Abstract
In this study, we looked at the inheritance of susceptibility and resistance to Schistosoma mansoni infection in the first generation of crossbred Biomphalaria alexandrina snails. Our ultimate goal is to use such information to develop a biological method of controlling schistosomiasis. We infected laboratory-bred snails with S. mansoni miracidia and examined cercarial shedding to determine susceptibility and resistance. Five parental groups were used: Group I contained 30 susceptible snails, Group II contained 30 resistant snails, Group III contained 15 susceptible and 15 resistant snails, Group IV contained 27 susceptible and three resistant snails and Group V contained three susceptible and 27 resistant snails. The percentage of resistant snails in the resulting progeny varied according to the ratio of susceptible and resistant parents per group; they are 7%, 100%, 68%, 45% and 97% from Groups I, II, III, IV and V, respectively. On increasing the percentage of resistant parent snails, the percentage of resistant progeny increased, while cercarial production in their susceptible progeny decreased.
Schistosoma mansoni; Biomphalaria alexandrina; susceptibility; resistance; biological control
ARTICLES
Inheritance of Schistosoma mansoni infection incompatibility in Biomphalaria alexandrina snails
Iman F Abou El Naga+ + Corresponding author: imanabouelnaga@hotmail.com ; Maha M Eissa; Shereen F Mossallam; Safaa I Abd El-Halim
Department of Parasitology, Faculty of Medicine, Alexandria University, Alexandria, Egypt
ABSTRACT
In this study, we looked at the inheritance of susceptibility and resistance to Schistosoma mansoni infection in the first generation of crossbred Biomphalaria alexandrina snails. Our ultimate goal is to use such information to develop a biological method of controlling schistosomiasis. We infected laboratory-bred snails with S. mansoni miracidia and examined cercarial shedding to determine susceptibility and resistance. Five parental groups were used: Group I contained 30 susceptible snails, Group II contained 30 resistant snails, Group III contained 15 susceptible and 15 resistant snails, Group IV contained 27 susceptible and three resistant snails and Group V contained three susceptible and 27 resistant snails. The percentage of resistant snails in the resulting progeny varied according to the ratio of susceptible and resistant parents per group; they are 7%, 100%, 68%, 45% and 97% from Groups I, II, III, IV and V, respectively. On increasing the percentage of resistant parent snails, the percentage of resistant progeny increased, while cercarial production in their susceptible progeny decreased.
Key words:Schistosoma mansoni - Biomphalaria alexandrina - susceptibility - resistance - biological control
Schistosomiasis is considered the second most important parasitic disease in the world, ranking next to malaria (Combes 1990). About 200 million people in the world are affected (WHO 1985). Africa, Latin America, South West and South East Asia are foci of the disease (WHO 1993). In Egypt, it is the most important endemic parasitic disease (Nunn & Tapp 2000). Approximately 70% of the adult chronic liver diseases and 35% of child liver diseases are due to schistosomiasis (El-Khoby et al. 2000). In 2007, about 3% of rural populations showed Schistosoma mansoni eggs in their stools (Bakr et al. 2007).
Over the last few decades, the epidemiological distribution of schistosomiasis in Egypt has changed; S. mansoni has replaced Schistosoma haematobium in the Nile delta and has become well established in Middle Egypt (Abdel-wahab et al. 2000). This has resulted in a marked decrease in the prevalence of S. haematobium infection, with an increase in S. mansoni infection rates. The spread of S. mansoni infection has serious effects on public health and has increased the burden of controlling schistosomiasis.
Various methods have been used to combat schistosomiasis, including treatment of patients, health education, improvement of sanitation, provision of safe water supplies to populations and snail control. Methods for controlling schistosome transmission by reducing snail populations have included chemical (molluscicides), physical and biological methods (Sturrock 2001).
One promising component of biological control is the introduction of parasite resistant snails into endemic areas to replace the resident susceptible snails and avoid the often destructive changes to the local ecosystem that accompany other methods of snail control (Sturrock 2001).
A preliminary step towards possible field trials in replacing susceptible with resistant snails is a laboratory study of the susceptibility patterns expected from progeny of interbreeding susceptible and resistant snail populations. This study would enable workers to predict the flow of resistance genes in the population and assess the feasibility of this snail control method (Lewis et al. 2002). It has been suggested that incompatibility to S. mansoni infection, a refractory characteristic of Biomphalaria alexandrina, could be hereditary, similar to susceptibility (Abdel-Hamid et al. 2006). Thus, it may be beneficial to select actively resistant snails and mass culture them to increase the proportion of alleles for incompatibility as a potential method for controlling schistosomiasis in natural populations. To better clarify the promise in this disease control methodology, we investigated the heritability of resistance in B. alexandrina snails.
MATERIALS AND METHODS
We bred the 100 mature snails in four transparent plastic aquaria. Each aquaria contained 5 L of well-aerated, aged and dechlorinated tap water (DTW), changed twice a week. During winter, they were kept at 26ºC and maintained at room temperature in the summer (Haroun 1996). Fresh lettuce leaves were supplied as food every couple of days; dead snails were regularly removed (Smithers & Terry 1965, Frandsen & Christensen 1984, Lewis et al. 2002).
Each aquarium contained pieces of foam that served as substratum for egg deposition (Azim & Watson 1948, Shoukry et al. 1997). Newly deposited egg masses were regularly collected using a scalpel, transferred to separate aquaria and inspected daily until hatching. After hatching, baby snails were reared for one month until they were juveniles (Shoukry et al. 1997). During this month, baby snails were fed using boiled lettuce leaves. White chalk pieces were added as a source of calcium for growth of the snail shell (Dettman et al. 1989).
Stools of infected, untreated children living in Abis village were collected, dispersed in physiological saline, strained and the filtrate centrifuged at 500 g for 3 min. We used the sediment containing S. mansoni eggs for infection of the B. alexandrina snails (Ragab et al. 2003).
We transferred the stool sediment to a petri dish containing about 500 mL of warm (35ºC) DTW and exposed it to direct sunlight for about 30 min to allow egg hatching. Using a fine capillary pipette, we aspirated 5-6 active, vigorously swarming miracidia and used them to infect each of the 40 juvenile snails, each placed in separate wells on tissue culture plates (Henning & Youssef 1976, El-Gindy et al. 1978, Shoukry et al. 1997). Four weeks later, we used infected B. alexandrina snails to harvest cercariae, by placing groups of 10 snails in 200 mL beakers, containing 50 mL DTW, under direct sunlight for about 1-2 h. After carefully shaking the cercarial suspensions, we aspirated 0.1 mL from each suspension and counted the cercariae using a dissecting microscope. We calculated the total number of harvested cercariae and then used them for animal infection (Pellegrino et al. 1962, Joy 1971).
Using the paddling technique, laboratory-bred Swiss strain albino mice were infected with 120 cercariae per mouse. Seven to eight weeks post-infection (Kogan et al. 1954), eggs obtained from the intestines and livers of infected mice (El-Gindy et al. 1985, Xu & Dresden 1989) were exposed to direct sunlight for approximately 30 min to stimulate miracidial hatching (Henning & Youssef 1976). To infect the juvenile snails, we aspirated 5-6 active, vigorously swarming miracidia (Henning & Youssef 1976, El-Gindy et al. 1978, Shoukry et al. 1997). Under the above described conditions, we transferred groups of roughly 25 snails to separate containers, each containing five litres of DTW. They were kept in darkness for about four weeks (Smithers & Terry 1965, Frandsen & Christensen 1984, Haroun 1996, Lewis et al. 2002).
Four weeks post-infection, the snails were checked individually for cercarial shedding twice per week for three weeks (McClelland 1965). Snails that shed cercariae were considered susceptible, while those that failed to shed cercariae were re-exposed to miracidia (El-Gindy et al. 1978, Shoukry et al. 1997). Four weeks later, the re-exposed snails were again tested for cercarial shedding, twice weekly for three weeks (McClelland 1965). Snails that failed to shed cercariae after the second miracidial exposure were considered resistant (Zanotti-Magalhaes & Magalhaes 1997).
To obtain the first generation (F1), 75 susceptible and 75 resistant snails were crossed in different proportions, as follows: Group I: 30 susceptible snails, Group II: 30 resistant snails, Group III: 15 susceptible, 15 resistant snails, Group IV: 27 susceptible, three resistant snails and Group V: three susceptible, 27 resistant snails.
Snails of each group were reared together in a separate aquarium containing about 4-5 L of DTW (Azim & Watson 1948, Kogan et al. 1954, Smithers & Terry 1965, Frandsen & Christensen 1984, Dettman et al. 1989, Lewis et al. 2002). To avoid fertilised eggs before the beginning of the experiment, we discarded egg batches from each of the five groups during the 1st four weeks. After the 4th week, the newly deposited batches were gently collected. Egg batches of each group were transferred to a separate container containing DTW. They were monitored daily until hatching.
After hatching, we used 150 snails in each experimental group, excluding dead snails from statistical analysis. Our analysis includes the 1st 100 snails that lived until the end of the experiment. The F1 in this study, the 150 baby snails from each group, was reared until they were juveniles. They were individually exposed to S. mansoni miracidia (Kogan et al. 1954, El-Gindy et al. 1978, Shoukry et al. 1997). Four weeks later, these juveniles were individually tested for cercarial shedding, returned to a separate container containing 250 mL of DTW and maintained in the same conditions described previously (Azim & Watson 1948, Kogan et al. 1954, Smithers & Terry 1965, Frandsen & Christensen 1984, Dettman et al. 1989, Lewis et al. 2002).
Cercarial shedding was done once weekly for each snail for four weeks (Pellegrino et al. 1962, Joy 1971). Snails that shed cercariae were considered susceptible, whereas any that did not shed, even after the second miracidial exposure, were considered resistant (Zanotti-Magalhaes & Magalhaes 1997).
Evaluation parameters of the F1 from different groups - (i) susceptibility rate: we determined the percentage of susceptible and resistant snails in each group (Shoukry et al. 1997, Lewis et al. 2002) and (ii) degree of susceptibility in each group: four weeks post-infection, we counted the number of cercariae shed from every susceptible snail in each group once weekly for four weeks (Pellegrino et al. 1962, Joy 1971). Total cercarial production per 100 snails (TCP/100 snails) and mean cercarial shedding per week were calculated for each group. We then used Frandsen's classes (Frandsen 1979) to assess the degree of susceptibility.
RESULTS
We recorded the susceptible and resistant traits expressed in the F1 from the various snail crosses (the 5 parent groups) of B. alexandrina snails, as follows.
Susceptibility and resistance rates in the F1 of the five groups -Table I and Fig. 1 show the percentages of susceptible and resistant snails in the F1 of the five parental groups. Among the F1 progeny from cross-bred Group I, containing only susceptible B. alexandrina parents, 93% of the snails were susceptible. Completely resistant F1 progeny were generated from Group II, in which all parents were resistant. The percentages of susceptible F1 snails resulting from mixed parental populations were 32%, 55% and 3% from parental Groups III, IV and V, respectively. The percentages of resistant snails were 7%, 100%, 68%, 45% and 97% in Groups I, II, III, IV and V, respectively.
Using Frandsen's classes (Frandsen 1979), we categorised the degree of susceptibility, as assessed according to the following parameters:
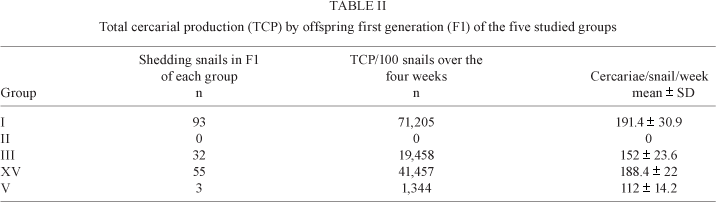
Cercarial production by susceptible snails among the offspring of the different groups - Shown in Table II, we calculated the total number of cercariae produced by the susceptible snails over a period of four weeks and the mean of cercariae shed by each susceptible snail in the different studied groups. From the Group I FI, in the third Frandsen class, the total number of cercariae produced by all snails over the four weeks (TCP) was 71,205. No cercariae (TCP = 0) were shed from F1 snails of Group II, which was in Frandsen class 0. The TCP of F1 snails in Group III was 19,458, placing them in Frandsen class 2. Both Groups IV and V were categorised as class 1 because their TCP values were 41,457 and 1,344 respectively.
We observed the highest cercarial production/snail/week in the Group I progeny at 191.4 ± 30.9 cercariae, followed by Group IV progeny at 188.4 ± 22, then Group III progeny that produced an average of 152 ± 23.6. Group V progeny showed the least cercarial production, at 112 ± 14.2 cercariae/snail/week, while Group II released no cercariae at all.
Mean number of cercariae shed by susceptible offspring in each group per week over the four-week interval - Table III and Fig. 2 show that the 2nd week of shedding yielded the highest mean number of cercariae shed by B. alexandrina snails in the progeny of Groups I, III and IV: 211.9 ± 41.3, 164.7 ± 25.9 and 203.2 ± 26.3 cercariae/snail, respectively. Data collected from Group V were not tabulated because they were not statistically significant, as only three snails shed cercariae. Yet we observed the highest mean (117 ± 13.1) in the 3rd week of shedding, followed by 115 ± 17.1 in the 2nd week. In the 1st and 4th weeks of shedding, the mean values were nearly the same: 108 ± 13.7 and 108 ± 13.1.
Table III shows the mean cercarial shedding for the different groups' progeny during the four weeks interval. In the 1st week, the mean values for the progeny in Groups I, III and IV were 182.8 ± 32.5, 146.2 ± 25.3 and 181.8 ± 22.6, respectively, where F = 21.13 and p = 0.000. During the 2nd week, 211.9 ± 41.3, 164.7 ± 25.9 and 203.3 ± 26.3 were the mean values of Group I, III and IV, respectively, where F = 22.024 and p = 0.000. For the 3rd week, the mean values of Group I, Group III and Group IV were 197.7 ± 36.8, 155.5 ± 27.5 and 192.8 ± 25.2, respectively, where F = 21.213 and p = 0.000. As for the 4th week, mean values of Group I, Group III and Group IV were 173.1 ± 27.3, 141.5 ± 22.6 and 175.8 ± 22.5, respectively, where F = 22.201 and p = 0.000. A post hoc test was calculated for the four-week interval and revealed a significant difference between Groups I and III, as well as between Groups III and IV.
DISCUSSION
Schistosomiasis presents significant economic and public health consequences in many developing countries and is considered a serious problem in Egypt (WHO 1985). Genetics is one form of biological control, first discussed by Newton (1952), who found that susceptibility of Biomphalaria glabrata to S. mansoni infection is hereditary. In 1958, Hubendick demonstrated that susceptible snail populations could be reduced by applying populations that are genetically resistant to the parasite. The flow of genetic resistance to S. mansoni in B. glabrata and Biomphalaria tenagophila was studied in the Western Hemisphere (Rosa et al. 2005, Coelho et al. 2008). However, little is known about the resistance genes in B. alexandrina.
Susceptibility of B. glabrata to S. mansoni infection is age dependant; in juvenile snails, susceptibility is controlled by at least four genes, each with multiple alleles, while in adulthood there is only one dominant gene that controls susceptibility (Richards & Shade 1987). Rosa et al. (2005) found that two dominant genes determine resistance in B. tenagophila. In the same year, El-Khayat et al. reported that genetics is one of the most important approaches for biological control of susceptibility of B. alexandrina to S. mansoni infection. In 2006, Abdel-Hamid et al. found that, similar to susceptibility, resistance is hereditary and is controlled by a genetic factor in B. alexandrina. In 2008, Coelho et al. introduced large numbers of resistant B. tenagophila into the field to cross-breed with the local snails. In their study, Coelho et al. applied molluscicides, which obliged the remaining local population to cross-breed with the resistant snails and generate S. mansoni resistant offspring. In light of such studies, we assessed the inheritance of S. mansoni resistance in B. alexandrina snails, using the progeny of interbreeding resistant and susceptible populations.
Analysis of these results revealed the appearance of resistant snails in the F1 progeny population in various snail crosses, which we attribute to a dominant resistance characteristic in B. alexandrina snails. Our result concurs with the Lewis et al. (2002) study using B. glabrata snails. Further, previous investigations documented dominant resistance heritability in B. glabrata snails (Richards & Merritt 1972).
Upon crossing susceptible and resistant snails in parental Groups III, IV and V, the resulting offspring contained resistant individuals in a higher percentage than in the parent groups. In B. glabrata, Lewis et al. (2002) generated almost comparable susceptibility phenotypes to those for B. alexandrina herein. The main difference between both studies was the 7% resistant progeny for B. alexandrina from completely susceptible parents, absent in the progeny of the analogous parental group for B. glabrata. A probable explanation for the 7% resistant B. alexandrina progeny is that the parent group includes snails that carry resistance genes and the resistance alleles increase among successive generations. Reinforcing this assumption, in 1997, Shoukry et al. reported the appearance of resistant B. alexandrina snails in the progeny of susceptible parents. Studying three successive B. alexandrina generations, they reported a decrease in susceptibility from one generation to the next among snail progeny originating from susceptible snails.
Containing completely resistant B. Alexandrina, all of the Group II offspring were resistant. Similarly, in 2002, Lewis et al. did not find any susceptible snails in the progeny of B. glabrata-resistant parents. However, whether or not susceptible progeny will appear as a result of crossing successive resistant generations requires further investigation. In 2006, Abdel-Hamid et al. validated this prediction using B. alexandrina, where they found that parent snails of susceptible progenies could be infection resistant.
In the current paper, susceptibility assessment by the TCP in each group revealed that the five groups fell within the lower degrees of Frandsen's categorisation (Frandsen 1979). According to Frandsen, Group I is compatible (class 3), as it produced the highest cercarial yield; this compatibility could be attributed to the snail crosses of this parental group that contained totally susceptible parents. Both Groups III and IV fell into class 2 of Frandsen (poorly compatible). Progeny of Group V were not very compatible (class 1). Group II lay within class 0, being completely resistant or incompatible; they shed no cercariae because their parents were completely resistant snails. Therefore, increasing the number of resistant parent snails decreases the degree of B. alexandrina susceptibility to S. mansoni infection. Notably, in 1996, Haroun collected B. alexandrina snails from two Egyptian Governorates and infected each group of snails with its homologous strain of S. mansoni miracidia. Haroun found that both groups lay within Frandsen's second, poorly compatible class.
Haroun's (1996) and our results show that B. alexandrina snails lie within the lower categories of compatibility compared to other Biomphalaria species studied by Frandsen in 1979. In Lewis et al. (2002), the B. glabrata parent group that contained totally susceptible snails did not give rise to any resistant progeny, while the analogous group in our B. alexandrina study yielded seven resistant snails. We reason that B. alexandrina may contain more resistance alleles in the susceptible population than those in other Biomphalaria species, thus accounting for its lower susceptibility and the appearance of resistant progeny from completely susceptible parents.
We used the mean cercarial shed/snail/week as our second parameter in assessing degree of B. alexandrina susceptibility to S. mansoni miracidia infection. This varied from one group to the other according to the percentage of resistant parents: the highest mean was from Group I progeny, having purely susceptible parents, followed by Group IV, containing only three resistant parents, then Group III, with 15 resistant parents. We observed the lowest cercarial production in Group V, consisting of 27 resistant parents. Comparing the mean cercarial shedding of Groups I, III and IV each week during the four weeks of shedding, there was a significant difference between both Groups I and III and Groups III and IV. In 2000, Knight et al. reported that even among susceptible snail stocks, certain individuals were more resistant than others.
Excluding Group II, which contained only resistant snails, we observed the highest cercarial count in each of the shedding groups in the 2nd week of shedding. Group V exhibited its highest cercarial count between the 2nd-3rd weeks of shedding. These results concur with Haroun's (1996) experiments that showed the highest cercarial production in shedding snails occurred mainly in the 2nd week of shedding.
Importantly, our results show that upon increasing the proportion of resistant parents, the percentage of their resistant progeny increased, while cercarial production in their susceptible progeny decreased. This study supports the introduction of parasite resistant B. alexandrina into endemic areas to replace the resident susceptible ones as a promising method of biological control for S. mansoni in Egypt. Future studies in the resistance and susceptibility of B. alexandrina to S. mansoni necessarily include the related genetic markers.
REFERENCES
Abdel-Hamid ZA, Rawi SM, Arafa AF 2006. Identification of genetic marker associated with the resistance to Schistosoma mansoni infection using random amplified polymorphic DNA analysis. Mem Inst Oswaldo Cruz 101: 863-868.
Abdel-wahab MF, Esmat G, Ramzy I, Narroz S, Medhat E, Ibrahim M, El-Boraey Y, Strickland GT 2000. The epidemiology of schistosomiasis in Egypt: Fayoum Governorate. Am J Trop Med Hyg 62: 55-64.
Azim M, Watson JM 1948. Comparative efficiency of various methods of infecting mice with Schistosoma mansoni. J Egypt Publ Helth Assoc 32: 121-128.
Bakr IM, Arafa NA, Ahmed MA, Mostafa ME, Mohamed MK 2007. Prevalence of Schistosoma mansoni and intestinal parasites in a rural population in Egypt and its relation to socio-demographic characteristics. Egypt J Com Med 25: 87-97.
Coelho PM, Rosa FM, Maciel E, Negrão-Correa DA, Carvalho OS, Caldeira RL, Jannotti-Passos LK, Moreira LA, Oliveira GC, Teles HM 2008. Transmission control of schistosomiasis mansoni by introduction of a resistant strain of Biomphalaria tenagophila in areas where transmission is maintained by this species. Acta Tropica 108: 245-248.
Combes C 1990. Where do human schistosomes come from? An evolutionary approach. Trends Ecol Evol 5: 334-337.
Dettman CD, Higgins-Opitz SB, Saikoolal A 1989. Enhanced efficacy of paddling method for schistosoma infection of rodents by a four-step pre-soaking procedure. Parasitol Research 76: 183-184.
El-Gindy IH, El-Gindy MS, Hammadi MJ 1978. Acquired susceptibility in Bulinus truncatus to bayluscide or mollutox due to repeated applications. J Egypt Soc Parasitol 8: 243-255.
El-Gindy MS, Arafa MS, Makled MK, Ismail MM, Elridi MM, Atia MM, El-Gamal RL 1985. Effect of some factors upon the susceptibility of Biomphalaria alexandrina to infection with Schistosoma mansoni. J Egypt Soc Parasitol 15: 231-235.
El-Khayat HMM, Saber MA, Abu El-Hassan A 2005. Study of the susceptibility of the Biomphalaria alexandrina collected from five localities in Egypt to infection with local strains of Schistosoma mansoni. Egypt J Schisto Infect End Dis 27: 39-50.
El-Khoby T, Galal N, Fenwick A, Barkat R, El-Hawey A, Nooman Z 2000. The epidemiology of schistosomiasis in Egypt: summary findings in nine governorates. Am J Trop Med Hyg 62: 88-99.
Frandsen F 1979. Discussion of the relationships between Schistosoma and their intermediate hosts, assessment of the degree of host-parasite compatibility and evaluation of schistosome taxonomy. Parasitol Research 58: 275-296.
Frandsen F, Christensen NQ 1984. An introductory guide to the identification of cercariae from African freshwater snails with special reference to cercariae of trematode species of medical and veterinary importance. Acta Tropica 41: 181-202.
Haroun NH 1996. Differences in susceptibility of Biomphalaria alexandrina to Schistosoma mansoni from Giza and Dakahlia Governorates, Egypt. J Egypt Soc Parasitol 26: 327-335.
Henning J, Youssef G 1976. Influence of diet on breeding and infectivity in mass cultivation of Biomphalaria glabrata. Egypt J Bilh 3: 45-55.
Hubendick B 1958. A possible method of schistosome-vector control by competition between resistant and susceptible strains. Bull WHO 8: 1113-1116.
Joy EJ 1971. The influence of day length upon the egg laying of Biomphalaria glabrata. Ann Trop Med Parasit 65: 573-578.
Knight M, Ongele E, Lewis FA 2000. Molecular studies of Biomphalaria glabrata, an intermediate host of Schistosoma mansoni. J Parasitol 30: 535-541.
Kogan IC, Short RB, Nez MM 1954. Maintenance of Schistosoma douthitti (Cort, 1941) in laboratory (Trematoda: Schistosomatidae). J Parasitol 40: 1-16.
Lewis FA, Patterson CN, Gizywarz C 2002. Parasite susceptibility phenotypes of F1 Biomphalaria glabrata progeny derived from interbreeding of Schistosoma mansoni resistant and susceptible snails. Parasitol Research 89: 98-101.
McClelland WJ 1965. The production of cercariae by Schistosoma mansoni and Schistosoma haematobium and methods for estimating the numbers of cercariae in suspension. Bull World Helth Organ 33: 270-276.
Newton WL 1952. The inheritance of susceptibility to infection with Schistosoma mansoni in Australorbis glabratus. Exp Parasitol 2: 525-541.
Nunn JF, Tapp E 2000. Tropical diseases in ancient Egypt. Trans R Soc Trop Med Hyg 94: 147-153.
Pellegrino J, Oliviera C, Favia J, Cunha AS 1962. New approach to the screening of drugs in experimental schistosomiasis mansoni in mice. Am J Trop Med Hyg 11: 201.
Ragab FMA, El-Khayat HMM, Mostafa BB, Gawish FA 2003. Difference in the susceptibility to certain molluscicides and Schistosoma mansoni infection of three forms of Egyptian Biomphalaria glabrata. J Egypt Soc Parasitol 33: 743-760.
Richards CS, Merritt JR 1972. Genetic factors in the susceptibility of juvenile Biomphalaria glabrata to Schistosoma mansoni infection. Am J Trop Med Hyg 21: 425-434.
Richards CS, Shade PC 1987. The genetic variation of compatibility in Biomphalaria glabrata for infection with Schistosoma mansoni. J Parasitol 73: 1146-1151.
Rosa FM, Godard ALB, Azevedo V, Coelho PMZ 2005. Biomphalaria tenagophila: dominant character of the resistance to Schistosoma mansoni in descendants of crossbreeding between resistant (Taim, RS) and susceptible (Joinville, SC) strains. Mem Inst Oswaldo Cruz 100: 19-23.
Shoukry NM, El-Assal FM, Soliman GN, Mansour NS 1997. Susceptibility of three successive snail generations from positive and negative laboratory bred Biomphalaria alexandrina from different localities in Egypt to infection with Schistosoma mansoni from Giza. J Egypt Soc Parasitol 27: 317-329.
Smithers SR, Terry RJ 1965. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology 55: 695-700.
Sturrock RF 2001. Schistosomiasis epidemiology and control: how did we get here and where should we go? Mem Inst Oswaldo Cruz 26: 17-27.
WHO - World Health Organization 1993. The control of schistosomiasis. Second report of the WHO Expert Committee. World Health Organ Tech Rep Ser 830: 1-86.
WHO - World Health Organization 1985. The control of schistosomiasis. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 782: 1-113.
Xu YZ, Dresden MH 1989. Schistosoma mansoni: egg morphology and hatchability. J Parasitol 75: 481-483.
Zanotti-Magalhaes EM, Magalhaes LA 1997. Relationship between pathogencity of Schistosoma mansoni in mice and the susceptibility of the vector mollusc. IV Infectiousness of miracidia. Rev Saude Publica 31: 488-494.
Received 3 August 2009
Accepted 25 February 2010
- Abdel-Hamid ZA, Rawi SM, Arafa AF 2006. Identification of genetic marker associated with the resistance to Schistosoma mansoni infection using random amplified polymorphic DNA analysis. Mem Inst Oswaldo Cruz 101: 863-868.
- Abdel-wahab MF, Esmat G, Ramzy I, Narroz S, Medhat E, Ibrahim M, El-Boraey Y, Strickland GT 2000. The epidemiology of schistosomiasis in Egypt: Fayoum Governorate. Am J Trop Med Hyg 62: 55-64.
- Azim M, Watson JM 1948. Comparative efficiency of various methods of infecting mice with Schistosoma mansoni J Egypt Publ Helth Assoc 32: 121-128.
- Bakr IM, Arafa NA, Ahmed MA, Mostafa ME, Mohamed MK 2007. Prevalence of Schistosoma mansoni and intestinal parasites in a rural population in Egypt and its relation to socio-demographic characteristics. Egypt J Com Med 25: 87-97.
- Coelho PM, Rosa FM, Maciel E, Negrão-Correa DA, Carvalho OS, Caldeira RL, Jannotti-Passos LK, Moreira LA, Oliveira GC, Teles HM 2008. Transmission control of schistosomiasis mansoni by introduction of a resistant strain of Biomphalaria tenagophila in areas where transmission is maintained by this species. Acta Tropica 108: 245-248.
- Combes C 1990. Where do human schistosomes come from? An evolutionary approach. Trends Ecol Evol 5: 334-337.
- Dettman CD, Higgins-Opitz SB, Saikoolal A 1989. Enhanced efficacy of paddling method for schistosoma infection of rodents by a four-step pre-soaking procedure. Parasitol Research 76: 183-184.
- El-Gindy IH, El-Gindy MS, Hammadi MJ 1978. Acquired susceptibility in Bulinus truncatus to bayluscide or mollutox due to repeated applications. J Egypt Soc Parasitol 8: 243-255.
- El-Gindy MS, Arafa MS, Makled MK, Ismail MM, Elridi MM, Atia MM, El-Gamal RL 1985. Effect of some factors upon the susceptibility of Biomphalaria alexandrina to infection with Schistosoma mansoni J Egypt Soc Parasitol 15: 231-235.
- El-Khayat HMM, Saber MA, Abu El-Hassan A 2005. Study of the susceptibility of the Biomphalaria alexandrina collected from five localities in Egypt to infection with local strains of Schistosoma mansoni. Egypt J Schisto Infect End Dis 27: 39-50.
- El-Khoby T, Galal N, Fenwick A, Barkat R, El-Hawey A, Nooman Z 2000. The epidemiology of schistosomiasis in Egypt: summary findings in nine governorates. Am J Trop Med Hyg 62: 88-99.
- Frandsen F 1979. Discussion of the relationships between Schistosoma and their intermediate hosts, assessment of the degree of host-parasite compatibility and evaluation of schistosome taxonomy. Parasitol Research 58: 275-296.
- Frandsen F, Christensen NQ 1984. An introductory guide to the identification of cercariae from African freshwater snails with special reference to cercariae of trematode species of medical and veterinary importance. Acta Tropica 41: 181-202.
- Haroun NH 1996. Differences in susceptibility of Biomphalaria alexandrina to Schistosoma mansoni from Giza and Dakahlia Governorates, Egypt. J Egypt Soc Parasitol 26: 327-335.
- Henning J, Youssef G 1976. Influence of diet on breeding and infectivity in mass cultivation of Biomphalaria glabrata. Egypt J Bilh 3: 45-55.
- Hubendick B 1958. A possible method of schistosome-vector control by competition between resistant and susceptible strains. Bull WHO 8: 1113-1116.
- Joy EJ 1971. The influence of day length upon the egg laying of Biomphalaria glabrata Ann Trop Med Parasit 65: 573-578.
- Knight M, Ongele E, Lewis FA 2000. Molecular studies of Biomphalaria glabrata, an intermediate host of Schistosoma mansoni J Parasitol 30: 535-541.
- Kogan IC, Short RB, Nez MM 1954. Maintenance of Schistosoma douthitti (Cort, 1941) in laboratory (Trematoda: Schistosomatidae). J Parasitol 40: 1-16.
- Lewis FA, Patterson CN, Gizywarz C 2002. Parasite susceptibility phenotypes of F1 Biomphalaria glabrata progeny derived from interbreeding of Schistosoma mansoni resistant and susceptible snails. Parasitol Research 89: 98-101.
- McClelland WJ 1965. The production of cercariae by Schistosoma mansoni and Schistosoma haematobium and methods for estimating the numbers of cercariae in suspension. Bull World Helth Organ 33: 270-276.
- Newton WL 1952. The inheritance of susceptibility to infection with Schistosoma mansoni in Australorbis glabratus Exp Parasitol 2: 525-541.
- Nunn JF, Tapp E 2000. Tropical diseases in ancient Egypt. Trans R Soc Trop Med Hyg 94: 147-153.
- Pellegrino J, Oliviera C, Favia J, Cunha AS 1962. New approach to the screening of drugs in experimental schistosomiasis mansoni in mice. Am J Trop Med Hyg 11: 201.
- Ragab FMA, El-Khayat HMM, Mostafa BB, Gawish FA 2003. Difference in the susceptibility to certain molluscicides and Schistosoma mansoni infection of three forms of Egyptian Biomphalaria glabrata. J Egypt Soc Parasitol 33: 743-760.
- Richards CS, Merritt JR 1972. Genetic factors in the susceptibility of juvenile Biomphalaria glabrata to Schistosoma mansoni infection. Am J Trop Med Hyg 21: 425-434.
- Richards CS, Shade PC 1987. The genetic variation of compatibility in Biomphalaria glabrata for infection with Schistosoma mansoni J Parasitol 73: 1146-1151.
- Rosa FM, Godard ALB, Azevedo V, Coelho PMZ 2005. Biomphalaria tenagophila: dominant character of the resistance to Schistosoma mansoni in descendants of crossbreeding between resistant (Taim, RS) and susceptible (Joinville, SC) strains. Mem Inst Oswaldo Cruz 100: 19-23.
- Shoukry NM, El-Assal FM, Soliman GN, Mansour NS 1997. Susceptibility of three successive snail generations from positive and negative laboratory bred Biomphalaria alexandrina from different localities in Egypt to infection with Schistosoma mansoni from Giza. J Egypt Soc Parasitol 27: 317-329.
- Smithers SR, Terry RJ 1965. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology 55: 695-700.
- Sturrock RF 2001. Schistosomiasis epidemiology and control: how did we get here and where should we go? Mem Inst Oswaldo Cruz 26: 17-27.
- WHO - World Health Organization 1993. The control of schistosomiasis. Second report of the WHO Expert Committee. World Health Organ Tech Rep Ser 830: 1-86.
- WHO - World Health Organization 1985. The control of schistosomiasis. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 782: 1-113.
- Xu YZ, Dresden MH 1989. Schistosoma mansoni: egg morphology and hatchability. J Parasitol 75: 481-483.
- Zanotti-Magalhaes EM, Magalhaes LA 1997. Relationship between pathogencity of Schistosoma mansoni in mice and the susceptibility of the vector mollusc. IV Infectiousness of miracidia. Rev Saude Publica 31: 488-494.
Publication Dates
-
Publication in this collection
19 Apr 2010 -
Date of issue
Mar 2010
History
-
Accepted
25 Feb 2010 -
Received
03 Aug 2009