Abstract
Infections due to protozoa of the genus Leishmania are a major worldwide health problem, with high endemicity in developing countries. The drugs of choice for the treatment of leishmaniasis are the pentavalent antimonials (SbV), which present renal and cardiac toxicity. Besides, the precise chemical structure and mechanism of action of these drugs are unknown up to date. In order to find new drugs against leishmaniasis, we have been studying extracts of Brazilian trees. In the present study, we have evaluated the effectiveness of an alkaloid extract of Aspidosperma ramiflorum Muell. Arg. (Apocynaceae), against the extracellular forms promastigotes of L. (L.) amazonensis and L. (V.) braziliensis. The alkaloid extract of A. ramiflorum was much more effective against L. (L.) amazonensis (LD50 < 47 µg/ml) than L. (V.) braziliensis. Based on these in vitro results against L. (L.) amazonensis new studies should be made to find the compounds with anti-leishmanial activity.
Apocynaceae; anti-leishmanial activity; Brazilian trees; - monoterpenoid indolic alkaloids; Aspidosperma ramiflorum; Leishmania amazonensis; - Leishmania braziliensis
ANTIMICROBIAL AND ANTIPANOSITIC DRUGS ACTIVITIES
Anti-leishmanial activity of alkaloidal extract from Aspidosperma ramiflorum
Izabel Cristina Piloto FerreiraI; Maria Valdrinez Campana LonardoniII; Gerzia MC MachadoIII; Leonor L LeonIII; Lucílio Gobbi FilhoI; Luís Henrique Bissoli PintoI; Arildo José Braz de OliveiraI, 1 1 Corresponding author. Fax +55-44-2636231. E-mail: ajboliveira@uem.br
ILaboratório de Química Farmacêutica e Síntese de Medicamentos, Departamento de Farmácia e Farmacologia
IILaboratório de Imunologia Clínica, Departamento de Análises Clínicas, Centro de Ciências da Saúde, Universidade Estadual de Maringá, Av. Colombo 5790, 87020-900 Maringá, PR, Brasil
IIIDepartamento de Imunologia, Instituto Oswaldo Cruz-Fiocruz, Rio de Janeiro, RJ, Brasil
ABSTRACT
Infections due to protozoa of the genus Leishmania are a major worldwide health problem, with high endemicity in developing countries. The drugs of choice for the treatment of leishmaniasis are the pentavalent antimonials (SbV), which present renal and cardiac toxicity. Besides, the precise chemical structure and mechanism of action of these drugs are unknown up to date. In order to find new drugs against leishmaniasis, we have been studying extracts of Brazilian trees. In the present study, we have evaluated the effectiveness of an alkaloid extract of Aspidosperma ramiflorum Muell. Arg. (Apocynaceae), against the extracellular forms promastigotes of L. (L.) amazonensis and L. (V.) braziliensis. The alkaloid extract of A. ramiflorum was much more effective against L. (L.) amazonensis (LD50 < 47 µg/ml) than L. (V.) braziliensis. Based on these in vitro results against L. (L.) amazonensis new studies should be made to find the compounds with anti-leishmanial activity.
Key words: Apocynaceae - anti-leishmanial activity - Brazilian trees - monoterpenoid indolic alkaloids - Aspidosperma ramiflorum - Leishmania amazonensis - Leishmania braziliensis
Leishmaniasis is a tropical disease caused by protozoa of the Leishmania genus. These protozoa cause a disease with different clinical forms, among them cutaneous, hyperergic, mucocutaneous, and anergic diffuse leishmaniasis (Leon et al. 1990a). The disease is endemic in some geographical areas of Brazil, where it constitutes a serious health problem (Lonardoni et al. 1999, Leon et al. 2002).
The drugs of choice for the treatment of leishmaniasis are the pentavalent antimonials (SbV), but they present renal and cardiac toxicity. A second choice for the treatment of the disease is a diamidine (pentamidine isethionate), which also has serious side effects (Korolkovas & Burckhalter 1988). However already in some trials alternative pharmaceutical formulations have been used to reduce the toxicity of these drugs (Frezard et al. 2000)
The lack of an effective anti-leishmanial drug led a renewed interest in the study of traditional remedies as sources for the development of new chemotherapeutic compounds with better activity and less toxicity. Several plants have been used for the treatment of parasitic diseases (Araujo et al. 1998). In order to find new drugs against leishmaniasis, we have studied alkaloidal extracts of Brazilian plants (Oliveira et al. 2002). Aspidosperma ramiflorum Muell. Arg. (Apocynaceae), commonly know as "guatambu", is a tree which grows from to 12-30 m in height and is native to the forests in Southeastern Brazil (Lorenzi 1992). Some alkaloids have been previously isolated from the stem bark (Reis et al. 1996), and the alkaloid extract of the bark showed antimicrobial activity against Gram positive and negative bacteria (Oliveira 1999). The aim of the present study is to investigate the anti-leishmanial activity of alkaloidal extracts from the stem bark of A. ramiflorum against L. (V.) braziliensis and L. (L.) amazonensis.
MATERIALS AND METHODS
Plant materials - A. ramiflorum Muell. Arg. was collected in the Horto Florestal de Maringá, July 2000, in Maringá, state of Paraná, Brazil. The plant was collected and identified by Prof. Dr Ismar Sebastião Moscheta and an exsiccatum deposited and authenticated at the Herbarium of the State University of Maringá, Maringá, Brazil.
Extraction of plant materials - Air-dried stem bark (1 kg) was extracted with 70% ethanol at room temperature. After removal of the ethanol, the crude extract was added to a 10% acetic acid solution (v/v) and kept at 5º overnight. After filtration, the aqueous phase was first extracted with chloroform (acid extract), then the pH raised to 10 and the resulting solution re-extracted with chloroform (basic extract). The two chloroform extracts were concentrated under reduced pressure, and then lyophilised yielding the acid (7.7 g) and basic fractions (11.6 g), which were both analyzed by thin layer chromatography (TLC) and high performance liquid chromatography (HPLC). The main bulk of the alkaloids was in the basic fraction which was called the alkaloidal extract and which was used for the assays.
Characterization of the alkaloidal extract - The alkaloidal extract from A. ramiflorum was analyzed and compared with isolated samples of alkaloids from the stem bark of the plant using TLC on silica gel GF254 developed with CHCl3:AcOEt:Triethylamine (49.5:49.5:1.0) in an NH3 atmosphere. For HPLC analysis, the crude extract was dissolved in CH2Cl2:MeOH (80:20) and 10 µl were injected onto a Waters µ-Bondapak RP-18 (reverse phase, 4.6 mm x 250 mm) column at 40º. Solvent A was 100 mmol l-1 ammonium formate in 0.12% octanesulfonic acid (v/v)/, formic acid and acetonitrile (88:4:8, v/v), while solvent B consisted of 100 mmol l-1 aqueous ammonium formate containing 0.12% octanesulfonic acid (v/v)/formic acid/acetonitrile (64:4:32, v/v). The separation was carried out using a mixture of solvent A and, a progressively increasing amount of B (0, 10, 40, 90, 100%) during 60 min. The flow rate was 1.3 ml min-1. The effluent was monitored with a photodiode-array detector with windows at 222 nm and 254 nm and also by mass spectral analysis of isolated eluates.
Culture and maintenance of the parasite - L. (V.) braziliensis promastigotes and L. (L.) amazonensis, MHOM/BR1987/M11272 and MHOM/BR/1977/LTB0016 promastigotes, were grown at 25º in Schneider's Drosophila medium supplemented with 10% (v/v) heat-inactivated fetal calf serum (FCS). Cells were harvested in the late log phase, resuspended in fresh medium, counted in Neubauer's chamber and adjusted to a concentration of 4 x 106/ml.
Anti-leishmanial in vitro assay with L. (V.) braziliensis and L. (L.) amazonensis promastigotes - The alkaloidal extract was added to the promastigote cultures, at 4 x 106/ml, as above, for screening (from 320 mg/ml to 0.125 mg/ml of extract) solubilized in dimethylsulfoxide (DMSO) (the highest concentration used was 1.6%, v/v) and incubated at 25º. After 24 h of incubation, the surviving parasites were counted in a Neubauer's chamber and compared with controls, which only had DMSO. All tests were done in triplicate and pentamidine isethionate (Eurofarma) was used as reference drug (Leon et al. 2002). The LD50/24 values were determined by linear regression analysis from this inhibition percentage using statistic error limits up 10%.
RESULTS AND DISCUSSION
The anti-leishmanial activity of plant extracts has been attributed to compounds belonging to diverse chemical groups, such as isoquinoline alkaloids, indole alkaloids, quinones, and terpenes (Araujo et al. 1998).
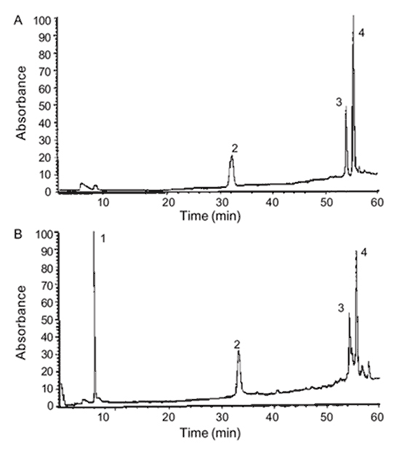
The alkaloidal extract of A. ramiflorum was chosen for assays because of the presence of bisindole monoterpene alkaloids (Fig. 1) with structures similar to alkaloids from Strychnos usambarensis, which have been reported to possess antiprotozoal activity (Angenot et al. 1991). The major constituents of A. ramiflorum alkaloidal extracts are: ramiflorine A (1) and ramiflorine B (2), whose presence was monitored by HPLC (Fig. 2) and TLC (Oliveira et. al. 2002).
Fig. 1: alkaloids from Aspidosperma ramiflorum and Strychnos usambarensis
Fig. 2. A: high performance liquid chromatography (HPLC) chromatogram of standards mixture isolated from Aspidosperma ramiflorum; B: HPLC chromatogram of alkaloidal extract. Peaks - 1: internal standard (tryptophol); 2: 10-methoxy-geissoschizol; 3: ramiflorine A; 4: ramiflorine B
In the present study, we evaluated the effectiveness of a crude alkaloid extract of A. ramiflorum against the extracellular form (promastigotes) of L. (L.) amazonensis and L. (V.) braziliensis. The alkaloid extract was more effective against the L. (L.) amazonensis (LD50 < 47 µg/ml) than L. (V.) braziliensis.
Although the mode of action of these alkaloids is not known, the fact that they are similar in structure to usambarine (3) and usabarensine (4), makes it possible that their modes of action may be similar, that is, they would acting as inhibitors of protein synthesis (Angenot et al. 1991).
This preliminary positive result suggests further work with isolated compounds to evaluate the individual activity of ramiflorine A (1) and ramiflorine B (2), as part of a continued search for new drugs with high activity and low side effects against diseases associated with protozoan parasites, such as leishmaniasis.
Received 13 November 2003
Accepted 6 February 2004
- Angenot L, Quentin-Leclercq J, Phillipson DJ, Warhurst DC, O'Neill MJ, Bray DH, Wright CW 1991. Antiamoebic and antiplasmodial activities of alkaloids isolated from Strychnos usambarensis Planta Medica 57: 337-340.
- Araujo CAC, Alegrio LV, Leon LL 1998. Antileshmanial activity of compounds extracted and characterized from Centrolobium sclerophyllum. Phytochemistry 49: 751-754.
- Frezard F, Michalick MSM, Soares CF, Demicheli C 2000. Novel methods for encapsulation of meglumine antimoniate into liposomes. Braz J Med Biol Res 33: 841-846.
- Leon LL, Gomes DCF, Alegrio LV, Lima MEF, Araújo CAC 2002. Synthetic derivatives of curcumin and their activity against Leishmania amazonensis. Arzneim Forsch/Drug Res 52: 120-124.
- Leon LL, Machado GMC, Carvalho-Paes LE, Grimaldi JG 1990a. Antigenic differences of Leishmania amazonensis isolates causing diffuse cutaneous leishmaniasis. Trans R Soc Trop Med Hyg 84: 678-680.
- Leon LL, Machado GMC, Carvalho-Paes LE, Grimaldi JG, Barral A 1990b. Antigenic differences among Leishmania amazonensis isolates and their relationship with distinct clinical forms of the disease. Mem Inst Oswaldo Cruz 87: 229-234.
- Korollkovas A, Burckhalter JH 1988. Química Farmacêutica, Guanabara, Rio de Janeiro, 39 pp.
- Lonardoni MVC, Silveira TGV, Arraes SMAA, Bertolini DA, Teodoro U 1999. Observações sobre o diagnóstico laboratorial e a epidemiologia da leishmaniose tegumentar no Estado do Paraná, sul do Brasil. Rev Soc Bras Med Trop 32: 413-423.
- Lorenzi H 1992. Árvores Brasileiras, Plantarum, Nova Odessa, São Paulo, Brasil, p. 21-26.
- Oliveira, AJB 1999. Estudo de Seis Espécies do Gênero Aspidosperma Utilizando GC, GC/MS e HPLC: Análise Qualitativa e Quantitativa. Teste Bioautográfico; Cultura de Tecidos e Células Vegetais e Rota de Preparação dos Compostos Diméricos Ramiflorina A e Ramiflorina B, PhD Thesis, Unicamp, Campinas.
- Oliveira AJB, Koike L, Shepherd SKL, Reis FAM 2002. Callus culture of Aspidosperma ramiflorum Muell. Arg.: growth and alkaloid production. Acta Scientiarum 23: 609-612.
- Reis FAM, Marques MFS, Filho HLF, Kato L 1996. Indole alkaloids from Aspidosperma ramiflorum Phytochemistry 41: 963-967.
Publication Dates
-
Publication in this collection
20 July 2004 -
Date of issue
May 2004
History
-
Accepted
06 Feb 2004 -
Received
13 Nov 2003