Abstract
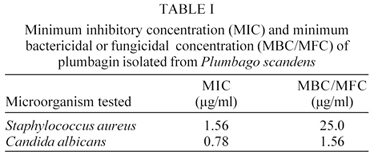
Plumbagin is a naturally occurring naphthoquinone isolated from roots of Plumbago scandens. The plant was collected at the Campus of Fundação Oswaldo Cruz, Rio de Janeiro, Brazil. P. scandens is used as a traditional medicine for the treatment of several diseases. The antimicrobial activity of plumbagin was evaluated using the macrodilution method. The compound exhibited relatively specific activity against bacteria and yeast. The minimum inhibitory concentration test showed the growth inhibiton of Staphylococcus aureus at a concentration of 1.56 µg/ml and of Candida albicans at a concentration of 0.78 µg/ml. These results suggest the naphthoquinone plumbagin as a promising antimicrobial agent.
Plumbago scandens; plumbagin; antimicrobial activity
ANTIMICROBIAL ACTIVITY
Antimicrobial activity in vitro of plumbagin isolated from Plumbago species
Selma Ribeiro de PaivaI,1 1 Corresponding author. Fax: +55-21-2456.2559. E-mail: s_paiva@far.fiocruz.br II; Maria Raquel FigueiredoII; Tânia Verônica AragãoIII; Maria Auxiliadora Coelho KaplanIV
IPrograma de Pós-Graduação em Biotecnologia Vegetal
IILaboratório de Química de Produtos Naturais
IIILaboratório de Microbiologia, Far-Manguinhos-Fiocruz, Rua Sizenando Nabuco 100, 21041-250 Rio de Janeiro, RJ, Brasil
IVNúcleo de Pesquisas de Produtos Naturais, UFRJ, Rio de Janeiro, RJ, Brasil
ABSTRACT
Plumbagin is a naturally occurring naphthoquinone isolated from roots of Plumbago scandens. The plant was collected at the Campus of Fundação Oswaldo Cruz, Rio de Janeiro, Brazil. P. scandens is used as a traditional medicine for the treatment of several diseases. The antimicrobial activity of plumbagin was evaluated using the macrodilution method. The compound exhibited relatively specific activity against bacteria and yeast. The minimum inhibitory concentration test showed the growth inhibiton of Staphylococcus aureus at a concentration of 1.56 µg/ml and of Candida albicans at a concentration of 0.78 µg/ml. These results suggest the naphthoquinone plumbagin as a promising antimicrobial agent.
Keywords:Plumbago scandens - plumbagin - antimicrobial activity
It is well known that infectious diseases account for high proportion of health problems, specially in the developing countries. Microorganisms have developed resistance to many antibiotics and this has created immense clinical problem in the treatment of infectious diseases (Davis 1994). This resistance has increased due to indiscriminated use of commercial antimicrobial drugs commonly used in the treatment of infectious diseases. This situation forced scientists to search for new antimicrobial substances from various sources, such as medicinal plants (Karaman et al. 2003).
Secondary metabolites produced by plants constitute a source of bioactive substances and nowadays the scientific interest has increased due to the search for new drugs from plant origin. Plumbago species are reported in the literature for its biological activities such as: antiparasitic (Chan-Bacab & Peña-Rodríguez 2001), insect antifeedant (Villavicencio & Perez-Escandon 1992), antitumoral (Devi et al. 1994) and others, some of them attributed to the presence of special chemical compounds, such as naphthoquinones.
Plumbagin is a naphthoquinone well distributed among Plumbago species, specially found in their roots (Van der Vijver 1972). This compound has been described in the literature and showed to possess a wide variety of bioactivities.
The microorganisms used for detecting antimicrobial activity were chosen for the following reasons: the bacterium Staphylococcus aureus was used due to its clinical relevance as a major cause of hospital acquired infections of surgical wounds and infections associated with indwelling medical devices. Besides, S. aureus rapidly develops resistance to many antimicrobial agents. Candida albicans is one of the most pervasive pathogenic fungi, especially infecting immuno-compromised hosts, in which it can invade various tissues (Esquenazi et al. 2002). Salmonella typhimurium can be found in a broad range of hosts as well as in the environment. Its infection is a serious public health problem in developing countries and represents a constant concern for the food industry. The severity and the outcome of a systemic Salmonella infection depends on the virulence of the bacteria strains, the infectious dose as well as the genetic makeup and immunological status of the host (Mastroeni 2002). The primary route by which humans acquire infection is by consumption of contaminated animal origin food. Escherichia coli is the best-known member of the normal microbiota of the human intestine and a versatile gastrointestinal pathogen. The varieties of E. coli that cause diarrhea are classified into named pathotypes, including enterotoxigenic, enteroinvasive, enteropathogenic, and enterohemorrhagic E. coli. Individual strains of each pathotype possess a distinct set of virulence-associated characteristics that determine the clinical, pathological and epidemiological features of the diseases they cause (Robins-Browne & Hartland 2002).
The present study was conducted to investigate antimicrobial properties of the naphthoquinone plumbagin isolated from roots of P. scandens against some bacteria and yeast.
MATERIALS AND METHODS
Plant collection - Roots of P. scandens were collected at the campus of Fundação Oswaldo Cruz, Rio de Janeiro, Brazil. A voucher of this plant was deposited at Rio de Janeiro Botanical Garden Herbarium (RB) under the number 340.340.
Plant extraction and fractionation - Roots of P. scandens were oven dried at 40°C and powdered (450 g). After they were exhaustively extracted with chloroform for 30 h in a Soxhlet apparatus. Evaporation of the solvent under reduced pressure gave 7.5 g of crude extract. A portion (5 g) of the crude chloroform extract from roots of P. scandens was subject to fractionation by column chromatography on silica gel, eluted with n-hexane, ethyl actetate, and methanol. The fraction PSRC1/13, eluted in hexane/ethyl acetate 2%, yielded 1.18 g of yellow needles identified as the naphthoquinone plumbagin by spectroscopic analysis and by comparison with literature data.
Instruments - 1H and 13C nuclear magnetic ressonance were performed on a Brüker AC200 spectrometer. GC/MS was performed on a Hewlett-Packard 6890 gas chromatograph equipped with a mass selective detector, model HP 5972 with data base.
Preparation of samples for testing - Plumbagin was dissolved in 30% dimethyl sulfoxide (DMSO). For the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) tests, plumbagin was first diluted to the highest concentration (50 µg/ml) and then serial twofold dilutions were performed in a concentration range from 0.098 µg/ml to 50 µg/ml in tubes containing Müeller-Hinton broth. The final concentration of DMSO in tubes was 3%, a value that did not cause interference in the antimicrobial activity. Pre-experimental procedures demonstrated that final DMSO concentration bellow 5% did not inhibit microorganims growth.
Microorganisms - The following microorganisms were used for detecting antibacterial activity: S. aureus (ATCC 6538), E. coli (ATCC 8739), S. typhimurium (ATCC 14028), and C. albicans (ATCC 10231).
Antimicrobial susceptibility testing - MIC of plumbagin was determined by macrodilution technique as described by the National Committee for Clinical Laboratory Standards (1993). The bacteria inoculum was prepared in 4 ml triptic soy broth and incubated at 37°C, over night. The C. albicans culture was grown in Sabouraud at 28°C for 24 h before being used. The cultures were diluted in Müeller-Hinton broth at a density adjusted to a 0.5 McFarland turbity standard [1 to 2 x108 colony-forming units (CFU)/ml]. The bacteria suspension was diluted 1:10 in Müeller-Hinton broth and 25 µl of it was added to 0.5 ml of the diluted sample. The yeast suspension was not diluted. The final inoculum was of approximately 5 x 105 CFU/ml. Controls with 0.5 ml of culture medium without the sample and other without microorganisms were used in the tests. Tubes were then incubated at 37°C for 24 h (bacteria) and at 28°C for 24/48 h (yeast). The activity was measured by the tubes turbidity. In tubes where the microorganism growth was not visible, a suspension aliquot of 0.1 ml was poured into pre-sterilized Petri dishes with Müeller-Hinton agar medium, in order to investigate the possible bactericidal activity of the sample. The antimicrobial testing was conducted in duplicate. MIC was the lowest concentration of antimicrobial agent that completely inhibited growth of the organism in the tubes, while MBC and the minimum fungicidal concentration (MFC) were defined as the lowest concentration of antimicrobial agent able to kill the organisms in their totality (maximum of 3 colonies).
RESULTS AND DISCUSSION
Traditionally, P. scandens has been used to treat many diseases, some of them caused by bacteria (Duke & Beckstrom-Sternberg 2002). The results from the current study revealed that the naphthoquinone plumbagin could be the main constituent from roots responsible for its activity. Ahmad et al. (1998) and Desta (1993) reported that aqueous and alcoholic extracts from roots of P. zeylanica showed antibacterial activities against S. aureus, Pseudomonas aeruginosa, Bacillus subutilis, Proteus vulgaris, and against the yeast C. albicans.
Plumbagin exhibited relatively specific antimicrobial activity. The growth of S. aureus and C. albicans was completely inhibited. However it was ineffective against the Gram-negative bacteria E. coli and S. typhimurium, demonstrating the specificity of plumbagin activity.
MIC and MBC/MFC tests against S. aureus and C. albicans were performed with the naphthoquinone plumbagin in order to evaluate its potential. The results are presented in Table I.
The results obtained for S. aureus were interesting. According to the National Committee for Clinical Laboratory Standards (2000), penicillin and oxacillin can be used to test the susceptibility or resistance of S. aureus strains. The MIC interpretative standards can be seen in Table II. Comparing the obtained results to the standards, the naphthoquinone plumbagin could be considered as a promising antimicrobial agent.
Plumbagin demonstrated a great potential against yeast. The discoveries of potential antifungal agents are encouraging in replacing the current commercial antifungal drugs that induce many types of toxicity in patients (Somchit et al. 2003). C. albicans, the agent of candidiasis, is an increasingly important disease that has a worldwide distribution due to the fact that it is a frequent opportunistic pathogen in AIDS patients.
Toxicological studies on this naphthoquinone and other compounds must be performed to ensure the safety for their use. The discovery of a potent remedy from plant origin will be a great advancement in fungal and bacterial infection therapies.
Plumbagin showed an interesting activity against the Gram-positive bacteria S. aureus and a very high inhibitory activity against C. albicans. These results indicate the naphthoquinone plumbagin as a potential antibiotic to be considered in this moment of great spread of C. albicans.
ACKNOWLEDGEMENTS
To Maria Antonieta Ferrrara from Far-Manguinhos-Fiocruz.
National Committee for Clinical Laboratory Standards 1993. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 3rd ed., Approved standard (M7-A3), Wayne, Pa.
National Committee for Clinical Laboratory Standards 2000. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 5th ed., Approved standard (M7-A5), Wayne, Pa.
Received 2 April 2003
Accepted 28 August 2003
- Ahmad I, Mehmood Z, Mohammad F 1998. Screening of some Indian medicinal plants for their antimicrobial properties. J Ethnopharmacol 62: 183-193.
- Chan-Bacab MJ, Peña-Rodríguez LM 2001. Plant natural products with leishmanicidal activity. Nat Prod Rep 18: 674-688.
- Davis J 1994. Inactivation of antibiotics and the dissemination of resistence genes. Science 264: 375-382.
- Desta B 1993. Ethiopian traditional herbal drugs. Part II. Antimicrobial activity of 63 medicinal plants. J Eth-nopharmacol 39: 129-139.
- Devi PU, Solomon FE, Sharada AC 1994. In vivo tumor inhibitory and radiosensitizing effects of an Indian medicinal plant, Plumbago rosea on experimental mouse tumors. Indian J Exp Biol 32: 523-528.
- Duke JA, Beckstrom-Sternberg SM 2002. Phytochemical Database, USDA ARS NGRL, Beltsville Agricultural Research Center, Beltsville, Maryland. http://www.ars-grin.gov/duke/ethnobot.html
- Esquenazi D, Wigg MD, Miranda MMFS, Rodrigues HM, Tostes JBF, Rozental S, Silva AJR, Alviano CS 2002. Antimicrobial and antiviral activities of polyphenolics from Cocos nucifera Linn. (Palmae) husk fiber extract. Res Microbiol 153: 647-652.
- Karaman I, Sahin F, Güllüce M, Ögütçü H, Sengul M, Adigüzel A 2003. Antimicrobial activity of aqueous and methanol extracts of Juniperus oxycedrus L. J Ethnopharmacol 2837: 1-5
- Mastroeni P 2002. Immunity to systemic Salmonella infections. Curr Mol Med 2: 393-406.
- Robins-Browne RM, Hartland EL 2002. Escherichia coli as a cause of diarrhea. J Gastroenterol Hepatol 17: 467-475.
- Somchit MN, Reezal I, Nur E, Mutalib AR 2003. In vitro antimicrobial activity of ethanol and water extracts of Cassia alata. J Ethnopharmacol 84: 1-4.
- Van der Vijver LM 1972. Distribution of plumbagin in the Plumbaginaceae. Phytochemistry 11: 3247-3248.
- Villavicencio MA, Perez-Escandon BE 1992. Plumbagin activity (from Plumbago pulchella Boiss. Plumbaginaceae) as a feeding deterrent for three species of Orthoptera. Folia Entomol Mex 86: 191-198.
Publication Dates
-
Publication in this collection
07 Jan 2004 -
Date of issue
Oct 2003
History
-
Accepted
28 Aug 2003 -
Received
02 Apr 2003