Abstract
The objective of this study was to evaluate the prevalence and dissemination of human astroviruses (HAstV) in the environment by analyzing urban sewage samples from a wastewater treatment plant in the city of Rio de Janeiro, Brazil. A one-year study was performed with a total of 48 raw and treated sewage composite samples, which were collected biweekly from an activated sludge plant. Virus particles were concentrated by the adsorption-elution method using negatively charged membranes associated to a Centriprep Concentrator® 50 (Nihon Millipore). HAstV were detected in 16.7% of the samples in raw and treated sewage by using both qualitative and quantitative reverse transcriptase-polymerase chain reactions (RT-PCR and qPCR, respectively). Positive untreated sewage sample exhibited mean values of 1.1 x 10(4) gEq/mL. The qPCR sensitivity was 18 gEq/reaction. Through utilization of qPCR, a HAstV recovery efficiency of 4.2% and 4.3% was demonstrated for raw and treated sewage samples, respectively. The presence of HAstV in both the raw and treated sewage samples demonstrated the dissemination of these viruses in the environment as well as viral permanence after sewage treatment. There was a reduction in the total and faecal coliform levels, indicating efficiency of the wastewater treatment plant.
astroviruses; wastewater; RT-PCR; qPCR
ARTICLES
Molecular detection of human astrovirus in an urban sewage treatment plant in Rio de Janeiro, Brazil
Flávia Ramos GuimarãesI; Fabiana Fioretti Martins FerreiraI; Carmen Baur VieiraI; Tulio Machado FumianI; Tatsuo ShuboII; José Paulo Gagliardi LeiteI; Marize Pereira MiagostovichI, + + Corresponding author: marizepm@ioc.fiocruz.br
ILaboratório de Virologia Comparada, Instituto Oswaldo Cruz-Fiocruz, Pavilhão Hélio & Peggy Pereira, Av. Brasil 4365, 21040-360 Rio de Janeiro, RJ, Brasil
IIAssessoria Técnica de Infra-estrutura e Meio Ambiente, Diretoria de Administração do Campus-Fiocruz, Rio de Jneiro, Brasil
ABSTRACT
The objective of this study was to evaluate the prevalence and dissemination of human astroviruses (HAstV) in the environment by analyzing urban sewage samples from a wastewater treatment plant in the city of Rio de Janeiro, Brazil. A one-year study was performed with a total of 48 raw and treated sewage composite samples, which were collected biweekly from an activated sludge plant. Virus particles were concentrated by the adsorption-elution method using negatively charged membranes associated to a Centriprep Concentrator® 50 (Nihon Millipore). HAstV were detected in 16.7% of the samples in raw and treated sewage by using both qualitative and quantitative reverse transcriptase-polymerase chain reactions (RT-PCR and qPCR, respectively). Positive untreated sewage sample exhibited mean values of 1.1 x 104 gEq/mL. The qPCR sensitivity was 18 gEq/reaction. Through utilization of qPCR, a HAstV recovery efficiency of 4.2% and 4.3% was demonstrated for raw and treated sewage samples, respectively. The presence of HAstV in both the raw and treated sewage samples demonstrated the dissemination of these viruses in the environment as well as viral permanence after sewage treatment. There was a reduction in the total and faecal coliform levels, indicating efficiency of the wastewater treatment plant.
Key words: astroviruses - wastewater - RT-PCR - qPCR
Recent developments in improved surveillance, routine screening and the application of sensitive molecular assays have increased recognition of enteric viruses as environmental contminants. Furthermore, the burden of human astroviruses (HAstV) infections has been well reported and recognized as important secondary etiologic agents of viral gastroenteritis (Wilhelmi et al. 2003).
Due to the growing importance of HAstV in cases of acute gastroenteritis among children, studies in Europe, USA, South America and Africa have investigated these viruses in the environment and demonstrated HAstV presence in rivers, reservoirs, residual waters and sludge (Gofti-Laroche et al. 2003, Le Cann et al. 2004, Meleg et al. 2006, Miagostovich et al. 2008).
HAstV are non-enveloped viruses with icosahedral symmetry of 28 nm in diameter, which belong to the Astroviridae family. The viral genome consists of a single-stranded, positive sense RNA molecule that is polyadenylated and comprised of approximately 6.8-7.2 kilobases, with three open reading frames (ORFs), designated ORF1a, ORF1b and ORF2 (Jonassen et al. 2003). HAstV are classified into eight genotypes (HAstV-1-HAstV-8), based on the phylogenetic analysis of ORF2. HAstV-1 has been described as the most prevalent genotype worldwide (Noel et al. 1995, Guix et al. 2002, Silva et al. 2006).
The objective of this study was to evaluate the prevalence and dissemination of HAstV in environmental samples using an adsorption-elution method for viral detection by using a negatively charged membrane technique, which was previously described for recovery of enteric viruses from seawater (Katayama et al. 2002). For this purpose, a one-year study was performed in an urban wastewater treatment plant in the city of Rio de Janeiro, since residual waters are the main source of pathogenic microorganisms. Therefore, such environments provide information regarding the different strains infecting human populations. Total and faecal coliforms were also investigated to characterize faecal contamination in the samples. To our knowledge, this is the first study demonstrating the circulation of HAstV in sewage samples of Brazil.
MATERIAL AND METHODS
Sewage samples - From January-December of 2005, 48 raw and treated sewage composite samples were collected biweekly from an activated sludge plant in the city of Rio de Janeiro, Brazil. Eight 250 mL aliquots were collected for each sample and a total of 2 L samples were stored in glass bottles. The samples were taken to the laboratory and immediately analysed for bacterial parameters. The samples were processed for viral concentration in the following 24 h and stored at -80ºC, until utilized for virus detection assays. Samples from influent and effluent were collected as positive and negative control.
Bacterial parameters - Total coliform (TC) and faecal coliform (FC) were measured using the Colilert®-18 Quanti-Tray®/2000 method (IDEXX Laboratories, Westbrook, USA).
Viral particle concentration method- HAstV were concentrated using an adsorption-elution method with negatively charged membranes, which included the insertion of an acid rinse step for removal of cations, as previously described (Katayama et al. 2002). Prior to process filtration, 1.2 MgCl2 was added in 2 L of water. The system was soaked briefly in a 10% bleach solution and rinsed in distilled water prior to each use. The eluate (10 mL) was re-concentrated to a final volume of 2 mL using a Centriprep Concentrator® 50 (Nihon Millipore).
Recovery efficiency of the method for HAstV concentration - In order to evaluate the recovery efficiency of HAstV from raw and untreated sewage, 100 µL of the 10% fecal suspension of HAstV genotype 1 strain (GenBank accession number DQ381498), prepared in Tris/HCl/Ca++ 0.01M pH 7.2 buffer, was spiked with HAstV. The negative control, without HAstV spiking, was also tested in order to certify the absence of a natural contamination. All assays for viral concentration were performed in triplicate (independent experiments) for both treated and untreated wastewater.
Extraction of viral RNA- Prior to extraction, 70 µL of Vertrel® (Sigma-Aldrich Corporation, St. Louis, Missouri, USA) was added to 140 µL of the sample and then centrifuged at 800 g for 10 min. Nucleic acid extraction from the supernatant was performed using a QIAmp Viral RNA Mini Kit® (Qiagen, Inc, Valencia, California, USA), following the manufacturer's protocol.
Reverse transcription reaction (RT)- The synthesis of cDNA was performed with RT using a random primer (PdN6, 50 A260 units, Amersham Biosciences, Chalfont St Giles, Buckinghamshire, UK) as previously described (Ferreira et al. 2008).
Enzymatic amplification- (i) Reverse transcription-Polymerase chain reaction (RT-PCR). Amplification was performed using 10-µL aliquots of the cDNA, as described in a previous study (Noel et al 1995). HAstV type 2 strain, obtained from faecal suspension at the Regional Reference Centre of Rotaviruses, was used as positive control. To exclude the possibility of cross-contamination, all reagents used for PCR were prepared in a laminar flow cabinet. A positive and a negative control were included in all PCR reactions. (ii) Quantitative Real-time PCR (qPCR). For specific detection and quantification of the HAstV genome, 5 µL of cDNA was also assayed. Amplification was performed in a 25 µL reaction mixture with the PCR Master Mix® (Applied Biosystems, Branchbug, New Jersey, USA). The reaction contained 5 µL of a cDNA sample or 10 µL of a quantified plasmid DNA, 1X TaqMan master mix, and the corresponding primers and TaqMan probes at the appropriate concentrations. HAstV genomes were quantified with 0.1 µM of the primers AV1 and AV2 and 0.15 µM of the fluorogenic probe AVS, as described by LeCann et al. (2004). Then, the uracil N-glycosilase in the core mix was activated (2 min at 50°C), followed by activation of the AmpliTaq Gold for 10 min at 95°C and 45 cycles (15 s at 94ºC and 1 min at 55°C), performed with an ABI 7500® (Applied Biosystem, California, USA). All samples were performed in duplicate. Positive and negative controls were included. The amount of DNA was defined as the average of the duplicate data obtained. The RT-PCR/qPCR reactions were performed in separate rooms from those used for viral isolation and processing of water samples. Table I shows the sequences of the primers used in both enzymatic amplification reactions.
RESULTS
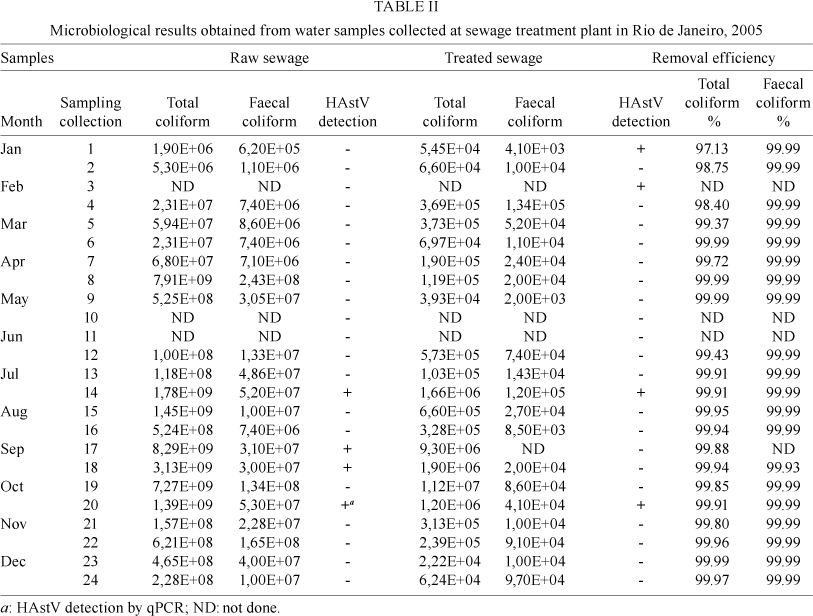
Detection and quantification of HAstV- The RT-PCR and qPCR protocol were both applied to 48 sewage samples: 24 from inflow and 24 from outflow. A total of seven HAstV strains (14.6%) were detectable by using qualitative RT-PCR, while qPCR detected one positive sample (2.1%) in a raw sewage with a titre of 1.1 x 104 G eq/mL. By using both methodologies, the total number of positive samples was determined to be 16.7% (8/48) of HAstV detected. The detection limit of the qPCR was 18 gEq/reaction. The standard curve demonstrated a correlation coefficient (R2) from 0.995-0.999 and slope varying from -3.64-4.0. The HAstV recovery efficiency was 4.2% and 4.3% for raw and treated sewage samples, respectively, as demonstrated by qPCR.
After sedimentation and biological secondary treatment (activated sludge), 99.9% of TC and 99.9% of FC were removed (Table II). The efficiency of HAstV removal could not be calculated, since positive samples could not be quantified from the raw sewage. HAstV were detected in both outflow and inflow water samples collected on the same day (Table II).
DISCUSSION
In Brazil, studies have been developed in order to evaluate the microbiological quality of water, with an emphasis on bacterial contamination (Alves et al. 2002, Nogueira et al. 2003). However, few studies have investigated the presence of human enteric viruses in water samples (Mehnert & Stewien 1993, Mehnert et al. 1997, Villar et al. 2006, Miagostovich et al. 2008). The absence of viral concentration methods of high recuperation efficiency and detection methods of low cost have been indicated as the primary reason for the low number of studies in the area of environmental virology. There is a growing demand for studies that establish fast and sensitive methods for the detection of viruses in environmental samples.
The recovery efficiency of the virus concentration method based on electrostatic interactions among viruses and an electronegative filter was previously evaluated for poliovirus (Katayama et al. 2002). Recently, the recovery of noroviruses and sapoviruses from sewage treatment plant also demonstrated the efficiency of this method for concentration and detection of viruses in water samples (Haramoto et al. 2006, 2008). In this study, the recovery of HAstV in both influent and effluent of the wastewater treatment plant was demonstrated using this methodology. However, the prevalence of HAstV (16.7%) was low when compared with other studies, with high indexes ranging from 43-100% and 82.3% from inflow and outflow wastewater samples, respectively (Nadan et al. 2003, Le Cann et al. 2004, Meleg et al. 2006). The high percentage of HAstV detected in sewage samples are usually explained due to the high seroprevalence (80-90%) of HAstV in the studied population, the high stability of these viruses in the environment, and the efficiency of the ultracentrifugation method that is traditionally used to concentrate and recover viral particles from wastewater (Le Cann et al. 2004). The low recovery of HAstV could be due to the low efficiency of the method used in this study and/or the low organic load supplied to the sewage treatment plant.
The prevalence of gastroenteritis associated with HAstV infection ranges from 2-11% and 2-26% in the developed and developing countries, respectively (Chikhin-Brachet et al. 2002, Cunlife et al. 2002, Dalton et al. 2002, Ratcliff et al. 2002). In Brazil, the prevalence of HAstV in the paediatric population ranged from 2-28% (Cardoso et al. 2002, Gabbay et al. 2005, Silva et al. 2006, Resque et al. 2007, Victoria et al. 2007, Soares et al. 2008). The Regional Reference Centre of Rotaviruses demonstrated a 7.8% prevalence of HAstV infection in the city of Rio de Janeiro in 2005 (unpublished data), which was lower than the prevalence detected from environmental samples in this study. According to studies based on HAstV detection in environmental samples, asymptomatic and/or mild digestive morbidity HAstV infections could create an underestimation of the real prevalence of infection by this virus in the population (Meleg et al. 2006). Studies with environmental samples have been suggested to replace those with clinical samples in order better determine the circulation of HAstV, since there is a higher prevalence of this virus in the environment than in clinical samples.
The presence of HAstV in both raw and treated samples demonstrates the resistance of these viruses to wastewater treatment and corroborates previous studies that also detected HAstV in inflow and outflow waters (Nadan et al. 2003, Le Cann et al. 2004, Meleg et al. 2006). This data suggest that HAstV resist sewage treatment at the activated sludge plant, remaining in the environment after being discharged into water body and is not related to the observed high removal efficiency of the total and faecal coliforms. Data obtained with these samples were previously published and demonstrated that the removal index of hepatitis A virus HAV (42.3%) was less than that for TC and FC (Villar et al. 2006). Molecular methods used for detecting HAstV cannot distinguish between infectious and non-infectious virions, although they provide a rapid and sensitive method to detect viruses as an alternative to overcome the limitations of conventional techniques, such as cell cultures, since HAstV are considered fastidious viruses. The detection of a single strand RNA genome in the environment has suggested the presence of infective viruses, since this molecule is not very stable in the environment (Meleg et al. 2006).
Although described as a more sensitive method that is recommended for investigation of environmental samples with low viral concentrations (Laverick et al. 2004), utilization of qPCR (Le Cann et al. 2004) did not present satisfactory results. Previous investigations have indicated lower sensitivity of the qPCR technique in comparison to traditional PCR (Noble et al. 2003, Grimm et al. 2004, Bastien et al. 2008).
To our knowledge, this is the first study demonstrating the detection of HAstV in a sewage treatment plant in Brazil. In a previous study, this methodology demonstrated the recovery of many human enteric viruses in the Amazon basin, with a 15.4% prevalence of HAstV in river waters (Miagostovich et al. 2008). This methodology, designed to both concentrate and detect HAstV in environmental samples, offers a new perspective for evaluation of viral circulation amongst the population via environmental dissemination.
ACKNOWLEDGEMENT
To Márcia Terezinha de Moraes e Souza, for supporting the cloning methodology, and Constança Britto, for helping with qPCR.
Received 15 August 2008
Accepted 19 November 2008
Financial support: Vice-Presidência de Serviços de Referência e Ambiente (Fiocruz), CNPq (472112/2004-0/303539/2004-6), CAPES.
- Alves NC, Odorizzi AC, Goulart F 2002. Análise microbiológica de águas minerais e de água potável de abastecimento. Rev Saude Publica 36: 749-751.
- Bastien P, Procop GW, Reischl U 2008. Quantitative Real-Time PCR is not more sensitive than "conventional" PCR. J Clin Microbiol 46: 1897-1900.
- Cardoso DD, Fiaccadori FS, Souza MB, Martins RM, Leite JP 2002. Detection and genotyping of astrovirus from children with acute gastroenteritis from Goiânia, Goiás, Brazil. Med Sci Monit 8: CR624-CR628.
- Chikhi-Brachet R, Bon F, Toubiana L, Pothier P, Nicolas JC, Flahault A, Kohli E 2002. Virus diversity in a winter epidemic of acute diarrhea in France. J Clin Microbiol 40: 4266-4272.
- Cunliffe NA, Dove W, Gondwe JS, Thindwa BD, Greensill J, Holmes JL, Bresee JS, Monroe SS, Glass RI, Broadhead RL, Molyneux ME, Hart CA 2002. Detection and characterization of human astroviruses in children with acute gastroenteritis in Blantyre, Malawi. J Med Virol 67: 563-566.
- Dalton RM, Roman ER, Negredo AA, Wilhelmi ID, Glass RI, Sanchez-Fauquier A 2002. Astrovirus acute gastroenteritis among children in Madrid, Spain. Pediatr Infect Dis J 21: 1038-1041.
- Ferreira MS, Xavier MP, Fumian TM, Victoria M, Oliveira SA, Pena LH, Leite JP, Miagostovich MP 2008. Acute gastroenteritis cases associated with noroviruses infection in the state of Rio de Janeiro. J Med Virol 80: 338-344.
- Gabbay YB, Luz CR, Costa IV, Cavalcante-Pepino EL, Sousa MS, Oliveira KK, Wanzeller AL, Mascarenhas JD, Leite JP, Linhares A C 2005. Prevalence and genetic diversity of astroviruses in children with and without diarrhea in São Luis, Maranhão, Brazil. Mem Inst Oswaldo Cruz 100: 709-714.
- Gofti-Laroche L, Gratacap-Cavallier B, Demanse D, Genoulaz O, Seigneurin JM, Zmirou D 2003. Are waterborne astrovirus implicated in acute digestive morbidity (EMIRA study)? J Clin Virol 27: 74-82.
- Grimm AC, Cashdollar JL, Williams EP, Fout GS 2004. Development of an astrovirus RT-PCR detection assay for use with conventional, real-time, and integrated cell culture/RT-PCR. Can J Microbiol 50: 269-278.
- Guix S, Caballero S, Villena C, Bartolome R, Latorre C, Rabella N, Simo M, Bosch A, Pinto RM 2002. Molecular epidemiology of astrovirus infection in Barcelona, Spain. J Clin Microbiol 40: 133-139.
- Haramoto E, Katayama H, Oguma K, Yamashita H, Tajima A, Nakajima H, Ohgaki S 2006. Seasonal profiles of human noroviruses and indicator bacteria in a wastewater treatment plant in Tokyo, Japan. Water Sci Technol 54: 301-308.
- Haramoto E, Katayama H, Phanuwan C, Ohgaki S 2008. Quantitative detection of sapoviruses in wastewater and river water in Japan. Lett Appl Microbiol 46: 408-413.
- Jonassen CM, Jonassen TO, Sveen TM, Grinde B 2003. Complete genomic sequences of astroviruses from sheep and turkey: comparison with related viruses. Virus Res 91: 195-201.
- Katayama H, Shimasaki A, Ohgaki S 2002. Development of a virus concentration method and its application to detection of enterovirus and norwalk virus from coastal seawater. Appl Environ Microbiol 68: 1033-1039.
- Laverick MA, Wyn-Jones AP, Carter MJ 2004. Quantitative RT-PCR for the enumeration of noroviruses (Norwalk-like viruses) in water and sewage. Appl Microbiol 39: 127-136.
- Le Cann P, Ranarijaona S, Monpoeho S, Le Guyader F, Ferré V 2004. Quantification of human astroviruses in sewage using real-time RT-PCR. Res Microbiol 155: 11-15.
- Mehnert DU, Stewien KE 1993. Detection and distribution of rotavírus in raw sewage and creeks in São Paulo, Brazil. Appl Environ Microbiol 59: 1140-143.
- Mehnert DU, Stewien KE, Harsi CM, Queiroz AP, Candeias JM, Candeias JA 1997. Detection of rotavirus in sewage and creek water: efficiency of the concentration method. Mem Inst Oswaldo Cruz 92: 97-100.
- Meleg E, Jakab F, Kocsis B, Bányai K, Melegh B, Szucs G 2006. Human astroviruses in raw sewage samples in Hungary. J Appl Microbiol 101: 1123-1129.
- Miagostovich MP, Ferreira FFM, Guimarães FR, Fumian TM, Diniz-Mendes L, Luz SLB, Silva LA, Leite JPG 2008. Molecular detection and characterization of gastroenteritis viruses occurring naturally on the water streams in Manaus, Central Amazônia, Brazil. Appl Environ Microbiol 74: 375-382.
- Nadan S, Walter JE, Grabow WO, Mitchell DK, Taylor MB 2003. Molecular characterization of astroviruses by reverse transcriptase PCR and sequence analysis: comparison of clinical and environmental isolates from South Africa. Appl Environ Microbiol 69: 747-753.
- Noble RT, Allen SM, Blackwood AD, Chu W, Jiang SC, Lovelace GL, Sobsey MD, Stewart JR, Wait DA 2003. Use of viral pathogens and indicators to differentiate between human and non-human fecal contamination in a microbial course tracking comparison study. J Water Health 1: 195-207.
- Noel J, Lee TW, Kurtz JB, Glass RI, Monroe SS 1995. Typing of human astroviruses from clinical isolates by enzyme immunoassay and nucleotide sequencing. J Clin Microbiol 33: 797-801.
- Nogueira G, Nakamura CV, Tognim MCB, Abreu Filho BA, Dias Filho BP 2003. Microbiological quality of drinking water of urban communities, Brazil. Rev Saude Publica 37: 232-236.
- Ratcliff RM, Doherty JC, Higgins GD 2002. Sensitive detection of RNA viruses associated with gastroenteritis by a hanging-drop single-tube nested reverse transcription-PCR method. J Clin Microbiol 40: 4091-4099.
- Resque HR, Munford V, Castilho JG, Schmich H, Caruzo TA, Rácz ML 2007. Molecular characterization of astrovirus in stool samples from children in São Paulo, Brazil. Mem Inst Oswaldo Cruz 102: 969-974.
- Silva PA, Cardoso DPP, Schreier E 2006. Molecular characterization of human astroviruses isolated in Brazil, including the complete sequences of astrovirus genotypes 4 and 5. Arch Virol 151: 1405-1417.
- Soares CC, Maciel de Albuquerque MC, Maranhão AG, Rocha LN, Ramírez ML, Benati FJ, Timenetsky MC, Santos N 2008. Astrovirus detection in sporadic cases of diarrhea among hospitalized and non-hospitalized children in Rio de Janeiro, Brazil, from 1998-2004. J Med Virol 80: 113-117.
- Victoria M, Carvalho-Costa FA, Heinemann MB, Leite JP, Miagostovich MP 2007. Genotypes and molecular epidemiology of human astroviruses in hospitalized children with acute gastroenteritis in Rio de Janeiro, Brazil. J Med Virol 79: 939-944.
- Villar LM, de Paula VS, Diniz-Mendes L, Lampe E, Gaspar AM 2006. Evaluation of methods used to concentrate and detect hepatitis A virus in water samples. J Virol Methods 137: 169-176.
- Wilhelmi I, Roman E, Sanchez-Fauquier A 2003. Viruses causing gastroenteritis. Clin Microbiol Infect 9: 247-262.
Publication Dates
-
Publication in this collection
13 Jan 2009 -
Date of issue
Dec 2008
History
-
Received
15 Aug 2008 -
Accepted
19 Nov 2008