Abstract
The diagnosis of visceral leishmaniasis (VL) generally requires the use of invasive tests for the collection of infected tissue (aspirates of bone marrow, spleen, liver or lymph nodes). This difficulty has led to the search for safer and less painful techniques to confirm the occurrence of the disease in children. Polymerase chain reaction (PCR) is a method that is advantageous in that it allows the use of peripheral blood samples for diagnosis. This paper reports the utilisation of PCR on peripheral blood samples to diagnose VL in 45 children in Mato Grosso do Sul, Brazil. This technique is compared with methods carried out using tissue collected by invasive procedures, including direct microscopy, culture and detection of Leishmania DNA by PCR in bone marrow aspirates. The results show that PCR of peripheral blood provides great sensitivity (95.6%) that is similar to that from the PCR of bone marrow aspirates (91.1%) and higher than that achieved with microscopy (80%) or culture (26.7%) methods. PCR of peripheral blood proved to be a suitable tool for the diagnosis of VL in children because it is highly sensitive and safe, with tissue collection being less invasive than in traditional tests.
leishmaniasis; PCR; peripheral blood; children
ARTICLES
Polymerase chain reaction of peripheral blood as a tool for the diagnosis of visceral leishmaniasis in children
Thiago Leite FragaI, + + Corresponding author: thiagofragavet@yahoo.com.br ; Yvone Maia BrustoloniII; Rosimar Baptista LimaIII; Maria Elizabeth Cavalheiros DorvalIV; Elisa Teruya OshiroIV; Janaina OliveiraI; Ana Lúcia Lyrio de OliveiraII; Claude PirmezIII
IPrograma de Pós-graduação em Doenças Infecciosas e Parasitárias, Departamento de Patologia, Universidade Federal de Mato Grosso do Sul, CP 549, 79070-900 Campo Grande, MS, Brasil
IIDepartamento de Pediatria, Universidade Federal de Mato Grosso do Sul, CP 549, 79070-900 Campo Grande, MS, Brasil
IIILaboratório de Imunopatologia, Instituto Oswaldo Cruz-Fiocruz, Rio de Janeiro, RJ, Brasil
IVDepartamento de Patologia, Universidade Federal de Mato Grosso do Sul, CP 549, 79070-900 Campo Grande, MS, Brasil
ABSTRACT
The diagnosis of visceral leishmaniasis (VL) generally requires the use of invasive tests for the collection of infected tissue (aspirates of bone marrow, spleen, liver or lymph nodes). This difficulty has led to the search for safer and less painful techniques to confirm the occurrence of the disease in children. Polymerase chain reaction (PCR) is a method that is advantageous in that it allows the use of peripheral blood samples for diagnosis. This paper reports the utilisation of PCR on peripheral blood samples to diagnose VL in 45 children in Mato Grosso do Sul, Brazil. This technique is compared with methods carried out using tissue collected by invasive procedures, including direct microscopy, culture and detection of Leishmania DNA by PCR in bone marrow aspirates. The results show that PCR of peripheral blood provides great sensitivity (95.6%) that is similar to that from the PCR of bone marrow aspirates (91.1%) and higher than that achieved with microscopy (80%) or culture (26.7%) methods. PCR of peripheral blood proved to be a suitable tool for the diagnosis of VL in children because it is highly sensitive and safe, with tissue collection being less invasive than in traditional tests.
Key words: leishmaniasis - PCR - peripheral blood - children
The quick and accurate diagnosis of visceral leishmaniasis (VL) enables early treatment and constitutes the key factor in the survival of the thousands of patients who contract the disease worldwide every year. However, confirmation of diagnosis usually requires direct visualization by microscopy or isolation of the aetiological agent in culture and is often difficult due to the shortage of parasites in clinical samples.
Recent improvements to the molecular approaches used to identify DNA sequences specific to certain pathogens have provided new options for the identification and characterisation of infectious agents, including those causing leishmaniasis. Knowledge of the Leishmania genome has allowed the development of sensitive and specific methods like polymerase chain reaction (PCR) that are used increasingly in the diagnosis of VL.
PCR has been shown to be a useful method for the diagnosis of VL because Leishmania DNA may be present in only minimal quantities in biological specimens (Pandey et al. 2009). Moreover, various biological samples may be employed in the reaction, including bone marrow (Cortes et al. 2004), skin biopsies (Stark et al. 2006), lymph nodes (Lachaud et al. 2002) or buffy coat (Lachaud et al. 2001). The possibility of utilising PCR on peripheral blood samples (Lachaud et al. 2000) makes this technique especially suitable for diagnosing the disease in children, who may be caused pain and discomfort, or may suffer potentially fatal accidents with the use of conventional invasive procedures (bone marrow, spleen and liver aspiration).
Although VL is a common disease in childhood, few studies have explored the use of PCR for its diagnosis in young children and these studies have excluded the use of peripheral blood, an easily obtainable biological material. Most reports include adults and children within the same sample (Katakura et al. 1998, Antinori et al. 2002, Disch et al. 2006). Only recently have there been studies that evaluate the use of PCR exclusively for the diagnosis of children (Cascio et al. 2002, Cruz et al. 2006, Kaouech et al. 2008). Moreover, the number of children in most cases is small and more studies are therefore needed to prove the value of the PCR method in the diagnosis of VL in children.
The objective of this study was to evaluate the efficacy of PCR of peripheral blood in diagnosing paediatric VL, comparing this technique with those that employ the invasive collection of samples, such as direct microscopy, culture tests and the detection of Leishmania DNA by PCR in bone marrow aspirates.
PATIENTS, MATERIALS AND METHODS
This study was based on a prospective analysis of children admitted for treatment of VL at the University Hospital of the Universidade Federal de Mato Grosso do Sul (NHU-UFMS) from March 2008-March 2009.
Children considered to be VL carriers presented characteristic clinical features (fever, hepatosplenomegaly and pancytopenia) that are associated with the detection of parasites in bone marrow aspirates [by means of direct microscopy or in NNN (Novy-MacNeal-Nicolle) culture] and/or had positive serology (> 1/80) as determined by indirect immunofluorescence (RIFI). Patients who had negative parasitological or serological test results but who presented a favourable response to specialised treatment were also considered to be VL carriers.
On admission, all patients submitted material for laboratory tests. In order to perform PCR, 2 mL of peripheral blood and 1 mL of bone marrow aspirate (obtained by sternal puncture) were collected in separate Eppendorf tubes (Perkin-Elmer, EUA) containing EDTA and were stored at -20ºC for later use. For parasitological tests (direct microscopy and culture), 2 mL of bone marrow aspirate were collected in a tube containing EDTA and were immediately sent for analysis. For serological analysis by RIFI, 2 mL of peripheral blood were used.
Direct microscopy - To identify Leishmania amastigotes, imprint smear slides were prepared from bone marrow aspirates, fixed with methanol, stained with Giemsa and analysed by specialists experienced in VL diagnosis. Four slides from each patient were analysed with a 10' eyepiece and a 100' oil objective. The entire smear was examined (more than 1000 microscopic fields) before a negative result was assigned.
Culture - Samples of bone marrow aspirates were cultivated in NNN medium with a liquid phase of Schneider medium supplemented with 20% fetal bovine serum. Cultures were incubated at 24ºC and examined weekly by microscopy for the presence of the parasite for a total of eight weeks.
PCR-DNA extraction - To isolate DNA from 300 μL of bone marrow aspirate or peripheral blood, the GenomicPrepTM Blood DNA Isolation Kit (Invitrogen) was used in accordance with the manufacturer's instructions.
Reaction - PCR was performed according to previously described procedures (Schubach et al. 1998, Pirmez et al. 1999). The DNA sequence amplified by PCR was the conserved region of the minicircle molecule (kinetoplastid mitochondrial DNA; kDNA). Briefly, a solution containing the following elements was added to Eppendorf tubes: 200 mM (2,5 μL) of each deoxynucleoside triphosphate (dCTP, dATP, dGTP, dTTP, Pharmacia, Upsalla, Sweden), 10X buffer (2,5 μL) (Invitrogen), 1,5 mM MgCl2 (1,5 μL), 200 ng (0,2 μL) of each primer (HM1: 5'- CCG CCC CTA TTT TAC ACC AAC CCC-3', HM2: 5'- GGG GAG GGG GCG TTC TGC GAA -3', HM3: 5'- GGC CCA CTA TAT TAC ACC AAC CCC -3', Gibco, São Paulo, SP) and 2,5 U (0,5 μL) of AmpliTaq Polymerase (Perkin-Elmer). A negative control without DNA in the mixture and a positive control containing 20 fg of Leishmania DNA were included in each experiment.
PCR was performed in a total reaction volume of 25 μL under the following conditions: 30 cycles of 30 s each consisting of denaturation at 94ºC, annealing at 54ºC and extension at 72ºC. A Perkin-Elmer 2400 automatic thermocycler was used. Amplified products (10 μL) were analysed by electrophoresis in 1% agarose gels stained with ethidium bromide (0.5 μg/mL) and visualised with UV. A 100-bp DNA ladder (Promega) was used as a marker. Samples were considered positive for Leishmania DNA when a PCR fragment of 120 bp could be detected.
PCR-RFLP (restriction fragment length polymorphism) - For Leishmania detection, the amplified products obtained through PCR of peripheral blood or bone marrow aspirates were analysed by RFLP according to the protocol proposed by Volpini et al. (2004). Briefly, PCR products (5 μL) were digested by the addition of 1 U of HaeIII (Invitrogen) for 3 h at 37ºC in the appropriate buffer. Restriction fragments were separated on a 10% polyacrylamide gel in the Mini-Protean 3 system and were visualised by silver staining (DNA Silver Staining Kit, Amersham Biosciences).
Statistical analysis - The Epi-Info 3.4.1 and Bioestat 5.0 programs were used for data analysis. The McNemar chi-square test was used to compare results. The level of significance adopted was 0.05.
Ethics - This study was approved by the Ethical Committee of the UFMS, protocol 1146. The guardians of each patient (generally parents or grandparents) were responsible for authorizing and signing informed consent documents.
RESULTS
In the period from March 2008-May 2009, 45 children from state of Mato Grosso do Sul were admitted at the NHU-UFMG, Brazil for treatment of VL.
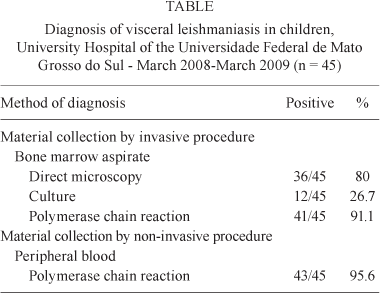
Of the 45 patients included in the study, 36 (80%) had a positive result from direct BMA microscopy, 12 (26.7%) had positive BMA cultures (Table) and 21 (46.7%) presented positive results in RIFI. Six children were scored negative by conventional tests but were included in the study because of favourable therapeutic evidence.
Twenty-three patients were male (51.1%) and 22 female (48.9%). Age ranged from five months to 13 years (mean = 3 years and 11 months). Thirty-eight children (84.4%) were treated with antimoniate of N-methylglucamine (20 mg/kg/day of Sbv) and seven (15.5%) received amphotericin B (1 mg/kg/day) (total = 20 mg/kg). Each group received treatment over a 20-day period. The Table compares the results from various methods used for the diagnosis of VL.
Among the six children included in the study whose results were negative by direct microscopy, culture and serology, PCR of peripheral blood gave a positive result for all and PCR of bone marrow aspirates was positive for four (Figure). RFLP analysis identified all strains as Leishmania infantum chagasi (Shaw 2006).
Comparison of PCR of peripheral blood with invasive methods
PCR in peripheral blood vs. direct microscopy of bone marrow aspirate - Thirty-five children (77.8%) presented positive results by both methods. Of the nine children with negative results from PCR of peripheral blood, eight had positive results from direct microscopy. There was a statistically significant discordance between the two methods (p = 0.039).
PCR of peripheral blood vs. PCR of bone marrow aspirate - Thirty-nine out of 45 patients (86.7%) were scored positive by both methods. The four children with negative results from PCR of bone marrow aspirates showed positive results from PCR of peripheral blood. A statistically significant agreement between the two methods was demonstrated (p = 0.688).
PCR of peripheral blood vs. culture of bone marrow aspirate - Eleven patients (24.4%) presented positive results with both methods. There was a statistically significant discordance between the two methods (p < 0.001). Of the 33 children with negative culture results, 32 had positive results from PCR of peripheral blood.
DISCUSSION
The collection of infected tissues through splenic puncture for parasitological diagnosis may result in fatal haemorrhagic complications (Siddig et al. 1988, Zijlstra et al. 1992) and demands a high level of expertise from the health professional that can be found only in a specialised centre. As a result, bone marrow aspirate is the most commonly utilised material for the diagnosis of paediatric VL (Kafetzis & Maltezou 2002).
For a long time, it was believed that parasites were absent or were very rare in the blood of patients with VL (Lachaud et al. 2000). Recently, molecular biology techniques were adapted for the detection of Leishmania DNA in peripheral blood (Adhya et al. 1995, Schaefer et al. 1995) from methods that had already been used in bone marrow aspirates in the early 1990s (Rodgers et al. 1990, Smith et al. 1992).
In this study, the high level of sensitivity of PCR in peripheral blood was demonstrated by its ability to detect parasite DNA in 95.6% of the samples. Other authors who evaluated the use of PCR with this kind of biological material exclusively in children reported sensitivities ranging from 79-100% (Cascio et al. 2002, Cruz et al. 2006, Kaouech et al. 2008).
The sensitivity of PCR in peripheral blood was as high as that of PCR in bone marrow aspirates (91.1%), a method currently ranked among the most sensitive. Recently, other researchers have confirmed our results, reporting the same efficacy when either bone marrow aspirates or peripheral blood were used for diagnosing VL by PCR techniques. Deborggraeve et al. (2008) recorded positive results in 92.1% of peripheral blood samples and in 92.9% of bone marrow aspirate. Likewise, Antinori et al. (2002) also detected pathogens with an efficiency of 98.5% for peripheral blood and 95.7% for bone marrow aspirates. There are other similar reports that confirm that PCR of peripheral blood is as reliable as PCR of bone marrow aspirate (Costa et al. 1996, Pizzuto et al. 2001, Cascio et al. 2002, Cruz et al. 2002, Fisa et al. 2002).
We have also analysed amastigotes by direct microscopy and the cultivation of promastigotes in culture, techniques considered to be the "gold standard" for diagnosing VL. We have demonstrated that PCR of peripheral blood is more sensitive for VL detection than microscopy of bone marrow aspirate (95.6% vs. 80%). Other studies in children corroborate these data. Cruz et al. (2006) analysed 25 individuals and identified positive results in 67% of the children by direct microscopy and 79% by PCR of peripheral blood. Kaouech et al. (2008) evaluated the efficacy of PCR in peripheral blood in 53 children in Tunisia and reported positive results for 100% of those tested with this method compared with 79% when direct microscopy of bone marrow aspirate was used. Piarroux et al. (1994) also recorded a greater sensitivity with PCR of peripheral blood (82%) when compared with direct microscopy (55%) and culture (55%) tests.
In our patients, the culture test demonstrated low sensitivity in detecting promastigotes. The value of using cultures for diagnosis may have been reduced because of the frequency of sample contamination. Cruz et al. (2006) also observed a slight elevation in the number of positive results from culture tests (44% of 25 children). As this method is also time-consuming, these results explain why the culture test is currently performed in only a few laboratories.
When the sensitivity of a method is evaluated, the inclusion of patients in the study in the absence of a goldstandard diagnostic technique may have important consequences. We included patients without parasitological confirmation, which constitutes one of the limitations of the present study.
The findings described here suggest that it may be advantageous to employ PCR of peripheral blood in place of traditional methods used for the diagnosis of VL, including direct microscopy and culture tests. PCR detection in peripheral blood can also be used instead of the highly sensitive PCR of bone marrow aspirates in diagnosing VL in children. This would avoid the collection of infected material through painful and sometimes hazardous invasive procedures.
Received 4 October 2009
Accepted 8 April 2010
- Adhya S, Chatterjee M, Hassan MQ, Mukherjee S, Sen S 1995. Detection of Leishmania in the blood of early kala-azar patients with the aid of the polymerase chain reaction. Trans R Soc Trop Med Hyg 89: 622-624.
- Antinori S, Calattini S, Longhi E, Bestetti G, Piolini R, Magni C, Orlando G, Gramiccia M, Acquaviva V, Foschi A, Corvasce S, Colomba C, Titone L, Parravicini C, Cascio A, Corbellino M 2002. Clinical use of polymerase chain reaction performed on peripheral blood and bone marrow samples for the diagnosis and monitoring of visceral leishmaniasis in HIV-infected and HIV-uninfected patients: a single-center, 8-year experience in Italy and review of the literature. Clin Infect Dis 44: 1602-1610.
- Cascio A, Calattini S, Colomba C, Scalamogna C, Galazzi M, Pizzuto M, Camilli R, Gramiccia M, Titone L, Corbellino M, Spinello A 2002. Polymerase chain reaction in the diagnosis and prognosis of Mediterranean visceral leishmaniasis in immunocompetent children. Pediatric 109: 27-31.
- Cortes S, Rolão N, Ramada J, Campino L 2004. PCR as a rapid and sensitive tool in the diagnosis of human and canine leishmaniasis using Leishmania donovani s.l. - specific kinetoplastid primers. Trans R Soc Trop Med Hyg 98: 12-17.
- Costa JM, Durand R, Deniau M, Rivollet D, Izri M, Houin R, Vidaud M, Bretagne S 1996. PCR enzyme-linked immunosorbent assay for diagnosis of leishmaniasis in human immunodeficiency virus-infected patients. J Clin Microbiol 34: 1831-1833.
- Cruz I, Cañavate C, Rubio JM, Morales MA, Chicharro C, Laguna F, Jiménez-Mejías M, Sirera G, Videla S, Alvar J 2002. A nested polymerase chain reaction (Ln-PCR) for diagnosing and monitoring Leishmania infantum infection in patients co-infected with human immunodeficiency virus. Trans R Soc Trop Med Hyg 96: 185-189.
- Cruz I, Chicharro C, Nieto J, Bailo B, Cañavate C, Figueras MC, Alvar J 2006. Comparison of new diagnostic tools for management of pediatric Mediterranean visceral leishmaniasis. J Clin Microbiol 44: 2343-2347.
- Deborggraeve S, Boelaert M, Rijal S, De Doncker, Dujardin J-C, Herdewijn P, Buscher P 2008. Diagnostic accuracy of a new Leishmania PCR for clinical visceral leishmaniasis in Nepal and its role in diagnosis of disease. Trop Med Int Health 13: 1378-1383.
- Disch J, Caligiome R, Maciel F, Oliveira M, Orsini M, Dias-Neto E, Rabello A 2006. Single-step duplex kDNA-PCR for detection of Leishmania donovani complex in human peripheral blood samples. Diag Microb Infect Dis 56: 395-400.
- Fisa R, Riera C, Ribera E, Gállego M, Portús M 2002. A nested polymerase chain reaction for diagnosis and follow-up of human visceral leishmaniasis patients using blood samples. Trans R Soc Trop Med Hyg 96: 191-194.
- Kafetzis DA, Maltezou HC 2002. Visceral leishmaniasis in paediatrics. Curr Opin Infect Dis 15: 289-294.
- Kaouech E, Kallel K, Toumi NH, Belhadj S, Anane S, Babba HE 2008. Pediatric visceral leishmaniasis diagnosis in Tunisia: comparative study between optimised PCR assays and parasitological methods. Parasite 15: 143-150.
- Katakura K, Kawazu S, Naya T, Nagakura K, Ito M, Aikawa M, Qu JQ, Guan LR, Zuo XP, Chai JJ, Chang KP, Matsumoto Y 1998. Diagnosis of kala-azar by nested PCR based on amplification of the Leishmania mini-exon gene. J Clin Microbiol 36: 2173-2177.
- Lachaud L, Chabbert E, Dubessay P, Reynes J, Lamothe J, Bastien P 2001. Comparison of various sample preparation methods for PCR diagnosis of visceral leishmaniasis using peripheral blood. J Clin Microbiol 39: 613-617.
- Lachaud L, Dereure J, Chabbert E, Reynes J, Mauboussin JM, Oziol E 2000. Optimized PCR using patient blood samples for diagnosis and follow-up of visceral leishmaniasis, with special reference to AIDS patients. J Clin Microbiol 38: 236-240.
- Lachaud L, Hammami SM, Chabbert E, Dereure J, Dedet JP, Bastien P 2002. Comparison of six PCR methods using peripheral blood for detection of canine visceral leishmaniasis. J Clin Microbiol 40: 210-215.
- Pandey BD, Pandey K, Kaneko O, Yanagi T, Hirayama K 2009. Relapse of visceral leishmaniasis after miltefosine treatment in a Nepalese patient. Am J Trop Med Hyg 80: 580-582.
- Piarroux R, Gambarelli F, Dumon H, Fontes M, Dunan S, Mary C, Toga B, Quilici M 1994. Comparison of PCR with direct examination of bone marrow aspiration, myeloculture and serology for diagnosis of visceral leishmaniasis in immunocompromised patients. J Clin Microbiol 32: 746-749.
- Pirmez C, Da Silva Trajano V, Paes-Oliveira Neto, Da-Cruz AM, Gonçalves-da-Costa SC, Catanho M, Degrave W, Fernandes O 1999. Use of PCR in diagnosis of human American tegumentary leishmaniasis in Rio de Janeiro, Brazil. J Clin Microbiol 37: 1819-1823.
- Pizzuto M, Piazza M, Senese D, Scalamogna C, Calattini S, Corsio L 2001. Role of PCR in diagnosis and prognosis of visceral leishmaniasis in patients coinfected with human immunodeficiency virus type 1. J Clin Microbiol 39: 357-361.
- Rodgers MR, Popper SJ, Wirth DF 1990. Amplification of kinetoplast DNA as a tool in the detection and diagnosis of Leishmania Exp Parasitol 71: 267-275.
- Schaefer KU, Schoone GJ, Gachihi GS, Muller AS, Kager PA, Meredith SE 1995. Visceral leishmaniasis: use of the polymerase chain reaction in an epidemiological study in Baringo district, Kenya. Trans R Soc Trop Med Hyg 89: 492-495.
- Schubach A, Haddad F, Oliveira-Neto MP, Degrave W, Pirmez C, Grimaldi Jr, Fernandes O 1998. Detection of Leishmania DNA by polymerase chain reaction in scars of treated human patients. J Infect Dis 178: 911-914.
- Shaw JJ 2006. Further thoughts on the use of the name Leishmania (Leishmania) infantum chagasi for the aetiological agent of American visceral leishmaniasis. Mem Inst Oswaldo Cruz 101: 577-579.
- Siddig M, Ghalib H, Shillington DC, Petersen EA 1988. Visceral leishmaniasis in the Sudan: comparative parasitological methods of diagnosis. Trans R Soc Trop Med Hyg 82: 66-68.
- Smyth AJ, Ghosh A, Hassan MQ, Basu D, De Bruijn MH, Adhya S, Mallik KK, Barker DC 1992. Rapid and sensitive detection of Leishmania kinetoplast DNA from spleen and blood samples of kala-azar patients. Parasitol 2: 183-192.
- Stark D, Pett S, Marriott D, Harkness J 2006. Post-kala-azar dermal leishmaniasis due to Leishmania infantum in a human immunodeficiency virus type 1-infected patient. J Clin Microbiol 44: 1178-1180.
- Volpini AC, Passos VMA, Oliveira GO, Romanha AJ 2004. PCR-RFLP to identify Leishmania (Viannia) braziliensis and L. (Leishmania) amazonensis causing American cutaneous leishmaniasis. Acta Tropica 90: 31-37.
- Zijlstra EE, Ali MS, El-Hassan AM, El-Toum IA, Satti M, Ghalib HW, Kager PA 1992. Kala-azar: a comparative study of parasitological methods and the direct agglutination test in diagnosis. Trans R Soc Trop Med Hyg 86: 505-507.
Publication Dates
-
Publication in this collection
21 May 2010 -
Date of issue
May 2010
History
-
Received
04 Oct 2009 -
Accepted
08 Apr 2010