Abstract
Despite efforts to eradicate American trypanosomiasis (AT) and Chagas disease from the Americas, there are still areas of active transmission that can eventually become a source of reinfection in previously controlled regions. Mexico could be one of those areas, where there are no formal preventive control programs despite the presence of communities infested by Triatominae bugs infected with Trypanosoma cruzi. This study explored the prevalence of T. cruzi infection in 405 habitants of 17 communities in the state of Colima, on the Pacific Mexican coast, through a seroepidemiological probabilistic survey. The results revealed a point seroprevalence of 2.4% positive for anti-T. cruzi. In addition, 2 clinical cases of chronic and 2 of acute Chagas disease were detected in the explored communities. These findings confirm the risk of active transmission of AT in Western Mexico, especially in rural and suburban communities infested with intra-domestic triatominae, where control programs should be implemented.
Chagas disease; American trypanosomiasis; Colima
EPIDEMIOLOGY
Active transmission of human chagas disease in Colima Mexico
Rafael Coll-CárdenasI; Francisco Espinoza-GómezII, 1 1 Corresponding author. Fax: +52-312 3-16-10-99. E-mail: fespin@cgic.ucol.mx ; Arcadio Maldonado-RodríguezII; Pedro A Reyes-LópezIII; Miguel Huerta-VieraI; Fabián Rojas-LariosII
ICentro Universitario de Investigaciones Biomédicas
IIFacultad de Medicina, Universidad de Colima, Av. Universidad 333, Colonia Las Víboras, Colima, Colima, México
IIIInstituto Nacional de Cardiología "Ignacio Chávez", Tlalpan DF, México
ABSTRACT
Despite efforts to eradicate American trypanosomiasis (AT) and Chagas disease from the Americas, there are still areas of active transmission that can eventually become a source of reinfection in previously controlled regions. Mexico could be one of those areas, where there are no formal preventive control programs despite the presence of communities infested by Triatominae bugs infected with Trypanosoma cruzi. This study explored the prevalence of T. cruzi infection in 405 habitants of 17 communities in the state of Colima, on the Pacific Mexican coast, through a seroepidemiological probabilistic survey. The results revealed a point seroprevalence of 2.4% positive for anti-T. cruzi. In addition, 2 clinical cases of chronic and 2 of acute Chagas disease were detected in the explored communities. These findings confirm the risk of active transmission of AT in Western Mexico, especially in rural and suburban communities infested with intra-domestic triatominae, where control programs should be implemented.
Key words: Chagas disease - American trypanosomiasis - Colima, Mexico
American trypanosomiasis (AT) is a parasitosis characteristically found in certain wild mammals on the American continent, caused by the protozoan Trypanosoma cruzi (Mastigophora, Kinestoplastidae). This parasitosis can eventually affect man causing Chagas disease manifested in an acute form, with persistent fever accompanied by cutaneous lesions (inoculation chagoma), ocular manifestations (Romaña's sign), lymphadenopathy, carditis or alterations to the central nervous system and is often confused with many other febrile diseases (WHO 2000, Moncayo 2003). Up to 30% of the people infected with AT can develop a chronic form of Chagas disease affecting the heart, digestive tract or peripheral nervous system, generally with an inexorable evolution towards fatal complications (Prata 2001). In general, at the beginning of the chronic phase of the disease exists the indeterminate form, in which anti-T. cruzi antibodies are present, but without any clinical manifestations. Most people affected by AT are discovered in this phase (Dias et al. 2003).
The most common route of infection in humans is by contact with defecations contaminated by T. cruzi, excreted by triatomine bugs, haematophagous insects belonging to the subfamily triatominae (Reduviidae, Hemiptera). Therefore the main risk factor for the transmission of Chagas disease is the presence of these insects, infected by T. cruzi in human dwellings (Cohen & Gurtler 2001), although recently the importance of transplacental transmission (Nisida et al. 1999) as well as through blood transfusions (Schmunis 1999) has been emphasized.
The presence of housing infested by triatominae continues to be a prevalent condition in extensive areas of Latin America, especially in rural or suburban communities, where it is estimated that there are 16,000,000 people infected with AT, of which possibly 3,000,000 suffer from a chronic form of Chagas disease. This has represented a large social burden for the entire region (WHO 2000), and for this reason, in 1998, the World Heath Organization approved the resolution WHA 51.14 with the purpose of eliminating the transmission of this disease from the American continent (WHO 1998).
Thanks to the South Cone and Andine countries initiatives, it has been possible to noticeably reduce the Chagas transmission in almost all of the South American subcontinent. However, in most northern regions of Latin America, including Mexico, the situation of Chagas disease is still uncertain. In accordance with the WHO classification of endemic countries, Mexico is placed in group 2, which refers to those countries that do not have formal control programs in spite of the presence of AT transmission (Moncayo 2003). This is fundamentally due to the fact that AT is not considered to be a public health problem and the epidemiological studies realized in the region appear to be incomplete (Sosa et al. 2003).
According to recent reviews on the state of the transmission of Chagas disease in Mexico, estimates show a prevalence of 0.3 in the large urban zones up to 15 % of infection by T. cruzi in rural communities of southern states of Chiapas and Oaxaca (Velasco Castrejón et al. 1992, Guzmán Bracho 2001, Flisser et al. 2002). By this we can assume that there are around 1,600,000 people who are carriers of this parasite infection, even though the recognition of clinical cases barely reaches 441 cases in the entire country (Dumonteil 1999). On the other hand, the incidence of this disease has scarcely been explored in some areas of the country, such as Jalisco where it reaches up to 2/1000 people annually (Contreras et al. 2000). Meanwhile the presence of the acute disease has been practically nil for the last 10 years (Lozano Kasten et al. 1993). This situation has conditioned the sanitary authorities to minimize the need to implement expensive control measures for a relatively insignificant health problem. However, it is possible that a significant amount of patients with Chagas disease are actually "masked" among patients with heart disease (in the chronic phase) or with a fever of uncertain origin (in the acute phase), and are not registered by the health systems, due to a lack of clinical suspicion, or lack of adequate diagnostic resources in areas infested by triatominae (Dumonteil 1999, Ramsey & Schofield 2003).
The state of Colima, located on the Mexican Pacific coast is an area where the presence of triatominae infected by T. cruzi has been identified in close contact with human dwelling areas (Espinoza et al. 2002), reason for which it is feasible to find active transmission of AT and cases of Chagas disease in this area. In spite of this assumption, there are only reports of one patient being diagnosed with Chagasic megaesophagus (Prieto et al. 2000) and other isolated anecdotal cases of unproven Chagasic cardiomyopathy in Colima. The objective of this study is to explore the prevalence of AT in the population of Colima, and at the same time identify the presence of Chagas disease among a group of inhabitants belonging to the studied communities. These results could contribute towards obtaining a more complete panorama of the actual state of this disease in Mexico and collaborate in this way, with the Central American eradication initiative. The recognition of active focal points of transmission of AT is very important in order to detain the persistent transmission of the disease, whether it be through blood donors, or the reinfestation of previously cleared zones by infected triatominae.
MATERIALS AND METHODS
The study consisted in a seroepidemiological probabilistic survey of a population base, taken along a north-south transect of the state of Colima (between 18º56' to 19º 23'NL, and 103º35' to 104º02' WL). The transect covers an area of approximately 600 km2 and includes the largest concentration of human settlements in the region (Figure). Seventeen communities located within this area, were selected and classified as urban or rural according to the criteria set by the Instituto Nacional de Estadística Geografía e Informática (INEGI 2000). They were also stratified, according to their ecological characteristics, in two categories: (a) high ecotope between 500 and 1200 m above sea level, with a semicalid humid climate ACw, and (b) low or coastal ecotope, less than 490 m above sea level with a hot and dry climate BS1 (h'), following Köepen's modified classification (CGSNEGI 1992). In the 17 communities, a total of 218 dwellings were surveyed. The communities and dwellings were selected randomly and in proportion to the number of inhabitants. In some rural localities, the number of samples was relatively high, due to the fact that several rural communities, dispersed within a radius of 5 km, were included in the same locality. In each dwelling, an entomological survey was conducted in search of triatominae, following the hour-man-house method proposed by Schofield (1978). The collected specimens were classified according to the criteria set by Lent and Wygodzinski (1979). At the same time, some of the inhabitants were surveyed and a 5 cc venous blood sample taken from the forearm was collected from each person. The blood was centrifuged, serum was obtained and stored at 20ºC until processing. The number of people surveyed was calculated based on an estimated national prevalence of 1.6% (Velasco Castrejón et al. 1992, Guzmán 2001) with an absolute precision of 1.2%, estimating this as a medium point between the large variation observed along the country (for example 0.3% in Colima to 17% in Chiapas) which provided the recommended number of 420 individuals (EPIDAT 1997). This phase of the study was performed during the period January 1998 to August 1999 and was part of a study on the epidemiology of Chagas disease in Colima.
During this survey, 61 people with clinical patterns suggestive of Chagas disease were included separately. These cases were both acute, manifested by fever of uncertain origin for more than a week, accompanied by cutaneous lesions such as probable inoculation chagoma (indurated, erythematous or brown nodules), lymphadenopathy or acute myocarditis; and chronic, among patients with chronic heart disease. These people were all inhabitants of the surveyed areas, referred by neighbors or by physicians from the community and their clinical status was verified by an internist who participated in the project. They were recorded at their address during the survey, or in public hospitals in the city of Colima, belonging to the Secretary of Health (SS) and the Mexican Institute of Social Security (IMSS).
In each case, the following data was registered: age, sex, current, and previous places of residency, as well as history of exposure to bugs at home. People older that 70 years old, with a history of diabetes mellitus, HIV infection, blood transfusions, or that had lived less than 5 years at their present address, were excluded. A venous blood sample was collected from each of them and the separated serum was stored at 20ºC before analysis.
In each of the stored serum samples, the presence of anti-T. cruzi antibodies was determined, with an indirect hemagglutination test, diluted with 2 mercaptoethanol (IHA, Chagatest, Wiener labs, Argentina) in triplicate testing. In the case of positive samples (titers equal to or higher than 1:8 with 2ME), a second confirmatory test was performed, with indirect immunofluorescence antigen test (IIF), or with Western Blot (WB), following the guidelines proposed by Monteón et al. (1997). Twelve serum samples were analyzed with the polimerase chain reaction (PCR RAPDS test), using TC3 and TC4 primers from kinetoplastic T. cruzi DNA, according to the recommendations made by Kirchhoff et al. (1996). In the suspected acute cases a blood smear by the microhematocrit concentration method (Feilij et al. 1983) and hemoculture of 1 ml of blood in LIT and biphasic NNN medium was obtained. These cultures were revised at 2, 6, 14, 30, and 60 days after incubation.
This project was approved by the Institutional Commission for Investigation and Bioethics of the Secretary of Health of Colima, as a minimal intervention protocol.
The data was analyzed with contingency tables using the EPIDAT 2.0 program (1997) and the correlation between the presence of AT and the ecological and demographic variables was estimated by Poisson regression with the PEPI v. 4.0 program (Abramson & Gahlinger 2001). In each case the confidence interval to 95% (CI), rate ratio (RR) and the regression coefficient is expressed.
RESULTS
Table I shows data corresponding to the 17 communities explored during the first phase of the study. In total, 405 blood samples were collected in the 218 homes surveyed, of which 10 resulted positive to IHA; 4 of them at titers higher than 1:16 and 6 with dilutions 1:8. Of the 12 serum samples analyzed with the PCR test, 4 resulted to be positive, all of which had titers of IHA 3 1:8 (2 in Colima-Villa de Álvarez, 1 in Cuauhtémoc, and the other in El Trapiche) while of the other 8 negative samples, only 1 resulted with IHA > 1:16 in the locality of Alcaraces, which was nonetheless included in the table. All of the positive cases were found in the high ecotope (10/321) while in the low coastal area there were not any positive serum samples among the 84 samples that were collected. The same Table I shows the entomological findings by locality, which have been presented in more detail in a previous publication (Espinoza et al. 2002), as well as the correlation between the index of triatominae per house and the presence of TA infection in each locality, analyzed by Poisson regression.
Table II shows the distribution of the sampled individuals by age group, gender, and those having previously received blood transfusions, with their respective correlation estimates by Poisson regression.
During the second phase of the study, between 1998 and 2000, 61 patients with clinical patterns suggestive of Chagas disease were detected. Of them, 23 corresponded to patients with chronic heart disease, 18 with fever of uncertain origin, and 20 people with cutaneous lesions suggestive of a chagoma at the site of presumed triatomine bite. Twenty-eight of these samples were obtained in the suburban areas of Colima-Villa de Álvarez, 5 in Cuauhtémoc, 8 in Coquimatlán, 8 in Armería, 2 in Joyitas, 2 in Cofradía de Juárez, 2 in Chiapa-Ocotillo, and 2 in Quesería. Thirty samples were taken in the community, 22 in the Regional Hospital of Colima, belonging to the Secretary of Health, and 9 in the General Hospital Zone No. 1 of the IMSS.
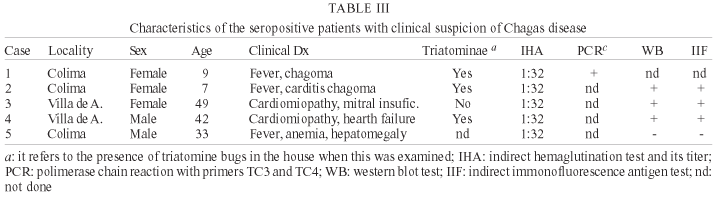
Of these 61 patients, the IHA test resulted positive (titer 3 1:32) in 5 cases (8.2%) and in 4 of them a confirmatory test was obtained, 1 by the PCR test and 3 by IIF and WB as shown on Table III. The parasite was not identified in any of the patients by means of blood smears or by hemoculture in NNN culture medium.
DISCUSSION
The present study confirms the presence of transmission of AT in the state of Colima, Mexico, with a point seroprevalence of 2.4%, noticeably higher than the expected for the national mean and than previous studies in the region (Velasco Castrejón et al. 1992). Although the indirect hemagglutination test, at titers of 1:8, is not considered in the diagnostic criteria for Chagas disease, the dilution with 2-ME increases considerably its sensitivity, meanwhile the cross-reactivity with other haemoflagelate infections in Mexico, like leishmaniasis or other trypanosomiasis is quite improbable. Therefore several authors validate these titers as positive indicators of anti-T. cruzi antibodies (Velasco Castrejón et al. 1992, Sánchez Guillén et al. 2002). The community cases detected by this means could reflect an asymptomatic infection, possibly in the indeterminate phase of the disease.
The presence of seropositive individuals showed a significant correlation with the proportion of triatominae per house in the explored communities, especially where more than two bugs per house were found. This appears to be a logical phenomenon when triatominae infected with T. cruzi are found inside the dwelling (Cohen & Gürtler 2001). However, until now this finding had not been documented for this part of the country, nor for the complex Triatoma phyllosoma species, endemic to all of the central-occidental region of Mexico, which have proven to be an effective vector for AT (Martínez Ibarra et al. 2001).
The significant coincidence of intra-domestic triatominae with the positive cases of AT, both asymptomatic and clinical, all of which are located in rural or periurban areas in the high ecotope, between the cities of Colima, Villa de Álvarez, and Cuauhtémoc, suggests that the transmission of AT is focalized in rural or recently urbanized human settlements in certain well defined geographic environments, where the natural nesting places for triatominae are disturbed (Ramsey & Schofield 2003). In this case the triatominae belonging to the phyllosoma complex are considered sylvatic species, but particularly able to adapt to human habitats (Lent & Wygodzinski 1979, Martínez Ibarra et al. 2001, Espinoza et al. 2002), especially in the semicalid ecotope between 400 and 900 m above sea level. This is why it is recommended that preventative programs be centered on such communities at risk, which should be identified at the first opportunity by entomological surveys, ecological characteristics, and by demographic growth projections, before the appearance of Chagas disease in people. Recognition of areas infested by sylvatic triatominae in the process of adaptation to human domestic environments and active transmission of Chagas disease in Mexico will contribute to more rational and efficient planning, through vector control campaigns and community education focused on risk areas. At the same time it could reduce the probability of the exportation of infections to areas that are, up until now, problem free.
Of the 10 individuals who resulted positive, 9 were women and 1 was a man, even when both groups had similar age and geographic distribution. Although not statistically significant, this suggests that women are more prone to become infected, possibly due to spending more time in the home, in contact with the insect vector. There was no significant correlation between age and infection. In this sense, it is remarkable the presence of one asymptomatic 2 years old girl. This finding, added to the presence of very suggestive cases of acute Chagas in another 2 girls, and in an adult with fever and hepatomegaly, although not confirmed by parasitemia, but positive for IIF and PCR, that are considered strong evidence of acute Chagas disease by some authors (Añez et al. 1999, Antas et al. 1999), verifies the assumption that there is active transmission of AT and human Chagas disease in Colima. For this reason, acute Chagas disease should be considered in the differential diagnosis of persistent fever, especially in cases accompanied by systemic manifestations in areas where domiciliated triatominae are detected.
The 2 cases of cardiomyopathy accompanied by heart failure, 1 of them with mitral valve insufficiency, possibly due to dilatation of the valvular ring or to a lesion of the papillary muscles, as has been reported in cases of chagasic cardiopathy (Almeida et al. 1992) indicate that chronic Chagas disease possibly plays an important role in the etiology of cardiac pathology in all of Western Mexico, an assumption previously addressed by other authors (Lozano Kasten et al. 1993). These findings emphasize the need for more extensive studies to identify Chagas disease among cardiac patients in this area, which up until now has not been routinely done by physicians, due to the low clinical suspicion and the difficulty to obtain a confirmatory diagnosis.
With these kinds of strategies our country would be able to increase its participation as a member of the Central American initiative to interrupt the transmission of Chagas disease, in accordance with the World Health Organization's recommendations.
ACKNOWLEDGMENTS
To Dr Victor Monteón Padilla, Instituto Nacional de Cardiología "Ignacio Chávez", Mexico DF; to Julio César Barragán Anaya for help in collecting field samples, and to Jennifer Danielle Unrau for her assistance in the translation to the English version.
Received 23 December 2003
Accepted 13 May 2004
Financial support: Regional Investigation System SIMORELOS, project 9602026
- Abramson JH, Gahlinger PM 2001. Computer Programs for Epidemiologic Analysis: PEPI v. 4.0, Sagebrush Press, Salt Lake, UT.
- Almeida EA de, Martin CB, Teixeira MA, Carvalhal Sdos S 1992. Pathogenesis of mitral valve prolapse in patients with chronic Chagas disease: study of papillary muscle myocarditis. Arq Bras Cardiol 58: 91-94.
- Antas PR, Medrano-Mercado N, Torrico F, Ugarte-Fernandez R, Gomez F, Correa Oliveira R, Chaves AC, Romanha AJ, Araujo-Jorge TC 1999. Early, intermediate, and late acute stages in Chagas' disease: a study combining anti-galactose IgG, specific serodiagnosis, and polymerase chain reaction analysis. Am J Trop Med Hyg 61: 308-314.
- Añez N, Carrasco H, Parada H, Crisante G, Rojas A, Gonzalez N, Ramirez JL, Guevara P, Rivero C, Borges R, Scorza JV 1999. Acute Chagas' disease in Western Venezuela: a clinical, seroparasitologic, and epidemiologic study. Am J Trop Med Hyg 60: 215-222.
- Cohen JE, Gürtler RE 2001. Modeling household transmission of American trypanosomiasis. Science 293: 694-698.
- CGSNEGI 1992. Carta Estatal de Climas para el Estado de Colima, Secretaría de Programación y Presupuesto, México.
- Contreras FT, Yerenas Mde L, Gutierrez MS, Anaya MR, Corder AJ 2000. Serological follow-up of Trypanosoma cruzi infection from 1987 to 1994 in individuals studies in 50 counties of the State of Jalisco, Mexico. Rev Soc Bras Med Trop 33: 591-596.
- Dias JCP, Silveira AC, Schofield CJ 2003. The impact of Chagas disease control in Latin America - A Review. Mem Inst Oswaldo Cruz 98: 603-612.
- Dumonteil E 1999. Update on Chagas' disease in Mexico. Salud Publica Mex 41: 322-327.
- EPIDAT 2 1997. O.P.S. Program EPIDAT version 2.0 for Windows, Xunta of Galicia and PAHO, Washington DC.
- Espinoza-Gomez F, Maldonado-Rodriguez A, Coll-Cardenas R, Hernandez-Suarez CM, Fernandez-Salas I 2002. Presence of triatominae (Hemiptera, Reduviidae) and risk of transmission of Chagas disease in Colima, Mexico. Mem Inst Oswaldo Cruz 97: 25-30.
- Feilij H, Muller L, Gonzalez Cappa SM 1983. Direct micromethod for diagnosis of acute and congenital Chagas' disease. J Clin Microbiol 18: 327-330.
- Flisser A, Velasco-Villa A, Martinez-Campos C, Gonzalez-Dominguez F, Briseno-Garcia B, Garcia-Suarez R, Caballero-Servin A, Hernandez-Monroy I, Garcia-Lozano H, Gutierrez-Cogco L, Rodriguez-Angeles G, Lopez-Martinez I, Galindo-Virgen S, Vazquez-Campuzano R, Balandrano-Campos S, Guzman-Bracho C, Olivo-Diaz A, de la Rosa J, Magos C, Escobar-Gutierrez A, Correa D 2002. Infectious diseases in Mexico. A survey from 1995-2000. Arch Med Res 33: 343-350.
- Guzman-Bracho C 2001. Epidemiology of Chagas disease in Mexico: an update. Trends Parasitol 17: 372-376.
- INEGI 2000. Anuario Estadístico del Estado de Colima, Instituto Nacional de Geografía e Informática y Gob., Edo. De Colima, México.
- Kirchhoff LV, Votava JR, Ochs DE, Moser DR 1996. Comparison of PCR and microscopic methods for detecting Trypanosoma cruzi J Clin Microbiol 34: 1171-1175.
- Lent H, Wygodzinsky P 1979. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas' disease. Bull Amer Museum Nat History 163 article 3, p. 1-520.
- Lozano Kasten F, Sanchez Cruz G, Gonzales Bartell M, Prata A, Lopes ER 1993. Acute Chagas' disease in an 80-year-old woman in Mexico. An anatomicopathological report. Rev Soc Bras Med Trop 26: 231-235.
- Martinez-Ibarra JA, Barcenas-Ortega NM, Nogueda-Torres B, Alejandre-Aguilar R, Lino Rodriguez M, Magallon-Gastelum E, Lopez-Martinez V, Romero-Napoles J 2001. Role of two Triatoma (Hemiptera: Reduviidae: Triatominae) species in the transmission of Trypanosoma cruzi (Kinetoplastida: Trypanosomatidae) to man in the west coast of Mexico. Mem Inst Oswaldo Cruz 96: 141-144.
- Moncayo A 2003. Chagas disease: current epidemiological trends after the interruption of vectorial and transfusional transmission in the Southern Cone countries. Mem Inst Oswaldo Cruz 98: 577-591.
- Monteon VM, Guzman-Rojas L, Negrete-Garcia C, Rosales-Encina JL, Reyes-López PA 1997. Serodiagnosis of American trypanosomosis by using nonpathogenic trypanosomatid antigen. J Clin Microbiol 35: 3316-3319.
- Nisida IV, Amato Neto V, Braz LM, Duarte MI, Umezawa ES 1999. A survey of congenital Chagas' disease, carried out at three health institutions in São Paulo City, Brazil. Rev Inst Med Trop São Paulo 41: 305-311.
- Prata A 2001. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis 1: 92-100.
- Prieto Díaz ChE, González VE, Medina ChJL 2000. Tratamiento quirúrgico del esófago chagásico: a propósito de un caso. Rev Med IMSS México 38: 349-54.
- Ramsey JM, Schofield CJ 2003. Control of Chagas disease vectors. Salud Publica Mex 45: 123-128.
- Sanchez-Guillen MC, Barnabe C, Guegan JF, Tibayrenc M, Velasquez-Rojas M, Martinez-Munguia J, Salgado-Rosas H, Torres-Rasgado E, Rosas-Ramirez MI, Perez-Fuentes R 2002. High prevalence anti-Trypanosoma cruzi antibodies, among blood donors in the State of Puebla, a non-endemic area of Mexico. Mem Inst Oswaldo Cruz 97: 947-952.
- Schmunis GA 1999. Prevention of transfusional Trypanosoma cruzi infection in Latin America. Mem Inst Oswaldo Cruz 94 (Suppl. 1): 93-101.
- Schofield C. J 1978. A comparison of sampling techniques for domestic populations of triatominae. Trans R Soc Trop Med Hyg 72: 949-955.
- Sosa-Jurado F, Mazariego-Aranda M, Hernandez-Becerril N, Garza- Murillo V, Cardenas M, Reyes PA, Hirayama K, Monteon VM 2003. Electrocardiographic findings in Mexican chagasic subjects living in high and low endemic regions of Trypanosoma cruzi infection. Mem Inst Oswaldo Cruz 98: 605-610.
- Velasco-Castrejon O, Valdespino JL, Tapia-Conyer R, Salvatierra B, Guzman-Bracho C, Magos C, Llausas A, Gutierrez G, Sepulveda J 1992. Seroepidemiología de la enfermedad de Chagas en México. Salud Publica Mex 34: 186-196.
- WHO 1998. 51st World Health Assembly, Resolution WHA 51.14, Geneve, Switzerland.
- WHO 2000. Control of Chagas disease, Technical Report Series 905, 2nd ed., Geneva, Switzerland.
Publication Dates
-
Publication in this collection
13 Aug 2004 -
Date of issue
June 2004
History
-
Received
23 Dec 2003 -
Accepted
13 May 2004