Abstract
An analysis of the dietary content of haematophagous insects can provide important information about the transmission networks of certain zoonoses. The present study evaluated the potential of polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis of the mitochondrial cytochrome B (cytb) gene to differentiate between vertebrate species that were identified as possible sources of sandfly meals. The complete cytb gene sequences of 11 vertebrate species available in the National Center for Biotechnology Information database were digested with Aci I, Alu I, Hae III and Rsa I restriction enzymes in silico using Restriction Mapper software. The cytb gene fragment (358 bp) was amplified from tissue samples of vertebrate species and the dietary contents of sandflies and digested with restriction enzymes. Vertebrate species presented a restriction fragment profile that differed from that of other species, with the exception of Canis familiaris and Cerdocyon thous. The 358 bp fragment was identified in 76 sandflies. Of these, 10 were evaluated using the restriction enzymes and the food sources were predicted for four: Homo sapiens (1), Bos taurus (1) and Equus caballus (2). Thus, the PCR-RFLP technique could be a potential method for identifying the food sources of arthropods. However, some points must be clarified regarding the applicability of the method, such as the extent of DNA degradation through intestinal digestion, the potential for multiple sources of blood meals and the need for greater knowledge regarding intraspecific variations in mtDNA.
blood meal analysis; cytochrome B; PCR-RFLP
Identification of the food source of arthropods allows a better understanding of the vector dynamics and transmission routes of diseases carried by vectors. It may be the best way to clarify which species are incriminated as zoonosis reservoirs and could therefore indicate better control strategies (Ribeiro 1999Ribeiro JMC 1999. Vector biology. In RL Guerrant, DH Walker, PF Weller (eds.), Tropical infectious diseases: principles, pathogens and practice, Churchill Livingstone, Philadelphia, p. 124-133.).
Traditionally, analysis of the dietary content of haematophagous arthropods has been carried out using immunological techniques (Mukabana et al. 2002bMukabana WR, Takken W, Knols BGJ 2002b. Analysis of arthropod blood meals using molecular genetic markers. Trends Parasitol 18: 505-509.). However, serological methods present two limitations: (i) it is impossible to distinguish species that are phylogenetically close (Silva et al. 2001Silva VC, Gomes RBB, Barral A, Costa CHN 2001. ELISA não dife- rencia sangue de cão doméstico de sangue de raposa. Rev Soc Bras Med Trop 34: 757.) and (ii) the analysis is restricted to one group of vertebrates because of the use of species-specific antiserum (Dias et al. 2003Dias FOP, Lorosa ES, Rebêlo JMM 2003. Fonte alimentar sanguínea e a peridomiciliação de Lutzomyia longipalpis (Lutz & Neiva, 1912) (Psychodidae, Phlebotominae). Cad Saude Publica 19: 1373-1380., Marassá et al. 2006Marassá AM, Consales CA, Galati EAB, Nunes VLB 2006. Identificação do sangue ingerido por Lutzomyia (Lutzomyia) longipalpis (Lutz & Neiva, 1912) e Lutzomyia (Lutzomyia) almerioi (Galati & Nunes, 1999) pela técnica imunoenzimática do ELISA de captura no sistema avidina-biotina. Rev Soc Bras Med Trop 39: 183-186.). Studies have identified a well-preserved DNA sequence in the cytochrome B (cytb) gene, which is present in mitochondrial DNA and codes for an electron-transporting protein (Meece et al. 2005Meece JK, Reynolds CE, Stockwell PJ, Jenson TA, Christensen JE, Reed KD 2005. Identification of mosquito blood meal source by terminal restriction fragment length polymorphism profile analysis of the cytochrome B gene. J Med Entomol 42: 657-667.). This DNA sequence exhibits few intraspecies variations, but sufficient interspecies variations, thereby increasing its specificity. Thus, a large number of hosts may be analysed using universal primers and phylogenetically close species may be distinguished using a molecular test based on the cytb gene (Boakye et al. 1999Boakye DA, Tang J, Truc P, Merriweather A, Unnasch TR 1999. Identification of blood meals in heamatophagous Diptera by cytochrome B heteroduplex analysis. Med Vet Entomol 13: 282-287., Chow-Shaffer et al. 2000Chow-Shaffer E, Sina B, Hawley WA, de Benedicts J, Scott TW 2000. Laboratory and field evaluation of polymerase chain reaction-based forensic DNA profiling for use in identification of human blood meal sources of Aedes aegypti (Diptera: Culicidae). J Med Entomol 37: 492-502., Lee et al. 2002Lee JH, Hassan H, Hill G, Cupp EW, Higazi TB, Mitchell CJ, Godsey MS, Unnasch TR 2002. Identification of mosquito avian-derived blood meals by polymerase chain reaction-heteroduplex analysis. Am J Trop Med Hyg 66: 599-604., Meece et al. 2005Meece JK, Reynolds CE, Stockwell PJ, Jenson TA, Christensen JE, Reed KD 2005. Identification of mosquito blood meal source by terminal restriction fragment length polymorphism profile analysis of the cytochrome B gene. J Med Entomol 42: 657-667., Steuber et al. 2005Steuber S, Abdel-Rady A, Clausen PH 2005. PCR-RFLP analysis: a promising technique for host species identification of blood meals from tsetse flies. Parasitol Res 97: 247-254., Muturi et al. 2011Muturi CN, Ouma JO, Malele II, Ngure RM, Rutto JJ, Mithöfer KM, Enyaru J, Masiga DK 2011. Tracking the feeding patterns of tsetse flies (Glossina Genus) by analysis of blood meals using mitochondrial cytochromes genes. PLoS ONE 6: e17284., Garlapati et al. 2012Garlapati RB, Abbasi I, Warburg A, Poché D, Poché R 2012. Identification of blood meals in wild caught blood fed Phlebotomus argentipes (Diptera: Psychodidae) using cytochrome B PCR and reverse line blotting in Bihar, India. J Med Entomol 49: 515-521., Tiwananthagorn et al. 2012Tiwananthagorn S, Bhutto AM, Baloch JH, Soomro FR, Kawamura Y, Nakao R, Aoshima K, Nonaka N, Oku Y, Katakura K 2012. Zoophilic feeding behaviour of phlebotomine sand flies in the endemic areas of cutaneous leishmaniasis of Sindh province, Pakistan. Parasitol Res 111: 125-133., Pettersson et al. 2013Pettersson E, Bensch S, Ander M, Chirico J, Sigvald R, Ignell R 2013. Molecular identification of blood meals and species composition in Culicoides biting midges. Med Vet Entomol 27: 104-112.).
The aim of the present study was to provide theoretical validation for using PCR-RFLP of the cytb gene as a technique to discriminate the blood meal source of Lutzomyia longipalpis. The restriction fragment profiles of some vertebrate species identified as possible sandfly food sources were analysed. It was found that the species could be differentiated according to the sizes of the restriction fragments.
The mitochondrial cytb gene fragments of the 11 species used in this study (Homo sapiens - HOSA, Rattus norvegicus - RANO, Didelphis marsupialis - DIMA, Canis famili- aris - CAFA, Felis catus - FECA, Sus domesticus - SUDO, Bos taurus - BOTA, Gallus gallus - GAGA, Equus caballus - EQCA, Cerdocyon thous - CETH and Pseudalopex vetulus - PSVE) were amplified in silico using the primers BM1 (5’CCCCTCAGAATGATATTTGTCCTCA3’) and BM2 (5’CCATCCAACATCTCAGCATGATGAAA3’) and Basic Local Alignment Search Tool software, which is available from the National Center for Biotechnology Information (GenBank). The sequence of the expected product (358 bp) was species-specific and the Aci I, Alu I, Hae III and Rsa I restriction sites were identified using Restriction Mapper software. The restriction profiles of the fox species P. vetulus and C. thous were the only two that were not analysed because it was not possible to identify the 358 bp cytb fragment in these species in GenBank. Each species had a restriction fragment profile that was distinct from the others. Thus, these profiles represented a unique “fingerprint”, which could be an important method for distinguishing between each species.
Biological samples (peripheral blood or cellular tissue) of the vertebrate species were collected and DNA was extracted. The mitochondrial cytb gene fragment (358 bp) was amplified using the BM1 and BM2 primers following the protocol of Meece et al. (2005)Meece JK, Reynolds CE, Stockwell PJ, Jenson TA, Christensen JE, Reed KD 2005. Identification of mosquito blood meal source by terminal restriction fragment length polymorphism profile analysis of the cytochrome B gene. J Med Entomol 42: 657-667. (Fig. 1). However, PSVE presented a unique problem: the polymerase chain reaction (PCR) amplified not only the 358 bp fragment, but also a fragment of approximately 615 bp. This fox species is rare and threatened, lives mainly in the central part of Brazil and was excluded from the study (Costa & Courtenay 2003Costa CHN, Courtenay O 2003. A new record of the hoary fox Pseudalopex vetulus in North Brazil. Mammalia 67: 593-594.). However, the presence of these two bands allowed for the differentiation of PSVE from the other animals.
: electrophoresis with polymerase chain reaction on the DNA extracted from peripheral blood and tissue samples from the vertebrate species amplified using the BM1 and BM2 primers (fragment of mitochondrial cytb ≅ 358 bp). L: DNA Leader 100 bp; NEG: negative control.
The amplified fragments were sequenced to determine the degree of conservation of the cytb gene. Comparing the sequenced loco-regional samples with the deposited GenBank sequences, most species presented few mismatches, with no compromised restriction sites and with degrees of similarity greater than 98% (Table I). There were no changes in the restriction sites of CAFA, HOSA, GAGA, RANO, SUDO or BOTA. However, EQCA and FECA gained Aci I and Rsa I restriction sites, respectively and DIMA lost two Alu I restriction sites (Table I, Supplementary data). Because the number of cytb sequences deposited in GenBank is limited, sequencing of this fragment in loco-regional host species becomes important for analysis using the PCR-restriction fragment length polymorphism (RFLP) technique. As cytb sequences continue to be deposited into these genetic databases and as the variations in mtDNA become better understood, sequencing of this fragment from loco-regional host species will be needed less frequently, thus reducing costs and increasing the applicability of the method.
To confirm the in silico analysis, the PCR products were submitted to enzymatic digestion using the Aci I, Alu I, Hae III and Rsa I enzymes. The restriction profiles of the species studied are shown in Fig. 2 and Table II. In practice, the restriction pattern of Aci I showed the expected fragments (244 bp and 113 bp) for DIMA, FECA and EQCA. Regarding the DNA of HOSA, a 244 bp band was found in addition to the expected fragments (189 bp, 113 bp and 55 bp). The expected restriction pattern of GAGA was observed; however, the 49 bp fragment could not be visualised because of its small size. The Aci I endonuclease did not cut the DNA of CAFA, BOTA, SUDO, RANO or CETH.
: polymerase chain reaction-restriction fragment length polymorphism on the DNA samples of interest amplified using the BM1 and BM2 primers and digested using Aci I, Alu I, Hae III and Rsa I (fragment of mitochondrial cytb ≅ 358 bp). L: DNA Leader 50 bp; 1: Aci I; 2: Alu I; 3: Hae III; 4: Rsa I.
When the DNA samples were digested with Alu I enzymes, the expected restriction fragments for SUDO (242 bp and 115 bp) and BOTA (190 bp, 114 bp and 53 bp) were confirmed. However, BOTA presented an additional nonspecific fragment (304 bp). No Alu I restriction site was found for DIMA, RANO, GAGA or HOSA. In addition to the expected fragments for EQCA and FECA, unexpected fragments were also observed: 242 bp and 115 bp for EQCA and 237 bp for FECA. CETH and CAFA presented bands of 190 and 167 bp, as expected (Fig. 2, Table II). As reported by other authors, nonspecific bands were also visualised in the present study (Zhang & Hewit 1996Zhang DX, Hewit GM 1996. Nuclear integrations: challenges for mitochondrial DNA markers. Trends Ecol Evol 11: 247-251., Partis et al. 2000Partis L, Croan D, Guo Z, Clark R, Coldham T, Murby J 2000. Evaluation of a DNA fingerprinting method for determining the species origin of meats. Meat Scie 54: 369-376., Steuber et al. 2005Steuber S, Abdel-Rady A, Clausen PH 2005. PCR-RFLP analysis: a promising technique for host species identification of blood meals from tsetse flies. Parasitol Res 97: 247-254.). One explanation for this phenomenon could be the co-amplification of cytb pseudogenes. Pseudogenes are non-functional copies of mtDNA fragments present in nuclear DNA. However, when the expected fragments are also viewed, identification of the species is not impaired.
Digestion with the Hae III endonuclease allowed differentiation between several species of vertebrates. GAGA and EQCA presented the same pattern of bands (159 bp, 124 bp and 74 bp); however, BOTA presented an extra fragment (290 bp). SUDO (130 bp, 153 bp and 74 bp) and HOSA (233 bp and 124 bp) showed the expected patterns. No Hae III restriction site was found for CAFA, CETH, DIMA or RANO. FECA presented both nonspecific and expected bands (Fig. 2, Table II).
Digestion with the Rsa I enzyme made it possible to differentiate CETH, RANO, GAGA and SUDO from the other host species. The restriction pattern for GAGA was as expected (208 bp and 149 bp). BOTA and DIMA presented single fragments of 322 bp and 326 bp, respectively. Fragments smaller than 31 bp were not visualised. Rsa I did not cut DNA extracted from HOSA, EQCA, SUDO, CETH or CAFA (Fig. 2, Table II).
The enzymes Aci I, Alu I, Hae III and Rsa I were sufficient for the differentiation of the species of interest, with the exception of CAFA and CETH, which presented the same restriction profile. A fifth enzyme or other genetic marker could be used to differentiate these two species. This differentiation is of great epidemiological interest because foxes and dogs share an important role as reservoirs of visceral leishmaniasis. As foxes have periurban habitats, this identification method could confirm whether foxes maintain the urban cycle (Costa & Vieira 2001Costa CHN, Vieira JBF 2001. Mudanças no controle da leishmaniose visceral no Brasil. Rev Soc Bras Med Trop 34: 223-228., Silva et al. 2001Silva VC, Gomes RBB, Barral A, Costa CHN 2001. ELISA não dife- rencia sangue de cão doméstico de sangue de raposa. Rev Soc Bras Med Trop 34: 757.).
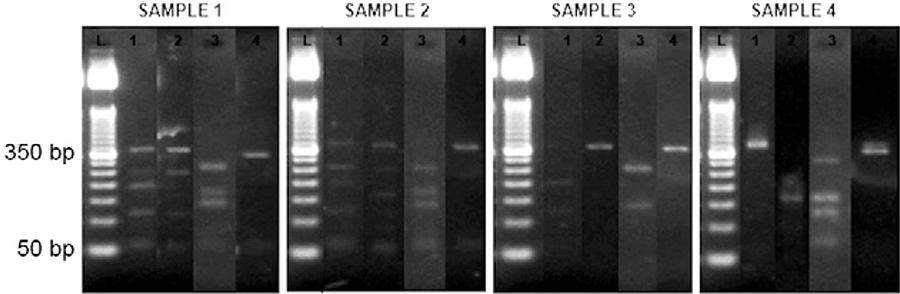
To verify whether PCR-RFLP of the cytb gene can be used to evaluate the blood meal source of sandflies, a total of 80 female specimens of Lu. longipalpis were caught in domestic and peridomestic environments using two electrically powered CDC light traps. DNA was extracted from the intestinal contents of sandflies and PCR amplification was performed using BM1 and BM2 primers. The 358 bp fragment of cytb was identified in 76 samples. Most of the sandflies had blood in their digestive tubes. Initially, this finding enabled analysis by means of sequencing or PCR-RFLP. Of the 76 samples, 10 were chosen randomly and their PCR products were individually digested using four restriction enzymes (Aci I, Alu I, Hae III and Rsa I). In four out of the 10 sandflies, the food sources were assumed (Fig. 3, Table III). The food sources of samples 1 and 2 were believed to be EQCA. The probable food sources of samples 3 and 4 were HOSA and BOTA, respectively. Some fragments could not be identified because of their size or due to the small amount of DNA present. It is possible that the low quantity DNA resulted in weak or even missing band signals, making it difficult to analyse the fragment profile. Theoretically, this technique could become less accurate if DNA was degraded via intestinal digestion (Mukabana et al. 2002aMukabana WR, Takken W, Killeen GF, Hawley WA, Knols BGJ 2002a. Extent of digestion affects success of amplifying human DNA from blood meals of Anopheles gambiae (Diptera: Culicidae). Bull Entomol Res 92: 233-239.). A detailed analysis of sandflies in the laboratory, using a known food source and performing measurements at specific time points, could resolve this issue.
: polymerase chain reaction-restriction fragment length polymorphism on the DNA samples from sandflies amplified using the BM1 and BM2 primers and digested using Aci I, Alu I, Hae III and Rsa I (fragment of mitochondrial cytb ≅ 358 bp). L: DNA Leader 50 bp; 1: Aci I; 2: Alu I; 3: Hae III; 4: Rsa I.
Therefore, the PCR-RFLP technique has the potential to be useful for phlebotomine blood meal source identification. However, some points must be clarified regarding the applicability of the method, such as the extent of DNA degradation through intestinal digestion, the potential for multiple sources of blood meals and the need for greater knowledge of intraspecific mtDNA variations.
Supplementary data
REFERENCES
- Boakye DA, Tang J, Truc P, Merriweather A, Unnasch TR 1999. Identification of blood meals in heamatophagous Diptera by cytochrome B heteroduplex analysis. Med Vet Entomol 13: 282-287.
- Chow-Shaffer E, Sina B, Hawley WA, de Benedicts J, Scott TW 2000. Laboratory and field evaluation of polymerase chain reaction-based forensic DNA profiling for use in identification of human blood meal sources of Aedes aegypti (Diptera: Culicidae). J Med Entomol 37: 492-502.
- Costa CHN, Courtenay O 2003. A new record of the hoary fox Pseudalopex vetulus in North Brazil. Mammalia 67: 593-594.
- Costa CHN, Vieira JBF 2001. Mudanças no controle da leishmaniose visceral no Brasil. Rev Soc Bras Med Trop 34: 223-228.
- Dias FOP, Lorosa ES, Rebêlo JMM 2003. Fonte alimentar sanguínea e a peridomiciliação de Lutzomyia longipalpis (Lutz & Neiva, 1912) (Psychodidae, Phlebotominae). Cad Saude Publica 19: 1373-1380.
- Garlapati RB, Abbasi I, Warburg A, Poché D, Poché R 2012. Identification of blood meals in wild caught blood fed Phlebotomus argentipes (Diptera: Psychodidae) using cytochrome B PCR and reverse line blotting in Bihar, India. J Med Entomol 49: 515-521.
- Lee JH, Hassan H, Hill G, Cupp EW, Higazi TB, Mitchell CJ, Godsey MS, Unnasch TR 2002. Identification of mosquito avian-derived blood meals by polymerase chain reaction-heteroduplex analysis. Am J Trop Med Hyg 66: 599-604.
- Marassá AM, Consales CA, Galati EAB, Nunes VLB 2006. Identificação do sangue ingerido por Lutzomyia (Lutzomyia) longipalpis (Lutz & Neiva, 1912) e Lutzomyia (Lutzomyia) almerioi (Galati & Nunes, 1999) pela técnica imunoenzimática do ELISA de captura no sistema avidina-biotina. Rev Soc Bras Med Trop 39: 183-186.
- Meece JK, Reynolds CE, Stockwell PJ, Jenson TA, Christensen JE, Reed KD 2005. Identification of mosquito blood meal source by terminal restriction fragment length polymorphism profile analysis of the cytochrome B gene. J Med Entomol 42: 657-667.
- Mukabana WR, Takken W, Killeen GF, Hawley WA, Knols BGJ 2002a. Extent of digestion affects success of amplifying human DNA from blood meals of Anopheles gambiae (Diptera: Culicidae). Bull Entomol Res 92: 233-239.
- Mukabana WR, Takken W, Knols BGJ 2002b. Analysis of arthropod blood meals using molecular genetic markers. Trends Parasitol 18: 505-509.
- Muturi CN, Ouma JO, Malele II, Ngure RM, Rutto JJ, Mithöfer KM, Enyaru J, Masiga DK 2011. Tracking the feeding patterns of tsetse flies (Glossina Genus) by analysis of blood meals using mitochondrial cytochromes genes. PLoS ONE 6: e17284.
- Partis L, Croan D, Guo Z, Clark R, Coldham T, Murby J 2000. Evaluation of a DNA fingerprinting method for determining the species origin of meats. Meat Scie 54: 369-376.
- Pettersson E, Bensch S, Ander M, Chirico J, Sigvald R, Ignell R 2013. Molecular identification of blood meals and species composition in Culicoides biting midges. Med Vet Entomol 27: 104-112.
- Ribeiro JMC 1999. Vector biology. In RL Guerrant, DH Walker, PF Weller (eds.), Tropical infectious diseases: principles, pathogens and practice, Churchill Livingstone, Philadelphia, p. 124-133.
- Silva VC, Gomes RBB, Barral A, Costa CHN 2001. ELISA não dife- rencia sangue de cão doméstico de sangue de raposa. Rev Soc Bras Med Trop 34: 757.
- Steuber S, Abdel-Rady A, Clausen PH 2005. PCR-RFLP analysis: a promising technique for host species identification of blood meals from tsetse flies. Parasitol Res 97: 247-254.
- Tiwananthagorn S, Bhutto AM, Baloch JH, Soomro FR, Kawamura Y, Nakao R, Aoshima K, Nonaka N, Oku Y, Katakura K 2012. Zoophilic feeding behaviour of phlebotomine sand flies in the endemic areas of cutaneous leishmaniasis of Sindh province, Pakistan. Parasitol Res 111: 125-133.
- Zhang DX, Hewit GM 1996. Nuclear integrations: challenges for mitochondrial DNA markers. Trends Ecol Evol 11: 247-251.
Publication Dates
-
Publication in this collection
07 May 2014 -
Date of issue
June 2014
History
-
Received
16 Aug 2013 -
Accepted
25 Feb 2014