Abstract
Candida infections are common infections and fluconazole is one of the most frequently administered antifungal agents in their treatment. The resistance developed against antifungal agents has necessitated the improvement of new treatments. This study focuses on the investigation of the effect of fluconazole and cytokines such as interferon-gamma (IFN-gamma), tumor necrosis factor-alpha (TNF-alpha), granulocyte-macrophage colony-stimulating factor (GM-CSF) on chemokine production and anticandidal activity of human monocytes. In the study it was observed that GM-CSF caused an increase in candidacidal activity of monocytes. Anticandidal activity of GM-CSF + IFN-gamma combination was not found to be more effective than GM-CSF or IFN-gamma alone. The presence of cytokine and fluconazole caused an increase in the levels of CCL3 and CCL4 chemokines. Accordingly, it was considered that chemokines could contribute to the efficacy of fluconazole in C. albicans infections. Besides, in order to strengthen the immune system some cytokines might be used in addition to antifungal agents for the treatment.
Candida albicans; fluconazole; cytokines; chemokines
ARTICLES
The effects of fluconazole and cytokines on human mononuclear cells
Isil FidanI, + + Corresponding author: isilfidan@yahoo.com ; Sevgi YukselI; Turgut ImirI; Ayse KalkanciI; Semra KustimurI; Mustafa N IlhanII
IDepartment of Medical Microbiology
IIDepartment of Public Health, Faculty of Medicine, Gazi University, Dekanlik Binasi 2.Kat Besevler/ANKARA 06500, Turkey
ABSTRACT
Candida infections are common infections and fluconazole is one of the most frequently administered antifungal agents in their treatment. The resistance developed against antifungal agents has necessitated the improvement of new treatments. This study focuses on the investigation of the effect of fluconazole and cytokines such as interferon-gamma (IFN-g), tumor necrosis factor-alpha (TNF-a), granulocyte-macrophage colony-stimulating factor (GM-CSF) on chemokine production and anticandidal activity of human monocytes. In the study it was observed that GM-CSF caused an increase in candidacidal activity of monocytes. Anticandidal activity of GM-CSF + IFN-g combination was not found to be more effective than GM-CSF or IFN-g alone. The presence of cytokine and fluconazole caused an increase in the levels of CCL3 and CCL4 chemokines. Accordingly, it was considered that chemokines could contribute to the efficacy of fluconazole in C. albicans infections. Besides, in order to strengthen the immune system some cytokines might be used in addition to antifungal agents for the treatment.
Key words:Candida albicans - fluconazole - cytokines - chemokines
For the recent few years, Candida albicans has been encountered rather increasingly in especially cancer and transplantation patients and considered as an important hospital infection agent (Ashman et al. 2004). The most important factor in invasive fungal infections is the changes in the immune response of the host (Farmaki & Roilides 2001). Even if treated with an appropriate antifungal agents, invasive fungal infections such as disseminated candidiasis cause high rates of mortality and morbidity especially in immunocompromised hosts.
Immunity against Candida infections develops as a result of a complex interaction between innate and adaptive immunities (Ashman et al. 2004). Peripheral blood mononuclear cells (PBMCs) play a very important role in the defence against Candida with both their phagocyte mechanisms and their other effects in immune system and they are effective to initiate cellular response. These cells carry mannose and b-glucan receptors on their surfaces for C. albicans and they also play an important role in the initiation of the cellular immune response against fungi (Anaissie et al. 2003). Cell-mediated immunity due to T cells and cytokines is the predominant host defense mechanism against mucosal and/or systemic C. albicans infections (Steele & Fidel 2002).
PBMC stimulation by microorganisms might result in increased production of cytokines such as TNF-a (tumor necrosis factor alpha), GM-CSF (granulocyte-macrophage colony-stimulating factor), and interferon-gamma (IFN-g). Then, phagocytosis and chemotaxis are enhanced and the host attempts to eliminate the microorganism. However, unless stimulated by cytokines, monocytes are found to have limited effect in host defenses (Baltch et al. 2001). Moreover, antifungal drugs may have limited immunomodulatory effects (Mencacci et al. 2000). Cytokines, effector cells, and antifungal agents may work synergistically to prevent fungal infections. For this reason, cytokines are considered for use as therapeutic agents along with antifungal agents especially in the prevention and treatment of invasive fungal infections.
Chemokines serve many important functions in the immune system (Saavedra et al. 1999). They are required for effective systemic cell-mediated immune responses (Huang & Levitz 2000). Especially, the stimulation of inflammatory chemokines by C. albicans in human PBMCs could be important to determine the role of T-cell mediated response in candidiasis. Moreover, we suggest that the immunomodulatory effects of fluconazole on the stimulation of chemokines can affect the inflammatory processes.
In this study, it was aimed to determine single or combined efficacy of fluconazole and cytokines on anticandidal activities of human PBMCs against C. albicans and on the production of chemokines. Thus, we could understand the mechanisms to prevent Candida infections and the efficacy of cytokines when used with antifungal agents.
MATERIALS AND METHODS
Preparation of human PBMC - Heparinized blood was collected from healthy human donor. Local Ethical Committee permissions were obtained. PBMCs were isolated from blood by sedimentation on a Ficoll-hypaque gradient (Sigma). PBMC were washed in phosphate buffered saline (PBS) three times and resuspended at a concentration of 2 x 106cells/ml in RPMI 1640 medium containing 2 mmol/l glutamine, 200 U/ml penicillin, 100 µg/ml streptomycin, supplemented with 10% fetal calf serum (FCS, Biochrom, Germany). Cell viability was 95% by the trypan blue exclusion test.
Microorganism - C. albicans ATCC 10231 was used in this study. C. albicans was grown on Sabouraud dextrose agar (SDA) for 48 h at 37ºC. For opsonization, several colonies were suspended in RPMI 1640 containing 10% fresh pooled normal human serum and were incubated for 30 min at 37ºC. The opsonized cells were centrifuged and resuspended at a concentration of 2 x 104 CFU/ml in RPMI 1640 containing 10% FCS.
Antifungal agents - Fluconazole was provided by Pfizer Laboratories (US). Antibiotic solutions were made fresh for each experiment and used immediately. The fluconazole MIC for C. albicans (ATCC 10231), determined according to NCCLS method M27-A2, was 2 µg/ml (NCCLS 2002).
Preparation of recombinant human cytokines - Recombinant GM-CSF, and TNF-a, IFN-g were obtained from Biosource (Camarillo, CA, US). All cytokines were made fresh for each experiment. For study, GM-CSF ve TNF-a were used at a concentration of 100 U/ml. IFN-g was used at a concentration of 1000 U/ml.
Study design - Human PBMC (2 x 106) were delivered to the wells of 24-well plates in a 1 ml volume and incubated to adhere for 2 h in 5% CO2 at 37ºC. Then, nonadherent cells were removed from the wells. The adherent monocyte layer was washed once with RPMI-1640. GM-CSF, TNF-a and IFN-g were added to duplicate wells single or in combination and incubated for 24 h in 5% CO2 at 37ºC. After 24 h, opsonized C. albicans (2 x 104 CFU/ml) was added to the wells. For phagocytosis, the plates were incubated for 1 h in 5% CO2 at 37ºC. Nonphagocytosed blastoconidia was aspirated and the cell layer was washed once with RPMI-1640. Cytokines were readministered to the monolayer and then 0.2 µg of fluconazole (0.1 times the MIC) was added to each wells. The plates were incubated for 0, 24, and 48 h in 5% CO2 at 37ºC and at the end of each selected time period the supernatants were removed and stored at -30ºC until used in ELISA. After the culture supernatants were removed, the monocytes were lysed with distilled water and lysates were plated in duplicate on Sabouraud dextrose agar. The plates were incubated for 24 h at 37ºC and colonies were counted (colony forming unit/ml = CFU/ml) (Baltch et al. 2001). Control wells contained: (1) RPMI-1640; (2) PBMC + C. albicans; (3) PBMC + fluconazole; (4) PBMC+ TNF-a; (5) PBMC + GM-CSF; (6) PBMC + IFN-g; (7) PBMC + GM-CSF + IFN-g.
The experiments were done in quadruplicate with monocytes from one donor.
Chemokine assays - Levels of chemokines such as CCL2 (monocyte chemotactic protein 1 = MCP-1), CCL3 (macrophage inflammatory protein 1a = MIP-1a) CCL4 (macrophage inflammatory protein 1b = MIP-1b), and CCL5 (regulated upon the activation of normal T cell expressed and secreted = RANTES) were determined by specific enzyme-linked immunosorbent assay (ELISA) techniques according to the manifacturer's instructions (Biosource). The concentration of chemokines was determined spectrophotometrically. The absorbance was read at 450 nm.
Statistical methods - The anticandidal activity results were analyzed by Mann-Whitney U test and Wilcoxon Signet Ranks. Changes in the chemokines between samples were analyzed using the one-way analysis of variance (ANOVA). The Bonferroni test was used as Post Hoc analysis, p < 0.05 was considered to be significant.
RESULTS
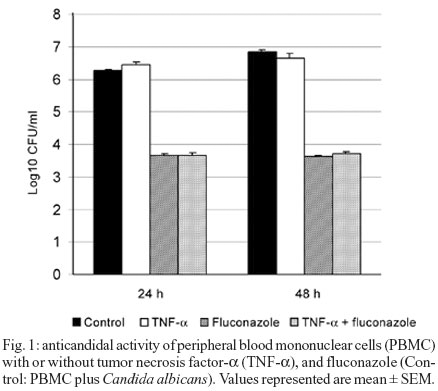
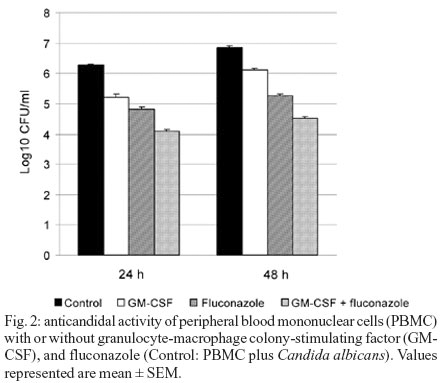
Effects of cytokines on the anticandidal activity of PBMC - The effect of GM-CSF, TNF-a, and IFN-g on anticandidal activities of mononuclear cells with the presence and absence of fluconazole in 0, 24, and 48 h are shown in Figs 1, 2, and 3 as Log10 CFU/ml.
When TNF-a alone was compared to the control well in 24 and 48 h, it was found that monocytes caused no significant changes in the anticandidal activity. The combination of flucanozole and TNF-a did not cause a change different from the one caused by the use of flucanozole alone (p > 0.05) (Fig. 1).
GM-CSF caused a significant decrease in the number of C. albicans both in 24 and 48 h compared to the control well when used alone (p < 0.05). GM-CSF, in combination with fluconazole, caused a significant decrease in the number of C. albicans both in 24 and 48 h compared to the wells where fluconazole was used alone (p < 0.05) (Fig. 2).
IFN-g caused a significant decrease in the number of live yeast both in 24 and 48 h compared to the control well (p < 0.05). However, in combination with fluconazole, IFN-g caused significantly more candidacidal activity both in 24 and 48 h than fluconazole when it was used alone (p < 0.05) (Fig. 3 ).
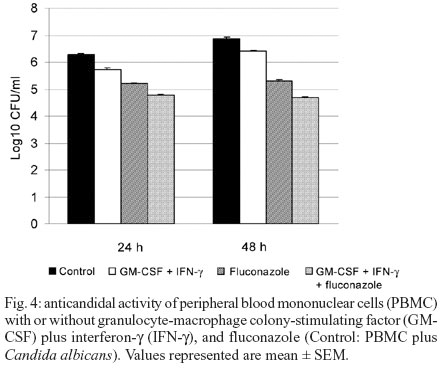
IFN-g and GM-CSF used in combination caused a significant decrease in the number of live C. albicans only in 24 and 48 h compared to the control well (p < 0.05). These two cytokines used in combination with fluconazole caused a significant decrease in the number of C. albicans both in 24 and 48 h compared to fluconazole when used alone (p < 0.05) (Fig. 4). However IFN-g and GM-CSF in combination did not cause a greater reduction in CFU/ml than either cytokines alone (Fig. 5).
The levels of chemokines in culture supernatants - The analysis of CCL3 levels in culture supernatants showed that while the addition of fluconazole in the presence of PBMCs caused a significant increase in the level of CCL3 in 24 h, the addition of C. albicans did not lead to any significant changes (p = 1). Moreover, the presence of TNF-a, GM-CSF, and IFN-g caused a significant increase in the level of CCL3. However, less increase was observed in the samples including the combination of GM-CSF and IFN-g. The addition of C. albicans and fluconazole caused more increase in CCL3 than cytokines alone did. After 48 h the same results were found as the ones obtained after 24 h and C. albicans did not cause any significant increases in the levels of CCL3 in the samples not containing fluconazole (p = 1) (Fig. 5).
The findings about CCL4 levels in culture supernatants revealed that the presence of both fluconazole and C. albicans increased the levels of CCL4 and only GM-CSF cytokines caused a significant increase compared to the samples including PBMC + C. albicans + fluconazole in 24 h (p < 0.05). The addition of C. albicans and fluconazole in the presence of cytokines caused a more remarkable increase in CCL4 level. As for 48 h, the co-existence of C. albicans and fluconazole brought about an increase in CCL4 levels as high as the samples including TNF-a, GM-CSF, and IFN-g (Fig. 6).
The other chemokines CCL2 and CCL5 whose levels were determined in their culture supernatants did not show any significant changes in their levels when there were PBMC, flucanozole, C. albicans or cytokines (p > 0.05) (data not shown).
Single or multiple cytokines without fluconazole or C. albicans did not show any significant differences from controls containing PBMC + C. albicans + fluconazole.
DISCUSSION
Increasing resistance against antifungal agents cause problems especially for the patients with compromised immune systems that are most likely to develop candidiasis and this makes it a must to perform further studies in order to find new alternatives in candidiasis treatment (Sheehan et al. 1999, Armstrong 2001, Maertens 2004).
Th1 cell response is associated with the resistance to fungal infections while Th2 response is associated with the susceptibility to fungi (Mencacci et al. 2000). Protective Th1 responses require the activation of a number of cytokines. Thus, it is considered that cytokines can be used as immunomodulatory agents in the treatment of candidiasis.
In our study, when TNF-a was used alone or in combination with fluconazole no significant changes were observed in anticandidal effect compared to control wells (p > 0.05). Louie et al. (1994) found that serums of mice infected with C. albicans exhibited increased TNF-a contents, but TNF-a had no anticandidal effect. In their in vitro studies with monocytes, Baltch et al. (2001) found that TNF-a had no effect on the growth of C. albicans.
In our study, the use of GM-CSF caused a significant increase in candidacidal activity of PBMCs in 24 and 48 h when used alone or in combination with fluconazole (p < 0.05). Yamamoto et al. (1997) observed that GM-CSF application caused an increase in the candidacidal activities of macrophages against C. albicans. In one of their clinical studies Vazques et al. (2000) observed that GM-CSF might be used as a support agent in patients with oropharyngeal candidiasis. Vora et al. (1998) indicated that GM-CSF increased the destructive effect of fluconazole and voriconazole against C. albicans.
We also observed that IFN-g increased the candidacidal effectiveness of PBMCs, with the presence or absence of fluconazole (p < 0.05). Marodi et al. (1993) indicated in their in vitro study that IFN-g increased the candidacidal activity of monocytes/macrophages in the presence of C. albicans. Bodasing et al. (2002) obtained positive results from administration of IFN-g and antifungal agents combination in the treatment of an HIV positive patient with recurrent resistant oropharyngeal candidiasis.
In this study, combination of IFN-g and GM-CSF was also found to have increased candidacidal activity significantly (p < 0.05). However, combined administration did not lead to a more significant decrease compared to their individual use. Thus, combination administration is not considered to be an advantage.
Baltch et al. (2005) reported that GM-CSF and IFN-g increased the intracellular anticandidal effect of voriconazole.
In the second part of our study, it was observed that the CCL3 chemokine levels increased significantly in the presence of fluconazole and cytokines. With the addition of both C. albicans and fluconazole, CCL4 increased as much as it did in the samples with cytokines especially in 48 h. As known, CCL3 and CCL4 are potent macrophage inflammatory chemokines. These chemokines are involved in acute and chronic inflammatory processes by attracting leukocytes to the site of inflammation or activating cell immune responses (Simitsopoulou et al. 2005). C. albicans might contribute to the cell-mediated immune responses by increasing the production of a number of chemokines (Huang & Levitz 2000). In our study fluconazole led to a significant increase in the levels of chemokines compared to control samples. The presence of cytokines caused a remarkable increase in the levels of CCL3 and CCL4, but CCL4 increased especially in the presence of fluconazole without cytokines. It was considered that fluconazole might contribute to the host response by increasing the levels of CCL3 and CCL4 chemokines. Since chemokines are considered to be crucial for the cell migration to the injured area the increase caused by fluconazole in the levels of CCL3 and CCL4 chemokines might be regarded as a different response to C. albicans.
Simitsopoulou et al. (2005) also indicated that some of lipid formulations of amphotericin B increased the levels of cytokine and chemokine in human monocytes.
The use of a single strain of C.albicans may affect the validity of the our results. However, we think that since the strain we studied is a reference strain, it will shed light on the results that will be obtained from other C. albicans strains.
Consequently, we think that fluconazole has an immunomodulatory effect and this effect may be apparent with the presence of cytokine. We believe that in future it will be inevitable to develop new treatments for the patients with disseminated candidiasis. But, it is certain that the results of some more studies are needed to be able use cytokines commonly in the treatments. Yet, we know that we carried out our studies in vitro and the conditions of in vivo may change some results. Still, the positive results obtained by some researchers in their clinical studies using cytokines and antifungal agents together encourages us for the results of our further studies.
Received 29 May 2006
Accepted 14 February 2007
Financial support: Gazi University Research Fund no. 01/2004-18
- Anaissie EJ, McGinnis MR, Pfaller MA 2003. The epidemiology of fungal infection. In Clinical Mycology, Churchill Livingstone, New York, p.1-19.
- Armstrong R 2001. The physiological role and pharmacological potential of nitric oxide in neutrophil activation. Int Immunopharmacol 1: 1501-1512.
- Ashman RB, Farah CS, Wanasaengsakul S, Hu Y, Pang G, Clancy RL 2004. Innate versus adaptive immunity in Candida albicans infection. Immunol Cell Biol 82: 196-204.
- Baltch AL, Bopp LH, Smith RP, Ritz WJ, Carlyn CJ, Michelsen PB 2005. Effects of voriconazole, granulocyte-macrophage colony-stimulating factor, and interferon g on intracellular fluconazole-resistant Candida glabrata and Candida krusei in human monocyte-derived macrophages. Diag Microb Infect Dis 52: 299-304.
- Baltch AL, Smith RP, Franke MA, Ritz WJ, Michelsen PB, Bopp LH 2001. Effects of cytokines and fluconazole on the activity of human monocytes against Candida albicans Antimicrob Agents Chemother 45: 96-104.
- Bodasing N, Seaton RA, Shankland GS, Pithie A 2002. Gamma-interferon treatment for resistant oropharyngeal candidiasis in an HIV-positive patient. J Antimicrob Chemother 50: 765-766.
- Farmaki E, Roilides E 2001. Immunotherapy in patients with systemic mycoses: a promising adjunct. Bio Drugs 15: 207-214.
- Huang C, Levitz SM 2000. Stimulation of Macrophage inflammatory protein-1a, macrophage inflammatory protein-1b, and RANTES by Candida albicans and Cryptococcus neo-formans in peripheral blood mononuclear cells from persons with and without human immunodeficiency virus infection. J Infect Dis 181: 791-794.
- Louie A, Baltch AL, Smith RP, Franke MA, Ritz WJ, Singh JK, Gordon MA 1994. Tumor necrosis factor alpha has a protective role in a murine model of systemic candidiasis. Infect Immun 62: 2761-2772.
- Maertens JA 2004. History of the development of azole derivatives. Clin Microbiol Infect 10 (Suppl. I): 1-10.
- Marodi L, Schreiber S, Anderson DC, MacDermott RP, Korchak HM, Johnston RB 1993. Enhancement of macrophage candidacidal activity by interferon-g. Increased phagocytosis, killing, and calcium signal mediated by a decreased number of mannose receptors. J Clin Invest 91: 2596-2601.
- Mencacci A, Cenci E, Bacci A, Bistoni F, Romani L 2000. Host immune reactivity determines the efficacy of combination immunotherapy and antifungal chemotherapy in candidiasis. J Infect Dis 181: 686-694.
- NCCLS 2002. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard, 2nd ed., p. 1-29, Wayne, Pennsylvania.
- Saavedra M, Taylor B, Lukacs N, Fidel PL 1999. Local production of chemokines during experimental vaginal candidiasis. Infect Immun 67: 5820-5826.
- Sheehan DJ, Hitchcock CA, Sibley CM 1999. Current and emerging azole antifungal agents. Clin Microbiol Rev 12: 40-79.
- Simitsopoulou M, Roilides E, Dotis J, Dalakiouridou M, Dudkova F, Andreadou E, Walsh TJ 2005. Differential expression of cytokines and chemokines in human monocytes induced by lipid formulations of amphotericin B. Antimicrob Agents Chemother 49: 1397-1403.
- Steele C, Fidel PL 2002. Cytokine and chemokine production by human oral and vaginal epithelial cells in response to Candida albicans Infect Immun 70: 577-583.
- Vazquez JA, Hidalgo JA, De Bono S 2000. Use of sargramostim (rh-GM-CSF) as adjunctive treatment of fluconazole-refractory oropharyngeal candidiasis in patients with AIDS: a pilot study. HIV Clin Trials 1: 23-29.
- Vora S, Purimetla N, Brummer E, Stevens DA 1998. Activity of voriconazole, a new triazole, combined with neutrophils or monocytes against Candida albicans: effect of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor. Antimicrob Agents Chemother 42: 907-910.
- Yamamoto Y, Klein TW, Tomioka M, Friedman H 1997. Differential effects of granulocyte/macrophage colony-stimulating factor (GM-CSF) in enhancing macrophage resistance to Legionella pneumophila vs Candida albicans Cell Immunol 176: 75-81.
Publication Dates
-
Publication in this collection
23 Feb 2007 -
Date of issue
Mar 2007
History
-
Accepted
14 Feb 2007 -
Received
29 May 2006