypostomus sp. 2, N. aff. paranensis, Ancistrus sp., P. aff. gracilis and G. aff. carapo, which do not fit available descriptions and are probably new to science.
The ichthyofaunistic inventory of the Goioerê River records the presence of 21 species of Characiformes, 17 Siluriformes, 2 Gymnotiformes and 4 Perciformes. The dominance of Characiformes followed by Siluriformes, found in both the species richness and the number of individuals, is in accordance with the general patterns for Neotropical fish diversity (LOWE-MCCONNELL, 1987; WINEMILLER, 1996; JEPSEN, 1997; LUIZ et al., 2003). AGOSTINHO et al. (1994) found the dominance in richness and number of individuals of Siluriformes in the Paraná River between the mouths of the Paranapanema and Iguaçu rivers. SILVANO et al. (2000) mentioned that the fish communities of the upper Juruá River possessed a large richness of Siluriformes, but in lower abundance compared to Characiformes, showing that this order did not dominate all of the ecosystems.
The differences in species richness, exclusive species (15 only registered in the Foz site), and the similarities among sites indicate that Paiquerê Falls (11 m high) is a barrier that separates the fish assemblages of the Goioerê River from the Piquiri River. However, in years of intense rain, large migratory species like Prochilodus lineatus (Valenciennes, 1836), Leporinus obtusidens (Valenciennes, 1836) and other reophilic species (good swimmers) are able to overpass it. This explains the certain degree of similarity between the Goioerê and Piquiri; however, genetic studies downstream and upstream from the falls are necessary to confirm this hypothesis. Paiquerê Falls is a barrier only for non-migratory species (bad swimmers), which cannot reach the upper parts of the Goioerê River. In fact, out of the 44 species recorded, 29 were collected at the sites above the falls; this also explains the high similarities among Gurucai, Olaria and Paiquerê.
These results suggest that this isolation was relatively recent. Besides limiting the dispersion of many species in the stretches above, this barrier also impedes the uses of the remaining habitats of the basin for fish from the Goioerê River. Therefore, the sustainability of the stocks above the falls depends exclusively on the success of the assemblage finding, in the existent habitats, the resources for survival and perpetuation.
The lowest species richness and abundance were found at Gurucaia. Two small species (A. altiparanae and A. aff. paranae) and three species of armored catfishes that are new to science were caught at this location. The causes of the low richness and abundance are varied, but they are commonly reported in the literature. Near headwaters, most organisms rely on allocthonous food sources (VANNOTE et al., 1980; PENCZACK et al., 1994; MATTHEWS, 1998; ABES & AGOSTINHO, 2001). However, in the specific case of Gurucaia, the site is located in a mosaic of rotational mechanized plantations of soybeans, wheat and corn, whose management brings impacts caused by the carrying of soil particles and agricultural inputs connected to these sediments by the rains. These characteristics may also contribute to explain the difference in richness and abundance in this stretch of the river.
Almost the same species richness was found at the middle sites (Olaria: 24 species; Paiquerê: 18); however, Olaria recorded the greatest abundance of individuals in the catches. The most abundant species (A. aff. paranae, C. modestus and A. altiparanae) spawn in several batches, have external fertilization and no parental care (VAZZOLLER, 1996; SENTEIO SMITH et al., 2003; AGOSTINHO et al., 2003). The presence of a small tributary on the left bank of this site may have contributed to these results because, despite being virtually devoid of riparian vegetation, it possesses more lentic waters, which allow the growth of juveniles, as verified in sporadic collections using a sieve (data not presented). The presence of L. platymetopon, the third most abundant species at Paiquerê, is curious because it occurs in small numbers in lotic environments and is extremely abundant in Paraná River floodplain lakes (DEI TOS et al., 1997; JÚLIO JR. et al., 2009). Its presence at this location is probably due to its introduction by fish farmers, because this species is commonly maintained in tanks in the region to consume the organic material at the bottom, avoiding sharp reduction in oxygen concentration (C. Dei Tos, pers. observ.).
The most abundant species at Foz were A. affinis, G. knerii and A. altiparanae. The first species is a detritivore abundant in sandy bottoms (LUZ-AGOSTINHO et al., 2006) and was not recorded in the stretches above the falls. At the sandy bottom site upstream, there is another parodontid (P. nasus - similar diet), while detrivorous scrapers like loricarids are among the most abundant species at the rocky bottom sites.
The piscivores were more diverse at Foz, and G. knerii was among the dominant ones. In addition, five others were recorded at this site. In the stretch upstream, the typically piscivorous species were O. pintoi, O. paranensis and H. aff. malabaricus. A. altiparanae, considered an insetivore-piscivore in some places (LUZ-AGOSTINHO et al., 2006), was present at all sites sampled in this river.
The Goioerê River is relatively long (about 123 km) and its ichthyofauna is subject to various environmental impacts from anthropogenic actions, such as large monocultures of soybeans and wheat that require mechanization and intense use of chemicals. In addition, a precariousness in the conservation of the marginal vegetation along the hydrographical basin was noted. There are areas that have been greatly affected by the use of soil (e.g. extraction of clay for the production of bricks and tiles has been observed in areas devoted to marginal vegetation). These impacts and the absence of marginal vegetation are extended to farms that maintain areas for cattle to drink water at the margins of the river. It is also evident that the dimension of these and other impacts and their degree of importance have not been determined for the basin and in fact may influence not only feeding and growth, but especially fish abundance in the areas of the spawning and growth of young individuals.
Perspectives for the fish communities above Paiquerê Falls. The richness and abundance of the ichthyofauna found in the Goioerê River furnish a more understandable picture of the diversity of this group in the basin, but it is not completely unveiled, because the ichthyofauna present in the 48 tributaries of the Goioerê River channel was not investigated. The data obtained in this research reveal a low abundance of naturally rare species.
Relevant questions concerning the factors that restrict the abundance of migratory species and those of commercial interest like P. lineatus and L. obtusidens and the reasons for such low diversity including abundance of species in the highest stretches of the basin, still lack answers. The variables or processes that control and structure the assemblages of fish in the basin, promoting substitutions or additions of species, as well as the role of these assemblages regarding the structure and effective processes in this river, also deserve explanations. Humans are altering the habitats along the Goioerê River and do not understand the consequences that the modifications can have over time on the viability of the existing biota. Agência Nacional de Energia Elétrica - (ANEEL) approved the Hydroelectric Inventory of the Goioerê River, which identified a potential of six Hydroelectric Power Stations (Água Limpa, Água Espraiada, Água Tranquila, Água Tremida, Água Clara and Água Nova), with the capacity of generating 65.0 MW each (GERALDO, 2002). If these small hydroelectric plants are approved in the region, this preliminary inventory of the ichthyofauna will reveal the importance of more detailed studies. These are some of the subjects and challenges that should be investigated so that management actions aiming at aquatic resource conservation can be taken rationally. However, these studies should be done with the participation of city halls, decision-making agencies, communities and public prosecutors, and also with an understanding of the state of conservation of the basin and what should be done to improve and preserve the fish biodiversity.
Acknowledgements. We appreciate the efforts and assistance of the following people: Júlia Vitório (Foundation for the Support of the Scientific and Technological Development of the Piquiri Valley - FADCT - Goioerê - state of Paraná), and José O. Ramos, Jaime Luiz L. Pereira and Carolina V. Minte-Vera from Universidade Estadual de Maringá (UEM). We are grateful to Carla S. Pavanelli, Weferson J. da Graça and Claudio H. Zawadzki from UEM, who contributed information on taxonomy and Elaine A. Luiz and Sybelle Bellay for statistical analyses. John Jervis Stanley Junior revised the English text. Two anonymous reviewers provided helpful comments on an earlier draft of the manuscript. Funding was provided by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (proc. 402.452/03-8). AAA and LCG also thank CNPq for the "Bolsa Produtividade em Pesquisa".
Received 10 January 2014
Accepted 28 March 2014
- ABES, S. S. & AGOSTINHO, A. A. 2001. Spatial patterns in fish distributions and structure of the ichthyocenosis in the Água Nanci stream, upper Paraná River basin, Brazil. Hydrobiologia 445(1-3):217-227.
- AGOSTINHO, A. A.; GOMES, L. C. & PELICICE, F. M. 2007. Ecologia e Manejo dos Recursos Pesqueiros em Reservatórios do Brasil Maringá, EDUEM. 501p.
- AGOSTINHO, A. A.; GOMES, L. C.; SUZUKI, H. I. & JÚLIO JR., H. F. 2003. Migratory Fishes of the Upper Paraná River Basin, Brazil. In: CAROLSFELD, J.; HARVEY, B.; ROSS, C. & BAER, A. eds. Migratory Fishes of South America: biology, fisheries and conservation status Vitoria, World Bank. p.19-98.
- AGOSTINHO, A. A.; JÚLIO JR., H. F. & BORGHETTI, J. R. 1992. Considerações sobre os impactos dos represamentos na ictiofauna e medidas para sua atenuação. Um estudo de caso: reservatório de Itaipu. Revista Unimar, 14(supl.):89-107.
- AGOSTINHO, A. A.; JÚLIO JR., H. F.; GOMES, L. C.; BINI, L. M. & AGOSTINHO, C. S. 1997. Composição, abundância e distribuição espaço-temporal da ictiofauna. In: VAZZOLER, A. E. A. DE M.; AGOSTINHO, A. A. & HAHN, N. S. eds. A planície de inundação do Alto rio Paraná: aspectos físicos, biológicos e socioeconômicos Maringá, EDUEM. p.179-208.
- AGOSTINHO, A. A.; JÚLIO JR., H. F. & PETRERE, JR., M. 1994. Itaipu reservoir (Brazil): impacts of the impoundment on the fish fauna and fisheries. In: COWX, I. G. ed. Rehabilitation of freshwater fisheries Oxford, Fishing News Books. p.171-184.
- AGOSTINHO, A. A.; PELICICE, F. M. & GOMES, L.C. 2008. Dams and the fish fauna of the Neotropical region: impacts and management to diversity and fisheries. Brazilian Journal of Biology 68(supl.):1119-1132.
- AGOSTINHO, A. A.; PELICICE, F. M. & JÚLIO JR, H. F. 2005a. Introdução de espécies de peixes em águas continentais brasileiras: uma síntese. In: ROCHA, O.; ESPÍNDOLA, E. L. G.; FENERICH-VERANI, N.; VERANI, J. R. & RIETZLER A. C. orgs. Espécies invasoras em águas doces: estudos de caso e propostas de manejo São Carlos, Ed. Universidade Federal de São Carlos. p.13-23.
- _____. 2006. Biodiversidade e introdução de espécies de peixes: Unidades de conservação. In: CAMPOS, J. B.; TOSSULINO, M. G. P. & MULLER, C. R. C. eds. Unidades de Conservação: ações para valorização da biodiversidade Curitiba, Instituto Ambiental do Paraná. p. 95-117.
- AGOSTINHO, A. A.; THOMAZ, S. M & GOMES, L. C. 2005b. Conservation of the Biodiversity of Brazil's Inland Waters. Conservation Biology 19(3):646-652.
- AGOSTINHO, A. A.; THOMAZ, S. M.; MINTE-VERA, C. V. & WINEMILLER, K. O. 2000. Biodiversity in the High Paraná River Floodplain. In: GOPAL, B.; JUNK, W. J. & DAVIS, J. A. eds. Biodiversity in wetlands: assessment, function and conservation Leiden, The Netherlands Backhuys Publishers. p.89-118.
- ALLAN, J. D. 1995. Stream Ecology: structure and function of running waters London, Chapman & Hall. 388p.
- ALLAN, J. D. & FLECKER, A. S. 1993. Biodiversity conservation in running waters. BioScience 43(1):32-43.
- ANDERSON, M. J.; GORLEY, R. N. & CLARKE, K. R. 2008. PERMANOVA + for PRIMER: Guide to Software and Statistical Methods. Plymouth, PRIMES-E. 214p.
- CLARKE, K. R. & GORLEY, R. N. 2006. PRIMER v6: user manual/tutorial Plymouth, Plymouth Maringe Laboratory. 190p.
- CUNICO, A. M.; AGOSTINHO, A. A. & LATINI, J. D. 2006. Influência da urbanização sobre as assembleias de peixes em três córregos de Maringá, Paraná. Revista Brasileira de Zoologia 23(4):1101-1110.
- DARNELL, R. M. 1970. Evolution and the Ecosystem. American Zoologist 10(1):9-15.
- DEI TOS, C.; AGOSTINHO, A. A. & SUZUKI, H. I. 1997. Population structure and reproductive biology of Loricariichthys platymetopon (Silurifomes, Pisces) in the upper river Paraná. Brazilian Archives of Biology and Technology 40(4):793-807.
- GERALDO, A. 2002. Superintendência de Gestão dos Potenciais Hidráulicos. Diário Oficial da União, Brasília, DF, 5, junho, 2002, n. 106, p. 73.
- JEPSEN, D. B. 1997. Fish species diversity in sand bank habitats of a Neotropical River. Environmental Biology of Fishes 49:449-460.
- JÚLIO JR., H. F.; DEI TOS, C.; AGOSTINHO, A. A. & PAVANELLI, C. S. 2009. A massive invasion of fish species after eliminating a natural barrier in the upper rio Paraná basin. Neotropical Ichthyology 7(4):709-718.
- LANGEANI, F.; CASTRO, R. M. C.; OYAKAWA, O. T.; SHIBATTA, O. A.; PAVANELLI, C. S. & CASATTI, L. 2007. Diversidade da Ictiofauna do Alto Rio Paraná: composição atual e perspectivas futuras. Biota Neotropica 7(3):181-197.
- LOWE-MCCONNELL, R. H. 1987. Ecological studies in tropical fish communities Cambridge, University Press. 382p.
- LUIZ, E. A.; GOMES, L. C.; AGOSTINHO, A. A. & BULLA, C. K. 2003. Influência de processos locais e regionais nas assembleias de peixes em reservatórios do Estado do Paraná, Brasil. Acta Scientiarum 25(1):107-114.
- LUZ-AGOSTINHO, C. D. G.; BINI, L. M.; FUGI, R.; AGOSTINHO, A. A. & JÚLIO JR., H. F. 2006. Food spectrum and trophic structure of the ichthyofauna of Corumbá reservoir, Paraná River Basin, Brazil. Neotropical Ichthyology 4(1):61-68.
- MAGURRAN, A. E. 1988. Ecological diversity and its measurement London, Croom Helm. 179p.
- MATTHEWS, W. J. 1998. Patterns in freshwater fish ecology Norwell, Kluwer Academic Publishers. 756p.
- NELSON, J. S. 2006. Fishes of the world 4 ed. New York, John Wiley and Sons. 601p.
- OKADA, E. K.; AGOSTINHO, A. A. & PETRERE JR., M. 1996. Catch and effort data and the management of the commercial fisheries of Itaipu Reservoir in the Upper Paraná River. In: COWX, I. G. org. Stock assessment in inland fisheries London, Fishing News Books/Blackwell Scientific Publications. p. 154-161.
- PENCZACK, T.; AGOSTINHO, A. A. & OKADA, E. K. 1994. Fish diversity and community structure in two small tributaries of the Paraná State, Brazil. Hydrobiologia 294:243-251.
- PIELOU, G. E. 1975. Ecological diversity New York, John Wiley and Sons. 165p.
- REIS, R. E.; KULLANDER, S. O. & FERRARIS, JR., C. J. 2003. Check list of freshwater fishes of South and Central America Porto Alegre, Edipucrs. 729p.
- SENTEIO SMITH, W.; PETRERE JR., M. & BARRELLA, W. 2003. The fish fauna in tropical rivers: The case of the Sorocaba River basin, SP, Brazil. Revista de Biología Tropical 51(3-4):769-782.
- SHELDON, A. L. 1988. Conservation of stream fishes: patterns of diversity, rarity, and risk. Conservation Biology 2(2):149-156.
- SILVANO, R. A. M.; AMARAL, B. D. & OYAKAWA, O. T. 2000. Spatial and temporal patterns of diversity and distribution of the Upper Juruá River fish community (Brazilian Amazon). Environmental Biology of Fishes 57:25-35.
- STATSOFT, INC. 2005. Statistica (data analysis software system), version 7.1 Available at: < http://www.statsoft.com/>. Accessed on: 24 April 2014.
- VANNOTE, R. L.; MINSHALL, G. W.; CUMMINS, K. W.; SEDELL, J. R. & CUSHING. C. E. 1980. The river continuum concept. Canadian Journal of Fisheries Aquatic Sciences 37:130-137.
- VAZZOLER, A. E. A. DE M. 1996. Biologia da Reprodução de Peixes Teleósteos: Teoria e Prática Maringá, EDUEM. 169p.
- WARD, J. V. & STANFORD, J. A. 1995. Ecological connectivity in alluvial river ecosystems and its disruption by flow regulation. Regulated River: Research & Management 11:105-109.
- WINEMILLER, K. O. 1996. Dynamic diversity in fish assemblages of tropical rivers. In: CODY, M. L. & SMALLWOOD, J. A. eds. Long-Term Studies of Vertebrate Communities San Diego, Academic Press. p.99-134.
- WINEMILLER, K. O.; AGOSTINHO, A. A. & CARAMASCHI, E. P. 2008. Fish ecology in tropical streams. In: DUDGEON, D. ed. Tropical Stream Ecology San Diego, Academic Press. p.107-146.
Variation of the ichthyofauna along the Goioerê River: an important tributary of the Piquiri-Paraná basin
Publication Dates
-
Publication in this collection
27 June 2014 -
Date of issue
Mar 2014
History
-
Accepted
28 Mar 2014 -
Received
10 Jan 2014