Abstract
Small mammals are found naturally infected by Schistosoma mansoni, becoming a confounding factor for control programs of schistosomiasis in endemic areas. The aims of this study were: to investigate the infection rates by S. mansoni on the water-rat Nectomys squamipes during four years in endemic areas of Sumidouro, state of Rio de Janeiro, using mark-recapture technique; to compare two diagnostic methods for schistosomiasis; and to evaluate the effects of the chemotherapy in the human infected population on the rodent infection rates. The rodent infection rates of S. mansoni increased when rodent population sizes were lower. Coprology and serology results presented the same trends along time and were correlated. Serology could detect recent infection, including the false negatives in the coprology. The chemotherapy in the humans could not interrupt the rodent infection. Rodents can increase the schistosomiaisis transmission where it already exists, they probably maintain the transmission cycle in the nature and can be considered as biological indicators of the transmission sites of this parasite since they are highly susceptible to infection. The water-rats may present different levels of importance in the transmission dynamics of S. mansoni infection cycle for each area, and can be considered important wild-reservoirs of this human disease.
diagnostic methods; population ecology; rodents; schistosomiasis
An ecological field study of the water-rat Nectomys squamipes as a wild reservoir indicator of Schistosoma mansoni transmission in an endemic area
Rosana GentileI,1 1 Corresponding author: rgentile@ioc.fiocruz.br ; Sócrates F Costa-NetoI; Margareth ML GonçalvesII; Simone T BoneckerI; Fabiano A FernandesIII; Juberlan S GarciaI; Magali G M BarretoIV; Marisa S SoaresIV; Paulo S D'AndreaI; José M PeraltaII; Luis ReyI
ILaboratório de Biologia e Controle da Esquistossomose, Departamento de Medicina Tropical
IILaboratório de Sorologia, Departamento de Imunologia, Instituto de Microbiologia, UFRJ, Rio de Janeiro, RJ, Brasil
IIIPrograma de Pós-Graduação em Biologia Animal, UFRGS, Porto Alegre, RS, Brasil
IVLaboratório de Avaliação e Promoção da Saúde Ambiental, Departamento de Biologia, Instituto Oswaldo Cruz-Fiocruz, Av. Brasil 4365, 21040-360 Rio de Janeiro, RJ, Brasil
ABSTRACT
Small mammals are found naturally infected by Schistosoma mansoni, becoming a confounding factor for control programs of schistosomiasis in endemic areas. The aims of this study were: to investigate the infection rates by S. mansoni on the water-rat Nectomys squamipes during four years in endemic areas of Sumidouro, state of Rio de Janeiro, using mark-recapture technique; to compare two diagnostic methods for schistosomiasis; and to evaluate the effects of the chemotherapy in the human infected population on the rodent infection rates. The rodent infection rates of S. mansoni increased when rodent population sizes were lower. Coprology and serology results presented the same trends along time and were correlated. Serology could detect recent infection, including the false negatives in the coprology. The chemotherapy in the humans could not interrupt the rodent infection. Rodents can increase the schistosomiaisis transmission where it already exists, they probably maintain the transmission cycle in the nature and can be considered as biological indicators of the transmission sites of this parasite since they are highly susceptible to infection. The water-rats may present different levels of importance in the transmission dynamics of S. mansoni infection cycle for each area, and can be considered important wild-reservoirs of this human disease.
Key words: diagnostic methods - population ecology - rodents - schistosomiasis
Small mammals are found naturally infected by Schistosoma mansoni, becoming a confounding factor for control programs of schistosomiasis in endemic areas (Barbosa et al. 1958, Antunes et al. 1971, Rey 1993). Among the extra-human definitive hosts of this parasite, rodents of the genera Nectomys and Holochilus are the most probable wild reservoirs taking into account: (1) their semi-aquatic habits (Ernest & Mares 1986), which make them highly exposed to infection; (2) their wide geographic distribution (Bonvicino 1994) coincident with the distribution of schistosomiasis in Brazil; (3) presence of infected individuals in most of the endemic areas where they were investigated, despite of the low human prevalence rodents frequently showing higher infection rates in relation to human populations (Rey 1993); (4) rodent tolerance to human presence, occurring near human dwellings. Several experimental studies also support the hypothesis that water-rats are probable wild reservoirs of S. mansoni, showing high susceptibility to infection (Borda 1972, Souza et al. 1992, Maldonado Jr. et al. 1994, Ribeiro et al. 1998), somatic development hypertrophy of adult worms (Machado-Silva et al. 1994), elimination of viable eggs with high infectivity potential (Picot 1992), high infection persistence (Silva et al. 1992), low pathogenicity with efficient peri-ovular modulation and low tissue aggression (Silva & Andrade 1989), and ability to close the transmission cycle in semi-natural conditions (Antunes et al. 1973, Carvalho et al. 1976, Kawazoe & Pinto 1983). Infection of small mammals other than Nectomys and Holochilus by S. mansoni occurs only occasionally, especially in areas of high transmission levels.
In high prevalence areas, a strong influence of human infection in the transmission of the parasite to the intermediary hosts is observed (Rey 1993). The contribution of rodents to those foci would be to increase the infection by human transmission. A previous study on the role of the water-rat N. squamipes in the transmission dynamics of schistosomiasis, carried out in an area with low intensity of infection in Sumidouro, state of Rio de Janeiro (RJ), showed that despite the low burden of S. mansoni found in rodents, their populations were always infected with S. mansoni (D'Andrea et al. 2000). For areas such as the former, the possibility that rodents maintain the parasite cycle should be considered. The presence of infected animals in sites not contaminated by human feces, inside an endemic area, suggests that the rodents can spread the parasite to local areas without human transmission, and consequently, complicating the control programs. Théron and Pointier (1995) demonstrated that, in the French Guianas, Rattus rattus could maintain the S. mansoni cycle in a wild focus, whereas in a semi-urban focus, both humans and rodents were involved in the cycle. The former was the sole study to assess the participation of rodents in the schistosome life-cycle without human presence.
The finding of wild rodent populations naturally infected by S. mansoni lead to the implementation of a long-term multidisciplinary study to evaluate the role of the water-rat N. squamipes in the dynamic transmission of schistosomiasis in Sumidouro, RJ, an endemic area for this parasitosis. In a study carried out at Pamparrão, D'Andrea et al. (2000) concluded that the rodent population was not influenced by the infection, neither in reproduction, nor in longevity. With these information, the aims of the present study were: to investigate the infection rates by S. mansoni on N. squamipes during a long term study in endemic areas of schistosomiais in Sumidouro, to compare two diagnostic methods for schistosomiasis; and, to evaluate the effects of the chemotherapy treatment in the human infected population on rodent infection rates. Population dynamics of the water-rat was also studied to lend support to the infection analysis.
MATERIALS AND METHODS
Study area - The municipality of Sumidouro is in the mountain region of the state of Rio de Janeiro on the northern slope of Serra dos Órgãos (22º02"S 42º 41"W), at 348 m above sea level, and 160 km NW of the city of Rio de Janeiro. The climate is humid-mesothermic, characterized by two climatic seasons: a hot and wet season from November to March, with the highest temperature in February and the highest rainfall rates in December, and a dry and cold season spanning from May to October with minimum temperatures in July (data provided by the Instituto Nacional de Meteorologia of Carmo Station).
Sumidouro has 14927 inhabitants (IBGE 2005), most of them (87.7%) living in the rural area. Since the beginnings of the XIX century, the economic development of this area has been based on agriculture. Nowadays, vegetable plantation and cattle breeding are the most important economic activities of the region. The full study was carried out mainly in the locality of Encanto, but observations of infection of S. mansoni in the populations of N. squamipes were also conducted in three other localities in Sumidouro: Bairro da Volta, Pamparrão, and Soledade.
Encanto has 37 residences (one is a lodging) and 137 inhabitants. The place does not have transport, health, and telephone or mobile services. The water supply and waste discharges flow into the Paquequer River, which is an affluent of the Paraíba do Sul River. This water source is used for the supply of corral, agriculture, and domestic activities. Encanto has a main river, which flows into the Paquequer River, originated by the union of two streams coming from other rural localities. Encanto's residences throw their drain into this river. Among the many streams of the region, one is used in agriculture, some of them are exclusive for fish raising and the rest is used for both activities. In Encanto, there is a lodging with a natural pool and a lake, which is supplied by the main river, and used by the guests. This locality has small Atlantic Forest fragments on the top of the mountain.
Bairro da Volta (22º02'53"S 42º40'06"W) is a small property near the center of Sumidouro, where the main activity is cattle breeding. Pamparrão (22º02'S 42º38'W) has about 80 inhabitants and the main activity is agriculture of vegetables. This area is slightly less disturbed than Encanto. Soledade (22º03'S 42º35'W) encloses a large area with several small rural properties and very disturbed vegetation.
Field methods - A capture-mark-recapture study of N. squamipes was carried out in Encanto during four-years (Sep/2001 to Nov/2005) every three months, except for May/2002, when there was no trapping. Three trapping lines were established in the locality of Encanto (22º01'07"S 42º38'01"W; 22º01'30"S 42º37'45"W; 22º02'37"S 42º37'13"). The rodents were live-caught in wiremesh live-traps (TomahawkÒ and ShermanÒ) placed on the floor along the streams, which is the most important habitat of N. squamipes (Gentile & Fernandez 1999). Traps were spaced 10 m apart, and baited with peanut butter mixed with banana, oat, and bacon on manioc pieces. Capture transects included 90 traps and were conducted during five consecutive days each trapping session, totalizing 450 trap-nights per session.
Transversal studies were conducted in the other localities. Animals were necropsied in Pamparrão and Soledade and mark-captured in Bairro da Volta. In Bairro da Volta and Pamparrão trappings were carried out in the same months of Encanto study. In Soledade three rodent collections were conducted: in October 2003, September 2004, and June 2005.
Species were identified based on morphology. Every individual was weighted, measured, marked with ear tags, and their sexual condition observed. The reproductive condition of females was assessed by groping and visual observation of abdomen dilation. For sexual maturity we considered the individuals, which had, at least, the minimum weight of males with scrotal testes, and females with open vagina. Animals were released at their respective trapping points after sample collection.
All procedures were in accordance with the Ethical Committee on Animal Use of Fundação Oswaldo Cruz (number P-0083-01). Animals were captured under authorization of the Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (licenses numbers 244/2001, 0123/2002, 061/2003, 124/2004, and 068/2005).
Diagnostic methods - Diagnostic of schistosome infection was based on coprologic and serologic exams. Surgical dischargeable hoods were placed at the bottom of each trap acting as a feces-collecting bag. Faecal samples were collected from every individual at each capture or recapture. The faecal samples from each individual were mixed during the capture session. Coprologic analysis was carried out using spontaneous sedimentation based on 2 to 5 g of faeces (Rey 2001). Blood samples from every animal were collected from the caudal vein at each trapping session and centrifuged. Serology was done using the ELISA (IgG) technique described for N. squamipes by Mello (2002). S. mansoni membrane extract were used as antigen. Rabbit anti-N. squamipes immunoglobulins were labeled with peroxidase and used as second antibody. Serum samples from experimentally infected and non infected animals were used as controls in all ELISA tests. Results were expressed in immunoenzimatic units (IU) which means the sample OD value divided by the cutoff OD value. Serum samples with values above one IU were considered as positive.
Snails studies - To study the natural infection of the snails, 14 collection points were established in Encanto, in several sites where schistosomiasis transmission was supposed to occur. The collections were carried out trimestraly, from March 2003 to March 2005. In Bairro da Volta, Pamparrão, and Soledade, collections were performed in the same period, in several points without regularity. Infection by S. mansoni was investigated in the laboratory by light exposure at every five days, for 45 days.
Human studies - Human diagnostic was carried out in Encanto during September 2002 on 135 individuals from Encanto. The diagnostic was based on Kato-Katz and Hoffman techniques (Katz et al. 1972, Rey 2001) performed as a single method after serological trial by ELISA. Each individual furnished from one to eight samples of feces, three on average. The human chemotherapy treatment of the infected population was performed during January and February 2003 with praziquantel (40 mg/kg). In June 2005 another diagnostic was conducted in 126 people of Encanto using the same techniques, followed by the treatment of the infected people, during July and September. In Bairro da Volta and Pamparrão human diagnostic was in April and June 2003, and treatment was in July 2003. In Soledade, the human diagnostic was done in August 2003 and the treatment was carried out in October 2003 and February 2004.
These procedures were in accordance with the Ethical Committee on Human Experimentation of Fundação Oswaldo Cruz (number 182-02).
Analytical methods - Rodent population sizes were estimated for each trapping session using the Minimun Number Known Alive method (Krebs 1966) considering a constant trapping effort along time. Survivorship and recruitment rates were estimated for each interval of trapping sessions with the Jolly-Seber model (Seber 1973), corrected for a 90-days interval period according to Fernandez (1995). Survivorship and recruitment were correlated with population sizes and with rainfall up to six months of time lag to analyze the population dynamics of the species using Spearman Rank correlation because data were not normally distributed. Age structure was analyzed based on Gentile et al. (2000) dividing animals in three age classes according to their weights and sexual conditions. This analysis was carried out only for the rodents from Encanto.
Prevalences were calculated as the proportion of infected animals in relation to the total number of animals analyzed for each trapping session. Incidences were considered as the number of new infected cases in the population. Prevalence was correlated with population sizes and rainfall up to six months lag using Spearman rank correlation because data were not normally distributed.
Serologic and coprologic diagnostic methods were compared for the rodents along time using Spearman rank correlation. Serologic results were also evaluated in relation to age and sex of the rodents. Animals trapped in other areas were perfused and used for comparison with serology.
Human prevalence was caculated based only on the coprologic analysis.
RESULTS
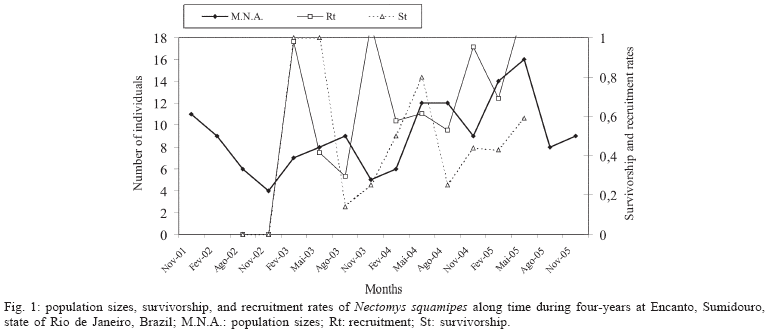
Rodent population - N. squamipes population size was declining during the first year of the study and increased after November/2002; the highest increases occurred in the first semester, except in 2002 (Fig. 1). The coefficient of variation (variance to mean ratio) was 36%. Population sizes were not correlated with survivorship (rs = 0.212, P > 0.25; N=10) or recruitment (rs = 0.04; P > 0.25; N = 10). Survivorship was inversely correlated with rainfall without time lag (rs = -0.720; 0.025 > P > 0.01) and positively correlated with five-months lag (rs= 0.773; 0.025 > P > 0.01). Survivorship rates were higher every May, and recruitment during the rainy seasons (Fig. 1). Reproduction occurred along the year predominantly in the end of the rainy seasons. Young individuals were found in February, May, and November, with predominance in February (Fig. 2). Most of the individuals were born during the rainy season.
Rodent prevalences varied from 0 to 100% in coprology and from 25 to 100% in serological method, with high infection rates most of the time (Fig. 3). Mean prevalence among the 18 sampling months was of 46.1% ± 28.1 for the coprologic method, 71.1% ± 26.2 for serology. The highest prevalences occurred from November 2002 to November 2003. There was a significant positive correlation between prevalences estimated using the two diagnostic methods (rs = 0.635; 0.005 > P > 0.0025; N = 18). The coprological method underestimated the serological prevalence in about 35%, mostly in the low prevalence months, and both presented the same trends along the time (Fig. 3). In February 2005, prevalences determined by coprological methods were higher than by serological ones; however, three individuals were not coprologically analyzed and one, serologically. S. mansoni prevalences estimated by serology were negatively correlated with rainfall for four months lag (rs = 0.441; 0.05 > P > 0.025; N = 16). N. squamipes population sizes were negatively correlated with prevalences estimated by serology (rs = 0.852; P < 0.0005; N = 18), indicating that rodent prevalences of S. mansoni increased when rodent population sizes were lower, mostly in the beginning of the rainy seasons (Fig. 3).
Incidences of S. mansoni in the rodent population varied from zero to seven individuals by serology, with an average of 3.6 ± 2.2, and constant oscillation was observed until the end of the study (Fig. 3). Incidences were not correlated with prevalences, neither with rodent population sizes (rs= 0.046, P > 0.25, N = 16; rs= 0.105, P > 0.25, N = 16, respectively).
S. mansoni serum antibody conversion was observed in five individuals. It occurred from November 2004 to February 2005 and from May to August 2005, and only one individual was young.
There was no difference in sex ratio between infected and non-infected animals (c2 = 0.16; P = 0.68; DF = 1).
Snail population - Snails of the species Biomphalaria glabrata were found in 10 collection sites and infected ones were found only once, in a small sewage deposit, with an infection rate of 29% (nine snails infected in 31 collected).
Human population - Human prevalence, in 2002, determined by coprologic examination was 19.3% with 26 positive individuals. In the second moment (2005), prevalence was 4.8% with six positive cases. Among them, two were new cases, three of them were reinfection or the infection was not cured, and one was not diagnosed in 2002.
After 18 months of human chemotherapy, prevalence among rodent population was reduced to zero when estimated by coprological diagnostic and to 25% by serology. However, the prevalence increased some months latter and returned to the same levels of the beginning of the study by the end of 2005 (Fig. 3).
Other localities - In Bairro da Volta no humans were infected, but only a few rodents had eggs in the stools. The highest prevalences in rodents occurred in February 2003, August 2003, and May 2005, but few animals were diagnosed. A continuous removal study was previously done in Pamparrão for two years (D'Andrea et al. in press), which drastically reduced the size of the population of N. squamipes. In this area, the prevalence of human infection was 13.4%. Rodent infection rate was always zero, except in September 2001, when one rodent was found infected, and in November 2005, when 75% of the individuals were infected. In Soledade, infection rates were 80, 80 and 85.7% in the serologic method, and 69.2, 66.7, and 14.3% in the coprologic method, for 2003, 2004, 2005, respectively. Unlike other areas, this locality had high infection rate in the human population (40%); and the rodent prevalence has not decreased even after one year of human treatment.
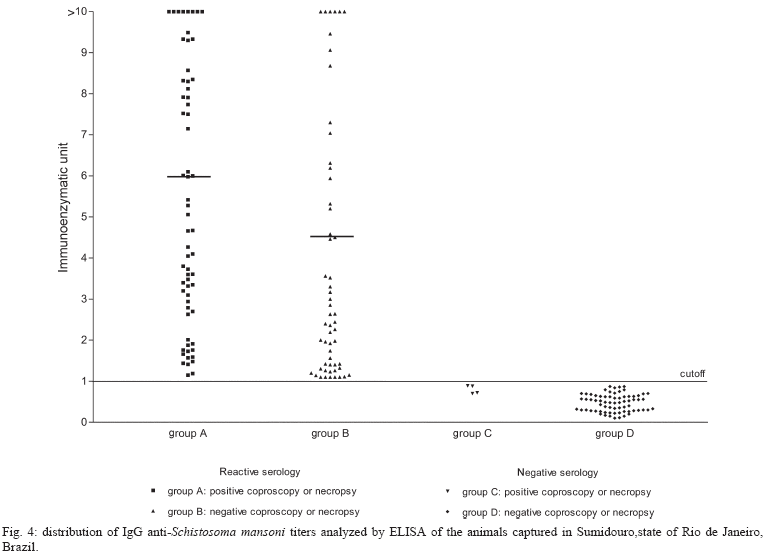
Comparing the serological results with coprological and perfusion results using all data from rodents, we observed distinct distribution in the ELISA titers. No differences in antibodies titers were found between individuals with coprology/necropsy negative and coprology/necropsy positive (Fig. 4). Four individuals were coprology/necropsy positive and serology negative (Fig. 4).
DISCUSSION
N. squamipes presented very high susceptibility to infection of S. mansoni, since rodent prevalences were always high, except one year after the human chemotherapy, despite the scarcity of infected snails detected. This result confirms those achieved in other endemic areas in Brazil, where infection rates were also high, over 30% (Rey 1993). The previous study carried out in Pamparrão also detected prevalence rates over 20% most of the time (D'Andrea et al. 2000). Théron et al. (1992) observed an overall prevalence of S. mansoni of 40% in R. rattus in the French Guiana.
The population dynamics of N. squamipes was in accordance with other studies where reproduction of animals occurred throughout the year but mostly during rainy periods, related to the close association of this rodent to resources found in water (Ernest & Mares 1986, Bergallo 1994, Gentile et al. 2000). They reproduce opportunistically, such that reproduction is triggered by resources availability according to rainfall pattern (Gentile et al. 2000), resulting in rapid population increases with higher survivorship rates a few months after the rainy periods, and young individuals are mostly observed in those periods.
The negative association trend observed between rodent population size and S. mansoni prevalence may be related to the rainfall pattern. The significant negative correlation observed between rodent prevalence and rainfall with four months lag indicates that rodent prevalence decreases in the end of the rainy season, and generally increases before the rainy periods. The snail populations are higher in the end of the dry season, because they are negatively influenced by the rainfall pattern (Giovanelli et al. 2001). The dry period is the most favorable one for S. mansoni transmission due to the low volume of the streams' water, which turns easier the meeting of the parasite with the intermediary host (Giovanelli et al. 2001). Rodent population sizes increase after the rains, when the snail population declines due to the torrents in the streams, as well as the prevalences of both hosts. Thus, the schistosome infection did not seem to affect the population dynamics of the rodents, agreeing with D'Andrea et al. (2000).
In spite of the low rodent infection rate about 18 months after the chemotherapy in the human population, this treatment could not interrupt the rodent infection rates, because after one year there was recrudescence in the rodent infection, while the human population prevalence was considerably reduced (from 19.3 to 4.8%). The high incidences and the serologic conversions observed in the last year of the study corroborate this idea, and indicate a continuous process of S. mansoni transmission in the area, despite the chemotherapy in the human population.
Comparing the two diagnostic methods for S. mansoni, we observed that during the mark-recapture study of the rodents, coprology and serology results presented the same trends along time and were significantly correlated, indicating that both are efficient and applicable to the study of the transmission dynamics of S. mansoni in rodent populations. However, serology is more efficient for prevalence analysis, especially when intensity of infection is low, because in this situation it is very difficult to find eggs in the feces.
The similarity in the reactive serology profile between individuals diagnosed coprology/necropsy negative in relation to coprology/necropsy positive, demonstrates that serology detect recent infection, including the false negatives in the coprology, since antibodies can be found after five days of the infection in laboratory experiments with N. squamipes (Mello 2002). The low titers of antibodies in most of these samples corroborates with this hypothesis. The four individuals of group C (serology negative with coprology/necropsy positive) can be interpreted as serology false negative results. This may occur since immune response can differ from one to another individual.
Although the studies carried out in the other localities of Sumidouro had few results for a definitive conclusion, we observed different patterns in the participation of the water-rat in S. mansoni transmission dynamics in each locality. In Bairro da Volta the rodents were able to maintain the S. mansoni infection even without infected humans, at least during a short period of time. In Pamparrão, the low rodent population size and the absence of rodent infection during three years after the removal procedure did not eliminate the infection transmission, since human prevalence was 13.4%. In Soledade, a high endemic area, we observed infected rodents far from human habitations, and the human and rodent transmission cycles seem not to be affecting each other. Théron et al. (1992) also observed different patterns on the role of R. rattus in S. mansoni transmission cycle in an endemic area of the French Guiana.
These results emphasize the importance of the water-rats as wild reservoirs of S. mansoni, and the facts that they can increase the schistosomiasis transmission where it already exists. Also they probably maintain the transmission cycle in the nature and can be considered as biological indicators of the transmission sites of this parasite since they are highly susceptible to infection. Nevertheless, water-rats present different levels of importance in the transmission of S. mansoni infection cycle for each area, even in a small scale region. Thus, they must be taken into account in the control programs of this infection.
ACKNOWLEDGEMENTS
To JWF Costa and the people from the Laboratório de Biologia e Controle da Esquistossomose-Fiocruz for helping in the field work, to the Municipal Secretary of Agriculture and Environment in Sumidouro, to SS Serafim for providing many operational facilities and a field base, to M Gusmão and T Figueiredo for helping in the human inquire, to RP Igreja for the human chemotherapy treatment, to AC Santana for technical assistance in the rodents' feces exams and in the field work, to MT Paulino and W Abreu for the human feces exams, to I Pimenta and W Valim for the snail collection and diagnosis, to the people of Encanto, Pamparrão, Bairro da Volta and Soledade in Sumidouro, who allowed us to carry out the field work in their properties, and participated in the schistosomiasis inquire, to C Bidau for the manuscript review, and to the Municipal Government, Office of Education and Culture and Office of Health and Social Promotion in Sumidouro, Rio de Janeiro, for many operational support.
Received 25 May 2006
Accepted 26 June 2006
Financial suport: Cnpq, Faperj, IOC, Papes-Fiocruz
- Antunes CMF, Milward-de-Andrade R, Katz N, Coelho PMZ 1971. Contribuição para o conhecimento do rato lava-pés, Nectomys squamipes, na epidemiologia da esquistossomose mansônica (Rodentia: Cricetidae). Rev Bras Malariol Doenc Trop 23: 203-204.
- Antunes CMF, Milward-de-Andrade R, Katz N, Coelho PMZ, Pelegrino J 1973. Hole of Nectomys squamipes in the epidemiology os Schistosoma mansoni infections. Ann Trop Med Parasitol 67: 67-73.
- Barbosa FS, Coelho MV, Coutinho-Abath E 1958. Infecção natural e experimental de alguns mamíferos de Pernambuco por Schistosoma mansoni Rev Bras Malariol Doenç Trop 10: 137-144.
- Borda CE 1972. Infecção Natural e Experimental de Alguns Roedores pelo Schistosoma mansoni, MSc Thesis, Universidade Federal de Minas Gerais, Belo Horizonte.
- Bergallo HG 1994. Ecology of a small mamal communit in an Atlantic Forest area of Southeastern Brazil. Stud Neotrop Fauna & Environm 29: 197-217.
- Bonvicino CR 1994. Especiação do Rato d'Água Nectomys squamipes (Rodentia, Cricetidae): Abordagem Cariológica, Morfológica e Geográfica, PhD Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro. 265 pp.
- Carvalho OS, Andrade RM, Cortês MIN 1976. Ciclo vital de Schistosoma mansoni através de Holochilus brasiliensis (Desmarest, 1818), em ambiente semi-natural (Trematoda: Schistosomatidae; Rodentia: Cricetidae). Rev Soc Bras Med Trop 10: 235-247.
- D'Andrea PS, Gentile R, Maroja LS, Fernandes FA, Coura RS, Cerqueira R. Small mammal populations of an agroecosystem in the Atlantic Forest domain, southeastern of Brazil. Braz J Biol (in press).
- D'Andrea PS, Maroja LS, Gentile R, Cerqueira R, Maldonado Jr A, Rey L 2000. The parasitism of Schistosoma mansoni (Digenea-Trematoda) in a naturally infected population of water rats, Nectomys squamipes (Rodentia-Sigmodontinae) in Brazil. Parasitology 120: 573-582.
- Ernest KA, Mares MA 1986. Ecology of Nectomys squamipes, the Neotropical Water rat, in central Brazil: home range, habitat selection, reproduction and behaviour. J Zool 210: 599-612.
- Fernandez FAS 1995. Métodos para estimativa de parâmetros populacionais por captura, marcação e recaptura. In PRP Neto, JL Valentin, FAZ Fernandez (eds), Tópicos em Tratamento de Dados Biológicos Oecologia Brasiliensis: Universidade Federal do Rio de Janeiro, Rio de Janeiro, p. 1-26.
- Gentile R, Fernandez FAS 1999. Influence of habitat structure on a streamside small mammal community in a Brazilian rural area. Mammalia 63: 29-40.
- Gentile R, D'Andrea PS, Cerqueira R, Maroja LS 2000. Population dynamics and reproduction of marsupials and rodents in a Brazilian rural area: a five-year study. Stud Neotrop Fauna & Environm 35: 1-9.
- Giovanelli A, Soares MS, D'Andrea PS, Gonçalves MML, Rey L 2001. Abundância e infecção do molusco Biomphalaria glabrata pelo Schistosoma mansoni no Estado do Rio de Janeiro, Brasil. Rev Saúde Públ 35: 523-530.
- Katz N, Chaves A, Pelegrino J 1972. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop São Paulo 14: 397-400.
- Kawazoe U, Pinto ACM 1983. Importância epidemiológica de alguns animais silvestres na esquistossomose mansônica. Rev Saúde Públ 17: 345-366.
- Krebs CJ 1966. Demographic changes in fluctuating populations of Microtus californicus Ecol Mongr 36: 239-273.
- Machado E, Silva JR, Galvão C, Presgrava OAF, Rey L, Gomes DC 1994. Host induced morphological changes of Schistosoma mansoni Sambon, 1907 male worms. Mem Inst Oswaldo Cruz 89: 411-416.
- Maldonado Jr A, Machado E, Silva JR, Rodrigues E, Silva R, Lenzi HL, Rey L 1994. Evaluation of the resistance to Schistosoma mansoni infection in Nectomys squamipes (Rodentia: Cricetidae), a natural host of infection in Brazil. Rev Inst Med Trop São Paulo 36: 193-198.
- Mello DGS 2002. Estudo comparativo entre Técnicas Parasitológica e Sorológica para o Diagnóstico da Esquistossomíase Mansônica em Nectomys squamipes (Rodentia, Muridae) Capturados no Município de Sumidouro, Rio de Janeiro, MSc Thesis, Universidade do Estado do Rio de Janeiro, Rio de Janeiro, 68 pp.
- Picot H 1992. Holochilus brasiliensis and Nectomys squamipes (Rodentia, Cricetidae) natural hosts of Schistosoma mansoni Mem Inst Oswaldo Cruz 87: 255-260.
- Rey L 1993. Non-human vertebrate hosts of Schistosoma mansoni and schistosomiasis transmission in Brazil. Res Rev Parasitol 53: 13-25.
- Rey L 2001. Parasitologia, 3Ş ed., Guanabara Koogan, Rio de Janeiro, 856 pp.
- Ribeiro AC, Maldonado Jr A, D'Andrea PS, Vieira GO, Rey L 1998. Suscetibility of Nectomys rattus (Pelsen 1883) to experimental infection with Schistosoma mansoni (Sambon 1907): a potential reservoir in Brazil. Mem Inst Oswaldo Cruz 93 (Suppl. I): 295-299.
- Seber GAF 1973. The Estimation of Animal Abundance and Related Parameters, Griffin Publication, London, 506 pp.
- Silva RR, Silva JRM, Faerstein NF, Lenzi HL, Rey L 1992. Natural infection of wild rodents by Schistosoma mansoni parasitological aspects. Mem Inst Oswaldo Cruz 87: 271-276.
- Silva TM, Andrade ZA 1989. Infecção natural de roedores silvestres pelo Schistosoma mansoni Mem Inst Oswaldo Cruz 84: 227-235.
- Souza VAM, Silva RR, Maldonado Jr A, Machado E, Silva JR, Rey L 1992. Nectomys squamipes (Rodentia - Cricetidae) as an experimental model for schistosomiasis mansoni. Mem Inst Oswaldo Cruz 87: 277-280.
- Théron A, Pointier JP 1995. Ecology, dynamics, genetics and divergence of trematode populations in heterogeneous environments: the model of Schistosoma mansoni in the insular focus of Guadeloupe. Res Rev Parasitol 55: 49-64.
- Théron A, Pointer JP, Morand S, Imbert-Establet D, Borel G 1992. Long-term dynamics of natural populations of Schistosoma mansoni among Rattus rattus in patchy environment. Parasitology 104: 291-298.
Publication Dates
-
Publication in this collection
12 Feb 2007 -
Date of issue
Oct 2006
History
-
Received
25 May 2006 -
Accepted
26 June 2006