ABSTRACT
Knickzones are originated from basaltic outcroppings, present runs, riffles and pools and are highly influenced by flood pulses, which maintain their natural dynamic. However, the construction of hydroelectric power plants alters or eliminate the dynamism of this area and can affect the resident fauna that may be dependent on it. The aim of this study was to evaluate the organization of a knickzone’s ichthyofauna considering the influence of seasonality and connectivity of habitats. The study was performed in a knickzone located in the Sapucaí-Mirim River, Southeast Brazil. We sampled four rocky pools connected to the river and three isolated pools, during rainy and dry conditions. The analysis of the two factors (connectivity and seasonality) and of their interaction showed a significant influence only for seasonality on ichthyofauna structure, with higher values of abundance in the rainy season. The species that most contributed to the high dissimilarity between seasons were Knodus moenkhausii (50% of contribution) and Astyanax bockmanni (21%). The former is the most abundant species in the rainy season and the later in the dry season. The alteration between low and high water level occurs frequently in knickzones, as it is a rocky shallow platform in the middle of a river, with floods occurring seasonally or in stochastic short-term periods. This hydrological seasonal dynamic, high limnological variability and complex interactions of different habitats (pools, runs and rapids) explain the particular ichthyofauna structure in such small area. Our results also indicate the potential importance of basaltic knickzones for regional fish diversity conservation, especially due to the imminent threat by intensive hydropower reservoir construction.
KEYWORDS
Basaltic substrate; conservation; rocky pool; Sapucaí-Mirim River; seasonal hydrologic pulses
RESUMO
Pedrais são originados de afloramentos basálticos, apresentando rápidos, corredeiras e poças e são altamente influenciados por pulsos de inundação que mantêm sua dinâmica natural. No entanto, a construção de usinas hidrelétricas altera ou elimina o dinamismo dessa área e pode afetar a fauna residente que pode ser dependente dela. O objetivo deste estudo foi avaliar a organização da ictiofauna de um pedral considerando a influência da sazonalidade e conectividade dos habitats. O estudo foi realizado em uma zona localizada no rio Sapucaí-Mirim, sudeste do Brasil. Foram amostradas quatro poças rochosas conectadas ao rio e três isoladas, em condições chuvosas e secas. A análise dos dois fatores (conectividade e sazonalidade) e de sua interação mostrou influência significativa apenas para a sazonalidade na estrutura da ictiofauna, com maiores valores de abundância na estação chuvosa. As espécies que mais contribuíram para a alta dissimilaridade entre as estações foram Knodus moenkhausii (50% de contribuição) e Astyanax bockmanni (21%). A primeira é a espécie mais abundante na estação chuvosa e a posterior na estação seca. A alteração entre baixo e alto nível de água ocorre com frequência em pedrais, pois é uma plataforma rochosa e rasa no meio de um rio, com inundações ocorrendo sazonalmente ou em períodos estocásticos de curto prazo. Esta dinâmica hidrológica sazonal, alta variabilidade limnológica e interações complexas de diferentes habitats (poças, rápidos e corredeiras) explicam a particular estrutura da ictiofauna em uma área tão pequena. Nossos resultados também indicam a importância potencial dos pedrais basálticos para a conservação regional da diversidade de peixes, especialmente devido à ameaça iminente da construção intensiva de reservatórios de hidrelétricas.
PALAVRAS-CHAVE
Substrato basáltico; conservação; poça rochosa; Rio Sapucaí-Mirim; pulsos hidrológicos sazonais
Seasonal hydrologic pulses strongly influence the ecology of tropical rivers (Correa & Winemiller, 2014Correa, S. B. & Winemiller, K. O. 2014. Flooding, fruiting phenology and resource partitioning among fishes in the Amazon. Ecology 95(1):210-224.; Fitzgerald et al., 2017Fitzgerald, D. B.; Winemiller, K. O.; Sabaj-Perez, M. H. & Sousa, L. M. 2017. Seasonal changes in the assembly mechanisms structuring tropical fish communities. Ecology 98(1):21-31.), introducing a temporal dynamic on the fish composition and structure in fluvial systems (Junk et al., 1989Junk, W. J.; Bayley, P. B. & Sparks, R. E. 1989. The flood pulse concept in river-floodplain systems. Canadian Special Publication Fisheries and Aquatic Sciences 106(1):110-127.; Driver & Hoeinghaus, 2016Driver, L. J. & Hoeinghaus, D. J. 2016. Spatiotemporal dynamics of intermittent stream fish metacommunities in response to prolonged drought and reconnectivity. Marine & Freshwater Research 67(11):1667-1679.), particularly in environments with strong seasonal or annual flood and drought cycles (Poff & Ward, 1989Poff, N. L. & Ward, J. V. 1989. Implications of streamflow variability and predictability for lotic community structure: A regional analysis of streamflow patterns. Canadian Journal of Fisheries and Aquatic Sciences 46(10):1805-1818.; Larned et al., 2010Larned, S. T.; Datry, T.; Arscott, D. B. & Tockner, K. 2010. Emerging concepts in temporary-river ecology. Freshwater Biology 55(4):717-738.). The flood pulse increases the lateral connectivity among habitats (opportunity for dispersion), the physical space for colonizers, and the availability of shelter and food resources (Thomaz et al., 2007Thomaz, S. M.; Bini, L. M. & Bozelli, R. M. 2007. Floods increase similarity among aquatic habitats in river-floodplain systems. Hydrobiologia 579(1):1-13.). As water level decreases, some habitats become isolated and the effect of biological interactions on fish communities can be intensified (Fernandes et al., 2009Fernandes, R.; Gomes, L. C.; Pelicice, F. M. & Agostinho, A. A. 2009. Temporal organization of fish assemblages in floodplain lagoons: the role of hydrological connectivity. Environmental Biology of Fishes 85(2):99-108.; Ferrareze & Nogueira, 2011Ferrareze, M. & Nogueira, M. G. 2011. Importance of lateral lagoons for the ichthyofauna in a large tropical reservoir. Brazilian Journal of Biology 71(4):807-820.), with harsh and variable abiotic conditions resulting in physiological stress upon the biota (Spranza & Stanley, 2000Spranza, J. J. & Stanley, E. H. 2000. Condition, growth, and reproductive styles of fishes exposed to different environmental regimes in a prairie drainage. Environmental Biology of Fishes 59(1):99-109.; Boulton, 2003Boulton, A. J. 2003. Parallels and contrasts in the effects if drought on stream macroinvertebrate assemblages. Freshwater Biology 48(7):1173-1185.).
Knickzones are river stretches often formed by changes in bed cover, channel geometry and erosion processes (Haykawa & Oguchi, 2009Hayakawa, Y. S. & Oguchi, T. 2009. GIS analysis of fluvial knickzone distribution in Japanese mountain watersheds. Geomorphology 111(1):27-37.; Dibiase et al., 2014Dibiase, R. A.; Whipple, K. X.; Lamb, M. P. & Heimsath, A. M. 2014. The role of waterfalls and knickzones in controlling the style and pace of landscape adjustment in the western San Gabriel Mountains, California. Geological Society of America Bulletin 127(3-4):539-559.). They are ecologically characterized by large rock outcrops forming a complex of habitats composed by riffles, runs and pools with distinct magnitudes and are highly influenced by flood pulses (Brambilla et al., 2018Brambilla, E. M.; Ruocco, A. M. C. & Nogueira, M. G. 2018. A contribution for the limnological knowledge of basaltic knickzones. Brazilian Journal of Biology 78(2):375-385.). The immersed or exposed condition of the substrate of a knickzone is highly variable and differences are mainly seasonal (summer-rainy and winter-dry periods), but can also occur stochastically throughout the year, in short-term periods of storms and dry spell events (Brambilla et al., 2018Brambilla, E. M.; Ruocco, A. M. C. & Nogueira, M. G. 2018. A contribution for the limnological knowledge of basaltic knickzones. Brazilian Journal of Biology 78(2):375-385.). The degree of connectivity of pools with the main river channel is also variable, with two connectivity conditions occurring during dry season: isolated pools with no connection with river flow and the connected ones with permanent connection with river flow. However, when the river water level is high, the entire substrate of the knickzone is fully immersed. After rainfall stops the water level quickly decreases and the degree of substrate exposed increases, consequently some pools quickly return to the isolated condition (Brambilla et al., 2018Brambilla, E. M.; Ruocco, A. M. C. & Nogueira, M. G. 2018. A contribution for the limnological knowledge of basaltic knickzones. Brazilian Journal of Biology 78(2):375-385.).
These unique ecosystems are currently threat by the construction of hydroelectric power plants, fact evidenced by the controversial construction of the huge Brazilian hydroelectric plant of Belo Monte, in Xingu River (Amazon Basin; 11,233 MW) (Winemiller et al., 2016Winemiller, K. O.; McIntyre, P. B.; Castello, L.; Fluet-Chouinard, E.; Giarrizzo, T.; Nam, S.; Baird, I. G.; Darwall, W.; Lujan, N. K.; Harrison, I.; Stiassny, M. L. J.; Silvano, R. A. M.; Fitzgerald, D. B.; Pelicice, F. M.; Agostinho, A. A.; Gomes, L. C.; Albert, J. S.; Baran, E.; Petrere Jr., M.; Zarfl, C.; Mulligan, M.; Sullivan, J. P.; Arantes, C. C.; Sousa, L. M.; Koning, A. A.; Hoeinghaus, D. J.; Sabaj, M.; Lundberg, J. G.; Armbruster, J.; Thieme, M. L.; Petry, P.; Zuanon, J.; Torrente Vilara, G.; Snoeks, J.; Ou, C.; Rainboth, W.; Pavanelli, C. S.; Akama, A.; van Soesbergen, A. & Sáenz, L. 2016. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science 351(6269):128-129.). This threat is a worldwide scenario, considering the existence of 8,600 dams primarily designed for electric generation (Zarfl et al., 2015Zarfl, C.; Lumsdon, A. E.; Berlekamp, J.; Tydecks, L. & Tockner, K. 2015. A global boom in hydropower dam construction. Aquatic Science 77(1):161-170.). Furthermore, studies about freshwater communities of knickzones are scarce (Muehlbauer & Doyle, 2012Muehlbauer, J. D. & Doyle, M. W. 2012. Knickpoint effects on macroinvertebrates, sediment, and discharge in urban and forested streams: urbanization outweighs microscale habitat heterogeneity. Freshwater Science 31(2):282-295.). For macroinvertebrates, it is known that knickzones support a high diversity and a unique filterer-dominated community not found elsewhere in stream reaches (Muehlbauer & Doyle, 2012Muehlbauer, J. D. & Doyle, M. W. 2012. Knickpoint effects on macroinvertebrates, sediment, and discharge in urban and forested streams: urbanization outweighs microscale habitat heterogeneity. Freshwater Science 31(2):282-295.). However, there are no studies about fish communities inhabiting knickzones (Muehlbauer & Doyle, 2012Muehlbauer, J. D. & Doyle, M. W. 2012. Knickpoint effects on macroinvertebrates, sediment, and discharge in urban and forested streams: urbanization outweighs microscale habitat heterogeneity. Freshwater Science 31(2):282-295.).
Therefore, the aim of this study was to evaluate the organization of the ichthyofauna in a Brazilian knickzone, considering the influence of seasonality and connectivity of the knickzone’s pools with the main river channel. Our hypothesis is that the structure of this ichthyofauna is influenced by these two factors, exhibiting differences between rainy and dry season and between connected and isolated pools, with lower values of abundance in dry season and isolated pools because of the longtime of disconnection with the main river channel.
MATERIAL AND METHODS
Study area. The study area is located in Sapucaí-Mirim River, a tributary of Grande River basin, between São Paulo and Minas Gerais States, Brazil. Currently, five small hydropower plants (SHP) are operating in this river basin, generating 70 MW, and another six potential sites were inventoried for future constructions (ANEEL, 2018ANEEL. 2018. Capacidade de Geração do Brasil. Available at: <Available at: http://www2.aneel.gov.br/aplicacoes/capacidadebrasil/capacidadebrasil.cfm
>. Accessed on: 16 January 2018.
http://www2.aneel.gov.br/aplicacoes/capa...
). Based on recent satellite images, at least eight knickzones can be recognized in this river. The selected knickzone (20°34’34.1”S, 47°47’06.5”W) has an area of 0.03 km², representing 0.00032% of the Sapucaí-Mirim basin, and was chosen for the study because it presents a naturally dynamic river flow (it is located in the upstream zone of Palmeiras SHP, but beyond its operational influence). In this knickzone, we selected four pools connected and three isolated from the river flow to study the ichthyofauna structure (See Brambilla et al., 2018 for more details and photos of sampling area).
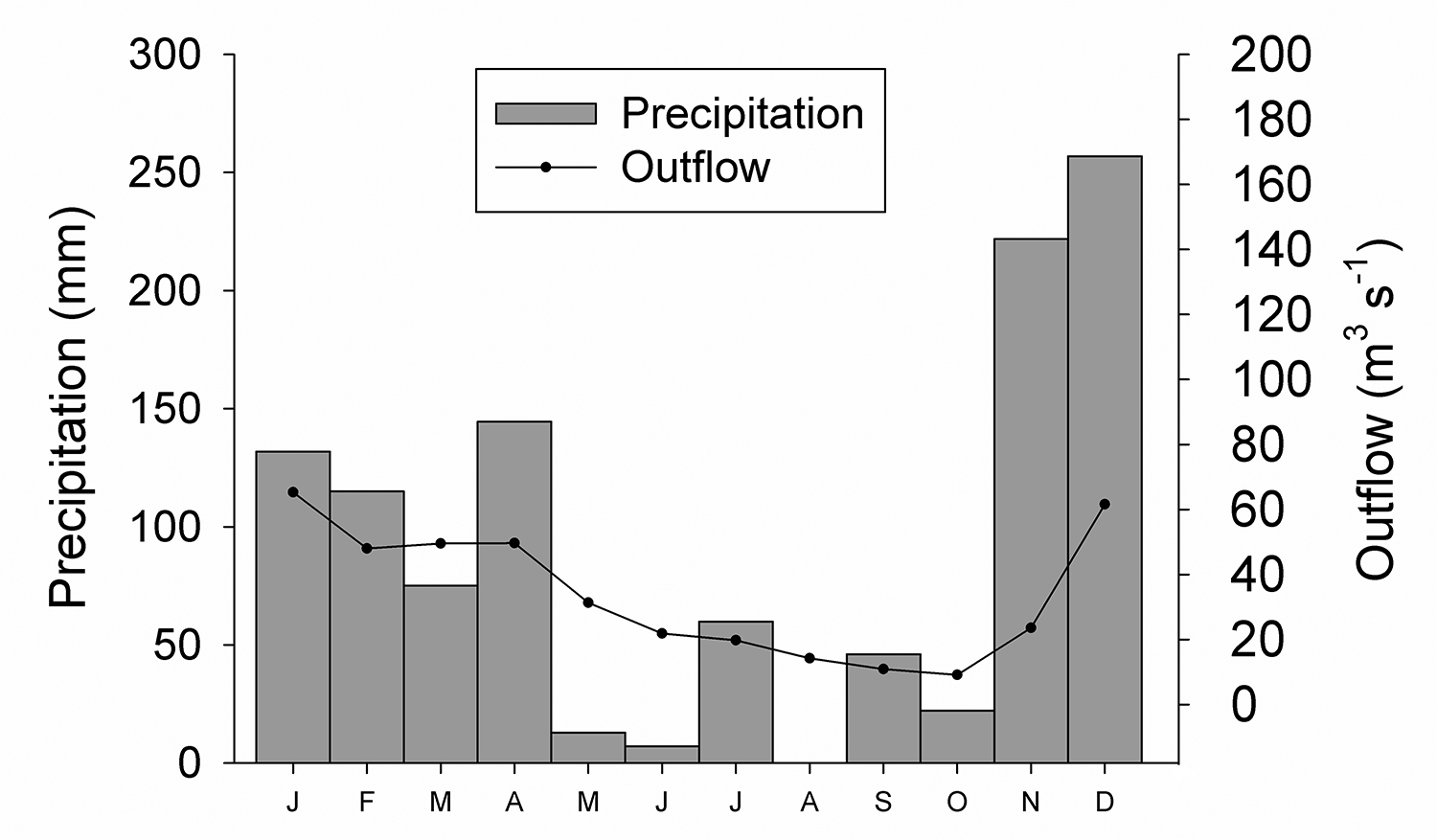
Sampling. Samplings were performed during one day of a dry season month (June/2014) and one day of a rainy season month (December/2014) (Fig. 1). The dry season sampling was performed approximately two months after the water level of the river starts decreasing (Fig. 1). In December, the outflow reached a peak of 90 m3 s-1 when, according to Brambilla et al. (2018Brambilla, E. M.; Ruocco, A. M. C. & Nogueira, M. G. 2018. A contribution for the limnological knowledge of basaltic knickzones. Brazilian Journal of Biology 78(2):375-385.), this knickzone become fully immersed. However, the rainy season sampling occurred two days after this high outflow peak when the water had already started to decrease and the river outflow reached 58 m3 s-1, with runs, riffles and pools already exposed (rainy condition). The pools sampled differed in relation to some characteristics, such as the presence of marginal herbaceous vegetation in contact with water, the amount of filamentous algae (estimated visually) and the volume of water (measured with graduated tape and ruler and calculated with the volume formula of the most similar geometric figure) (Tab. I).
Physical characteristics of the pools studied in a basaltic knickzone of the Sapucai-Mirim River, sampled in a dry and rainy season. C- connected pool, I- isolated pool.
In each pool, several sampling methods were used, including seine, sieve (mesh size 0.5 cm) and electrofishing, in order to obtain a representative sample of the ichthyofauna. The sampling effort was five passes of seine and sieve and 30 minutes of electrofishing on each pool (during each season). Sampled fish were immediately euthanized in a hyper concentrated solution of eugenol, fixed in formalin 10%, and subsequently transferred to 70% ethanol.
In laboratory, fish were identified according to morphological and meristic features (Castro et al., 2004Castro, R. M. C.;. Casatti, L.; Santos, H. F.; Melo, A. L. A.; Martins, L. S. F.; Ferreira, K. M.; Gibran, F. Z.; Benine, R. C.; Carvalho, M.; Ribeiro, A. C.; Abreu, T. X.; Bockmann, F. A.; Pelição, G. Z.; Stopiglia, R. & Langeani, F. 2004. Estrutura e composição da ictiofauna de riachos da bacia do rio Grande no Estado de São Paulo, sudeste do Brasil. Biota Neotropica 4(1):1-39.; Graça & Pavanelli, 2007Graça, W. J. & Pavanelli, C. S. 2007. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: EDUEM. 216p.; Langeani & Rego, 2014Langeani, F. & Rego, A. C. L. 2014. Guia ilustrado dos peixes da bacia do Rio Araguari. Uberlândia: Grupo de Mídia Brasil Central. 194p.). The identifications were checked through scientific collections and confirmed by specialists (Dr. Francisco Langeani Neto from the State University of São Paulo - Campus São José do Rio Preto and Dr. Cláudio Zawadzki from the State University of Maringá). The specimens were deposited in two collections (LBP - Laboratório de Biologia e Genética de Peixes, UNESP, Botucatu; NUP -Núcleo de Pesquisas em Limnologia, Ictiologia e Aqüicultura, UEM, Maringá).
The specimens that presented standard length values lower than the first sexual maturation size reported in literature for the species (Nakatami et al., 2001Nakatani, K.; Agostinho, A. A.; Baumgartner, G.; Bialetzki; A., Sanches, P. V.; Makrakis, M. C. & Pavanelli, C. S. 2001. Ovos e larvas de peixes de água doce: desenvolvimento e manual de identificação. Maringá, EDUEM. 378p.; Graça & Pavanelli, 2007Graça, W. J. & Pavanelli, C. S. 2007. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: EDUEM. 216p.; Langeani & Rego, 2014Langeani, F. & Rego, A. C. L. 2014. Guia ilustrado dos peixes da bacia do Rio Araguari. Uberlândia: Grupo de Mídia Brasil Central. 194p.) were considered juveniles, development period characterized by whole formation of fins and scales until sexual maturation (Nakatami et al., 2001Nakatani, K.; Agostinho, A. A.; Baumgartner, G.; Bialetzki; A., Sanches, P. V.; Makrakis, M. C. & Pavanelli, C. S. 2001. Ovos e larvas de peixes de água doce: desenvolvimento e manual de identificação. Maringá, EDUEM. 378p.).
Analyses. In order to discriminate the fish assemblage structure considering connectivity and seasonality, it was used a Non-Metric Multidimensional Scaling (NMDS) (Legendre & Legendre, 2012Legendre, P. & Legendre, L. 2012. Numerical Ecology. 2ed. Amsterdan: Elsevier. 853p.). The ordination analysis was applied to a Bray-Curtis similarity matrix derived from fish abundance data transformed in log (x+1). A Permutation Multivariate Analysis of Variance (PERMANOVA) (Anderson, 2001Anderson, M. J. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology 26(1):32-46.) based on the Bray-Curtis similarity matrix, derived from fish abundance data transformed in log (x+1), was used to test for differences in fish assemblage between seasons (rainy and dry), connectivity (isolated and connected) and the interaction between both factors. When the PERMANOVA pseudo-F is significant (p < 0.01), a Permutation Analysis of Multivariate Dispersions (PERMDISP) is applied to the same data set to confirm if the differences found are really related to the factors analyzed (pseudo-F not significant in the PERMDISP results; p > 0.01) or only related to the dispersion or heterogeneity of samples (in this case with a pseudo-F significant, p < 0.01) (Anderson, 2006Anderson, M. J. 2006. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62(1):245-253.; Warton et al., 2012Warton, D. I.; Wright, S. T. & Wang, Y. 2012. Distance-based multivariate analyses confoundlocation and dispersion effects. Methods in Ecology and Evolution 3(1):89-101.). A Similarity Percentage Routine (SIMPER) was used to determine the contribution of individual taxa to the average dissimilarity (typifying species) (Clarke & Warwick, 2001Clarke, K. R. & Warwick, R. M. 2001. Change in marine communities: an approach to statistical analysis and interpretation. Plymouth: Plymouth Marine Laboratory. 380p.). Analyses were performed in PRIMER v6.0 software.
RESULTS
The ichthyofauna of the studied knickzone of Sapucaí-Mirim River was composed by 23 species, distributed in 11 families (Tab. II). The most frequent order was Characiformes (5 families, 11 species), followed by Siluriformes (3 families, 6 species) and Perciformes (1 family, 4 species). The orders Cyprinodontiformes and Gymnotiformes appeared with only one family and one species each.
The rainy season presented 447 specimens of 20 species, the dry season 161 specimens of 12 species. Connected pools harbored 399 specimens of 22 species and isolated pools 209 specimens of 9 species. Most species (82%) were small in size (smaller than 20 cm of standard length), representing 98% of total abundance, and the remaining were medium-sized (between 20-50 cm of standard length). The most abundant species were Knodus moenkhausii (Eigenmann & Kennedy, 1903) and Astyanax bockmanni Vari & Castro, 2007 during both the rainy and dry seasons (Tab. II). Juveniles of Astyanax bockmanni, Knodus moenkhausii, and Geophagus brasiliensis (Quoy & Gaimard, 1824) were collected in both seasons and juveniles of Schizodon nasutus Kner, 1858, Astyanax lacustris (Lütken, 1875), Steindachnerina insculpta (Fernández-Yépez, 1948), Hoplias malabaricus (Bloch, 1794) and Poecilia reticulata Peters, 1859 were collected only in the rainy season.
Taxonomic list and abundance of the fish species sampled in connected (C) and isolated (I) pools, during a dry (D) and rainy (R) season, in a basaltic knickzone of the Sapucaí-Mirim River. Voucher number of specimens deposited in two collections (LBP, Laboratório de Biologia e Genética de Peixes, UNESP, Botucatu; NUP, Núcleo de Pesquisas em Limnologia, Ictiologia e Aqüicultura, UEM, Maringá). * Non-native species. Taxonomic list based on Eschmeyer et al. (2018Eschmeyer, W. N.; Fricke, R. & van der Laan, R. 2018. Catalog of fishes: genera, species, references. Available at: <Available at: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp >. Accessed on: 16 Feb 2018.
http://researcharchive.calacademy.org/re... ).
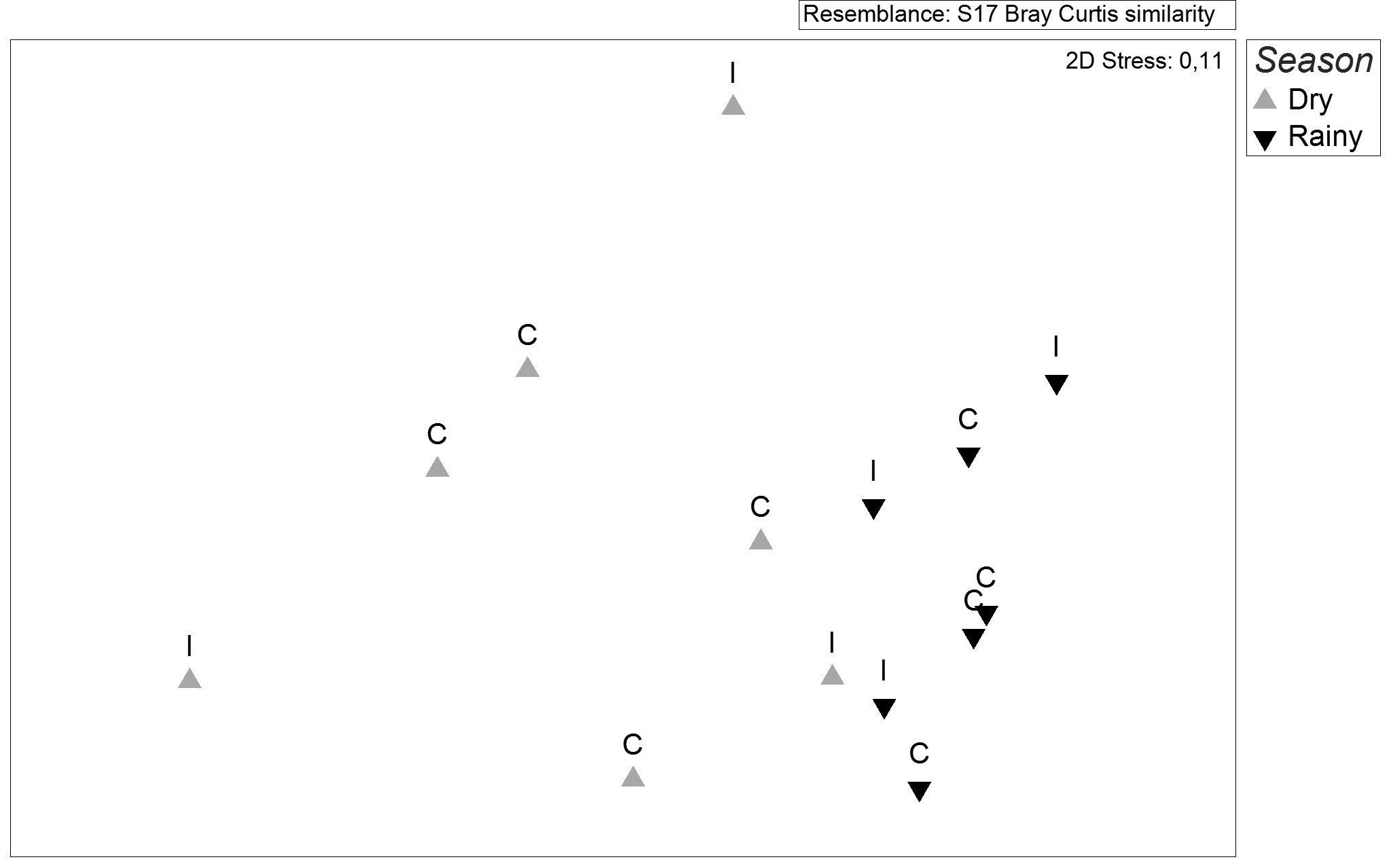
The ordination analysis (NMDS), applied to fish abundance data, suggested a separation of the ichthyofauna assemblage between dry and rainy seasons and not between condition of connectivity, with rainy-season samples more aggregate than dry-season ones (Fig. 2). Supporting NMDS results, the PERMANOVA indicated differences in fish structure only for the factor seasonality (pseudo-F = 3.94, p = 0.008), but neither for connectivity (pseudo-F = 1.25, p = 0.278) nor for the interaction of the two factors (pseudo-F = 0.51, p = 0.845). The not-significant results of the PERMDISP (pseudo-F = 10.04, p = 0.02) confirmed that the seasonal difference was really related to the factor analyzed and not for data dispersion or heterogeneity of samples. The dissimilarity between seasons was of 81.6%, according to SIMPER analysis, which also evidences the difference between dry and rainy seasons. The species that most contribute to this dissimilarity were Knodus moenkhausii (50% of contribution) and Astyanax bockmanni (21%), the former being the most abundant species sampled in the rainy season and the later the most abundant sampled in the dry season (Tab. II). The SIMPER analysis also showed a higher average similarity percentage between pools during the rainy (44.7%) than during the dry season (18.8%), what agree with the NMDS results of more aggregation of rainy season samples (Fig. 2).
Non-metric multidimensional plots of the abundance of fish assemblage sampled in isolated (I) and connected (C) pools during the rainy and dry season in the Sapucaí-Mirim River knickzone, Southeast Brazil.
DISCUSSION
The orders Characiformes and Siluriformes were the most representative in the studied knickzone. This is the common pattern for the Upper Paraná basin (Lowe-McConnell, 1975Lowe-McConnell, R. H. 1975. Fish communities in tropical freshwater: their distribution, ecology and evolution. London and New York, Longman. 337p.; Agostinho & Júlio Jr., 1999Agostinho, A. A. & Júlio Jr., H. F. 1999. Peixes da bacia do alto Paraná. In: Lowe-McConnell, R. H. ed. Estudos ecológicos de comunidades de peixes tropicais. São Paulo: Edusp, p. 374-400.; Langeani et al., 2007Langeani, F.; Castro, R. M. C.; Oyakawa, O. T.; Shibatta, O. A.; Pavanelli, C. S. & Casatti, L. 2007. Diversidade da ictiofauna do alto rio Paraná: composição atual e perspectivas futuras. Biota Neotropica 7(3):1-17. ), which is also recurrent in the Sapucai-Grande basin (Castro et al., 2004Castro, R. M. C.;. Casatti, L.; Santos, H. F.; Melo, A. L. A.; Martins, L. S. F.; Ferreira, K. M.; Gibran, F. Z.; Benine, R. C.; Carvalho, M.; Ribeiro, A. C.; Abreu, T. X.; Bockmann, F. A.; Pelição, G. Z.; Stopiglia, R. & Langeani, F. 2004. Estrutura e composição da ictiofauna de riachos da bacia do rio Grande no Estado de São Paulo, sudeste do Brasil. Biota Neotropica 4(1):1-39.; Oliveira et al., 2016Oliveira, A. K.; Garavello, J. C.; Cesario, V. V. & Cardoso, R. T. 2016. Fish fauna from Sapucaí-Mirim River, tributary of Grande River, upper Paraná River basin, Southeastern Brazil. Biota Neotropica 16(1):1-9.).
The ichthyofauna structure of the studied knickzone differs from that reported in other stretches of Sapucaí-Mirim River (Castro et al., 2004Castro, R. M. C.;. Casatti, L.; Santos, H. F.; Melo, A. L. A.; Martins, L. S. F.; Ferreira, K. M.; Gibran, F. Z.; Benine, R. C.; Carvalho, M.; Ribeiro, A. C.; Abreu, T. X.; Bockmann, F. A.; Pelição, G. Z.; Stopiglia, R. & Langeani, F. 2004. Estrutura e composição da ictiofauna de riachos da bacia do rio Grande no Estado de São Paulo, sudeste do Brasil. Biota Neotropica 4(1):1-39.; Oliveira et al., 2016Oliveira, A. K.; Garavello, J. C.; Cesario, V. V. & Cardoso, R. T. 2016. Fish fauna from Sapucaí-Mirim River, tributary of Grande River, upper Paraná River basin, Southeastern Brazil. Biota Neotropica 16(1):1-9.). In this knickzone, it was sampled 23% of the total fish species richness known for this river and five new species records were reported (Bryconamericus turiuba Langeani, Lucena, Pedrini & Tarelho-Pereira, 2005, Knodus moenkhausii, Planaltina britskii Menezes, Weitzman & Burns, 2003, Hypostomus fluviatilis (Schubart, 1964) and Pseudostegophilus paulensis Miranda Ribeiro, 1918). As the sample methodologies used in this study were the same of the other studies, probably those new records are related to the particular physical and limnological characteristics of this habitat (Brambilla et al., 2018Brambilla, E. M.; Ruocco, A. M. C. & Nogueira, M. G. 2018. A contribution for the limnological knowledge of basaltic knickzones. Brazilian Journal of Biology 78(2):375-385.), which may support specific ecological requirements such as food resources, shelters and reproduction sites. Additionally, the presence of juveniles of some species reinforces the importance of this macrohabitat, indicating that they can be used for complete life cycle of these species. The knickzone contains plentiful shelters, reducing pressure from predators (Schlosser, 1987Schlosser, I. J. 1987. A conceptual framework for fish communities in small warm water streams. In: Matthews, W.J. & Heins, D.C. (eds). Community and evolutionary ecology of North American stream fishes. Norman, University of Oklahoma, p.17-24.) and providing suitable conditions for rearing grounds (Bain et al., 1989Bain, M. B.; Finn, J. T. & Booke, H. E. 1989. Stream flow regulation and fish community structure. Ecology 69(2):382-392.; Gore et al., 1989Gore, J. A.; Nestler, J. M. & Layzer, J. B. 1989. Instream flow predictions and management options for biota affected by peaking-power hydroelectric operations. Regulated Rivers 3(1):35-48.; Flebbe & Dolloff, 1995Flebbe, P. A. & Dolloff, C. A. 1995. Trout use of woody debris and habitat in Appalachian wilderness streams of North Carolina. North American Journal of Fisheries Management 15(3):579-591.).
The initial hypothesis of this study was corroborated only in part, since only the seasonality, was proven to have influence on the structure of the fish assemblage in the studied knickzone. The effect of seasonal hydrologic pulses was evident in all analyses, with a separation between dry and rainy periods, the second with higher values of abundance. A similar temporal pattern was also observed in Neotropical floodplains (Ortega et al., 2015Ortega, J. C. G.; Dias, R. M.; Petry, A. C.; Oliveira, E. F. & Agostinho, A. A. 2015. Spatio-temporal organization patterns in the fish assemblages of a Neotropical floodplain. Hydrobiologia 745(1):31-41., Siqueira-Souza et al., 2016Siqueira-Souza, F. K.; Freitas, C. E.; Hurd, L. E. & Petrere Jr., M. 2016. Amazon floodplain fish diversity at different scales: do time and place really matter? Hydrobiologia 776(1):99-110.), Tropical marginal lakes (Ferrareze & Nogueira, 2011Ferrareze, M. & Nogueira, M. G. 2011. Importance of lateral lagoons for the ichthyofauna in a large tropical reservoir. Brazilian Journal of Biology 71(4):807-820.) and Amazonian rapids (Fitzgerald et al., 2017Fitzgerald, D. B.; Winemiller, K. O.; Sabaj-Perez, M. H. & Sousa, L. M. 2017. Seasonal changes in the assembly mechanisms structuring tropical fish communities. Ecology 98(1):21-31.). In the studied knickzone the factor seasonality (dry and rainy seasons) clearly influenced the ichthyofauna structure, both composition and abundance.
The dry period starts with the decrease of rains and, consequently, with water retraction (low hydrometric level). In this hydrological phase, areas located in higher elevations or more distant from the river channel become disconnected and aquatic organisms can remain confined within these habitats for a variable period of time (Humphries & Baldwin, 2003Humphries, P. & Baldwin, D. S. 2003. Drought and aquatic ecosystem: an introduction. Freshwater Biology 48(7):1141-1146.; Lake, 2003Lake, P. S. 2003. Ecological effects of perturbation by drought in flowing waters. Freshwater Biology 48(7):1161-1172.). During this isolation, stressful abiotic conditions intensify progressively until the following flood (Tockner et al., 2000Tockner K.; Malard, F. & Ward, J. V. 2000. An extension of the flood pulse concept. Hydrological Processes 14(16-17):2861-2883.). At the same time, biotic interactions, such as competition and predation, are expected to become more intense, mainly among individuals restricted to habitats of small proportions like lagoons and pools. Therefore, non-random patterns of species co-occurrence (aggregation/segregation) are expected between these natural disturbance events, due to harsh abiotic/biotic conditions that lead some species to local extinctions (Arrington et al., 2005Arrington, D. A.; Winemiller, K. O. & Layman, C. A. 2005. Community assembly at the patch scale in a species rich tropical river. Oecologia 144(1):157-167.).
When the rainy period starts, it is expected that communities shift from structured patterns in low-water periods to random patterns in high-water periods. Fish assemblages can display a progressive increase in organization following hydrometric variations (Arrington et al., 2005Arrington, D. A.; Winemiller, K. O. & Layman, C. A. 2005. Community assembly at the patch scale in a species rich tropical river. Oecologia 144(1):157-167.; Fernandes et al., 2009Fernandes, R.; Gomes, L. C.; Pelicice, F. M. & Agostinho, A. A. 2009. Temporal organization of fish assemblages in floodplain lagoons: the role of hydrological connectivity. Environmental Biology of Fishes 85(2):99-108.). The increase in water level expands the area available for dispersal and provides the connection between isolated sites and the main channels of rivers, consequently resetting the organizational process of assemblages (Ortega et al., 2015Ortega, J. C. G.; Dias, R. M.; Petry, A. C.; Oliveira, E. F. & Agostinho, A. A. 2015. Spatio-temporal organization patterns in the fish assemblages of a Neotropical floodplain. Hydrobiologia 745(1):31-41.). In this situation, a reduction of competition and predation occurs, which can play a major role in maintaining high fish diversity over a larger scale (Fitzgerald et al., 2017Fitzgerald, D. B.; Winemiller, K. O.; Sabaj-Perez, M. H. & Sousa, L. M. 2017. Seasonal changes in the assembly mechanisms structuring tropical fish communities. Ecology 98(1):21-31.). Additionally, increased input of terrestrial resources during the rainy season together with decreased species density should enhance fitness via greater supply of energy for growth, reproduction and migration (Fitzgerald et al., 2017Fitzgerald, D. B.; Winemiller, K. O.; Sabaj-Perez, M. H. & Sousa, L. M. 2017. Seasonal changes in the assembly mechanisms structuring tropical fish communities. Ecology 98(1):21-31.).
This alteration between low and high water levels occurs frequently in knickzones. Floods in such rocky shallow platform in the middle of the river occur even seasonally or in stochastically, in short-term periods. This hydrological dynamic results in a high limnological variability and complex interactions among the different habitats (pools, runs and rapids), in terms of depth, area, volume, different rocky substrates, presence of marginal vegetation and connectivity with the river flow (Brambilla et al., 2018Brambilla, E. M.; Ruocco, A. M. C. & Nogueira, M. G. 2018. A contribution for the limnological knowledge of basaltic knickzones. Brazilian Journal of Biology 78(2):375-385.). Certainly, all this temporal and spatial interactions in a relatively small area corresponding to the knickzones, contribute to explain the particular ichthyofauna structure and high species richness observed in our study.
Although the results did not point for the influence of connectivity on the ichthyofauna structure, Brambilla et al. (2018Brambilla, E. M.; Ruocco, A. M. C. & Nogueira, M. G. 2018. A contribution for the limnological knowledge of basaltic knickzones. Brazilian Journal of Biology 78(2):375-385.) found influence of this factor on limnological parameters. The connection of each pool with the river flow promotes a water renovation, which decrease harsh abiotic situations, increasing the dissolved oxygen concentration and reducing extreme values of pH and temperature. However, this connection may not be strong enough like flood events to reset the organizational process of assemblages and, consequently, alter the structure of the ichthyofauna in knickzones, evidence also found in lakes of Pantanal wetland (Penha et al., 2017Penha, J.; Landeiro, V. L.; Ortega, J. C. G. & Mateus, L. 2017. Interchange between flooding and drying, and spatial connectivity control the fish metacommunity structure in lakes of the Pantanal wetland. Hydrobiologia 797(1):115-126.) and in upper Parana River floodplain (Vasconcelos et al., 2014Vasconcelos, L. P.; Alves, D. C. & Gomes, L. C. 2014. Spatial and temporal variations among fish with similar strategies: patterns of reproductive guilds in a floodplain. Hydrobiologia 726(1):213-228.).
This study shows the importance of knickzone as a different environment for the river ichthyofauna. We observed a high fish richness and abundance considering the proportionally small area of the knickzone and even the presence of species exclusive to this macrohabitat if compared to the Sapucaí-Mirim River fish assemblage. There were also evidences that this rocky habitat is a nursery and growth area for some species. Additionally, these characteristics indicate the knickzones have a high importance to fish conservation, mainly in a context of threats to river biodiversity by the construction of hydroelectric power plants. It is difficult to avoid the construction of dams, but if this kind of macrohabitat could be preserved when a dam construction occurs, an extinction or decrease of some fish species can be avoided. Besides that, it would be important to preserve the natural flood and dry cycles of river (the most influential factor in fish structure of knickzones). Sabo et al. (2017Sabo, J.; Ruhi, A.; Holtgrieve, G.; Elliott, V.; Arias, M.; Ngor, P.B.; Räsänen, T. & Nam, S. 2017. Designing river flows to improve food security futures in the lower Mekong basin. Science 358(6468): eaao 1053.) showed that maintaining flow regimes would improve fisheries, but can also benefit other fish species besides those commercially exploited. Thus, knickzones should be considered as strategic environments in the regional planning for biodiversity conservation, especially due to their eminent threat by intensive hydropower reservoir construction.
Acknowledgments
We thank M.Sc. Marco Aurelio Pessotto and M.Sc. Diogo Souza for fieldwork help, Lilian Casatti for loan the electric fisheries sampling device, Doug Kane for text revision of the manuscript and CNPq for the scholarship grant to EMB.
REFERENCES
- Agostinho, A. A. & Júlio Jr., H. F. 1999. Peixes da bacia do alto Paraná. In: Lowe-McConnell, R. H. ed. Estudos ecológicos de comunidades de peixes tropicais. São Paulo: Edusp, p. 374-400.
- Anderson, M. J. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology 26(1):32-46.
- Anderson, M. J. 2006. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62(1):245-253.
- ANEEL. 2018. Capacidade de Geração do Brasil. Available at: <Available at: http://www2.aneel.gov.br/aplicacoes/capacidadebrasil/capacidadebrasil.cfm >. Accessed on: 16 January 2018.
» http://www2.aneel.gov.br/aplicacoes/capacidadebrasil/capacidadebrasil.cfm - Arrington, D. A.; Winemiller, K. O. & Layman, C. A. 2005. Community assembly at the patch scale in a species rich tropical river. Oecologia 144(1):157-167.
- Bain, M. B.; Finn, J. T. & Booke, H. E. 1989. Stream flow regulation and fish community structure. Ecology 69(2):382-392.
- Boulton, A. J. 2003. Parallels and contrasts in the effects if drought on stream macroinvertebrate assemblages. Freshwater Biology 48(7):1173-1185.
- Brambilla, E. M.; Ruocco, A. M. C. & Nogueira, M. G. 2018. A contribution for the limnological knowledge of basaltic knickzones. Brazilian Journal of Biology 78(2):375-385.
- Castro, R. M. C.;. Casatti, L.; Santos, H. F.; Melo, A. L. A.; Martins, L. S. F.; Ferreira, K. M.; Gibran, F. Z.; Benine, R. C.; Carvalho, M.; Ribeiro, A. C.; Abreu, T. X.; Bockmann, F. A.; Pelição, G. Z.; Stopiglia, R. & Langeani, F. 2004. Estrutura e composição da ictiofauna de riachos da bacia do rio Grande no Estado de São Paulo, sudeste do Brasil. Biota Neotropica 4(1):1-39.
- Clarke, K. R. & Warwick, R. M. 2001. Change in marine communities: an approach to statistical analysis and interpretation. Plymouth: Plymouth Marine Laboratory. 380p.
- Correa, S. B. & Winemiller, K. O. 2014. Flooding, fruiting phenology and resource partitioning among fishes in the Amazon. Ecology 95(1):210-224.
- Dibiase, R. A.; Whipple, K. X.; Lamb, M. P. & Heimsath, A. M. 2014. The role of waterfalls and knickzones in controlling the style and pace of landscape adjustment in the western San Gabriel Mountains, California. Geological Society of America Bulletin 127(3-4):539-559.
- Driver, L. J. & Hoeinghaus, D. J. 2016. Spatiotemporal dynamics of intermittent stream fish metacommunities in response to prolonged drought and reconnectivity. Marine & Freshwater Research 67(11):1667-1679.
- Eschmeyer, W. N.; Fricke, R. & van der Laan, R. 2018. Catalog of fishes: genera, species, references. Available at: <Available at: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp >. Accessed on: 16 Feb 2018.
» http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp - Fernandes, R.; Gomes, L. C.; Pelicice, F. M. & Agostinho, A. A. 2009. Temporal organization of fish assemblages in floodplain lagoons: the role of hydrological connectivity. Environmental Biology of Fishes 85(2):99-108.
- Ferrareze, M. & Nogueira, M. G. 2011. Importance of lateral lagoons for the ichthyofauna in a large tropical reservoir. Brazilian Journal of Biology 71(4):807-820.
- Fitzgerald, D. B.; Winemiller, K. O.; Sabaj-Perez, M. H. & Sousa, L. M. 2017. Seasonal changes in the assembly mechanisms structuring tropical fish communities. Ecology 98(1):21-31.
- Flebbe, P. A. & Dolloff, C. A. 1995. Trout use of woody debris and habitat in Appalachian wilderness streams of North Carolina. North American Journal of Fisheries Management 15(3):579-591.
- Graça, W. J. & Pavanelli, C. S. 2007. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: EDUEM. 216p.
- Gore, J. A.; Nestler, J. M. & Layzer, J. B. 1989. Instream flow predictions and management options for biota affected by peaking-power hydroelectric operations. Regulated Rivers 3(1):35-48.
- Hayakawa, Y. S. & Oguchi, T. 2009. GIS analysis of fluvial knickzone distribution in Japanese mountain watersheds. Geomorphology 111(1):27-37.
- Humphries, P. & Baldwin, D. S. 2003. Drought and aquatic ecosystem: an introduction. Freshwater Biology 48(7):1141-1146.
- Junk, W. J.; Bayley, P. B. & Sparks, R. E. 1989. The flood pulse concept in river-floodplain systems. Canadian Special Publication Fisheries and Aquatic Sciences 106(1):110-127.
- Lake, P. S. 2003. Ecological effects of perturbation by drought in flowing waters. Freshwater Biology 48(7):1161-1172.
- Langeani, F.; Castro, R. M. C.; Oyakawa, O. T.; Shibatta, O. A.; Pavanelli, C. S. & Casatti, L. 2007. Diversidade da ictiofauna do alto rio Paraná: composição atual e perspectivas futuras. Biota Neotropica 7(3):1-17.
- Langeani, F. & Rego, A. C. L. 2014. Guia ilustrado dos peixes da bacia do Rio Araguari. Uberlândia: Grupo de Mídia Brasil Central. 194p.
- Larned, S. T.; Datry, T.; Arscott, D. B. & Tockner, K. 2010. Emerging concepts in temporary-river ecology. Freshwater Biology 55(4):717-738.
- Legendre, P. & Legendre, L. 2012. Numerical Ecology. 2ed. Amsterdan: Elsevier. 853p.
- Lowe-McConnell, R. H. 1975. Fish communities in tropical freshwater: their distribution, ecology and evolution. London and New York, Longman. 337p.
- Muehlbauer, J. D. & Doyle, M. W. 2012. Knickpoint effects on macroinvertebrates, sediment, and discharge in urban and forested streams: urbanization outweighs microscale habitat heterogeneity. Freshwater Science 31(2):282-295.
- Nakatani, K.; Agostinho, A. A.; Baumgartner, G.; Bialetzki; A., Sanches, P. V.; Makrakis, M. C. & Pavanelli, C. S. 2001. Ovos e larvas de peixes de água doce: desenvolvimento e manual de identificação. Maringá, EDUEM. 378p.
- Oliveira, A. K.; Garavello, J. C.; Cesario, V. V. & Cardoso, R. T. 2016. Fish fauna from Sapucaí-Mirim River, tributary of Grande River, upper Paraná River basin, Southeastern Brazil. Biota Neotropica 16(1):1-9.
- Ortega, J. C. G.; Dias, R. M.; Petry, A. C.; Oliveira, E. F. & Agostinho, A. A. 2015. Spatio-temporal organization patterns in the fish assemblages of a Neotropical floodplain. Hydrobiologia 745(1):31-41.
- Penha, J.; Landeiro, V. L.; Ortega, J. C. G. & Mateus, L. 2017. Interchange between flooding and drying, and spatial connectivity control the fish metacommunity structure in lakes of the Pantanal wetland. Hydrobiologia 797(1):115-126.
- Poff, N. L. & Ward, J. V. 1989. Implications of streamflow variability and predictability for lotic community structure: A regional analysis of streamflow patterns. Canadian Journal of Fisheries and Aquatic Sciences 46(10):1805-1818.
- Sabo, J.; Ruhi, A.; Holtgrieve, G.; Elliott, V.; Arias, M.; Ngor, P.B.; Räsänen, T. & Nam, S. 2017. Designing river flows to improve food security futures in the lower Mekong basin. Science 358(6468): eaao 1053.
- Schlosser, I. J. 1987. A conceptual framework for fish communities in small warm water streams. In: Matthews, W.J. & Heins, D.C. (eds). Community and evolutionary ecology of North American stream fishes. Norman, University of Oklahoma, p.17-24.
- Siqueira-Souza, F. K.; Freitas, C. E.; Hurd, L. E. & Petrere Jr., M. 2016. Amazon floodplain fish diversity at different scales: do time and place really matter? Hydrobiologia 776(1):99-110.
- Spranza, J. J. & Stanley, E. H. 2000. Condition, growth, and reproductive styles of fishes exposed to different environmental regimes in a prairie drainage. Environmental Biology of Fishes 59(1):99-109.
- Thomaz, S. M.; Bini, L. M. & Bozelli, R. M. 2007. Floods increase similarity among aquatic habitats in river-floodplain systems. Hydrobiologia 579(1):1-13.
- Tockner K.; Malard, F. & Ward, J. V. 2000. An extension of the flood pulse concept. Hydrological Processes 14(16-17):2861-2883.
- Vasconcelos, L. P.; Alves, D. C. & Gomes, L. C. 2014. Spatial and temporal variations among fish with similar strategies: patterns of reproductive guilds in a floodplain. Hydrobiologia 726(1):213-228.
- Warton, D. I.; Wright, S. T. & Wang, Y. 2012. Distance-based multivariate analyses confoundlocation and dispersion effects. Methods in Ecology and Evolution 3(1):89-101.
- Winemiller, K. O.; McIntyre, P. B.; Castello, L.; Fluet-Chouinard, E.; Giarrizzo, T.; Nam, S.; Baird, I. G.; Darwall, W.; Lujan, N. K.; Harrison, I.; Stiassny, M. L. J.; Silvano, R. A. M.; Fitzgerald, D. B.; Pelicice, F. M.; Agostinho, A. A.; Gomes, L. C.; Albert, J. S.; Baran, E.; Petrere Jr., M.; Zarfl, C.; Mulligan, M.; Sullivan, J. P.; Arantes, C. C.; Sousa, L. M.; Koning, A. A.; Hoeinghaus, D. J.; Sabaj, M.; Lundberg, J. G.; Armbruster, J.; Thieme, M. L.; Petry, P.; Zuanon, J.; Torrente Vilara, G.; Snoeks, J.; Ou, C.; Rainboth, W.; Pavanelli, C. S.; Akama, A.; van Soesbergen, A. & Sáenz, L. 2016. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science 351(6269):128-129.
- Zarfl, C.; Lumsdon, A. E.; Berlekamp, J.; Tydecks, L. & Tockner, K. 2015. A global boom in hydropower dam construction. Aquatic Science 77(1):161-170.
Publication Dates
-
Publication in this collection
29 Nov 2018 -
Date of issue
2018
History
-
Received
21 Feb 2018 -
Accepted
10 Aug 2018