Abstract
This study was designed to assess the effect of GB virus (GBV)-C on the immune response to human immunodeficiency virus (HIV) in chronically HIV-infected and HIV- hepatitis C virus (HCV)-co-infected patients undergoing antiretroviral therapy. A cohort of 159 HIV-seropositive patients, of whom 52 were HCV-co-infected, was included. Epidemiological data were collected and virological and immunological markers, including the production of interferon gamma (IFN-γ) and interleukin (IL)-2 by CD4, CD8 and Tγδ cells and the expression of the activation marker, CD38, were assessed. A total of 65 patients (40.8%) presented markers of GBV-C infection. The presence of GBV-C did not influence HIV and HCV replication or TCD4 and TCD8 cell counts. Immune responses, defined by IFN-γ and IL-2 production and CD38 expression did not differ among the groups. Our results suggest that neither GBV-C viremia nor the presence of E2 antibodies influence HIV and HCV viral replication or CD4 T cell counts in chronically infected patients. Furthermore, GBV-C did not influence cytokine production or CD38-driven immune activation among these patients. Although our results do not exclude a protective effect of GBV-C in early HIV disease, they demonstrate that this effect may not be present in chronically infected patients, who represent the majority of patients in outpatient clinics.
CD38; cytokines; GBV-C/HGV; HIV; immune activation; Tγδ
ARTICLES
Influence of GB virus C on IFN- γ and IL-2 production and CD38 expression in T lymphocytes from chronically HIV-infected and HIV-HCV-co-infected patients
Giovana Lotici Baggio-ZappiaI, + + Corresponding author: giovana.lotici@unifesp.br ; Aline de Jesus BarbosaI; Milena Karina Coló BrunialtiI; Reinaldo SalomãoI; Celso Francisco Hernandes GranatoI, II
IDisciplina de Infectologia, Laboratório de Virologia e Imunologia, Universidade Federal de São Paulo, Rua Pedro de Toledo 669 10º andar, 04039-032 São Paulo, SP, Brasil
IIFleury Medicina Diagnóstica, São Paulo, SP, Brasil
ABSTRACT
This study was designed to assess the effect of GB virus (GBV)-C on the immune response to human immunodeficiency virus (HIV) in chronically HIV-infected and HIV- hepatitis C virus (HCV)-co-infected patients undergoing antiretroviral therapy. A cohort of 159 HIV-seropositive patients, of whom 52 were HCV-co-infected, was included. Epidemiological data were collected and virological and immunological markers, including the production of interferon gamma (IFN-γ) and interleukin (IL)-2 by CD4, CD8 and Tγδ cells and the expression of the activation marker, CD38, were assessed. A total of 65 patients (40.8%) presented markers of GBV-C infection. The presence of GBV-C did not influence HIV and HCV replication or TCD4 and TCD8 cell counts. Immune responses, defined by IFN-γ and IL-2 production and CD38 expression did not differ among the groups. Our results suggest that neither GBV-C viremia nor the presence of E2 antibodies influence HIV and HCV viral replication or CD4 T cell counts in chronically infected patients. Furthermore, GBV-C did not influence cytokine production or CD38-driven immune activation among these patients. Although our results do not exclude a protective effect of GBV-C in early HIV disease, they demonstrate that this effect may not be present in chronically infected patients, who represent the majority of patients in outpatient clinics.
Key words: CD38 - cytokines - GBV-C/HGV - HIV - immune activation - Tγδ
Co-infection with GB virus (GBV-C) has been associated with decreased mortality rates and improved outcomes in human immunodeficiency virus (HIV)-seropositive patients, even after progression to acquired immunodeficiency syndrome (AIDS) (Heringlake et al. 1998, Yeo et al. 2000). Williams et al. (2004) evaluated a cohort of 271 men included in the Multicenter Acquired Immunodeficiency Syndrome Cohort Study and demonstrated that GBV-C viremia is associated with increased life expectancy among HIV-seropositive patients. In that study, researchers also found an intermediate protective role for antibodies specific for the E2 protein of GBV-C. Studies conducted in the highly active anti-retroviral therapy (HAART) era have demonstrated that GBV-C is associated with lower rates of HIV rebound after HAART-driven virological success (Antonucci et al. 2005, Souza et al. 2006). Several mechanisms have been proposed to explain the putative effect of GBV-C on the course of HIV disease, such as increased secretion of the chemokines regulated on activation, normal T cell expressed and secreted (Nattermann et al. 2003), macrophage inflammatory protein (MIP)-1α, MIP-1@@β and stromal cell-derived factor-1, reduced expression of the chemokine receptors CCR5 and CXCR4 (Xiang et al. 2004), preservation of the Th1 cytokine profile (Sathar et al. 2004), activation of the interferon (IFN) gene system (Capobianchi et al. 2006), increased frequency of plasmacytoid dendritic cells expressing CD80 (Lalle et al. 2008, Baggio-Zappia & Granato 2009) and decreased T cell activation (Maidana-Giret et al. 2009). In contrast, other studies have failed to demonstrate an influence of GBV-C on the course of HIV infection (Quiros-Roldan et al. 2002, Jung et al. 2005, Van der Bij et al. 2005, Haji Molla Hoseini et al. 2007), while at least one report concluded that GBV-C viremia tended to worsen rather than improve mortality among the co-infected patients (Brust et al. 2002). For example, Birk's group (Birk et al. 2002) studied 157 patients recently infected with HIV, of whom 36 (23%) were GBV-C RNA-positive, and demonstrated no significant differences between GBV-C RNA-positive and GBV-C RNA-negative patients in the time required to progress to a CD4 T cell lymphocyte count below 200 cells/µL, the time to AIDS diagnosis or the time to AIDS-related death. Furthermore, controlling for known prognostic factors, such as age, sex, year of HIV seroconversion, use of antiretroviral therapy and pneumocystis pneumonia prophylaxis did not affect any of the endpoints in this study. Studying another Swedish cohort, Björkman et al. (2004) followed 230 patients with serum samples obtained within two years of HIV diagnosis until initiation of antiretroviral therapy, death or their last visit, with a median follow-up period of 4.3 years. At inclusion, 62 (27%) patients had GBV-C viremia and 69 (30%) had anti-E2 antibodies. The authors found that baseline GBV-C status was not associated with overall mortality, HIV-related mortality or development of AIDS.
Thus, whether GBV-C viremia is associated with a better prognosis among HIV-seropositive patients remains unclear. Co-infection with other viruses, such as hepatitis B virus (HBV) and hepatitis C virus (HCV), makes the analysis of the interaction of GBV-C with HIV even more complex. Few studies have evaluated the effect of GBV-C on the disease caused by HCV and even fewer have assessed the effects of triple infection. Importantly, immune response in this profile was not evaluated. These considerations provide new perspectives for the investigation of a potential beneficial role of GBV-C viremia in HIV-seropositive patients co-infected with HCV.
This study was thus designed to assess the effect of GBV-C viremia and the presence of GBV-C E2 antibodies on immune responses in chronically HIV-infected and HIV-HCV-co-infected patients. The impact of GBV-C on HIV and HCV viremia, TCD4 and TCD8 cell counts, HIV-specific and nonspecific induction of type-I cytokines and expression of CD38 were evaluated.
PATIENTS, MATERIALS AND METHODS
Patients and methods - A cohort of 159 chronically HIV-infected patients, of whom 52 were HCV co-infected, from the Centre of Immune Deficiencies Control of the São Paulo Hospital, state of São Paulo, Brazil were prospectively included. Demographic data were collected and are summarised in Table. Blood samples were collected to evaluate virological and immunological parameters and to test the patients for active GBV-C replication and for the presence of E2 antibodies. Written informed consent was obtained, and the study was approved by the Institutional Review Board (protocol 1296/05).
Diagnosis of GBV-C infection - Active GBV-C infection is demonstrated by the presence of plasma viral RNA and the resolution of the infection is mediated by antibodies directed against the viral envelope protein E2 (Alter et al. 2004). The simultaneous presence of viral RNA and antibodies specific for GBV-C E2 is a rare and possibly transient event (Thomas et al. 1997). Diagnosis of active GBV-C infection was carried out by real-time polymerase chain reaction (qPCR) using methods standardised in our laboratory. The 5'-untranslated region of GBV-C was chosen because it is a conserved region among all viral isolates around the world. A total of 119 sequences available in GenBank were aligned in Sequencher 4.5 software (Gene Codes Corporation) to design the primers used for qPCR as previously described (Barbosa et al. 2009). Samples were tested for the presence of GBV-C E2 antibodies using a commercial enzymatic immunoassay (Roche Diagnostics GmbH).
CD4 and CD8 T cell counts - CD4 and CD8 T cell counts were carried out using a FACSCaliburTM flow cytometer with monoclonal antibodies from Becton Dickinson Biosciences (BD Biosciences, San Jose, CA).
Virological parameters - HIV viral loads (VL) were assessed in the Laboratory of Retrovirology of the Federal University of São Paulo using the branched DNA HIV-1 RNA 3.0 assay (Bayer Diagnostics, Tarrytown, NY) with a detection limit of 50 copies/mL. HCV VLs were assessed in central laboratories of the São Paulo Hospital using a PCR assay (Amplicor 2.0 HCV monitor, Roche Diagnostics Systems, Basel, Switzerland).
Cell separation and stimulation for cytokine production - Peripheral blood mononuclear cells (PBMCs) were isolated using the Ficoll-Paque method (GE Bio-Science, Uppsala). PBMCs were stored in liquid nitrogen at a final concentration of 1 x 107/mL in vials containing 1 mL of fetal calf serum (FCS) (Gibco) with 10% dimethyl sulfoxide (EMD Biosciences, La Jolla, CA) until use. Thawed cells were resuspended at a concentration of 1 x 106 cells/mL in Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco, Grand Island, NY) supplemented with 10% FCS, transferred to a polypropylene tube and stimulated with 20 ng/mL phorbol myristate acetate (PMA) and 1 mM ionomycin (Sigma) as a positive control or with 2 µg/µL of a pool of peptides derived from the Nef HIV-1 protein (Cat#5189, NIH) and 5 µg/µL of HIV-1III-B viral lysate (Advanced Biotechnologies, Columbia, Maryland) for specific stimulation. All samples received 1 µL co-stimulatory molecules CD28/CD49d at a final concentration of 100 µg/mL. Brefeldin-A (Sigma) was added to inhibit protein secretion.
Surface and intracellular staining for cytokine detection - After 15-16 h of incubation, cells were washed twice in 10 mL staining buffer [phosphate buffered saline supplemented with 0.1% sodium azide (Sigma) and 1% FCS]. CD8-PerCP, CD3-APC-Cy7 and T cell receptor (TCR)γδ-FITC antibodies (BD Biosciences) were added and samples were incubated in the dark at room temperature (RT) for 30 min. Cells were washed and incubated with 750 µL permeabilisation buffer [BD FACSTM lysing solution (BD Biosciences) containing 0.05% Tween 20] in the dark at RT for 10 min. IFN-γ-PECy7 and interleukin (IL)-2-APC antibodies (BD Biosciences) were added and cells were incubated in the dark at RT for 30 min. Cells were washed with staining buffer and resuspended in a solution of 1% paraformaldehyde for flow cytometry.
CD38 activation marker staining - Thawed cells were washed with staining buffer and the concentration of viable cells was adjusted to 1 x 106 cells/mL. After washing, 20 µL of pooled CD8-FITC/CD38-PE/CD3-PerCP antibodies (QuantiBRITE PETM, BD Biosciences) was added and cells were incubated for 15 min at RT in the dark. Cells were washed and resuspended in a solution of 1% paraformaldehyde for flow cytometry.
Cytokine detection - To detect cytokine expression, a FACSCanto flow cytometer (BD Biosciences) was used to acquire 70,000 events in the lymphocyte gate. Data were analysed using FlowJo software (Tree Star). The CD8+ T cell population was defined based on positive staining for CD3 and CD8, whereas the CD4+ T cell population was defined as CD8- CD3+. γδ T cells were defined based on positive staining for γδ TCR combined with the SSC parameter. Results are expressed as the percentages of cells producing IL-2 and IFN-γ.
CD38 activation marker - Expression of CD38 was quantitatively evaluated using the QuantiBRITE PETM system by acquiring 70,000 events in the lymphocyte gate using a FACSCaliburTM flow cytometer (BD Biosciences). Data were analysed using CellQuestTM software (BD Biosciences). CD8+ T cell and CD4+ T cell populations were defined as previously described. The QuantiBRITE PETM system involves the use of phycoerythrin (PE)-labelled beads with known numbers of molecules of PE conjugated to each bead. Beads were used to generate a curve of mean fluorescence intensity versus the number of molecules of PE/bead. This curve was used to determine the number of CD38 molecules per cell in samples stained with a PE-labelled anti-CD38 antibody. Results are expressed as the mean number of molecules of PE/cell.
Statistical analysis - Kolmogorov-Smirnov tests were performed to determine whether distributions were normal. Continuous variables were described as means plus standard deviations and discrete variables were described as percentages. Differences among the groups were assessed by analysis of variance plus Tukey's post hoc test. A p value of < 0.05 was considered significant. Analysis was performed using the SPSS package 13.0 and GraphPad Prism 5.0.
RESULTS
The mean age of the entire cohort was 43 ± 9 years, 98 (61.6%) were men, 15 (9.4%) were intravenous drug users and 38 (23.8%) were alcohol abusers. The mean time of HIV infection diagnosis was 9.7 ± 4.8 years, while the mean time of HCV diagnosis was 4.8 ± 4.06 years. Of the 159 patients, 136 (85.5%) were undergoing HAART, with a mean period of HAART of 7 ± 3 years (Table).
GBV-C markers - Of the 159 included patients, 65 (40.8%) presented markers of GBV-C infection. Of these 65 patients, 31 (19.4%) presented GBV-C viremia and 34 (21.4%) were positive for E2 antibodies. After GBV-C testing, patients were separated into six distinct groups based on their infection profiles.
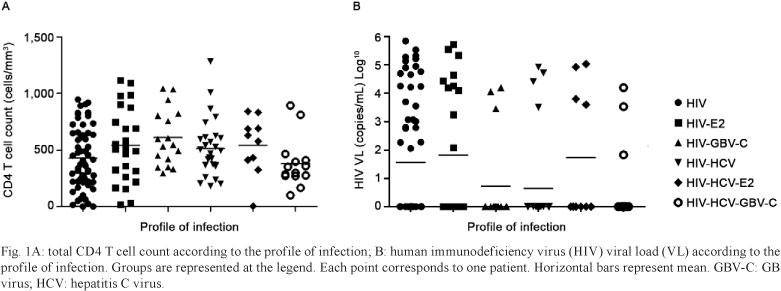
CD4 and CD8 T cell counts - The mean CD4 T cell count of the entire cohort was relatively high [489 (267) cells/mm3] and variable, ranging from 4-1,280 cells/mm3 (Fig. 1). The mean CD8 T cell count for the entire cohort was also elevated [862 (442) cells/mm3] and variable, ranging from 117-2,507 cells/mm3. No differences were observed in CD4 and CD8 T cell counts among patients with different infection profiles (Table).
Virological parameters - Of the 159 patients included in the study, 136 were undergoing HAART. Of these 136 patients, 106 (77.9%) presented undetectable HIV VLs. The mean VL of the patients with detectable HIV viremia was 3.9 (1.19) copies/mL log10, and VLs ranged from 1.83-5.84. No significant differences were observed in HIV VLs among patients with different infection profiles (p = 0.075) (Fig. 1). HIV VLs did not correlate with GBV-C VLs (p = 0.709). Furthermore, the proportion of patients with undetectable HIV VLs did not significantly differ among the groups evaluated (χ2 = 8.11, p = 0.150) and exclusion of those patients who were not undergoing HAART did not affect these results (χ2 = 8.71; p = 0.121). Evaluation of GBV-C viremia and the presence of E2 antibodies demonstrated no significant differences in HCV VLs among the HIV-HCV-co-infected patients (p = 0.260) and no correlation was observed between GBV-C and HCV VLs (p = 0.238).
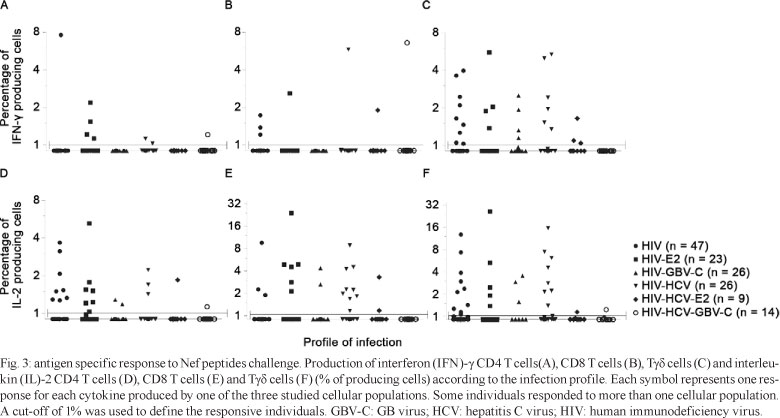
Intracellular detection of cytokines in T lymphocyte subsets - PBMCs from 135 patients were challenged with PMA/ionomycin and Nef peptides and PBMCs from 80 patients were challenged with HIV-1 viral lysate. The percentage of TCD4, TCD8 and Tγδ cells producing IFN-γ and IL-2 was significantly increased after nonspecific stimulation with PMA/ionomycin when compared with the respective unstimulated controls; however, the presence of GBV-C viremia or E2 antibodies did not affect the percentage of cytokine-producing cells (Fig. 2). Furthermore, no changes in these results were observed when the nine HIV-HCV-co-infected patients who were receiving treatment with interferon/ribavirin were excluded from the analysis (data not shown). As expected, the production of cytokines after HIV-specific stimulation was less robust than that elicited by PMA/ionomycin stimulation.
The percentage of cytokine-producing cells after antigen-specific stimulation was normalised to that observed in unstimulated control cells. Normalised samples in which = 1% of cells produced cytokines in response to antigen specific stimulation were considered responsive (Trigona et al. 2003, Chen et al. 2005). Of the 135 patients challenged with Nef, 66 (48.8%) presented antigen-specific responses in at least one of the three cellular populations, with a total of 119 responses when the three cell populations and the two cytokines were considered individually. Among the responders, 24 (36.4%) were HIV-monoinfected, 14 (21.2%) were HIV-HCV-co-infected, 13 (19.7%) were HIV-E2+, eight (12.1%) were HIV-GBV-C-co-infected, five (7.6%) were HIV-HCV-E2+ and two (3%) were HIV-HCV-GBV-C-triple-infected. No differences were observed in any of the lymphocyte populations in the proportion of cells responding to Nef peptides by producing IFN-γ (p = 0.676, p = 0.566, p = 0.540) or IL-2 (p = 0.764, p = 0.321, p = 0.123) among patients with different infection profiles (Fig. 3).
Of the 80 patients challenged with HIV-1 viral lysate, 35 (43.7%) presented specific responses in at least one of the three lymphocyte populations. Of these patients, eight (22.8%) were HIV-monoinfected, 14 (40%) were co-infected with HCV, six (17.1%) were HIV-E2+, one (2.8%) was HIV-GBV-C viremic, four (11.4%) were HIV-HCV-E2+ and two (5.7%) were triple-infected, with a total of 66 responses (Fig. 4). No differences in IFN-γ (p = 0.567, p = 0.766, p = 0.546) and IL-2 (p = 0.786, p = 0.564, p = 0.856) production were observed in T CD4, T CD8 and Tγδ populations among the patient groups.
CD38 activation marker - Expression of CD38 on CD4 and CD8 T cells was positively correlated with HIV VLs (p = 0.001, p = 0.017). The expression of CD38 on CD4 and CD8 T cells did not correlate with GBV-C VLs (p = 0.588, p = 0.753). No differences in CD38 expression on CD4 or CD8 T cells were observed among patients with different infection profiles (Fig. 5).
DISCUSSION
Epidemiological studies have associated the presence of GBV-C RNA with slower progression to AIDS, decreased mortality rates and increased survival of HIV-seropositive patients after the development of AIDS. Our results, however, demonstrate that neither GBV-C viremia nor the presence of E2 antibodies influenced these parameters in either HIV-seropositive or chronically HIV-HCV-co-infected patients.
Our results thus demonstrate that GBV-C does not affect CD4 and CD8 T cell counts or HIV VLs. Previous studies of the impact of GBV-C viremia on CD4 T cell counts and HIV VLs among HIV-seropositive patients have provided inconsistent results. Some studies have demonstrated increased CD4 T cell counts and decreased HIV VLs among HIV-GBV-C-co-infected patients when compared to those in HIV-monoinfected patients (Heringlake et al. 1998, Lefrere et al. 1999, Xiang et al. 2001, Li et al. 2006). Other studies, however, have not corroborated these findings, as no effect of GBV-C viremia on these parameters was observed. These discrepancies could be a consequence of differences in the populations included, the stage of the HIV disease, the presence of other infections such as HBV and HCV or the standard of care of HIV therapy. Our study evaluated a cohort of chronically infected patients of whom the majority was undergoing HAART therapy. In addition, patients carrying HCV were evaluated as a separate group. The mean time of HIV infection diagnosis among the entire cohort was 9.7 years, while the mean time of HCV infection diagnosis among all co-infected patients was 4.8 years. Careful evaluation of the previous studies assessing the effect of GBV-C viremia on HIV infection reveals that some studies did not report the time of HIV infection diagnosis and others included patients at different stages of the HIV disease. Taken together, the findings reported in these studies suggest that any potentially positive effect of GBV-C viremia on HIV infection may not be extended to all HIV-seropositive patients and may be influenced by the stage of HIV disease. Considering that the patients in our study were chronically infected and that GBV-C viremia could be rapidly reversed in these immunosuppressed patients, it is reasonable to hypothesise that the patients in our study were first infected with HIV and then acquired GBV-C. This sequence of viral infections may preclude some putative protective effects of GBV-C. In an in vitro study, Xiang et al. (2001) previously demonstrated that HIV replication was inhibited more efficiently when GBV-C infection preceded HIV infection.
Antiretrovirals influence the parameters evaluated in this study and could thus be a confounding factor. In this study, 85% of the included patients were undergoing HAART and the proportion of untreated patients was not significantly different among the groups stratified for analysis based on the infection profile. In addition, the profile of antiretroviral treatment did not consistently differ among the included patients. Previous studies have suggested that GBV-C viremia influences the response to antiretroviral therapy. Rodriguez et al. (2003) showed that HIV-GBV-C-co-infected patients had an increased incidence of undetectable HIV VLs after HAART initiation. In contrast, Brumme et al. (2002) also evaluated the effect of GBV-C viremia on patients undergoing HAART and found lower HIV VLs at inclusion; however, no effect on the time at which virological success was achieved was observed. Our results suggest that GBV-C did not influence HIV replication in chronically infected patients, as no differences in the proportion of patients undergoing HAART that achieved virological success were observed among patients with different infection profiles. In addition, GBV-C viremia did not influence HCV replication, as no differences in HCV VLs were observed.
The central focus of this study was to evaluate the effect of GBV-C viremia and the presence of E2 antibodies on the immune response in HIV-infected and HIV-HCV-co-infected patients. To this end, we evaluated the production of the Th1 cytokines IFN-γ and IL-2 by CD4 and CD8 T cells and Tγδ cells after specific immune stimulation. To the best of our knowledge, this study provides the first analysis of the activation of Tγδ cells in patients with these infection profiles. The role of GBV-C viremia in the maintenance of an intact Th1 profile among HIV-seropositive patients was first proposed by Nunnari et al. (2003), who evaluated a panel of cytokines in a cohort of asymptomatic patients and found a positive influence of GBV-C viremia. Taking into account the results obtained by Nunnari et al. (2003), we hypothesised that GBV-C viremia could preserve the cellular immune response to HIV and expected to observe increased production of the Th1 cytokines IFN-γ and IL-2 in the co-infected patients.
Stimulation with PMA and ionomycin triggers a strong production of cytokines in vitro and is often used to evaluate immune responses when specific stimulation is not feasible because of the capacity of these stimuli to amplify the profile of the responses seen in vivo (Vitale et al. 2000, Eylar et al. 2001). In the present study, we found increased production of IFN-γ and IL-2 after nonspecific stimulation with PMA and ionomycin, demonstrating that the capacity to respond with synthesis of type-1 cytokines is preserved in these patients; however, no differences in the production of cytokines by TCD4, TCD8 and Tγδ cells were observed in patients with different infection profiles, regardless of the presence of GBV-C viremia or E2 antibodies.
Specific cellular immune responses mediated by T cells play an important role in controlling HIV infection and efficient T cell responses can delay disease progression. The T cell antigen-specific repertoire declines throughout the course of HIV infection, with dramatic changes in the CD8 T cell phenotype (Alter et al. 2004). Data from studies of HIV-infected patients have demonstrated a decline in specific cellular immune responses as the disease progresses and these changes have been associated with the HIV VL (Legrand et al. 1997, Kousignian et al. 2003). Our results demonstrated a positive correlation between the HIV VL and Nef-specific responses in CD4 and CD8 T cells, suggesting a certain degree of preservation of immunity to HIV antigens. This preservation of immunity was independent of GBV-C, as no differences were observed in these parameters among patients with different infection profiles. Some patients demonstrated efficient Tγδ cell responses to specific stimulation with viral lysate. In addition to IFN-γ, Tγδ cells produced high levels of IL-2; however, production of these cytokines did not differ among patients with different infection profiles, demonstrating no influence of GBV-C on Tγδ cell responses.
In an attempt to assess the influence of GBV-C viremia or the presence of E2 antibodies on HIV disease, we quantitatively assessed the expression of CD38 in CD4 and CD8 T cells. A recent report showed decreased levels of CD38 expression among GBV-C viremic patients (Maidana-Giret et al. 2009). In contrast, our findings do not corroborate these results; instead, we demonstrated that GBV-C viremia did not decrease cellular activation in chronically HIV-infected or triple-infected patients. These divergent findings could be a result of the stage of the HIV disease in the included patients, as the patients included in our study were chronically infected, and those included in previous studies were acutely infected. In addition, the methodologies used in these studies differed greatly. While the authors of the previous studies expressed their results as the percentage of CD38+ cells, our results are quantitative and are presented as the mean number of CD38 molecules expressed per cell. Expression of CD38 was widely variable among the patients, reflecting the variable immune status characteristic of chronically infected patients.
Our results suggest that neither GBV-C viremia nor E2 antibodies appear to influence viral replication of HIV or HCV or CD4 T cell counts in chronically infected patients. Furthermore, GBV-C infection did not influence the immune response as measured by cytokine production or CD38-driven immune activation in these patients. Our results do not exclude a protective effect of GBV-C in HIV disease but show that this effect is not present in chronically infected patients, who represent the majority of patients seen in outpatient clinics. Several factors, such as differences in the study populations, the stage of HIV infection and the time of GBV-C acquisition, may account for the discrepant results reported by different research groups.
ACKNOWLEDGEMENTS
To Roche Diagnostics, for providing the ELISA µPLATE anti-HGenv kit, and NIH, to provide the HIV-1 Nef peptide set used in the specific stimulation assay.
Received 20 January 2011
Accepted 19 July 2011
Financial support: FAPESP (05/57611-5, 05/58901-7), CNPq, CAPES
- Alter G, Tsoukas CM, Rouleau D, Côté P, Routy JP, Sékaly RP, Bernard NF 2004. Assessment of longitudinal changes in HIV-specific effector activity in subjects undergoing untreated primary HIV infection. AIDS 18: 1979-1989.
- Antonucci G, Girardi E, Cozzi-Lepri A, Capobianchi MR, Morsica G, Pizzaferri P, Ladisa N, Sighinolfi L, Chiodera A, Solmone M, Lalle E, Ippolito G, Monforte A, HepaICoNA Study Group, ICoNA Study Group 2005. Response to HAART and GB virus type C coinfection in a cohort of antiretroviral-naive HIV-infected individuals. Antivir Ther 10: 109-117.
- Baggio-Zappia GL, Granato CFH 2009. HIV-GB virus C co-infection: an overview. Clin Chem Lab Med 47: 12-19.
- Barbosa AJ, Baggio-Zappia GL, Dobo C, Alvez-Souza VK, Lanzara GA, Silva ID, Lanzoni VP, Granato CFH 2009. Analysis of GB virus C infection among HIV-HCV coinfected patients. Rev Soc Bras Med Trop 42: 591-593.
- Birk M, Lindback S, Lidman C 2002. No influence of GB virus C replication on the prognosis in a cohort of HIV-1-infected patients. AIDS 16: 2482-2485.
- Björkman P, Flamholc L, Nauclér A, Molnegren V, Wallmark E, Widell A 2004. GB virus C during the natural course of HIV-1 infection: viremia at diagnosis does not predict mortality. AIDS 18: 877-886.
- Brumme ZL, KJ Chan, Dong WW, Mo T, Wynhoven B, Hogg RS, Montaner JS, O'Shaughnessy MV, Harrigan 2002. No association between GB virus-C viremia and virological or immunological failure after starting initial antiretroviral therapy. AIDS 16: 1929-1933.
- Brust D, Jagannatha S, Herpin B, Miller K, Metcalf J, Lau D, Alter H, Lane HC, Falloon J, Fauci A 2002. Hepatitis G virus (HGV) infection does not prolong survival of patients with early-stage HIV disease: importance of baseline HIV viral load as a predictor of mortality. Int Conf AIDS 14: WeOrC1378.
- Capobianchi MR, Lalle E, Martini F, Poccia F, D'Offizi G, Antonucci G, Abbate I, Dianzani 2006. Influence of GBV-C infection on the endogenous activation of the IFN system in HIV-1 co-infected patients. Cell Mol Biol 52: 3-8.
- Chen H, Reichman R, Keefer M, McDermott MP, Jin X 2005. Establishment of an alternative intracellular cytokine staining assay for HIV/AIDS clinical studies. J Virol Methods 123: 131-140.
- Eylar EH, Lefranc CE, Yamamura Y, Báez I, Colón-Martinez SL, Rodriguez N, Breithaupt TB 2001. HIV infection and aging: enhanced interferon- and tumor necrosis factor-alpha production by the CD8+ CD28- T subset. BMC Immunol 2: 10.
- Haji Molla Hoseini M, Pourfathollah AA, Mohraz M, Soheili Z, Amini S, Aghaiepour M, Samiee S, Nikoogoftar M, Meshkani R 2007. Evaluation of circulating natural type 1 interferon-producing cells in HIV/GBV-C and HIV/HCV coinfected patients: a preliminary study. Arch Med Res 38: 868-875.
- Heringlake S, Ockenga J, Tillmann HL, Trautwein C, Meissner D, Stoll M, Hunt J, Jou C, Solomon N, Schmidt RE, Manns MP 1998. GB virus C/hepatitis G virus infection: a favorable prognostic factor in human immunodeficiency virus-infected patients? J Infect Dis 177: 1723-1726.
- Jung S, Knauer O, Donhauser N, Eichenmuller M, Helm M, Fleckenstein B, Reil H 2005. Inhibition of HIV strains by GB virus C in cell culture can be mediated by CD4 and CD8 T-lymphocyte derived soluble factors. AIDS 19: 1267-1272.
- Kousignian I, Autran B, Chouquet C, Calvez V, Gomard E, Katiama C, Rivière Y, Costagliola D 2003. Markov modelling of changes in HIV-specific cytotoxic T-lymphocyte responses with time in untreated HIV-1 infected patients. Stat Med 22: 1675-1690.
- Lalle E, Sacchi A, Abbate I, Vitale A, Martini F, D'Offizi G, Antonucci G, Castilletti C, Poccia F, Capobianchi MR 2008. Activation of interferon response genes and of plasmacytoid dendritic cells in HIV-1 positive subjects with GB virus C co-infection. Int J Immunopathol Pharmacol 21: 161-171.
- Lefrere JJ, Roudot-Thoraval F, Morand-Joubert L, Petit JC, Lerable J, Thauvin 1999. Carriage of GB virus C/hepatitis G virus RNA is associated with a slower immunologic, virologic, and clinical progression of human immunodeficiency virus disease in coinfected persons. J Infect Dis 179: 783-789.
- Legrand E, Pellegrin I, Neau D, Pellegrin JL, Ragnaud JM, Dupon M, Guillemain B, Fleury HJ 1997. Course of specific T lymphocyte cytotoxicity, plasma and cellular viral loads, and neutralizing antibody titers in 17 recently seroconverted HIV type 1-infected patients. AIDS Res Hum Retroviruses 13: 1383-1394.
- Li CP, Collini P, Danso K, Owusu-Ofori S, Dompreh A, Candotti D, Opare-Sem O, Allain JP 2006. GB virus C and HIV-1 RNA load in single virus and co-infected West African individuals. AIDS 20: 379-386.
- Maidana-Giret MT, Silva TM, Sauer MM, Tomiyama H, Levi JE, Bassichetto KC, Nishiya A, Diaz RS, Sabino EC, Palacios R, Kallas EG 2009. GB virus type C infection modulates T-cell activation independently of HIV-1 viral load. AIDS 23: 2277-2287.
- Nattermann J, Nischalke HD, Kupfer B, Rockstroh J, Hess L, Sauerbruch T, Spengler 2003. Regulation of CC chemokine receptor 5 in hepatitis G virus infection. AIDS 17: 1457-1462.
- Nunnari G, Nigro L, Palermo F, Attanasio M, Berger A, Doerr HW, Pomerantz RJ, Cacopardo B 2003. Slower progression of HIV-1 infection in persons with GB virus C co-infection correlates with an intact T-helper 1 cytokine profile. Ann Intern Med 139: 26-30.
- Quiros-Roldan E, Maroto MC, Torti C, Moretti F, Casari S, Pan A, Carosi G 2002. No evidence of benefical effect of GB virus type C infection on the course of HIV infection. AIDS 16: 1430-1431.
- Rodriguez BI, Woolley I, Lederman MM, Sdunek D, Hess G, Valdez H 2003. Effect of GB virus C coinfection on response to antiretroviral treatment in human immunodeficiency virus-infected patients. J Infect Dis 187: 504-507.
- Sathar MA, York DF, Gouws E, Coutsoudis A, Coovadia HM 2004. GB virus type C coinfection in HIV-infected African mothers and their infants, KwaZulu Natal, South Africa. Clin Infect Dis 38: 405-409.
- Souza IE, Zhang W, Diaz RS, Chaloner K, Klinzman D, Stapleton JT 2006. Effect of GB virus C on response to antiretroviral therapy in HIV-infected Brazilians. HIV Med 7: 25-31.
- Thomas DL, Nakatsuji Y, Shih JW, Alter HJ, Nelson KE, Astemborski JA, Lyles CM, Vlahov D 1997. Persistence and clinical significance of hepatitis G virus infections in injecting drug users.J Infect Dis 176: 586-592.
- Trigona WL, Clair JH, Persaud N, Punt K, Bachinsky M, Sadasivan-Nair U, Dubey S, Tussey L, Fu TM, Shiver J 2003. Intracellular staining for HIV-specific IFN-gamma production: statistical analyses establish reproducibility and criteria for distinguishing positive responses. J Interferon Cytokine Res 23: 369-377.
- Van der Bij AK, Kloosterboer N, Prins M, Boeser-Nunnink B, Geskus RB, Lange JM, Coutinho RA, Schuitemaker H 2005. GB virus C coinfection and HIV-1 disease progression: The Amsterdam Cohort Study. J Infect Dis 191: 678-685.
- Vitale M, Caruso A, Licenziati S, Rodella L, Fiorentini S, Zauli G, Castelli F, Manzoli FA, Turano A 2000. Differential production of IFN-gamma, analyzed at the single-cell level, by specific subsets of human NK and T cells from healthy and HIV(+) subjects. Cytometry 39: 189-194.
- Williams CF, Klinzman D, Yamashita TE, Xiang J, Polgreen PM, Rinaldo C, Liu C, Phair J, Margolick JB, Zdunek D, Hess G, Stapleton JT 2004. Persistent GB virus C infection and survival in HIV-infected men. N Engl J Med 350: 981-990.
- Xiang J, George SL, Wunschmann S, Chang Q, Klinzman D, Stapleton T 2004. Inhibition of HIV-1 replication by GB virus C infection through increases in RANTES, MIP-1alpha, MIP-1beta and SDF-1. Lancet 363: 2040-2046.
- Xiang JS, Wunschmann S, Diekema DJ, Klinzman D, Patrick KD, George SL, Stapleton JT 2001. Effect of coinfection with GB virus C on survival among patients with HIV infection. N Engl J Med 345: 707-714.
- Yeo AE, Matsumoto A, Hisada M, Shih JW, Alter HJ, Goedert JJ 2000. Effect of hepatitis G virus infection on progression of HIV infection in patients with hemophilia. Multicenter Hemophilia Cohort Study. Ann Intern Med 132: 959-963.
Publication Dates
-
Publication in this collection
10 Oct 2011 -
Date of issue
Sept 2011
History
-
Received
20 Jan 2011 -
Accepted
19 July 2011